Abstract
The ability of feed related measures to prevent or reduce post weaning diarrhoea (PWD) was examined in a split litter study including 30 pigs from 6 litters allotted into 5 groups. Four groups were exposed to 3 pathogenic strains of E. coli via the environment at weaning. Three of them were given zinc oxide, lactose+fibres or non-pathogenic strains of E. coli as probiotics. The challenged and the unchallenged control groups were given a standard creep feed. Diarrhoea was observed in all challenged groups but not among uninfected animals, and the incidence of diarrhoea was lower in the group given non-pathogenic E. coli compared to all other challenged groups. The severity of PWD also differed between litters. When corrected for mortality due to PWD, a decreased incidence of diarrhoea was also seen in the groups given zinc oxide or lactose+fibres. The dominating serotype of E. coli within faecal samples varied from day to day, also among diarrhoeic pigs, indicating that diarrhoea was not induced by one single serotype alone. The diversity of the faecal coliform populations decreased in all piglets during the first week post weaning, coinciding with an increased similarity between these populations among pigs in the challenged groups. This indicated an influence of the challenge strains, which ceased during the second week. The group given lactose+fibres was least affected with respect to these parameters. In conclusion feed related measures may alleviate symptoms of PWD.
Keywords: E. coli, lactose, zinc oxide, probiotic, prevention.
Introduction
Weaning is one of the most dangerous situations in the life of a pig and introduces a number of stress factors. Some of these may be of infectious origin, such as E. coli [12], rotavirus [42], Clostridium perfringens [8]. Other stressors are of non-infectious origin such as an abrupt separation from the sow and a sudden change of feed from sow milk to a cereal based creep feed. The latter also include withdrawal of the protective IgA that is secreted in milk [23] and act locally in the intestine of the piglets. Sometimes piglets are mixed at weaning, which will amplify the stress by fights that will last until a social rank is established [46]. Taken together, these stress factors may affect the immune functions negatively post weaning [4,2,40,51]. This will coincide in time with alterations of the intestinal population, in terms of a less diversified faecal coliform flora, which is induced by the weaning [26,30,32,22].
The drawbacks of weaning described above may contribute to outbreaks of post weaning diarrhoea (PWD), and such outbreaks are often related to infections with E. coli. However, many authors have suggested PWD to mirror a syndrome rather than a specific infection, because single infections/provocations have failed to induce PWD [44,13,50,37,29,28,32]. Consequently efforts aiming to reduce the negative impact of the weaning have been practised. The introduction of age segregated rearing systems that provides a good environment and a low pathogen load have been proven efficient in preventing PWD [29], and the importance of using relevant feeding systems to weaned piglets have been discussed [41].
High protein concentration enhances growth [10] and preheating of the feed facilitates feed utilization [11]. However, such a feed may also contribute to PWD in several ways. The protective influence of chewing and saliva is reduced. The gastric passage rate is increased and the feed has a high acid binding capacity, resulting in a decreased effect of hydrochloric acid and proteolytic enzymes [5,47]. Also individual ingredients, such as soya [18,36], have occasionally been proven provocative. Consequently, an interest has been paid to feed composition.
By adding pure lactose to the feed the abrupt switch of general energy source at weaning may be moderated and by using non-heated meal feed with extra dietary fibres the intestinal passage time may be prolonged [20]. Other efforts to prevent PWD have included admixture of ingredients that stabilise the intestinal flora around weaning. For instance high amounts of feed administered zinc oxide preserve the intestinal flora post weaning by preventing certain clones of bacteria to increase in number on behalf of other clones [22]. However, it should be noted that such administrations never should exceed 14 days due to the toxicity of zinc [19]. Also antibiotics may prevent PWD, but antibiotics as feed ingredients have been prohibited in Sweden since 1986. The European Communities (EC) has followed this example regarding 8 out of 12 permitted substances 1999 (Council directive 70/524/EEC on Feed additives) and the future of the remaining substances are to be discussed. Yet another strategy to prevent PWD has been to introduce non-pathogenic microorganisms, aiming to obstruct colonisation of pathogenic microorganism of indigenous or exotic origin by competition for nutrients and receptor sites [27,49].
The aim of the present study was to scrutinize the efficacy of some strategies aiming to prevent development of PWD in pigs exposed to pathogenic strains of E. coli in a way that previously had been proven to induce PWD. These strategies included feed composition, admixture of zinc oxide and administration of non-pathogenic bacteria.
Materials and methods
Animals, initial health status and experimental design
The animals originated from a conventional herd free from diseases according to the A-list of International office of epizootics, Aujeszky's disease, Atrophic rhinitis, Transmissible gastro-enteritis, Porcine epidemic diarrhoea, Porcine reproductive and respiratory syndrome, Brachyspira hyodysenteriae and Salmonellosis. To further reduce the pathogen load, sows were given antiparasitic treatment prior to farrowing (Ivomec® vet, MSD, Rahway, N J, USA). They were also vaccinated to prevent erysipelas and parvovirus (Nordpremum® Plus vet, Pharmacia & Upjohn Animal Health, Helsingborg, Sweden), as well as neonatal infections with E. coli in the offspring (Piliguard vet, Scanvet, Fredensborg, Denmark). No haemolytic strains of E. coli were found in the faeces from any of the 30 piglets one week before weaning.
The present study included 30 piglets, representing 6 litters (1 to 6) designated to 5 experimental groups (A to E) with 6 pigs at weaning. Each group included one pig from each litter of origin. Each animal was given a group, litter identification, i.e. pigs with the same letter were group mates, and pigs with the same number were littermates.
All groups were fed ad libitum through feeding automates (Piggomat, Skälby Maskin, Enkö-ping, Sweden). Group A was left as an uninfected control group and offered a preheated standard feed (Startgris Fiber, Lantmännen, Svalöv, Sweden). The other groups were exposed to 3 pathogenic strains of E. coli as described below. Group D was left as an infected control group, while group B was offered a feed with 2,500 ppm ZnO and group C was offered a non-heated meal feed with lactose and fibres (produced by Nibble, Tillberga, Sweden). Group E was also offered the standard feed, but each pig was given an oral dose with 106 colony forming units (CFU) of each of 60 defined non-pathogenic strains of E. coli 15 min prior to the challenge with pathogenic strains of E. coli (Table 1).
Table 1.
The experimental design of a study aiming to scrutinise the effect of different feed related prophylactic measures in pigs exposed to three pathogenic serotypes of E. coli via the environment. The pigs were weaned on living day 35.
| Exposed to | Feed | ||||||
| Group | Pathogenic E. coli | Non-pathogenic E. coli | Structure | Heat processed (75°C for 20 sek) | Protein (%) | ZnO (2500 ppm) | Lactose |
| A | - | - | Pelletedc | Yes | 15.5 | - | - |
| B | Yesa | - | Pelletedc | Yes | 15.5 | Yes | - |
| C | Yesa | - | Meald | - | 14.5 | - | Yes |
| D | Yesa | - | Pelletedc | Yes | 15.5 | - | - |
| E | Yes | Yesb | Pelletedc | Yes | 15.5 | - | - |
a) E. coli O147; K89, STb at the day of weaning;
E. coli O141; K85, STb, VT2 and E. coli O149; K91, K88, STa, STb, LT three days post weaning
b) A mixture of 106 CFU of each of 60 defined non pathogenic strains of E. coli given per os.
c) Startgris Fiber, Lantmännen, Svalöv, Sweden
d) Meal feed with lactose, dietary fibres and char cole (Nibble, Tillberga, Sweden)
When initiating the trial, the groups were housed in separated rooms at the National Veterinary Institute (NVI) with separated urine and manure handling. The rooms were free from draught, illuminated for 14 h per day and kept at 20°C. To prevent spread of E. coli (including probiotic strains) to previously not exposed pigs, the groups were always visited in alphabetical order. Boots and tools were designated to and kept within each room.
Inducement of post weaning diarrhoea
PWD was induced as briefly described below with a model earlier used [32,33]. At the day of weaning (living day 35) the animals were transported for 1 h in a joint closed horse trailer to the NVI. All but the control pigs were exposed to pathogenic strains of E. coli via the environment. One h before the arrival of the animals a broth with a pathogenic strain of E. coli (O147; K89, STb) was spread to a density of 2 × 106CFU per square meter on the floor of empty and previously disinfected pens. In the pen for the control group a sterile BHI-broth was used. One h after the arrival of the piglets, the pens were bedded with sawdust and the animals were given access to feed and water. Three days post weaning the animals were exposed a second time in the same way with a broth comprising both E. coli O141 (K85, STb, VT2) and E. coli O149 (K91, K88, STa, STb, LT).
The trial was terminated 14 days post weaning by sacrificing the animals. At that time the intestinal epithelium of all piglets was tested for presence of receptors to the adhesion factor F4/K88 post mortem [7]. All 3 E. coli strains used were previously tested positive for toxins using PCR-technique [32,33], and by loop tests [43] they were proven pathogenic [32,33].
Health status
The health status of the animals was inspected at least 3 times per day, with special attention to faecal consistency. If the consistency allowed a collected sample to adapt to the shape of any container it was characterised as "diarrhoea". A watery consistence was denoted "watery diarrhoea". These terms are separated in table 2, but the common term "diarrhoea" is used for both types of loose stool in the text. The results are expressed as number of pigs with diarrhoea (at any time) per group, and as number of pig days with diarrhoea per group. The latter corresponds to the sum of all days with diarrhoea per pig within group, and is also compared to total number of pig days at risk.
Table 2.
Results obtained from 6 control animals and 24 piglets exposed to three pathogenic strains of E. coli at weaning on living day 35. The different prophylactic regimes used in the in the study are described in Table 1. Demonstration of rotavirus and/or the challenge strains of E. coli in faeces are shown on daily bases. Days with diarrhoea are shaded.
| F4 | Sampling day (Day 0 = day of weaning) | |||||||||||||||
| Piglet | Receptor | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| A:1 | Neg | |||||||||||||||
| A:2 | Neg | |||||||||||||||
| A:3 | Pos | R | ||||||||||||||
| A:4 | Pos | |||||||||||||||
| A:5 | Pos | |||||||||||||||
| A:6 | Pos | |||||||||||||||
| B:1 | Pos | 12 | 7 | 9 | 9 | 9 | 9,1 | 1,9,7 | 1 | 1 | 1,7 | 7,1,9 | 1 | 1 | ||
| B:2 | Neg | 7 | 7 | 7 | 1,9 | 1,7 | 1,9,7 | 1 | 1 | 1,7 | 1 | 1 | 1 | |||
| B:3 | Pos | R | 7, R | 7, R | 7, R | 9,1,7 | 7,9 | 1 | 9,1,7 | 1,9 | 1 | 1,7 | 1 | 1 | ||
| B:4 | Pos | R | 7, R | 7, R | 7,1 | 7,1 | 9,7 | 7,1,9 | 7,1 | 1,7 | 1,7 | 1 | 1 | 1 | ||
| B:5 | Pos | 9 | 1 | 1 | 1 | 1 | 1,7,9 | 1 | 1 | 1,7,9 | 7 | |||||
| B:6 | Pos | 7 | 7 | 9 | 1,7,9 | 7,1 | 1,7 | 1,7,9 | 1,7 | 1 | 1 | 1 | 1 | R | ||
| C:1 | Neg | 7 | 7 | 1,7,9 | 1,7 | 1,7 | 7 | 7,1 | 1,7 | 7 | 7,1 | 7 | 7 | 7 | ||
| C:2 | Neg | 1,7,9 | 1,7,9 | 1 | 1 | 1 | 1 | 1,7 | 7,1 | 7 | 7,1 | 7 | ||||
| C:3 | Pos | R | R | R | 7 | 9 | 9 | 9 | 9 | 9 | 1,9 | 7,1 | 1 | 7,9 | 7 | R |
| C:4 | Pos | 7 | 7 | 7 | 1,7,9 | 1,7 | 1,7 | 1,7 | 9,1 | 1,7 | 1 | 1,7 | 1,7 | |||
| C:5 | Pos | 7 | 7 | 1,9 | 1,9 | 9,1 | 1,7 | 1,7,9 | 7,1,9 | 1,7 | 7 | 7,9 | 9,7 | |||
| C:6 | Pos | 7 | 7 | 7 | 1,7 | 1,7 | 1 | 1 | 1 | 1,7 | 7,1 | 7 | 7 | 7 | Dead | |
| D:1 | Pos | 7 | 9 | 9 | 9,1 | 9,7,1 | 7 | 7,1,9 | 7 | 7 | 7 | |||||
| D:2 | Neg | 7 | 7 | 9 | 1 | 1 | 1 | 1 | 1,7 | 1 | 7,1 | 7 | 7 | |||
| D:3 | Pos | 7 | 9,1 | 1,9 | 1,7,9 | 1,7 | 1,9 | 1,7 | 1,7,9 | 7,1 | 7 | 1,7 | ||||
| D:4 | Pos | R | R | 7, R | 9,1 | 7 | 1 | 1 | 1,7 | 7,1 | ||||||
| D:5 | Neg | 7 | 7 | 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | 9 | 9 | ||
| D:6 | Pos | R | 7 | 7 | 7 | 1,9,7 | 7,9 | -------------Piglet (D6) dead from day 6 post weaning------------- | ||||||||
| E:1 | Neg | 7 | 7 | 1,9 | 9,1 | 9,1 | 1,9 | 1 | 1 | 7,1 | 9 | 9 | ||||
| E:2 | Neg | 1 | 1,9 | 1 | 1 | 1 | 1 | 7,1 | ||||||||
| E:3 | Pos | 7 | 1 | 1,9 | 1,9 | 1 | 1 | 1 | 9 | |||||||
| E:4 | Pos | R | R | 9,1 | 9 | 9 | 9 | 1 | 1 | 1 | 9 | 1 | 1 | |||
| E:5 | Neg | 1,9 | 1,9 | 1 | 1 | 1 | 1 | 1,7,9 | 9 | 9 | 9 | 9 | ||||
| E:6 | Pos | 1 | 1 | 1 | 1,9 | 1 | 1 | 1 | 1 | 9,7 | ||||||
Diarrhoea: light grey = "diarrhoea"; dark grey = "watery diarrhoea".
1, 7 and 9 = presence of E. coli O141, O147 and O149 respectively. The most frequent serotype is given first. If bolded the challenge strains comprised more than 25 % of the total coliform flora.
R = presence of rotavirus.
Daily weight gain, feed intake and feed conversion
From 7 days before weaning the piglets were weighed once a week on an electronic scale (Epescale 1045, Alfa-Laval, Södertälje, Sweden) and the daily weight gains (DWG) were calculated as gram gained per day. The weight of given, as well as consumed, creep feed was noted. Feed conversion ratios were calculated as kg feed consumed per kg weight gained.
Sampling procedures
Rectal samples for microbial analyses were collected daily at 9 a.m. with cotton swabs and transported to the laboratory in Aimes transport medium (Copan Italia, Brescia, Italy). The presence of Brachyspira spp was investigated in all animals at weaning and on day 7 post weaning. The occurrence of Isospora suis was analysed on day 5 post weaning in faeces collected from the pen floor (3× 10 g per pen). All microbial analyses were initiated within 2 h after sampling with the exception of detection of rotavirus. These samples were stored at -20°C until analysed all at one single occasion. After the termination of the study the entire Ileum from all animals were stored in -80°C for analysis of Lawsonia intracellularis by PCR.
Detection of the challenge strains
Faecal samples were spread on blood agar plates (blood agar base No. 2; LabM, Salford, England + 5% horse blood) and incubated for 18 h at 37°C. No haemolytic strains of E. coli were determined prior to weaning why occurrence of β-haemolytic E. coli were denoted as potential isolates of the challenge strains. They were estimated as percentage of the total number of coliforms, and tested for presence of capsule antigen (n = 5 per pig and day) by agglutination with rabbit serum [45]. If positive (K85 = O141; K89 = O147; K91 = O149), they were considered as a reisolated challenge strain.
Detection of other pathogenic microorganisms
The presence of Brachyspira spp was investigated by culturing on Fastidious Anaerobe agar, (LabM LAB 90, Salford, England) for 6 days under anaerobic conditions at 37°C [9]. Rotavirus was detected by an ELISA demonstrating group A rotavirus antigen in faecal samples [6]. Isospora suis was analysed using a modified version of a flotation/McMaster technique [48] used in routine diagnostics at NVI. The presence of Lawsonia intracellularis was analysed by PCR [17].
Biochemical fingerprinting
Faecal samples were spread on MacConkey agar (Oxoid, Basingstoke, Hampshire, England) and incubated for 18 h at 37°C. From each sample 24 colonies of coliforms were picked randomly and inoculated on PhP-RE plates (Pheneplates®, PhPlate AB, Stockholm, Sweden). Each isolate was spread to 11 different substrates on a microtiter plate and the absorption values (A650) were measured with a photometer (Titertek Multiscan MCC/340, Labsystems OY, Helsinki, Finland) after 16, 40 and 64 h of incubation at 37°C. The ability to utilise the various substrates was compared and isolates showing similarity coefficients higher than 97.5 were regarded as identical [24] and assigned to the same biochemical phenotype (BPT).
The phenotypic diversity of the coliform populations was measured as Simpson's index of diversity [16]. Diversity is high (maximum value of 1) for a population constituting many different and evenly distributed BPTs and low (minimum value of 0) if one BPT is dominant. The mean diversities of the faecal coliform populations of each sampling occasion post weaning are presented as a percentage of each group mean value on the day of weaning.
The floras of different piglets were compared using "Population Similarity" as described by [25]. In this model the similarity, expressed as a SP- value, is high (maximum value of 1) if the 2 compared populations are identical and low (minimum value of 0) if they are totally different. Within each experimental group and sampling occasion all piglet floras were compared to each other giving a matrix of SP-values. From this matrix a mean SP-value for each group and sampling occasion was calculated. Further, within each group all isolates from each sampling occasion, i.e. days 3, 7, 10 and 14, were compared to the flora at weaning.
Statistical analyses
The significance of differences between groups or litters, respectively, was calculated with the Mann-Whitney U test. The significance of differences within groups or litters over time was determined by the Wilcoxon signed-rank test. The significance of differences regarding clinical signs between groups or litters, respectively, was calculated by χ2-tests.
Results
Reisolation of challenge strains
None of the pathogenic strains of E. coli used for challenge was found in any faecal sample collected prior to the study, or in any faecal sample collected from the control pigs. In contrast, pathogenic E. coli challenge strains were frequently isolated from all exposed pigs (Table 2). The distribution in faeces of these 3 challenge strains differed between experimental groups (Table 2). In group E, given a mixture of non-pathogenic E. coli strains prior to challenge, the proportion of E. coli O147 was lower (p < 0.001) and the extent of E. coli O141 higher (p < 0.001–0.05), compared to all other groups. Also the litter of origin influenced this distribution. In litter 2, where no animal expressed the F4 receptor in their jejunal epithelium (Tables 2 &3a), the shedding of O149 was low compared to litters 3, 4 and 5 (p < 0.001–0.05). On the contrary, the proportion of O141 in litter 2 was high compared to litters 3 and 4 (p < 0.01–0.05). The dominating serotype within each faecal sample varied from day to day, also among diarrhoeic pigs (Table 2).
Table 3a.
Incidence of diarrhea in one uninfected control group and four groups exposed to pathogenic serotypes of E. coli in connection with weaning (I). One infected group was left as an infected control group, while the other three groups were given feed related prophylactics (for details see Table 1). Comparisons with the infected control group are hatched. The table also shows (II) the incidence of diarrhoea in exposed pigs (n = 24) with respect to litter of origin (1–6). For both categories the presence of the F4-receptor in the intestine is given.
| Group/Litter | Ratio F4 Pos/Neg | Pigs | Days | |||||||||
| At risk | With diarrhea | At risk | With diarrhea | Significance of difference | ||||||||
| (n) | (n) | (%) | (n) | (n) | (%) | |||||||
| I) Group | B | C | D | E | ||||||||
| A: Uninfected control | 4/2 | 6 | 0 | 0% | 84 | 0 | 0% | *** | *** | *** | *** | |
| B: ZnO | 5/1 | 6 | 5 | 83% | 84 | 38 | 45% | ** | ||||
| C: Meal feed | 4/2 | 6 | 5 | 83% | 83 | 30 | 36% | * | ||||
| D: Infected control | 4/2 | 6 | 6 | 100% | 75 | 37 | 49% | ** | ||||
| E: Probiotic | 3/3 | 6 | 6 | 100% | 84 | 20 | 24% | |||||
| II) Litter (exposed pigs) | 2 | 3 | 4 | 5 | 6 | |||||||
| 1 | 2/2 | 4 | 4 | 100% | 56 | 24 | 43% | * | ||||
| 2 | 0/4 | 4 | 4 | 100% | 56 | 13 | 23% | * | ** | |||
| 3 | 4/0 | 4 | 4 | 100% | 56 | 25 | 45% | |||||
| 4 | 4/0 | 4 | 4 | 100% | 56 | 18 | 32% | * | ||||
| 5 | 2/2 | 4 | 3 | 75% | 56 | 20 | 36% | |||||
| 6 | 4/0 | 4 | 4 | 100% | 46 | 25 | 51% | |||||
Significant differences: * = p < 0.05; ** = p < 0.01 and *** = p < 0.001 (only shown at the row with the lowest group or litter number)
Rotavirus, Brachyspira spp, Lawsonia intracellularis and Isospora suis
As shown in Table 2, rotavirus was demonstrated on 20 sampling occasions in samples collected from 7 piglets representing all experimental groups. All except 2 of these samples were colleted during the first 4 days post weaning. These rotavirus positive piglets did all originate from litters 3, 4 and 5. Two additional piglets in litter 2 excreted rotavirus 7 days before weaning, but not after weaning. Neither Brachyspira spp, Lawsonia Intracellularis nor Isospora suis were detected in any sample collected.
Health status
All control pigs remained healthy throughout the study. Diarrhoea was recorded in 5 to 6 piglets in each group exposed to pathogenic E. coli (Tables 2 and 3).
When presented as pig days with diarrhoea, all groups exposed to pathogenic E. coli showed a higher incidence of diarrhoea (p < 0.001) than the uninfected control group (Tables 3a &3b). The incidence of diarrhoea in group E (given non-pathogenic strains of E. coli at weaning) was significantly (p < 0.05-0.01) lower when compared to all other groups exposed to the challenge strains (Table 3a). Also the onset of clinical signs was slower and milder in this group, with 5 pig days of diarrhoea during the first week post weaning (Table 2). The corresponding figures were: 22 days in Group B (p < 0.001) and 18 days in Groups C and D (p < 0.01).
As shown in Table 2, diarrhoea was seen in correlation to shed of rotavirus in 5 out of 7 rotavirus positive pigs (B3, B4, C3, D4 and E4). However, 4 of these 5 diarrhoeic piglets also shed E. coli O147. One animal in the control group (A3) excreted rotavirus on one occasion (at weaning), without any correlation to diarrhoea.
Another piglet (D6) was rotavirus positive at weaning, but did not show any clinical signs of diarrhoea at that time. E. coli O147 was demonstrated in faeces of that pig during day 1 to 3. From day 4 all 3 challenge strains were demonstrated and the pig developed diarrhoea. On day 6 post weaning it died in PWD. Another pig from the same litter (C6, offered the meal feed) also died due to PWD. This pig died on day 13 post weaning after having had PWD for 9 days (Table 2).
All but one of the challenged pigs expressed diarrhoea during the observation period). However, when the results were stratified according to litter the number of pig days with diarrhoea was lower in litter 2 (lacking the F4-receptor) than in litters 1, 3 (p < 0.05) and 6 (p < 0.01; Table 3a). The pigs that died due to PWD originated from litter 6 and would presumably have contributed to a higher number of days with diarrhoea in their groups if they had survived. In spite of this, litter 6 had the highest incidence of days with diarrhoea, 51% (Table 3a). This difference between litter 6 and all other groups was most evident during the second week post weaning (p < 0.05). When the results from litter 6 were excluded (Table 3b) a lower incidence of diarrhoea (p < 0,05) was revealed in groups B (ZnO) and C (meal feed) when compared to group D (infected control) during the second week post weaning.
Table 3b.
Group wise incidence of diarrhoea when the litter with mortality (litter 6) were excluded from the animals presented in table 3a. Comparisons with the infected control group are hatched.
| Group | Incidence of Days with Diarrhoea (%)and Significance of differences between groups | |||||||||||||||
| Day 1–7 | Day 8–14 | Whole period, Day 1–14 | ||||||||||||||
| (%) | B | C | D | E | (%) | B | C | D | E | (%) | B | C | D | E | ||
| A: Uninfected control | (n = 5) | 0% | *** | *** | *** | * | 0% | *** | *** | *** | *** | 0% | *** | *** | *** | *** |
| B: ZnO | (n = 5) | 43% | *** | 26% | * | 34% | p = 0.06 | |||||||||
| C: Meal feed | (n = 5) | 43% | ** | 23% | * | 33% | * | |||||||||
| D: Infected control | (n = 5) | 49% | *** | 51% | 50% | ** | ||||||||||
| E: Probiotic | (n = 5) | 11% | 43% | 27% | ||||||||||||
Significant differences: * = p < 0.05; ** = p < 0.01 and *** = p < 0.001. (only shown at the row with the lowest group or litter number)
DWG and Feed consumption
The highest DWG, both during the first (154 ± 73 gram per day) and the second (354 ± 39 gram per day) week post weaning was recorded in the uninfected Group A. In the slowest growing group (C) the corresponding figures were 29 ± 113 gram per day and 226 ± 213 gram per day respectively. The highest DWG among infected groups was seen in Group E, 136 ± 98 gram per day during the first week post weaning and 314 ± 104 gram per day during the second week post weaning.
The DWG reflected the feed consumption. During the first week post weaning the mean feed consumption ranged from 343 gram per pig and day in the group given meal feed (Group C) to 388 gram per pig in Groups A and E. During the second week post weaning the highest feed consumption was recorded in Group A (617 gram per day) and the lowest (524 gram per day) in the infected control group (D). No significant differences in DWG between experimental groups or between litters were recorded.
Biochemical fingerprinting
The mean diversities of the faecal coliform populations of each sampling occasion post weaning are presented as a percentage of the mean values obtained within group at the day of weaning (Fig. 1). The diversity of the faecal coliform population decreased in all piglets during the first week following weaning. However, the flora was less affected in group C (given a meal feed with lactose and fibres). During the second week post weaning the diversity in Group C continued to decrease slightly, thereby reaching a similar level as groups A, B and D which in turn had regained an increased diversity of the coliform flora during the second week post weaning. Group E (given non-pathogenic E. coli strains at weaning) developed a less diversified intestinal coliform flora during the first week post weaning, and remained at that level during the second week. (Fig. 1).
Figure 1.
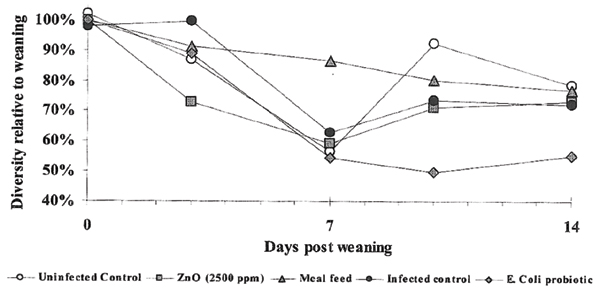
The diversity of the faecal coliform flora in one uninfected control group and four groups exposed to pathogenic serotypes of E. coli in connection with weaning. The results are presented as mean diversity values within group in relation to the mean diversity of that group at weaning.
A comparison of the coliform floras between the members within each experimental group and sampling occasion revealed a mean SP-value of around 0.1 (range 0.08 to 0.11) at weaning and mixing (Fig. 2). In all groups exposed to pathogenic strains of E. coli the mean SP value increased to a maximum level (0.24 to 0.46) on day 7 post weaning, indicating a more homologous flora within the groups at that time. Thereafter it decreased again to a level similar to that at weaning. This was obtained on day 14 post weaning (Fig. 2). In the uninfected group (Group A) the similarity within group was relatively constant over time.
Figure 2.
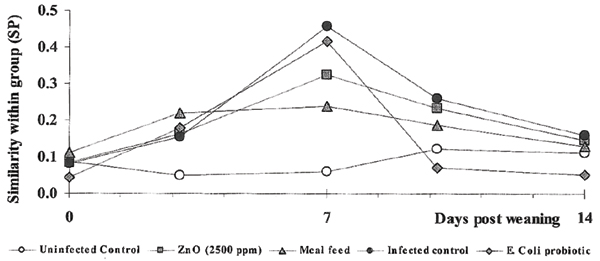
Similarity (SP) within group between the individual faecal coliform populations at each sampling occasion. The study comprises one uninfected control group and four groups exposed to pathogenic serotypes of E. coli in connection with weaning. One infected group was left as an infected control group, while the other three groups were given feed related prophylactics (for details see Table 1).
The total coliform population for each group sampling (i.e. all isolates from all piglets within the group taken together) was compared to the total coliform population of that group at weaning. Overall a decreasing similarity to the flora at weaning was seen with time (Fig. 3). This indicates development of an altered intestinal coliform flora following weaning.
Figure 3.
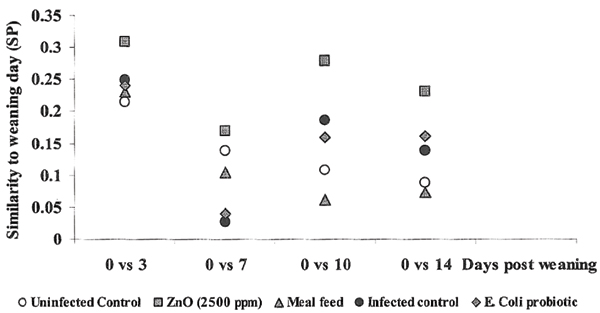
Similarity (SP) between the total faecal coliform population of each group and sampling occasion compared to the total coliform population of that group at weaning. The study comprises one uninfected control group and four groups exposed to pathogenic serotypes of E. coli in connection with weaning. One infected group was left as an infected control group, while the other three groups were given feed related prophylactics (for details see Table 1).
Discussion
Diarrhoea was detected in all groups exposed to pathogenic strains of E. coli but not in the control group. This confirmed earlier observations that the present combination of 3 pathogenic strains of E. coli can induce PWD [33]. Interestingly, and as found before [37,21,34], the dominating serotype within each animal varied from day to day (Table 2), indicating that a diarrhoeic pig does not necessarily excrete one single or a even dominant serotype of E. coli throughout a session of diarrhoea. Also concurring earlier observations [34], a genetic predisposition to develop PWD was indicated as the number of days with diarrhoea varied significantly between litters, and as both pigs that died emanated from the most affected litter (litter 6). In this context it was also notable that pigs from litter 2 (that lacked the F4-recepteor) were not fully protected against PWD. Still, this litter expressed fewest pig days with diarrhoea, and E. coli O149 (F4+) was only demonstrated occasionally during the first 3 days post exposure to that strain (performed on day 3 post weaning; Tables 1 &2).
The negative impact of PWD was indicated by a higher DWG in the healthy control group. However, due to the low number of pigs and to large variations in initial weight, differences in weight gain and feed intake were not significant. In spite of the fact that PWD was induced in all challenged groups, many of the parameters measured varied between different feeding regimes, as discussed below.
The diversity in the faecal coliform flora decreased in all groups, including the unexposed control group, during the first week post weaning. This effect of weaning has previously been observed in healthy as well as in diarrhoeic pigs [26,21,22,31-33]. In the pigs exposed to pathogenic strains of E. coli this disturbance may last long [32], probably due to a continuous influence of those strains. In the present study the diversity was somewhat restored in all but one group at the end of the second week post weaning. Group E (that was given non-pathogenic strains of E. coli at weaning) conserved a low diversity among the coliform intestinal flora throughout the study. This phenomenon may be associated to an initially good colonisation of at least some of the strains of E. coli given as probiotic at weaning as indicated by the increasing similarity (SP-values) between the faecal coliform populations of the pigs in this group during the first week post weaning (Fig. 2). The decrease in similarity between the faecal populations of the pigs during the subsequent week may indicate that these probiotic strains fade away. However, it may also actually indicate a good and evenly distributed colonisation of the probiotic strains, which ought to be further evaluated (the 60 probiotic strains outnumber the 24 CFU collected per pig and sampling occasion for biochemical fingerprinting; 144 CFU were analysed per group and day). Still, that might be of less importance as also the similarity between the intestinal coliform populations between pigs in the infected control group ceased, indicating a decreased influence of the challenge strains with time.
The use of non-pathogenic strains of a disease provoking bacteria, both as a prophylaxis and as treatment of infections, has earlier been proven useful [52,35,3]. Enteropathogenic strains of E. coli are dependent on receptor specific adhesions. Therefore, a competitive inhibition of these receptor sites by non-pathogenic strains of E. coli could decrease adhesion of pathogenic strains of E. coli and thereby prevent or reduce PWD. Group E was given a defined mixture of 60 strains of non-pathogenic E. coli at weaning. Interestingly, the incidence of diarrhoea was significantly lower in this group compared to all other challenged groups (Tables 2 &3b). This was most obvious during the first week post weaning (Tables 2, 3a &3b). The proportion of E. coli O147 was also lower than in the other infected groups.
Feed supplemented with 2500 ppm ZnO has previously been shown to prevent or decrease the severity of PWD [14,15]. [22] reported a less affected diversity of the coliform flora post weaning and an improved DWG in healthy piglets given ZnO supplemented feed for 2 weeks post weaning. A high diversity is suggested to indicate a stabile ecosystem [39] and a stabile microflora is considered to have a higher colonisation resistance [1]. In this study, however, the ZnO treated group did not differ from the infected control group with respect to diversity of the coliform flora post weaning, nor with respect to pig days with diarrhoea. However, it ought to be remembered that the pig from the most affected litter of the infected control group already died on day 6 post weaning due to PWD. That death probably decreased the overall percentage of pig days with diarrhoea in the infected control group, since that pig probably would have expressed diarrhoea for more days if it had survived (Table 2). If the results from litter 6 were excluded (Table 3b) the incidence of diarrhoea was lower in Group B compared to the infected control (Group D) during the second week post weaning.
Heat-processing of the feed increases the passage rate through the digestive channel, which in turn will reduce the effect of protective elements such as digestive enzymes and hydrochloric acid. This may result in a large number of ingested microorganisms entering the intestine in a viable form and thereby increasing the risk for infections with pathogenic microorganisms. A non-heated meal feed rich in dietary fibres would instead prolong the gastric passage [20]. Group C was given such a feed (enriched with lactose) and the decline in diversity of the faecal coliform flora was less pronounced in this group with no accentuated dip on day 7, which indicates that the flora was less dominated by the challenge strains used (Fig. 1). Also the comparably low similarity between the coliform floras of different piglets in this group at each sampling occasion indicates that the pigs managed to reduce the external influence on their intestinal flora (Fig. 2). The incidence of diarrhoea was lower (p < 0.05-0.01) than in the infected control group when the results were corrected for mortality (i.e. when litter 6 was excluded; Table 3b). Regarding the comparably low DWG in Group C (especially during the first week post weaning), respect should be paid to the fact that the pigs were offered pellets during suckling and that they consumed fairly little of the non-heated meal feed. This feed also contained a lower amount of protein than the standard diet given to the other groups.
This study confirmed that the post weaning period is dangerous, and our results further suggest that the intestinal floras that develop post weaning with time will differ completely from those present before weaning (Fig. 3), as earlier proven by [21]. However, many strains of E. coli could be regarded as intestinal transients [26,37,21], which further points to the complex interaction between different microorganisms in the intestine. Efforts must therefore always be undertaken to facilitate the life for piglets at weaning. These measures include a good environment, a low pathogen load, and the results obtained in this study indicate the importance of well-designed creep feeds. The different feeding regimes and the use of good colonisers of non-pathogenic strains of E. coli appeared able to influence the intestinal coliform microflora of piglets following weaning and thereby influence the severity of infections gained at that time.
It is possible that the results obtained would have been different if for instance the probiotic strains of E. coli had been administered repeatedly or well before weaning, as well as if the pigs offered the non-heated meal feed with lactose had been adapted to that food before weaning. However, the design of the study prioritized genotype (litter of origin) and phenotype (conditions during the suckling period), factors of importance to the health status. Further it must be noted that we managed to standardise some important factors that could contribute to development of PWD, such as temperature, draught, and also had a low general pathogen load in this experiment. The efficacy of the measures studied in practise must therefore be further evaluated, and the results obtained may well differ between different herds due to unspecific management errors.
Acknowledgments
Acknowledgements
We thank Sigbrit Mattsson for the skilful work concerning all analyses regarding the coliform populations, Helena Reineck-Bosaeus for the detection of rotavirus, Ulla Zimmerman for the detection of Brachyspira, Brittlouise Ljungström for the analyses regarding Isospora suis and Dr Inger Edfors-Lilja for the investigation concerning presence of F4 receptors on the jejunal epithelium. This study was financed by grants from the Swedish Council of Forestry and Agricultural Research and from The Swedish Meat producing Farmers R&D Program.
References
- Atlas RM. Diversity of microbial communities. Adv Microbial Ecol. 1984;7:1–47. [Google Scholar]
- Bailey M, Clarke CJ, Wilson AD, Williams NA, Stokes CR. Depressed potential for interleukin-2 production following early weaning of piglets. Vet Immunol Immunopathol. 1992;34:197–207. doi: 10.1016/0165-2427(92)90164-L. [DOI] [PubMed] [Google Scholar]
- Barrow PA, Page K. Inhibition of colonisation of the alimentary tract in young chickens with Campylobacter jejuni by pre-colonisation with strains of C. jejuni. FEMS Microbiol Letters. 2000;182:87–91. doi: 10.1111/j.1574-6968.2000.tb08879.x. [DOI] [PubMed] [Google Scholar]
- Blecha F, Pollmann DS, Nichols DA. Weaning pigs at an early age decreases cellular immunity. J Animal Sci. 1983;56:396–400. doi: 10.2527/jas1983.562396x. [DOI] [PubMed] [Google Scholar]
- Bolduan G. Acid-binding capacity of pig feeds. Muhle + Mischfuttertechnik. 1992;129:87–89. [Google Scholar]
- de Verdier Klingenberg K, Esfandiari J. Evaluation of a one-step test for rapid, in practice detection of rotavirus in farm animals. Vet Rec. 1996;138:393–395. doi: 10.1136/vr.138.16.393. [DOI] [PubMed] [Google Scholar]
- Edfors-Lilja I, Gustafsson U, Duval-Iflah Y, Ellergren H, Johansson M, Juneja RK, Marklund L, Andersson L. The porcine intestinal receptor for Escherichia coli K88ab, K88ac: regional localization on chromosome 13 and influence of IgG response to the K88 antigen. Anim Genet. 1995;26:237–242. doi: 10.1111/j.1365-2052.1995.tb03250.x. [DOI] [PubMed] [Google Scholar]
- Estrada Correa A, Taylor DJ. Enterotoxigenic Clostridium perfringens type A as a cause of diarrhoea in weaned pigs. Proc Int Pig Vet Soc Rio de Janeiro, Brazil. 1988;10:138. [Google Scholar]
- Fellström C, Petterson B, Uhlén M, Gunnarsson A, Johansson K-E. Phylogeny of Serpulina based on sequence analyses of the 16S rRNA gene and comparison with a scheme involving biochemical classification. Res Vet Sci. 1995;59:5–9. doi: 10.1016/0034-5288(95)90022-5. [DOI] [PubMed] [Google Scholar]
- Gracia MI, Medel P, Castellanos I, Mateos GG. Effect of replacement of fish meal LT by a soya protein concentrate on productivity of early weaned pigs. VIII jornadas sobre prod animal. 1999;20:448–450. [Google Scholar]
- Graham H, Fadel JG, Newman CW, Newman RK. Effect of pelleting and beta-glucanase supplementation on the ileal and fecal digestibility of a barley-based diet in the pig. J Anim Sci. 1989;67:1293–1298. doi: 10.2527/jas1989.6751293x. [DOI] [PubMed] [Google Scholar]
- Hampson DJ. In: Postweaning Escherichia coli Diarrhoea in Pigs. in Escherichia coli in Domestic Animals and Humans. Gyles CL, editor. CABI Int., Walingford; 1994. pp. 171–191. [Google Scholar]
- Hampson DJ, Hinton M, Kidder DE. Coliform numbers in the stomach and small intestine of healthy pigs following weaning at three weeks of age. J Comp Pathol. 1985;95:353–362. doi: 10.1016/0021-9975(85)90039-8. [DOI] [PubMed] [Google Scholar]
- Holm A. E. coli associated diarrhoea in weaner pigs: zinc oxide added to the feed as a preventive measure. Proc Int Pig Vet Soc, Lausanne, Switzerland. 1990;11:154. [Google Scholar]
- Holm A. Zinkkoncentration i vaev hos slagtesvin. Tillsaetning av zinkoxid till foder. (Zinc concentration in pigs at slaughter. Effect of creep feeding with high levels of zinc oxide) Dan Vet Tidsskr. 1993;76:10–11. (In Danish) [Google Scholar]
- Hunter PR, Gaston MA. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J Clin Microbiol. 1988;26:2465–2466. doi: 10.1128/jcm.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M, Fellström C, Heldtander M, Gunnarsson A. The prevalence of Lawsonia intracellularis in Swedish pigs submitted for autopsy. Proc Int Pig Vet Soc Congr Melbourne, Australia. 2000;16:74. [Google Scholar]
- Jager LP, Zijlstra FJ, Hoogendoorn A, Nabuurs MJ. Enteropooling in piglets induced by soya-peptone mediated via an increased biosynthesis of prostanoids. Vet Res Commun. 1986;10:407–412. doi: 10.1007/BF02214003. [DOI] [PubMed] [Google Scholar]
- Jensen-Waern M, Melin L, Lindberg R, Johannisson A, Petersson L, Wallgren P. Dietary zinc oxide in weaned pigs-effects on performance, tissue concentrations, morphology, neutrophil functions and faecal microflora. Res Vet Sci. 1998;64:225–231. doi: 10.1016/S0034-5288(98)90130-8. [DOI] [PubMed] [Google Scholar]
- Johansen HN, Bach Knudsen KE. Effects of reducing the starch content in oat-based diets with cellulose on jejunal flow and absorption of glucose over an isolated loop of jejunum in pigs. B J Nutr. 1994;72:717–729. doi: 10.1079/BJN19940074. [DOI] [PubMed] [Google Scholar]
- Katouli M, Lund A, Wallgren P, Kühn I, Söderlind O, Möllby R. Phenotypic Characterization of Intestinal Escherichia coli of Pigs during Suckling Post-weaning and Fattening Periods. Appl Environ Microbiol. 1995;61:778–783. doi: 10.1128/aem.61.2.778-783.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katouli M, Melin L, Jensen-Waern M, Wallgren P, Möllby R. The effect of zinc oxide supplementation on the stability of the intestinal flora with special reference to composition of coliforms in weaned pigs. J Appl Microbiol. 1999;87:564–573. doi: 10.1046/j.1365-2672.1999.00853.x. [DOI] [PubMed] [Google Scholar]
- Klobasa F, Werhahn E, Butler JE. Regulation of humoral immunity in the piglet by immunoglobulin of maternal origin. Res Vet Sci. 1981;31:195–206. [PubMed] [Google Scholar]
- Kühn I. Biochemical fingerprinting of Escherichia coli. a simple method for epidemiological investigations. J Microbiol Methods. 1985;3:159–170. doi: 10.1016/0167-7012(85)90043-0. [DOI] [Google Scholar]
- Kühn I, Allestam G, Stenström TA, Möllby R. Biochemical fingerprinting of water coliform bacteria, a new method for measuring phenotypic diversity and for comparing different bacterial populations. Appl Environ Microbiol. 1991;57:3171–3177. doi: 10.1128/aem.57.11.3171-3177.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn I, Katouli M, Lund A, Wallgren P, Möllby R. Phenotypic diversity and stability of the intestinal coliform flora in piglets during the first 3 months of age. Microbial Ecol Health Disease. 1993;6:101–107. [Google Scholar]
- Kyriakis SC, Tsiloyiannis VK, Vlemmas J, Sarris K, Tsinas AC, Alexopoulos C, Jansegers L. The effect of probiotic LSP 122 on the control of post-weaning diarrhoea syndrome of piglets. Res Vet Sci. 1999;67:223–228. doi: 10.1053/rvsc.1999.0308. [DOI] [PubMed] [Google Scholar]
- Madec F, Bridoux N, Bounaix S, Cariolet R, Duval Iflah Y, Hampson DJ, Jestin A. Experimental models of porcine post-weaning colibacillosis and their relationship to post-weaning diarrhoea and digestive disorders as encountered in the field. Vet Microbiol. 2000;72:295–310. doi: 10.1016/S0378-1135(99)00202-3. [DOI] [PubMed] [Google Scholar]
- Madec F, Bridoux N, Bounaix S, Jestin A. Measurement of digestive disorders in the piglet at weaning and related risk factors. Prev Vet Med. 1998;35:53–72. doi: 10.1016/S0167-5877(97)00057-3. [DOI] [PubMed] [Google Scholar]
- Melin L, Jensen-Waern M, Johannisson A, Ederoth M, Katouli M, Wallgren P. Development of Selected Faecal Microfloras and of Phagocytic and Killing Capacity of Neutrophils in Young Pigs. Vet Microbiol. 1997;54:287–300. doi: 10.1016/S0378-1135(96)01286-2. [DOI] [PubMed] [Google Scholar]
- Melin L, Katouli M, Jensen-Waern M, Wallgren P. The influence of zincoxide of the intestinal microflora of piglets at weaning. Proc Int Pig Vet Soc Congr Bologna, Italy. 1996;14:465. [Google Scholar]
- Melin L, Katouli M, Lindberg A, Fossum C, Wallgren P. Weaning of piglets. Effects of an exposure to a pathogenic strain of Escherichia coli. J Vet Med B. 2000;47:663–675. doi: 10.1046/j.1439-0450.2000.00393.x. [DOI] [PubMed] [Google Scholar]
- Melin L, Mattsson S, Wallgren P. Challenge with pathogenic strains of E. coli at weaning (I. Clinical signs and reisolation of challenge strains) Proc Int Pig Vet Soc Congr Melbourne, Australia. 2000;16:22. [Google Scholar]
- Melin L. Thesis. Department of Large Animal Clinical Sciences, Swedish University of Agricultural Sciences, Uppsala; 2001. Weaning of Pigs with Special Focus on the Intestinal Health. [Google Scholar]
- Methner U, Barrow PA, Berndt A, Steinbach G. Combination of vaccination and competitive exclusion to prevent Salmonella colonization in chickens: experimental studies. Int J Food Microbiol. 1999;49:1–2. doi: 10.1016/S0168-1605(99)00051-3. [DOI] [PubMed] [Google Scholar]
- Nabuurs MJA. Thermostable factor(s) in soya producing a net excess of secretion in the ligated gut test in pigs. Vet Res Commun. 1986;10:399–405. doi: 10.1007/BF02214002. [DOI] [PubMed] [Google Scholar]
- Nabuurs MJ, van Zijderveld FG, de Leeuw PW. Clinical and microbiological field studies in The Netherlands of diarrhoea in pigs at weaning. Res Vet Sci. 1993;55:70–77. doi: 10.1016/0034-5288(93)90037-g. [DOI] [PubMed] [Google Scholar]
- Pielou EC. Ecological Diversity. Wiley Interscience, New York; 1975. pp. 129–135. [Google Scholar]
- Poulsen HD. Zinc oxide for weanling piglets. Acta Agri Scand Section A, Animal Science. 1995;45:159–167. [Google Scholar]
- Puppe B, Tuchscherer M, Tuchscherer A, Vega Lopez MA, Bailey M, Telemo E, Stokes CR, Bosi P. The effect of housing conditions and social environment immediately after weaning on the agonistic behaviour, neutrophil/lymphocyte ratio, and plasma glucose level in pigs Effect of early weaning on the development of immune cells in the pig small intestine Modulation of immune response and barrier function in the piglet gut by dietary means. Livestock Prod Sci. 1997;48:157–164. doi: 10.1016/S0301-6226(97)00006-7. [DOI] [Google Scholar]
- Rantzer D. Thesis. Department of Agricultural Biosystems and 0Technology, Swedish University of Agricultural Sciences, Alnarp; 1997. Weaning of Pigs. Exocrine pancreas secretion and the influence of preweaning housing and postweaning strategic feeding. [Google Scholar]
- Saif LJ, Rosen BI, Parwani AV. Animal Rotaviruses in Viral Infections of the Gastrointestinal Tract, Marcel Dekker, Inc. 1994. pp. 335–367.
- Smith HW, Halls S. Observations by the ligated intestinal segment and oral inoculation methods on Escherichia coli infections in pigs, calves, lambs and rabbits. J Pathol Bacteriol. 1967;93:499–529. doi: 10.1002/path.1700930211. [DOI] [PubMed] [Google Scholar]
- Smith HW, Jones JET. Observations on the alimentary tract and its bacterial flora in healthy and diseased pigs. J Pathol Bacteriol. 1963;86:387–412. doi: 10.1002/path.1700860214. [DOI] [PubMed] [Google Scholar]
- Söderlind O. Studies on Escherichia coli in pigs. II Serological investigations Zentralbl Veterinarmed [B] 1971;18:569–590. doi: 10.1111/j.1439-0450.1971.tb01661.x. [DOI] [PubMed] [Google Scholar]
- Spencer BT, Howell PG, Hillman K, Murdoch TA, Spencer RJ, Stewart CS. Some husbandry factors influencing weaning stresses in piglets. J S Afr Vet Assoc. 1989;60:62–64. [PubMed] [Google Scholar]
- Spencer BT, Tilley TJ, Poomvises P, Ingkaninun P. Low acid binding feed for weaner piglets. Proc Int Pig Vet Soc Congr Bangkok, Thailand. 1994;13:279. [Google Scholar]
- Thienpont D, Rochette F, van Parijs OFJ. Diagnosing Helmenthiasis through corpological examination. Jansen Research Foundation, Beerse, Belgium; 1979. pp. 40–41. [Google Scholar]
- Underdahl NR. The effect of feeding Streptococcus faecium upon Escherichia coli induced diarrhea in gnotobiotic pigs. Prog Food Nutr Sci. 1983;7:5–12. [PubMed] [Google Scholar]
- Wathes CM, Miller BG, Bourne FJ. Cold stress and post-weaning diarrhea in piglets inoculated orally or by aerosol. Anim Prod. 1989;49:483–496. [Google Scholar]
- Wattrang E, Wallgren P, Lindberg Å, Fossum C. Signs of infections and reduced immune functions at weaning of conventionally reared and specific pathogen free pigs. Zentralbl Veterinarmed [B] 1998;45:7–17. doi: 10.1111/j.1439-0450.1998.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Winberg J, Herthelius-Elman M, Möllby R, Nord CE. Pathogenesis of urinary tract infection-experimental studies of vaginal resistance to colonization. Pediatr Nephrol. 1993;7:509–514. doi: 10.1007/BF00852528. [DOI] [PubMed] [Google Scholar]


