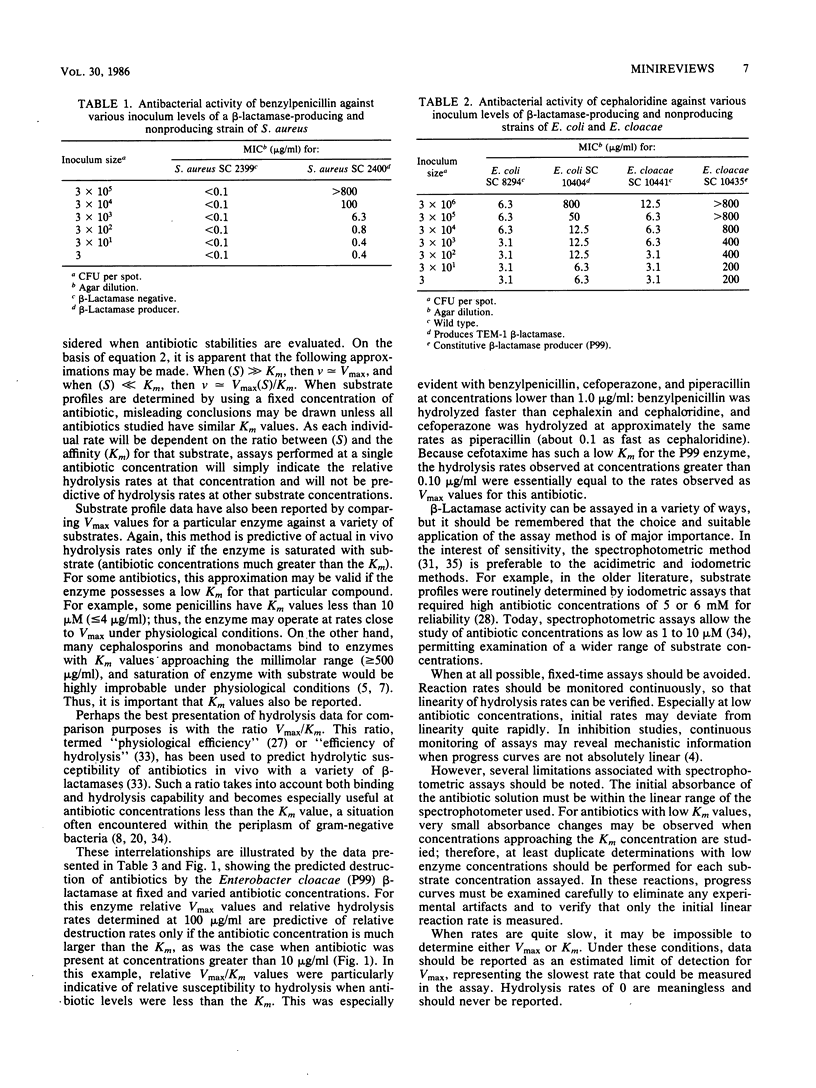
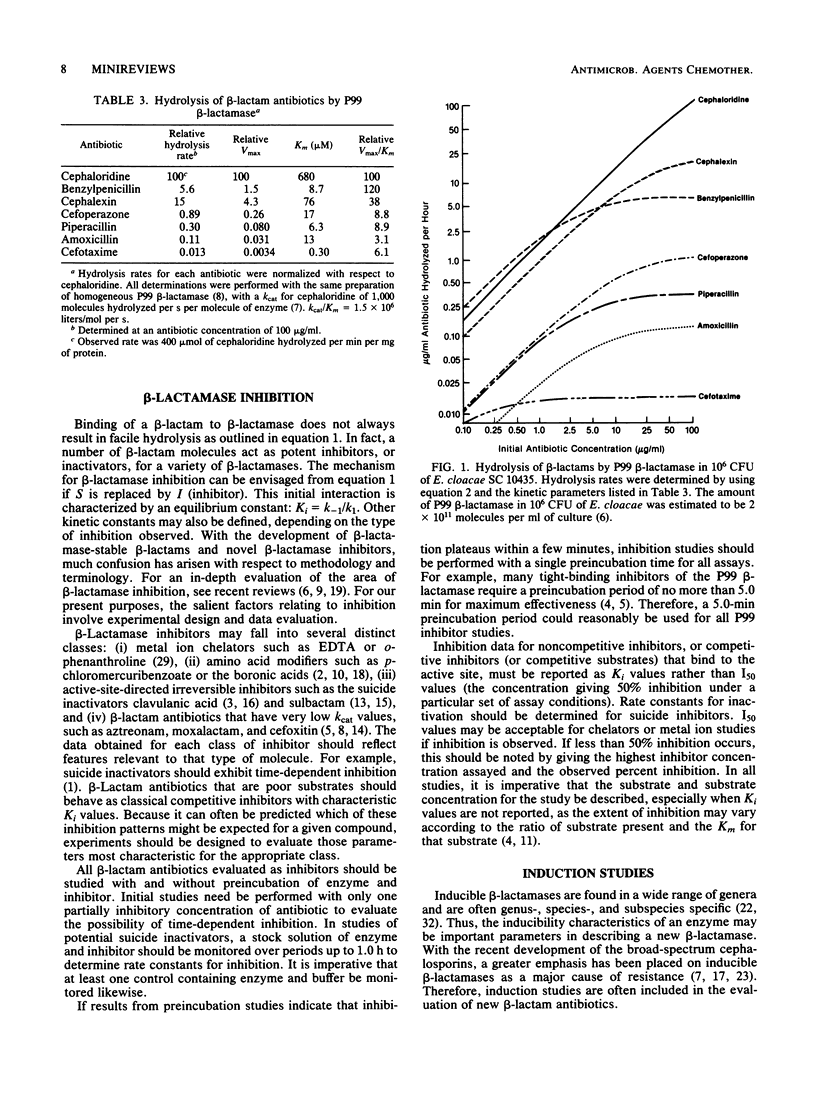
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beesley T., Gascoyne N., Knott-Hunziker V., Petursson S., Waley S. G., Jaurin B., Grundström T. The inhibition of class C beta-lactamases by boronic acids. Biochem J. 1983 Jan 1;209(1):229–233. doi: 10.1042/bj2090229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Butterworth D., Cole M., Hanscomb G., Hood J. D., Reading C., Rolinson G. N. Naturally-occurring beta-lactamase inhibitors with antibacterial activity. J Antibiot (Tokyo) 1976 Jun;29(6):668–669. doi: 10.7164/antibiotics.29.668. [DOI] [PubMed] [Google Scholar]
- Bush K., Freudenberger J. S., Sykes R. B. Interaction of azthreonam and related monobactams with beta-lactamases from gram-negative bacteria. Antimicrob Agents Chemother. 1982 Sep;22(3):414–420. doi: 10.1128/aac.22.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K. Screening and characterization of enzyme inhibitors as drug candidates. Drug Metab Rev. 1983;14(4):689–708. doi: 10.3109/03602538308991405. [DOI] [PubMed] [Google Scholar]
- Bush K., Sykes R. B. beta-Lactamase inhibitors in perspective. J Antimicrob Chemother. 1983 Feb;11(2):97–107. doi: 10.1093/jac/11.2.97. [DOI] [PubMed] [Google Scholar]
- Bush K., Tanaka S. K., Bonner D. P., Sykes R. B. Resistance caused by decreased penetration of beta-lactam antibiotics into Enterobacter cloacae. Antimicrob Agents Chemother. 1985 Apr;27(4):555–560. doi: 10.1128/aac.27.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright S. J., Waley S. G. Purification of beta-lactamases by affinity chromatography on phenylboronic acid-agarose. Biochem J. 1984 Jul 15;221(2):505–512. doi: 10.1042/bj2210505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright S. J., Waley S. G. beta-Lactamase inhibitors. Med Res Rev. 1983 Oct-Dec;3(4):341–382. doi: 10.1002/med.2610030402. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English A. R., Retsema J. A., Girard A. E., Lynch J. E., Barth W. E. CP-45,899, a beta-lactamase inhibitor that extends the antibacterial spectrum of beta-lactams: initial bacteriological characterization. Antimicrob Agents Chemother. 1978 Sep;14(3):414–419. doi: 10.1128/aac.14.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J., Belasco J. G., Khosla S., Knowles J. R. beta-Lactamase proceeds via an acyl-enzyme intermediate. Interaction of the Escherichia coli RTEM enzyme with cefoxitin. Biochemistry. 1980 Jun 24;19(13):2895–2901. doi: 10.1021/bi00554a012. [DOI] [PubMed] [Google Scholar]
- Fisher J., Charnas R. L., Bradley S. M., Knowles J. R. Inactivation of the RTEM beta-lactamase from Escherichia coli. Interaction of penam sulfones with enzyme. Biochemistry. 1981 May 12;20(10):2726–2731. doi: 10.1021/bi00513a004. [DOI] [PubMed] [Google Scholar]
- Fisher J., Charnas R. L., Knowles J. R. Kinetic studies on the inactivation of Escherichia coli RTEM beta-lactamase by clavulanic acid. Biochemistry. 1978 May 30;17(11):2180–2184. doi: 10.1021/bi00604a024. [DOI] [PubMed] [Google Scholar]
- Gootz T. D., Sanders C. C. Characterization of beta-lactamase induction in Enterobacter cloacae. Antimicrob Agents Chemother. 1983 Jan;23(1):91–97. doi: 10.1128/aac.23.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack G. W., Richmond M. H. A comparative study of eight distinct beta-lactamases synthesized by gram-negative bacteria. J Gen Microbiol. 1970 Apr;61(1):43–61. doi: 10.1099/00221287-61-1-43. [DOI] [PubMed] [Google Scholar]
- Livermore D. M. Do beta-lactamases 'trap' cephalosporins? J Antimicrob Chemother. 1985 May;15(5):511–514. doi: 10.1093/jac/15.5.511. [DOI] [PubMed] [Google Scholar]
- Matthew M., Harris A. M. Identification of beta-lactamases by analytical isoelectric focusing: correlation with bacterial taxonomy. J Gen Microbiol. 1976 May;94(1):55–67. doi: 10.1099/00221287-94-1-55. [DOI] [PubMed] [Google Scholar]
- Minami S., Matsubara N., Yotsuji A., Araki H., Watanabe Y., Yasuda T., Saikawa I., Mitsuhashi S. Induction of cephalosporinase production by various penicillins in enterobacteriaceae. J Antibiot (Tokyo) 1983 Oct;36(10):1387–1395. doi: 10.7164/antibiotics.36.1387. [DOI] [PubMed] [Google Scholar]
- Nordström K., Sykes R. B. Induction kinetics of beta-lactamase biosynthesis in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1974 Dec;6(6):734–740. doi: 10.1128/aac.6.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonogi K., Kuno M., Higashide E. Induction of beta-lactamase in Proteus vulgaris. J Gen Microbiol. 1986 Jan;132(1):143–150. doi: 10.1099/00221287-132-1-143. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R. PURIFICATION AND PROPERTIES OF PENICILLINASES FROM TWO STRAINS OF BACILLUS LICHENIFORMIS: A CHEMICAL, PHYSICOCHEMICAL AND PHYSIOLOGICAL COMPARISON. Biochem J. 1965 Mar;94:666–675. doi: 10.1042/bj0940666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. W., Chanter K. V., Harris A. M., Kirby S. M., Marshall M. J., O'Callaghan C. H. Comparison of assay techniques for beta-lactamase activity. Anal Biochem. 1973 Jul;54(1):9–16. doi: 10.1016/0003-2697(73)90241-8. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Abraham E. P. Zinc as a cofactor for cephalosporinase from Bacillus cereus 569. Biochem J. 1966 Jan;98(1):11C–13C. doi: 10.1042/bj0980011c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino Y., Kobayashi F., Inoue M., Mitsuhashi S. Purification and properties of inducible penicillin beta-lactamase isolated from Pseudomonas maltophilia. Antimicrob Agents Chemother. 1982 Oct;22(4):564–570. doi: 10.1128/aac.22.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuni A. A direct spectrophotometric assay and determination of Michaelis constants for the beta-lactamase reaction. Anal Biochem. 1975 Jan;63(1):17–26. doi: 10.1016/0003-2697(75)90185-2. [DOI] [PubMed] [Google Scholar]
- Sutherland R. Classification of beta-lactamase-producing bacteria. Scott Med J. 1982;27(Spec No):S3–S9. doi: 10.1177/00369330820270S102. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Bonner D. P., Bush K., Georgopapadakou N. H., Wells J. S. Monobactams--monocyclic beta-lactam antibiotics produced by bacteria. J Antimicrob Chemother. 1981 Dec;8 (Suppl E):1–16. doi: 10.1093/jac/8.suppl_e.1. [DOI] [PubMed] [Google Scholar]
- Vu H., Nikaido H. Role of beta-lactam hydrolysis in the mechanism of resistance of a beta-lactamase-constitutive Enterobacter cloacae strain to expanded-spectrum beta-lactams. Antimicrob Agents Chemother. 1985 Mar;27(3):393–398. doi: 10.1128/aac.27.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waley S. G. A spectrophotometric assay of beta-lactamase action on penicillins. Biochem J. 1974 Jun;139(3):789–790. doi: 10.1042/bj1390789. [DOI] [PMC free article] [PubMed] [Google Scholar]


