Abstract
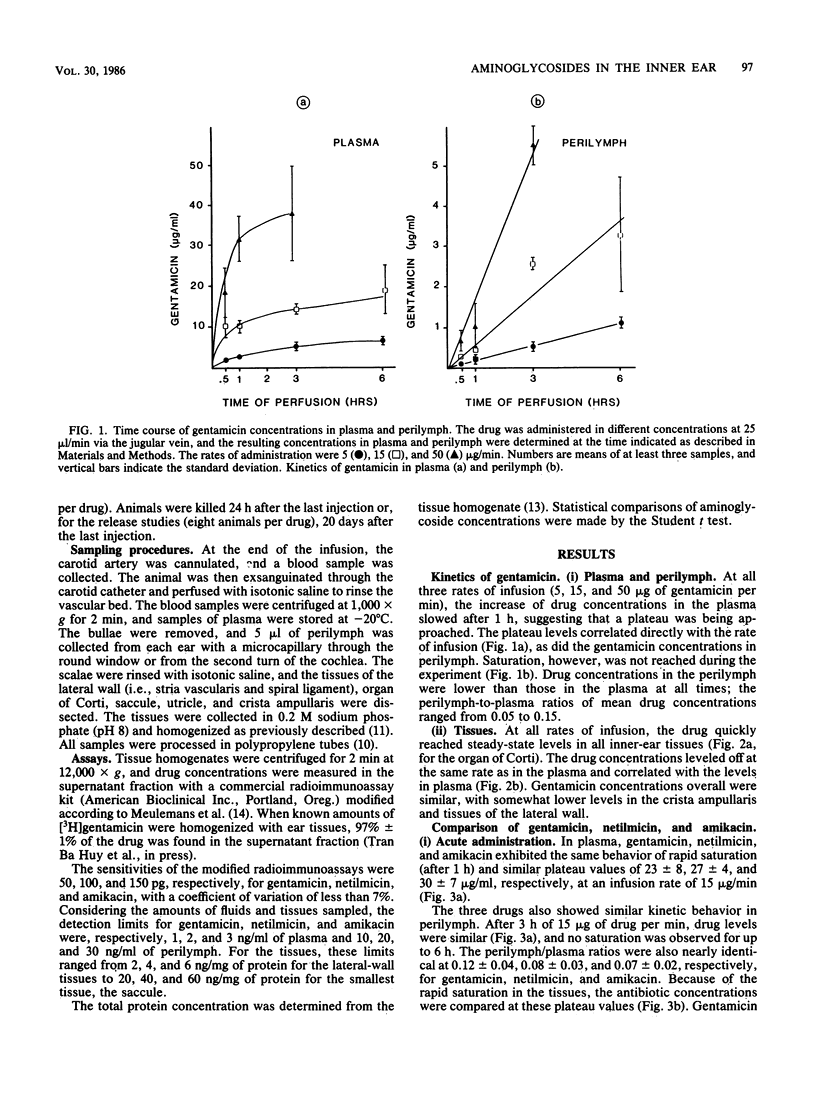
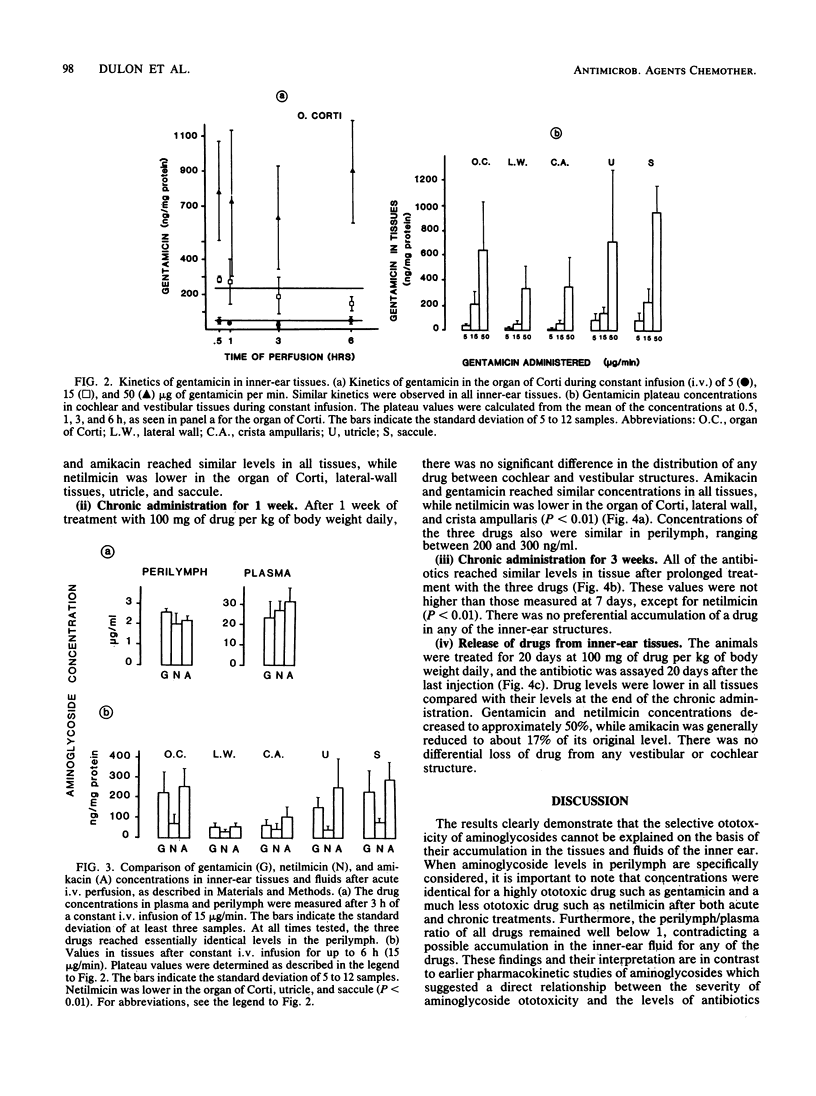
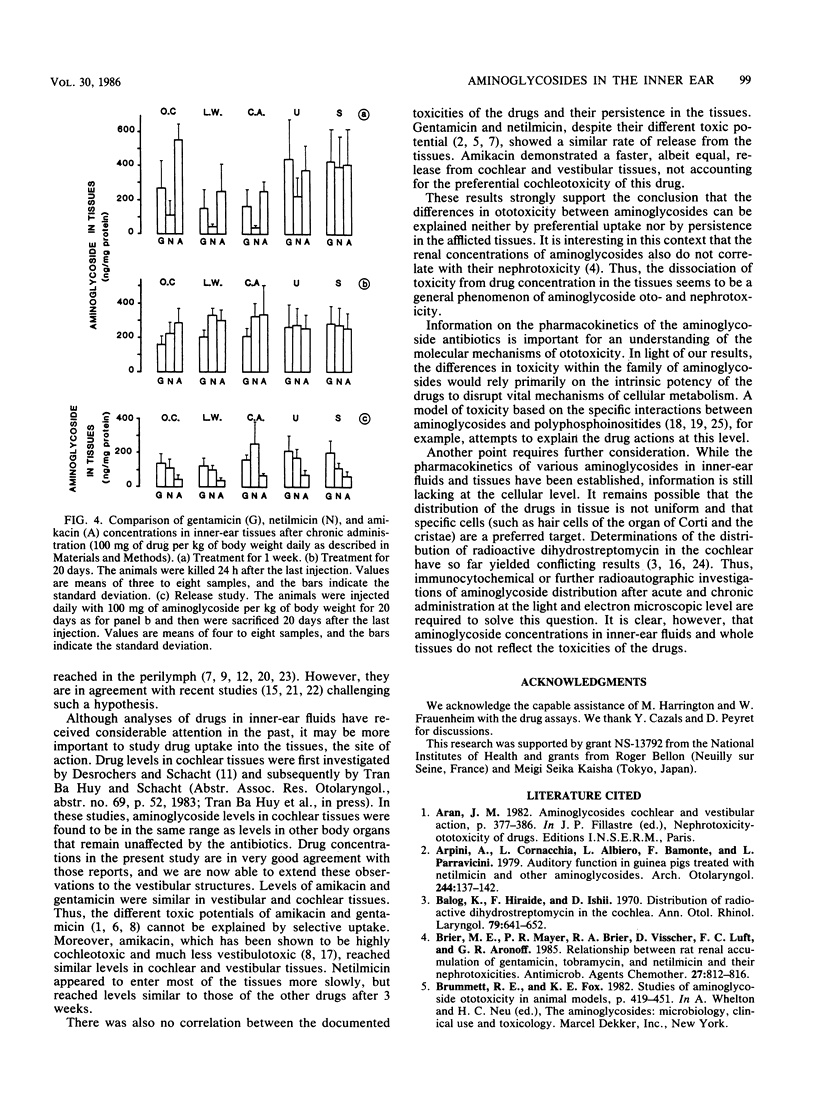
The kinetics of the entry of three aminoglycosides into inner-ear tissues of the guinea pig after acute and chronic administration were compared: gentamicin toxic to the cochlea and the vestibule, amikacin preferentially cochleotoxic, and netilmicin of low ototoxic liability. During constant intravenous infusion, levels of the three drugs in plasma tended to reach a plateau after 1 h, while levels in perilymph did not reach a plateau within 6 h. The drug concentrations in both vestibular and cochlear tissues quickly reached saturation. Amikacin and gentamicin concentrations were similar in vestibular and cochlear tissues, while netilmicin values were somewhat lower. After 1 week of chronic treatment (100 mg of drug per kg of body weight daily subcutaneously), levels of gentamicin and amikacin in tissue were similar to each other and were not significantly different between cochlear and vestibular tissues. Netilmicin concentrations again were somewhat lower in the tissues, but identical to those of the other drugs in the perilymph. After 3 weeks of treatment, all of the drugs were equally distributed in the inner-ear tissues. Release of the drug from the tissues after the 3-week treatment was faster for amikacin (83% decrease after 20 days) than for netilmicin and gentamicin (approximately 50% decrease). There was no correlation, under any of the experimental conditions, between the drug concentrations and their degrees of toxicity. These results demonstrate that selective aminoglycoside ototoxicity cannot be explained by a preferential uptake or accumulation of drugs in the afflicted tissues or in the perilymph.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arpini A., Cornacchia L., Albiero L., Bamonte F., Parravicini L. Auditory function in guinea pigs treated with netilmicin and other aminoglycoside antibiotics. Arch Otorhinolaryngol. 1979;224(1-2):137–142. doi: 10.1007/BF00455238. [DOI] [PubMed] [Google Scholar]
- Balogh K., Jr, Hiraide F., Ishii D. Distribution of radioactive dihydrostreptomycin in the cochlea. An autoradiographic study. Ann Otol Rhinol Laryngol. 1970 Jun;79(3):641–652. doi: 10.1177/000348947007900329. [DOI] [PubMed] [Google Scholar]
- Brier M. E., Mayer P. R., Brier R. A., Visscher D., Luft F. C., Aronoff G. R. Relationship between rat renal accumulation of gentamicin, tobramycin, and netilmicin and their nephrotoxicities. Antimicrob Agents Chemother. 1985 May;27(5):812–816. doi: 10.1128/aac.27.5.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummett R. E., Fox K. E., Bendrick T. W., Himes D. L. Ototoxicity of tobramycin, gentamicin, amikacin and sisomicin in the guinea pig. J Antimicrob Chemother. 1978 May;4 (Suppl A):73–83. doi: 10.1093/jac/4.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- Brummett R. E., Fox K. E., Brown R. T., Himes D. L. Comparative ototoxic liability of netilmicin and gentamicin. Arch Otolaryngol. 1978 Oct;104(10):579–584. doi: 10.1001/archotol.1978.00790100033007. [DOI] [PubMed] [Google Scholar]
- Christensen E. F., Reiffenstein J. C., Madissoo H. Comparative ototoxicity of amikacin and gentamicin in cats. Antimicrob Agents Chemother. 1977 Aug;12(2):178–184. doi: 10.1128/aac.12.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M., Parravicini L., Assael B. M., Cavanna G., Radwanski E., Symchowicz S. Comparative pharmacokinetics of aminoglycoside antibiotics in guinea pigs. Antimicrob Agents Chemother. 1982 Dec;22(6):1017–1021. doi: 10.1128/aac.22.6.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers C. S., Schacht J. Assay of aminoglycosides is influenced by tissue homogenization technique. Experientia. 1981 Dec 15;37(12):1357–1358. doi: 10.1007/BF01948411. [DOI] [PubMed] [Google Scholar]
- Desrochers C. S., Schacht J. Neomycin concentrations in inner ear tissues and other organs of the guinea pig after chronic drug administration. Acta Otolaryngol. 1982;93(1-6):233–236. doi: 10.3109/00016488209130877. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meulemans A., Manuel C., Tran Ba Huy P. Radioimmunoassay of gentamicin in microliter and nanoliter samples of biological fluids. Chemotherapy. 1981;27(1):29–33. doi: 10.1159/000237951. [DOI] [PubMed] [Google Scholar]
- Ohtani I., Ohtsuki K., Aikawa T., Sato Y., Anzai T., Ouchi J., Saito T. Low ototoxicity and its mechanism of netilmicin. ORL J Otorhinolaryngol Relat Spec. 1985;47(2):84–89. doi: 10.1159/000275749. [DOI] [PubMed] [Google Scholar]
- Portmann M., Darrouzet J., Coste Ch. Distribution within the cochlea of dihydrostreptomycin injected into the circulation. An autoradiographic and electron microscopic study. Arch Otolaryngol. 1974 Dec;100(6):473–475. doi: 10.1001/archotol.1974.00780040487014. [DOI] [PubMed] [Google Scholar]
- Reiffenstein J. C., Holmes S. W., Hottendorf G. H., Bierwagen M. E. Ototoxicity studies with BB-K8, a new semisynthetic aminoglycoside antibiotic. J Antibiot (Tokyo) 1973 Feb;26(2):94–100. doi: 10.7164/antibiotics.26.94. [DOI] [PubMed] [Google Scholar]
- Schacht J. Isolation of an aminoglycoside receptor from guinea pig inner ear tissues and kidney. Arch Otorhinolaryngol. 1979;224(1-2):129–134. doi: 10.1007/BF00455236. [DOI] [PubMed] [Google Scholar]
- Stupp H., Küpper K., Lagler F., Sous H., Quante M. Inner ear concentrations and ototoxicity of different antibiotics in local and systemic application. Audiology. 1973 Sep-Dec;12(5):350–363. doi: 10.3109/00206097309071650. [DOI] [PubMed] [Google Scholar]
- Tran Ba Huy P., Manuel C., Meulemans A., Sterkers O., Amiel C. Pharmacokinetics of gentamicin in perilymph and endolymph of the rat as determined by radioimmunoassay. J Infect Dis. 1981 Mar;143(3):476–486. doi: 10.1093/infdis/143.3.476. [DOI] [PubMed] [Google Scholar]
- Tran Ba Huy P., Meulemans A., Wassef M., Manuel C., Sterkers O., Amiel C. Gentamicin persistence in rat endolymph and perilymph after a two-day constant infusion. Antimicrob Agents Chemother. 1983 Feb;23(2):344–346. doi: 10.1128/aac.23.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voldrich L. The kinetics of streptomycin, kanamycin and neomycin in the inner ear. Acta Otolaryngol. 1965 Sep;60(3):243–248. [PubMed] [Google Scholar]
- Wang B. M., Weiner N. D., Takada A., Schacht J. Characterization of aminoglycoside-lipid interactions and development of a refined model for ototoxicity testing. Biochem Pharmacol. 1984 Oct 15;33(20):3257–3262. doi: 10.1016/0006-2952(84)90087-x. [DOI] [PubMed] [Google Scholar]
- von Ilberg C., Spoendlin H., Arnold W. Autoradiographical distribution of locally applied dihydrostreptomycin in the inner ear. Acta Otolaryngol. 1971 Feb-Mar;71(2):159–165. doi: 10.3109/00016487109125345. [DOI] [PubMed] [Google Scholar]


