Abstract
Background
Recent reports suggest that endotoxin exposure can blunt phagocyte functions. The aim of this study was to examine whether lung phagocytic cells have altered host defense function in young cystic fibrosis (CF) patients, and to explore the contribution of neutrophil elastase (NE) and surfactant proteins to these effects.
Methods
BALF cells from CF children (N=12) and disease controls (N=12) were analyzed by flow cytometry for mCD14 and HLA-DR expression and phagocytosis. The effects of exogenous surfactant protein A and D (SP-A,D) and proteases on BALF cells in short-term culture were assessed experimentally.
Results
Expression of the surface markers mCD14 and HLA-DR, and phagocytosis, were all blunted on CF phagocytes compared to disease controls (p<0.05). In CF phagocytes, SP-A enhanced both phagocytosis and mCD14 expression (p<0.05). Both CF BALF and NE reduced phagocytosis and expression of mCD14 and HLA-DR (p<0.05) by non-CF phagocytes; the latter effect was attenuated by protease inhibitor.
Conclusion
CF airway phagocytes appear to have altered host defense functions that could contribute to poor bacterial clearance. These impairments can be reproduced by incubation of non-CF cells with NE, while SP-A can partially reverse them. Decreasing protease activity and increasing collectin activity may be beneficial in early CF.
Keywords: Cystic Fibrosis, phagocytosis, neutrophil elastase, CD14, HLA-DR, Surfactant protein-A
1. Introduction
Chronic bacterial airways infection and subsequent intense neutrophilic inflammation with release of intracellular proteases are considered the main contributors to bronchiectasis and end-stage lung disease in cystic fibrosis (CF) [1, 2]. Recent research has demonstrated the additional importance of inflammation and immune response in CF airway disease [3, 4, 5]. Observations raising concern about primary or secondary immunologic abnormalities in CF include the excessive inflammation in infancy, the persistence of neutrophilic inflammation, and the inability to clear bacteria despite this intense response. Clearance of lung infection involves antigen recognition, presentation to effector cells, phagocytosis and killing. Macrophages and polymorphonuclear neutrophils (PMN) are the prototypic cells of innate immune response. They play a central role in innate immunity through expression of receptors that recognize and mediate responses to microbial pathogens and through their phagocytic function. Examples of these receptors include membrane-bound CD14 (mCD14), a component of the receptor for lipopolysaccharide (LPS), and MHC Class II molecules or HLA-DR, which plays a role in antigen presentation to T lymphocytes [6].
Previous studies have yielded conflicting data as to whether lung phagocytes are modified in CF disease with respect to phagocytic capacity. Studies of BAL neutrophils and macrophages showed similar phagocytic capacity as those of normal children [7], or these functions were decreased in presence of CF serum [8]. Other studies showed decreased phagocytosis of P. aeruginosa [9] or indirect evidence of decreased phagocytosis by accumulation of apoptotic cells in sputa and that this may be related to neutrophil elastase [10]. Expression of immune cell-surface receptors was also examined in terminal CF by measuring antigen presenting function of macrophages derived at the time of transplant. Cells from CF subjects had decreased antigen presenting capacity and decreased lymphocyte activation compared to non-CF [11]. A study in cftr knock-out animals showed increased expression of the co-stimulatory molecule B7 (CD80) in these animals and evidence of increased expression of these surface markers in BALF cells of CF patients [12]. It is possible that phagocytosis and antigen presentation are compromised secondary to the chronic infection. Even at an early stage, CF airways are rich in neutrophils and the neutrophil-derived proteases such as neutrophil elastase (NE). There is evidence that these proteases degrade immunoglobulins [13], complement and other cell-surface receptors [14, 15] and surfactant proteins [16]. Surfactant proteins A and D (SP-A and SP-D) are pattern recognition proteins that facilitate removal of microorganisms by serving as opsonins in macrophage phagocytosis [17]. In CF lungs, SP-A and SP-D levels are depleted in a manner that appears to be inversely related to the degree of neutrophilic inflammation in the airways [18, 19, 20].
Recent findings in our laboratory and others suggest that exposure of lung phagocytic cells to endotoxin may alter several important phagocyte functions and increase risk for infection. We have found that inhalation of endotoxin by volunteers results in acutely increased expression of HLA-DR and other dendritic cell markers (CD80, CD86) by sputum macrophages, but reduced phagocytic capacity [21, 22]. Muehlstedt et al. [23] have shown decreased HLA-DR expression in lung cells prior to acquisition of nosocomial pneumonias. Thus, the interplay between innate host defense elements, bacterial products, and proteolytic enzymes are all factors potentially affecting risk for infection and the phenotypic expression of disease.
In the absence of an ideal animal model [24], an improved understanding of how inflammation and lung disease develop in CF depends on data derived using lung cells from young CF patients. Most previous studies of innate immune cell functions in CF have been carried out using cells from peripheral blood or from older patients with longstanding lung disease. Currently there are few data that directly compare the functional characteristics of lung phagocytes (macrophages, monocytes, neutrophils) recovered from pediatric CF patients with less severe disease, to non-CF disease controls of similar age. We therefore obtained BALF cells from children with CF and from non-CF children undergoing bronchoscopies for clinical indications, for assays of phagocytic function and surface receptor expression in short term culture. Our goals were (1) to determine whether functions of cellular host defense response are altered in CF compared to other states of lung infection, and (2) to evaluate in vitro if addition of surfactant proteins can restore these alterations. The specific phagocyte functions to be studied were chosen based on our previous studies of the effects of endotoxin inhalation on sputum phagocytes [21, 22, 25].
2. Materials and Methods
2.1. Study subjects
A total of 24 infants and children who underwent clinically indicated bronchoscopies participated in the study. Exclusion criteria were the use of inhaled or systemic steroids or high dose ibuprofen in CF patients during the 6 weeks preceding the bronchoscopy. Patients in whom mycobacteria or viruses were detected in the bronchoalveolar lavage fluid (BALF) were excluded from the data analysis. Informed consent was obtained from all parents and assent from patients older than 6 years of age.
2.2. Bronchoscopy
Bronchoscopies were performed according to our clinical routine as has been described previously [22]. Bronchoalveolar lavage (BAL) was performed in the most affected region, as determined by infiltrates on chest radiograph or by findings seen during bronchoscopy. The clinician performing the procedure made the choice of location for BAL. Venipuncture was performed at the time of bronchoscopy to obtain peripheral blood cells for assays similar to those performed on BALF cells.
2.3. Total and Differential Cell Count and Cytokine Levels
Cells and cytokines were recovered from freshly recovered BALF as has been described previously [22]. Cytospin slides were stained with modified Wright-Giemsa stain and a differential cell count was determined by counting a total of 200 consecutive cells, including epithelial cells but excluding red blood cells. Cytokines in cell-free BALF supernatants were measured using commercially available ELISA kits (R&D Systems, Minneapolis MN. USA)
2.4 Microbiology
Cultures were performed in the UNC Hospitals Clinical Microbiology Laboratory, as described in our previous report [26]. Briefly, specimens were cultured for gram-positive and gram-negative bacteria, and cultures and stains for viruses (including cytomegalovirus, respiratory syncytial virus, influenza A and B, Para influenza 1–3, and adenovirus), fungi and mycobacteria were carried out for all patients. For bacterial pathogens, a cut off value of 50,000 colony-forming units (cfu)/ml was chosen for classification of “infected” versus “non-infected” specimens, consistent with our previous reports.
2.5 Flow Cytometry, Immunofluorescence Staining and Phagocytosis
Flow cytometric methodologies for BALF and blood cells for cell-surface marker staining [27] and phagocytosis [28] have been described in previous reports. Briefly, flow cytometry was performed with a FACSORT (Becton Dickinson) using an Argon-ion laser (wavelength = 488 nm). Gain and amplitude settings were set to accommodate both blood and BAL samples. Gates for blood cells, as well as previously established gates for BALF cells [27], were used as general reference gates to establish the best possible gates for blood and BALF leukocyte identification. Gating of viable macrophages, monocytes, neutrophils, and lymphocytes in blood and BALF was based on light scatter (FSC/forward scatter, SSC/side scatter) properties and positive expression (% positive cells) for CD45 (pan leukocyte marker) to distinguish leukocyte populations from non-leukocyte cells and cell debris. Antibodies known to be highly expressed (MFI) on specific cell types such as CD16 (neutrophils), HLA-DR (macrophages), mCD14 (monocytes) and CD3 (lymphocytes) were used to gate and distinguish those cell populations of interest. FACS analysis showed very little overlap between the cell populations of interest, but some overlap could not be ruled out: 98% of neutrophils were CD16 bright positive, 95% were both CD14 and HLA-DR negative; 80% of monocytes were CD14 positive, 100% were CD3 negative and 10% were CD16 dim positive; 85% of macrophages were HLA-DR bright positive and 6% were CD16 positive; and 100% of lymphocytes were CD3 positive and 100% were CD14 negative.
For immunofluorescence staining, fluorescein (FITC) and phycoerythrin (PE) conjugated non-specific antibodies of the same isotope as the receptor antibodies were used as controls to establish background fluorescence and non-specific antibody binding. The (arithmetic) mean fluorescence intensity (MFI) of the cells stained with control antibody was subtracted from the MFI of the cells stained with receptor antibodies to provide a measure of receptor-specific MFI. Relative cell size and density/granularity were quantified by analyzing light scatter properties, namely FSC for cell size, and SSC for cell density/granularity, and recording the mean fluorescent intensities for each. Immunofluorescence staining was performed for mCD14, CD11b/CR3, CD64/FcγRI, CD16/FcγRIII, MHC Class II/HLA-DR, CD45, and CD3.
For phagocytosis, Saccharomyces cerevisiae zymosan A BioParticles (Molecular Probes, Inc; Eugene, OR) conjugated to FITC were opsonized with IgG opsonizing reagent (purified rabbit polyclonal IgG containing RIA-grade bovine serum albumin to block non-specific binding) specific for zymosan particles. Incubation was for 45 minutes at 37°C and then the zymosan particles were washed with RPMI 1640 two times before adjusting the particle concentration to 2 x 106/mL. Blood cells were isolated using Lymphoprep solution (Axis-Shield PoC AS, Oslo, Norway) and Percoll gel density centrifugation (Pharmacia, Uppsala, Sweden). Gradients and cell isolation was performed for monocytes and neutrophils. BAL cells and purified blood cells (2 x 106/mL) were exposed to the yeast cell walls at a ratio of 1:10 for one hour at 37°C in the presence of human serum (20 μL) before tubes were placed on ice. Next 200 μL of 2% paraformaldehyde was added to each tube and the tubes were stored at 4°C in the dark and analyzed by flow cytometry (FACSORT) within 24 hours of particle exposure. Particle uptake was identified and displayed on histogram plots as a rightward shift in FITC stained cells (FL1, x-axis) in the phagocyte populations. Phagocytosis was determined by assessing the cells in the zymosan-exposed population showing increased mean fluorescence intensity (MFI) compared to cells that had not been exposed (control population, ave. MFI < 5) to zymosan particles. The percent positive cells were the proportion of cells that right-shifted out of the control region into the zymosan treated region as displayed on histogram plot analysis.
2.6. In Vitro Measurements
Freshly isolated BALF cells (0.5 x 106 cells/ml) from CF subjects were incubated overnight (37°C) in 500 μl RPMI with 10% FBS, Pen/Strep, and Gentamicin (GIBCO, Invitrogen Life Technologies, Carlsbad, CA), Amphotericin B, (Sigma, St. Louis, Mo) with or without 100 ng/ml E. coli LPS (Sigma, St. Louis, Mo.). Cells were recovered the next day, counted under a hemacytometer and viability recorded using Trypan Blue staining. Cells were then prepared for flow cytometric assays to measure phagocytosis and cell-surface phenotypes as described above.
Where indicated, CF BALF cells were incubated overnight in the presence of 20 ug/ml of either SP-A or SP-D, washed, and then subjected to surface marker and phagocytosis measurements. These surfactant proteins preparations were kind gifts from the laboratory of Dr. Jeffrey Whitsett, Cincinnati Childrens Hospital, Cincinnati, OH. SP-A was derived from patients with alveolar proteinosis. SP-D was purified from BAL of SP-A −/− mice who overexpress SP-D and this SP-D is structurally very similar to human SP-D. Purification was done by fractionation and maltosyl-agarose column as previously described [29, 30, 31, 32]. Endotoxin concentration was <1 ng/ml for SP-D and < 0.06 U/ml for SP-A conditions. In other experiments, non-CF BALF cells were incubated overnight with cell-free CF BALF supernatant (250 μl final concentration pooled from approximately equal volumes of BALF from 4 of the studied CF patients), protease inhibitor (Aprotinin, 1 μM, and protease inhibitor cocktail tablets, 1 tablet dissolved in 1.5ml H2O, Roche Applied Sciences, Indianapolis, IN) or neutrophil elastase (5.7 μg/ml final concentration, Elastin Products Co., Owensville, MO). Cells from these experiments were washed then subjected to surface marker and phagocytosis measurements.
2.7. Data Analysis
For a given response measure, differences between CF patients and non-CF controls, and differences between treatment and baseline, were compared using unpaired and paired non-parametric tests (Mann-Whitney U) with the overall alpha level set at 0.05. Data are presented as mean ± SEM unless otherwise noted. All analyses were performed using GraphPad Prism 3.0.
3. Results
3.1. Subject and Clinical Data
Indications for bronchoscopy in the CF patients were worsening respiratory symptoms suggestive of lower airway infection. The 12 children with CF (8 males, 4 females) had a mean age of 5.9 ± 1.2 yrs. Eight were heterozygote and 4 homozygote for the ΔF508 mutation. Eleven of the CF patients had > 50,000 cfu/ml of one or more bacterial pathogens in BAL (S. aureus, M. catharrhalis, H. influenzae, E. coli, P. aeruginosa). The 12 non-CF controls consisted of 9 males and 3 females with a mean age of 4.8 ± 1.3 yrs. Indications for bronchoscopy in non-CF patients (N=12) included recurrent pneumonia or bronchitis, upper airway obstruction, hemoptysis, chronic cough and tracheostomy evaluation. Five non-CF subjects had BALF infection according to our definitions, with one or more organisms (M. catarrhalis, S. pneumoniae and H. influenzae).
3.2. Differential Cell Counts and Cytokines
BALF yield was not different between CF and non-CF children. Total and differential cell counts showed significantly greater total number of cells and proportion of neutrophils compared in CF to non-CF disease controls (Table 1), consistent with previous reports. Total protein and cytokine concentrations of IL-6 and IL-8 were significantly higher, and GM-CSF significantly reduced, in CF compared to non-CF samples. No differences in levels of IL-4, IL-10 and IFN-γ were observed between CF patients and non-CF controls (Table 2).
Table 1.
Total and Differential Cells counts (mean ± SEM) Recovered in CF and Non-CF BALF
| Subjects | TCC/ml (x 1000) | %PMN | %Mac | %Mono | %Lym | % EPi | Cell Viability (%)Pre 24h Incubation | Cell Viability (%) Post 24h Incub. |
|---|---|---|---|---|---|---|---|---|
| CF Subjects (N=12) | 4423* (2014) | 59* (8) | 25 (7) | 6 (1) | 2 (0.4) | 8 (1) | 71 (4) | 75 (2) |
| Non-CF Subjects (N=12) | 1007 (368) | 22 (7) | 29 (7) | 12 (4) | 4 (1) | 29 (6) | 61 (3) | 66 (8) |
significantly different than non-CF controls (p<0.05); TCC = Total Cell Count; PMN = polymorphonuclear neutrophil; Mac = Macrophage; Mono = Monocyte; Lym = Lymphocyte; Epi = Epithelial cell
Table 2.
Cytokine Levels Recovered in CF and Non-CF BALF (mean ± SEM)
| Subjects | IL-1β (pg/ml) | IL-4 (pg/ml) | IL-6 (pg/ml) | IL-8 (pg/ml) | IL-10 (pg/ml) | IFN-γ (pg/ml) | GM-CSF (pg/ml) | Total Protein (ug/ml) | sCD14 (pg/ml) | TNF-α (pg/ml) |
|---|---|---|---|---|---|---|---|---|---|---|
| CF (N=12) | 88 (23) | 18 (6) | 101* (26) | 12,690* (2837) | 66 ( 10) | 101 (29) | 245* (64) | 1218* (342) | 9470 (2481) | 48 (15) |
| Non-CF (N=12) | 137 (78) | 11 (4) | 23 (9) | 1327 (757) | 45 ( 6) | 87 (29) | 812 (193) | 238 (87) | 6646 (991) | 57 (14) |
significantly different from non-CF controls (p<0.05)
sCD14 = soluble CD14 measured in BAL supernatants
3.3. Cell Surface Receptors
The cell surface markers HLA-DR and mCD14 were significantly decreased on CF vs. non-CF BALF macrophages and monocytes (Figure 1). Other cell-surface marker molecules (CD11b, CD64, CD16) were not different on BALF macrophages or monocytes between CF and non-CF subjects (Table 3). In non-CF subjects, the presence of infection significantly enhanced mCD14 expression on monocytes (124 ± 20 vs. 70 ± 11, p< 0.05) but not macrophages (data not shown). We stratified CF patients according to the presence/absence of P. aeruginosa. Macrophages and monocytes showed similar levels of mCD14 expression with or without P. aeruginosa infection. Comparison of all surface markers on blood monocytes and neutrophils showed no difference between CF and non-CF subjects (Table 3).
Figure 1.
mCD14 (A) and HLA-DR (B) expression (MFI) on CF (N=12, solid bar) and non-CF (N=12, open bar) BALF macrophages and monocytes. mCD14 and HLA-DR expression were significantly (*p<0.05) decreased in CF versus non-CF subjects.
Table 3.
Cell Surface Marker Expression (MFI) on BALF and Blood Cells from CF and Non-CF Subjects (mean ± SEM)
| CF Subjects | CD11b/CR3 | mCD14 | CD64/FcγRI | CD16/FcγRIII | HLA-DR |
|---|---|---|---|---|---|
| BALF Mac | 48 (8) | 28* (5) | 26 (6) | 148 (23) | 106* (37) |
| BALF Mono | 22 (3) | 28* (4) | 11 (5) | 40 (9) | 15* (3) |
| BALF PMN | 27 (2) | 12 (1) | 6 (2) | 95 (18) | 9 (4) |
| Blood Mono | 10 (1) | 96 (12) | 5 (1) | 25 (4) | 8 (2) |
| Blood PMN | 8 (1) | 7 (1) | 0 | 518 (59) | 0 |
| Non CF Controls | |||||
| BALF Mac | 41 (23) | 53 (6) | 35 (4) | 119 (13) | 416 (118) |
| BALF Mono | 22 (10) | 80 (14) | 12 (2) | 28 (6) | 105 (26) |
| BALF PMN | 35 (15) | 11 (2) | 9 (2) | 110 (27) | 30 (26) |
| Blood Mono | 13 (1) | 109 (19) | 5 (1) | 26 (7) | 9 (1) |
| Blood PMN | 9 (1) | 7 (1) | 0 | 544 (55) | 0 |
significantly different from non-CF, p < 0.05; MFI = mean fluorescent intensity. Mac = Macrophage; Mono = Monocyte; PMN = Polymorphonuclear Neutrophil
3.4. Comparison of phagocytosis in CF vs. non-CF neutrophils
Adequate BALF samples for phagocytosis measurements were obtained from 11 CF and 6 non-CF patients. Macrophages and neutrophils recovered from CF BALF showed blunted phagocytosis compared to phagocytes recovered from non-CF BALF as measured by mean fluorescence intensity (MFI) (Figure 2A). Phagocytosis, as measured by the percentage of cells (macrophages, neutrophils) that took up fluorescently labeled zymosan particles, was also significantly decreased in CF versus non-CF subjects (Figure 2B). For CF subjects (dark bar), the mean (± SEM) % of macrophages and neutrophils that took up zymosan particles was 78% (5%) and 52% (6%), respectively. By comparison, the mean (± SEM) % of cells that took up zymosan particles for non-CF subjects (open bar) was 91% (2%) for macrophages and 76%, (6%) for neutrophils. Particle uptake was also demonstrated as a right-ward shift on histogram analysis (Figures 2C and 2D). A representative histogram from a non-CF (2C) and CF subject (2D) subject shows that CF subjects had a smaller proportion of cells that took up particles and a greater proportion of cells that remained in the control region (M1), where no particle uptake occurred. Unlike BALF cells, phagocytic activity (MFI) on blood monocytes and neutrophils did not differ between CF and non-CF subjects, respectively (Mono: 273 ± 176 vs. 345 ± 73; neutrophils: 1194 ± 322 vs. 1355 ± 181).
Figure 2.

Phagocytosis of zymosan particles by macrophages and neutrophils recovered from BALF of CF (N=11, solid bar) and non-CF (N=6, open bar) subjects as measured by both mean fluorescence intensity (MFI) (2A) and % cells (macrophages, neutrophils) that took up zymosan particles (2B). Phagocytosis on macrophages and neutrophils is significantly (* p<0.05) decreased in the CF (solid bar) vs. non-CF (open bar) subject groups. Figures 2C and 2D show representative histograms of a non-CF and CF subject, respectively. Macrophage phagocytosis after zymosan exposure is shown as the dark histogram. No zymosan exposure is shown as the grey histogram. The M2 region indicates zymosan uptake by macrophages and M1 (control region) indicates no zymosan uptake.
3.5 Effects of SP-A and SP-D on CF BALF Cells
BALF phagocytes from CF subjects (N=12) were incubated in short term culture with and without SP-A and SP-D, and phagocytosis and surface marker expression were measured. Both SP-A and SP-D significantly enhanced macrophage phagocytosis compared to control, with SP-A showing a more robust response (25% increase) than SP-D (13% increase) (Figure 3A). Incubation with LPS served as a negative control and produced an expected decrease (10%) in phagocytosis.
Figure 3.
BALF cell culture experiments measuring phagocytosis (3A) and mCD14 expression (3B) on macrophages obtained from 12 CF subjects 24h after incubation with SP-A, SP-D, or LPS. Phagocytosis (MFI, % change from control) was significantly increased following SP-A and SP-D incubation (*p<0.05). mCD14 expression (MFI, % change from control) was significantly increased following incubation with SP-A (*p<0.05).
With respect to surface marker expression, incubation with SP-A, but not SP-D, produced a significant increase in mCD14 expression (p<0.05) on CF BALF phagocytes (Figure 3B). For HLA-DR, expression tended to be enhanced by both SP-A and SP-D on macrophages and monocytes, but these changes were not statistically significant (data not shown).
3.6.Effects of CF BALF, NE and PI on non-CF BALF Cells
Figure 4 shows the effects of cell-free CF BALF supernatant, NE and PI on non-CF cell phagocytosis (A), mCD14 (B) and HLA-DR (C) expression. Macrophages, monocyte and neutrophil phagocytosis from non-CF subjects was significantly blunted following incubation with cell-free CF BALF supernatant (N=5, p<0.05) (4A). Incubation with NE significantly decreased neutrophil phagocytosis on these subjects by 47% (N=4, p<0.05, data not shown), and trends for reduced phagocytosis by NE were observed for macrophages and monocytes, but these were not statistically significant. In addition, PI significantly attenuated (77%) the decrement in monocyte phagocytosis induced by cell-free CF BALF supernatant (N=4, p<0.05, data not shown).
Figure 4.
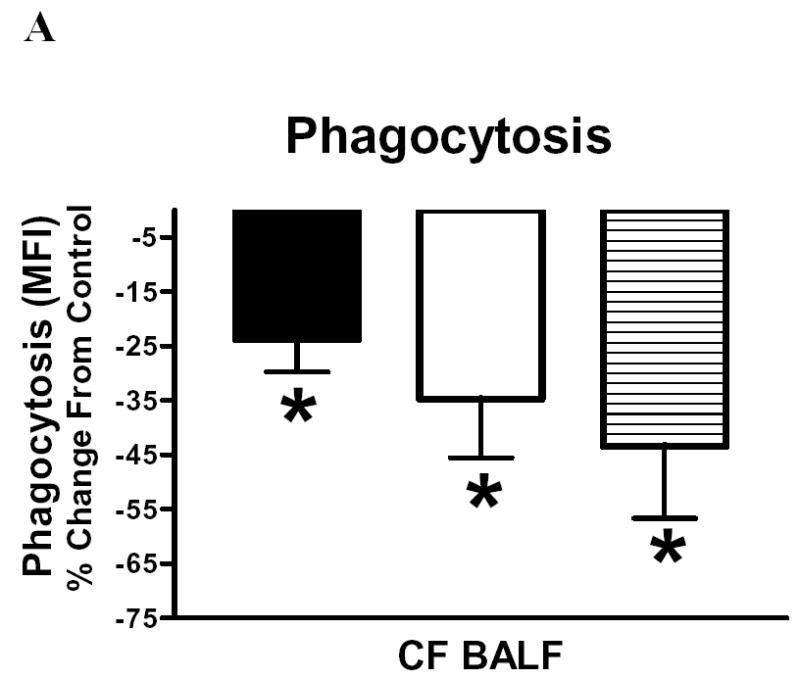
BALF cell culture experiments measuring phagocytosis (4A), mCD14 (4B) and HLA-DR (4C) expression on non-CF BALF cells after in vitro incubations with cell free CF BALF, neutrophil elastase (NE) and these conditions with addition of proteinase inhibitor (PI). 4A: Phagocytosis was measured in macrophages (solid bar), monocytes (open bar) and neutrophils (horizontal hatch). Phagocytosis (MFI, % change from control) was significantly (*p<0.05) reduced for macrophages, monocytes and neutrophils following incubation with CF BALF (N=5); other conditions NS and not shown. 4B and 4C show surface marker expression after BALF + PI (N=5), NE (N=4) and NE + PI (N=4). Both, mCD14 (4B) and HLA-DR (4C) expression (MFI, as % change from control) were significantly decreased following incubation with CF BALF and NE (*p<0.05). PI significantly (#p<0.05) attenuated the NE-induced decrement in mCD14 and HLA-DR expression, but not the CF BALF-induced decrement.
CF BALF and NE significantly modified cell surface marker expression on macrophages and monocytes from non-CF subjects. Figure 4 shows that expression of mCD14 and HLA-DR was decreased following incubation with cell-free BALF supernatant (N=5) or with NE (N=4) (p<0.05) (Figure 4B, 4C). The NE-induced decrements in mCD14 (4B) and HLA-DR (4C) were significantly attenuated by PI by 75% and >100%, respectively. However, addition of PI did not significantly attenuate decrements in mCD14 and HLA-DR expression induced by cell-free CF BALF supernatant. This was in contrast to the attenuation of the BALF induced decrement seen in monocyte phagocytosis.
4. Discussion
We examined whether host defense responses of lung phagocytic cells recovered by BALF were altered in young CF subjects compared to BALF phagocytes from non-CF disease controls. We observed that mCD14 (LPS receptor) and HLA-DR (MHC Class II) expression were significantly reduced on CF macrophages and monocytes and that phagocytosis was significantly blunted. In the lung, the ability to effectively mount a host defense response against gram-negative (LPS)/positive organisms involves recognition mechanisms, as well as communication with adjacent trans-membrane cell surface receptors like TLR4 and TLR2, to initiate downstream signal transduction. Decreased expression of mCD14 may lead to a weak initiation of a series of signal transduction steps that ordinarily would induce the necessary production of immune-enhancing factors like GM-CSF, which promotes clearance of LPS-bearing pathogens by suppressing surfactant protein degradation [33]. In CF lungs, these immune enhancing factors may be under-produced as we found in our CF cohort, where GM-CSF levels were reduced compared to non-CF BALF. In the case of HLA-DR, our observation of reduced HLA-DR expression on CF BALF macrophages supports an earlier report by Knight and colleagues [11] who obtained BALF macrophages from terminal CF patients and found they were unable to present antigen when compared to disease controls. Although neutrophil elastase is not known to cleave or degrade HLA-DR, it does bind directly to a macrophage receptor where it is internalized and has a relatively long and potentially toxic intracellular life. This intracellular milieu may affect the internal mobilization of HLA-DR antigens and down-regulate its expression on the cell surface. Additionally, endotoxin tolerance resulting from the persistent presence of endotoxin in the airways, may have played a role in our decreased HLA-DR surface expression. Evidence suggests that LPS tolerance can reduce mRNA expression of HLA-DM and the chaparonic invariant chain [34]. It is also possible, that the presence of some HLA-DR dim neutrophils in the macrophage gated population, may have contributed to the overall reduced HLA-DR expression we observed on CF macrophages.
Previous studies have indirectly suggested that phagocytosis is impaired in the CF lung by demonstrating that CF airways have increased numbers of apoptotic cells indicating defective clearance mechanisms, [10] or by showing the effects of proteases on nonspecific phagocytosis from non-CF or from peripheral blood [35, 36]. In the current study we showed by direct means (uptake of IgG-opsonized zymosan particles), that phagocytosis is significantly reduced on lung phagocytes in pediatric CF lung disease. These changes were not found when peripheral blood phagocytes were assayed suggesting that factors local to the lung microenvironment, rather than genetic factors alone, may be responsible for the effects observed. This notion was supported by our observation that the findings were mimicked after incubation with neutrophil elastase in vitro.
Early evidence that soluble factors in the airway milieu of CF lungs may be contributing to reduced phagocytosis was reported by Thomassen and colleagues [8] who found reduced phagocytosis of CF macrophages in the presence of CF serum, suggesting that soluble factors may be inhibiting macrophage function. We found that BALF from young CF subjects with clinically active disease and NE were capable of reducing phagocytosis by non-CF BALF cells, and that the attenuation induced by NE (but not CF BALF) was reversed by protease inhibitors. This is consistent with a previous report where a 10% solution of CF lung fluid and NE inhibited phagocytosis of apoptotic Jurkat cells by a human monocyte-derived macrophage (HMDM) cell line [35]. In that study, an elastase inhibitor reversed phagocytosis inhibition. The lack of complete restoration of phagocytosis by addition of protease inhibitors to CF BALF could be secondary to other BALF factors causing defective phagocytosis. Alternatively, technical factors such as the choice of PI, and use of human cells vs. cell lines could explain the differences.
Further studies will be needed to define the specific mechanism of reduced phagocytosis we observed in CF lung cells, and the causal relationships among reduced CD14, HLA-DR, and phagocytosis, if any, remain unclear. The observation that protease inhibitor attenuated effects of BALF supernatants on phagocytosis but not on mCD14 or HLA-DR expression may suggest that the decrease in phagocytosis seen here are not directly linked to these surface markers. Other mechanisms causing defective phagocytosis may include cleavage of cell-surface receptors that mediate phagocytosis, such as complement receptor (CD11b/CR3) or FcγRI (CD64) for IgG-mediated phagocytosis, by proteases. Although we did not observe any difference in CD11b or CD64 expression between CF and non-CF subjects before or after incubation with CF BALF or NE, other phagocytosis receptors such as the phosphatidylserine receptor, are vulnerable to cleavage by CF airway fluid (in a NE-dependent manner), and may have contributed to the phagocytic response seen here [10]. Alternatively, cell activation processes involved in the ingestion of bound particles may have been affected by the presence of proteases producing attenuated function.
Here we also showed that incubation of CF phagocytes with physiologic concentrations of SP-A or SP-D significantly enhanced phagocytosis, with SP-A having a more robust effect than SP-D. The greater response to SP-A may be explained by the fact that SP-A enhances phagocytosis through multiple mechanisms: as a pattern recognition molecule that binds and opsonizes microorganisms, by directly acting on macrophages to enhance phagocytic capacity, and by modulation of cytokine production [37]. Both SP-A and SP-D are found in abundance in healthy lungs and play a significant role in pulmonary clearance of infectious microorganisms, maintenance of homeostasis and resolution of inflammation. In CF lungs however, both SP-A and SP-D [18, 19] are depleted in the presence of bacterial infection, and in inverse proportion to the degree of neutrophilic inflammation. With respect to the latter, it appears that proteases released by activated neutrophils directly modify the levels of lung collectins [16]. Other proteases in CF lungs besides NE are capable of degrading SP-A/SP-D and likely contribute to reduced phagocytosis. These include cathepsin G, proteinase-3, and metalloproteases produced by P. aeruginosa [38]. Regardless of the source of degradation, deficient levels of surfactant proteins as seen in SP-A/D knockout mice [39], lead to reduced phagocytic capacity of lung phagocytes. Thus our observation of partial restoration of phagocytosis and mCD14 by surfactant protein A, shows that some of the defects are reversible in cells from young CF patients.
Another fact to consider regarding the effect of SPA/D on CD14 expression is that SP-A/D differentially affect the LPS-CD14 interaction depending on the type of LPS present (smooth vs. rough). For example, both SP-A and SP-D inhibit binding of smooth LPS to CD14, but SP-A enhances and SP-D inhibits, binding of rough LPS to CD14 [40]. The LPS used in this study (E. coli) resembles rough LPS, hence the partial restoration we observed with SP-A on CD14 and phagocytosis, the latter increases with up-regulated CD14 expression, is consistent with SP-A’s ability to enhance the LPS-CD14 interaction.
There were potential limitations associated with our study design. Our study was limited to symptomatic children undergoing clinically indicated bronchoscopies. Thus, we are unable to determine whether the changes we observed are specific for clinical exacerbation of CF lung disease, or are a more general part of CF pathophysiology. A related potential limitation of this study was lack of BALF from healthy age-matched controls, for ethical reasons. It is unclear to what degree data from a heterogeneous mixture of non-CF disease controls reflect data from true normals. In this regard, it is interesting to note that data from our non-CF children were similar to those obtained in a previous study from our laboratory of healthy young adults using similar protocols for measuring phagocytosis and cell-surface marker expression (HLA-DR, mCD14) on BALF cells [27].
In summary, in young children with CF lung disease, there is evidence of decreased host defense function of lung phagocytes that is likely due, in part, to the presence of neutrophil elastase in the airway milieu. Furthermore, in vitro, SP-A enhances phagocytosis and expression of immune cell-surface phenotypes (mCD14, HLA-DR) on CF phagocytes. These findings lend support to the concept that chronic neutrophilic inflammation can have multiple direct or indirect effects on the functions of other cells and pathways central to host defense in the lung. In CF, treatment strategies aimed at restoring protease/antiprotease balance and activity of lung collectins are rational adjuncts to strategies aimed at improving the primary defects in ion/fluid balance at the airway surface.
Acknowledgments
The authors wish to thank Paula Murphy, Justin Hubbard, and Fernando Dimeo for their technical assistance with this study. We also thank the staff of the UNC Pediatric Bronchoscopy laboratory for their assistance.
Footnotes
Abstracts containing part of these data have been presented at: International Conference of the American Thoracic Society, (Seattle, WA May 2003) and at the North American Cystic Fibrosis Conference, (Anaheim, CA Oct. 2003).
References
- 1.Chmiel JF, Berger M, Konstan MW. The role of inflammation in the pathophysiology of CF lung disease. Clin Rev Allergy Immunol. 2002;23:5–27. doi: 10.1385/CRIAI:23:1:005. [DOI] [PubMed] [Google Scholar]
- 2.Bruce MC, Poncz L, Klinger JD, Stern RC, Tomashefski JF, Jr, Dearborn DG. Biochemical and pathologic evidence for proteolytic destruction of lung connective tissue in cystic fibrosis. Am Rev Respir Dis. 1985;132:529–535. doi: 10.1164/arrd.1985.132.3.529. [DOI] [PubMed] [Google Scholar]
- 3.Cantin A. Cystic fibrosis lung inflammation: early, sustained, and severe. Am J Respir Crit Care Med. 1995;151(4):939–941. doi: 10.1164/ajrccm.151.4.7697269. [DOI] [PubMed] [Google Scholar]
- 4.Doring G, Worlitzsch D. Inflammation in cystic fibrosis and its management. Paediatr Respir Rev. 2000;1(2):101–106. doi: 10.1053/prrv.2000.0030. [DOI] [PubMed] [Google Scholar]
- 5.Berger M. Inflammatory mediators in cystic fibrosis lung disease. Allergy Asthma Proc. 2002;23(1):19–25. [PubMed] [Google Scholar]
- 6.Moss RB. Pulmonary defenses. In: Hilman B, editor. In Pediatric Respiratory Disease. WB Saunders: Philadelphia; 1993. pp. 12–35. [Google Scholar]
- 7.Cassino RJ, Sordelli DO, Macri CN, Kohan M, Dillon MH, Pivetta OH. Pulmonary nonspecific defense mechanisms in cystic fibrosis. I. Phagocytic capacity of alveolar macrophages and neutrophils. Pediatr Res. 1980;14:1212–1215. doi: 10.1203/00006450-198011000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Thomassen MF, Demko CA, Wood RE, et al. Ultrastructure and function of alveolar macrophages from cystic fibrosis patients. Pediatr Res. 1980;14:715–721. doi: 10.1203/00006450-198005000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Speert DP, Wong SY, Macdonald M, Sargeant R. Modulation of macrophage function for defence of the lung against Pseudomonas aeruginosa. Behring Inst Mitt. 1997;98:274–282. [PubMed] [Google Scholar]
- 10.Vandivier RW, Fadok VA, Hoffmann PR, et al. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest. 2002;109:661–670. doi: 10.1172/JCI13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knight RA, Kollnberger S, Madden B, Yacoub M, Hodson ME. Defective antigen presentation by lavage cells from terminal patients with cystic fibrosis. Clin Exp Immunol. 1997;107:542–547. doi: 10.1046/j.1365-2249.1997.d01-954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soltys J, Bonfield T, Chmiel J, Berger M. Functional IL-10 deficiency in the lung of cystic fibrosis (cftr(−/−)) and IL-10 knockout mice causes increased expression and function of B7 costimulatory molecules on alveolar macrophages. J Immunol. 2002;168:1903–1910. doi: 10.4049/jimmunol.168.4.1903. [DOI] [PubMed] [Google Scholar]
- 13.Fick RB, Baltimore RS, Squier Su, Reynolds HY. IgG proteolytic activity of Pseudomonas aeruginosa in cystic fibrosis. J Infect Dis. 1985;151(4):589–598. doi: 10.1093/infdis/151.4.589. [DOI] [PubMed] [Google Scholar]
- 14.Tosi MF, Zakem H, Berger M. Neutrophil elastase cleaves C3bi on opsonized pseudomonas as well as CR1 on neutrophils to create a functionally important opsonin receptor mismatch. J Clin Invest. 1990;86:300–308. doi: 10.1172/JCI114699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doring G, Frank F, Boudier C, Herbert S, Fleischer B, Bellone G. Cleavage of lymphocyte surface antigens CD2, CD4, and CD8 by polymorphonuclear leukocyte elastase and cathepsin G in patients with cystic fibrosis. J Immunol. 1995;154(9):4842–4850. [PubMed] [Google Scholar]
- 16.Rubio F, Cooley J, Accurso FJ, Remold-O'Donnell E. Linkage of neutrophil serine proteases and decreased surfactant protein-A (SP-A) levels in inflammatory lung disease. Thorax. 2004;59(4):318–323. doi: 10.1136/thx.2003.014902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandivier RW, Ogden CA, Fadok VA, et al. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol. 2002;169 (7):3978–3986. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- 18.Noah TL, Murphy PC, Alink JJ, et al. Bronchoalveolar lavage fluid surfactant protein-A and surfactant protein-D are inversely related to inflammation in early cystic fibrosis. Am J Respir Crit Care Med. 2003;168 (6):685–691. doi: 10.1164/rccm.200301-005OC. [DOI] [PubMed] [Google Scholar]
- 19.Griese M, Essl R, Schmidt R, et al. BEAT Study Group. Pulmonary surfactant, lung function, and endobronchial inflammation in cystic fibrosis. Am J Respir Crit Care Med. 2004;170:1000–1005. doi: 10.1164/rccm.200405-575OC. [DOI] [PubMed] [Google Scholar]
- 20.Postle AD, Mander A, Reid KB, et al. Deficient hydrophilic lung surfactant proteins A and D with normal surfactant phospholipid molecular species in cystic fibrosis. Am J Respir Cell Mol Biol. 1999;20 (1):90–98. doi: 10.1165/ajrcmb.20.1.3253. [DOI] [PubMed] [Google Scholar]
- 21.Alexis N, Lay JC, Almond M, Peden DB. Inhalation of low dose endotoxin by human volunteers favors a local TH2 response profile and primes airway phagocytes in vivo. J Allergy Clin Immunol. 2004;114:1325–1331. doi: 10.1016/j.jaci.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Alexis NE, Lay JC, Almond M, Patel D, Peden DP. Acute LPS Inhalation in Healthy Volunteers Induces Dendritic Cell Maturation in vivo. J Allergy Clin Immunol. 2005;115:345–350. doi: 10.1016/j.jaci.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 23.Muehlstedt SG, Lyte M, Rodriguez JL. Increased IL-10 production and HLA-DR suppression in the lungs of injured patients precede the development of nosocomial pneumonia. Shock. 2002;17(6):443–450. doi: 10.1097/00024382-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Scholte BJ, Davidson DJ, Wilke M, De Jonge HR. Animal models of cystic fibrosis. J Cyst Fibrosis. 2004;(Suppl 2):183–190. doi: 10.1016/j.jcf.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 25.Alexis NE, Peden DB. Blunting eosinophilic inflammation results in a decreased airway neutrophil response to inhaled LPS in atopic asthmatics: A role for CD14. J Allergy Clin Immunol. 2001;108:577–580. doi: 10.1067/mai.2001.118511. [DOI] [PubMed] [Google Scholar]
- 26.Muhlebach MS, Stewart PW, Leigh MW, Noah TL. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med. 1999;160:186–191. doi: 10.1164/ajrccm.160.1.9808096. [DOI] [PubMed] [Google Scholar]
- 27.Alexis NE, Ghio A, Soukup J, Becker S. Sputum phagocytes are functional and activated: A flow cytometric comparison with cells in bronchoalveolar lavage and peripheral blood. Clinical Immunology. 2000;97:21–32. doi: 10.1006/clim.2000.4911. [DOI] [PubMed] [Google Scholar]
- 28.Alexis NE, Soukup J, Nierkens S, Becker S. Association between airway hyperreactivity and bronchial macrophage dysfunction in individuals with mild asthma. Am J Physiol Lung Cell Mol Physiol. 2001;280(2):L369–375. doi: 10.1152/ajplung.2001.280.2.L369. [DOI] [PubMed] [Google Scholar]
- 29.Ikegami M, Elliott J, Na CL, Korfhagen T, Whitsett JA. Surfactant protein D influences surfactant ultrastructure and uptake by alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 2004;31(2):193–199. doi: 10.1152/ajplung.00142.2004. [DOI] [PubMed] [Google Scholar]
- 30.Strong P, Kishore U, Morgan C, Bernal AL, Singh M, Reid KBM. A novel method of purifying surfactant proteins A and D from the lung lavage of alveolar proteinosis patients and from pooled amniotic fluid. J Immunol Methods. 1998;220:139–149. doi: 10.1016/s0022-1759(98)00160-4. [DOI] [PubMed] [Google Scholar]
- 31.LeVine AM, Hartshorn K, Elliott J, Whitsett J, Korfhagen T. Absence of SP-A modulates innate and adaptive defense responses to pulmonary influenza infection. Am J Physiol Lung Cell Mol Physiol. 2002;282(3):L563–572. doi: 10.1152/ajplung.00280.2001. [DOI] [PubMed] [Google Scholar]
- 32.Haagsman HP, Hawgood S, Sargeant T, Buckley J, White RT, Drickamer K, Benson BJ. The major surfactant protein, SP-A 28–36, is a calcium-dependent carbohydrate-binding protein. J Biol Chem. 1987;262:13877–13880. [PubMed] [Google Scholar]
- 33.Yoshida M, Ikegami M, Reed JA, Chroneos ZC, Whitsett JA. GM-CSF regulates protein and lipid catabolism by alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2001;280:L379–386. doi: 10.1152/ajplung.2001.280.3.L379. [DOI] [PubMed] [Google Scholar]
- 34.Wolk K, Kunz S, Crompton NE, Volk HD, Sabat R. Multiple mechanisms of reduced major histocompatibility complex class II expression in endotoxin tolerance. J Biol Chem. 2003;278(20):18030–18036. doi: 10.1074/jbc.M207714200. [DOI] [PubMed] [Google Scholar]
- 35.Vandivier RW, Fadok VA, Ogden CA, et al. Impaired clearance of apoptotic cells from cystic fibrosis airways. Chest. 2002;121 (Suppl 3):89S. doi: 10.1378/chest.121.3_suppl.89s. [DOI] [PubMed] [Google Scholar]
- 36.Fick RB, Jr, Naegel GP, Matthay RA, Reynolds HY. Cystic fibrosis pseudomonas opsonins. Inhibitory nature in an in vitro phagocytic assay. J Clin Invest. 1981;68:899–914. doi: 10.1172/JCI110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright JR. Immunomodulatory functions of surfactant. Physiol Rev. 1997;77:931–962. doi: 10.1152/physrev.1997.77.4.931. [DOI] [PubMed] [Google Scholar]
- 38.Mariencheck WI, Alcorn JF, Palmer SM, Wright JR. Pseudomonas aeruginosa elastase degrades surfactant proteins A and D. Am J Respir Cell Mol Biol. 2003;28(4):528–537. doi: 10.1165/rcmb.2002-0141OC. [DOI] [PubMed] [Google Scholar]
- 39.LeVine AM, Whitsett JA, Gwozdz JA, et al. Distinct effects of surfactant protein A or D deficiency during bacterial infection on the lung. J Immunol. 2000;165(7):3934–3940. doi: 10.4049/jimmunol.165.7.3934. [DOI] [PubMed] [Google Scholar]
- 40.Sano H, Chiba H, Iwaki D, Sohma H, Voelker DR, Kuroki Y. Surfactant proteins A and D bind CD14 by different mechanisms. J Biol Chem. 2000;275(29):22442–22451. doi: 10.1074/jbc.M001107200. [DOI] [PubMed] [Google Scholar]






