Abstract
Nitric oxide (NO) has been implicated in mediation of cerebral vasodilation during neuronal activation and, specifically, in pharmacological activation of N-methyl-D-aspartate (NMDA) and kainate receptors. Possible mediators of cerebral vasodilation to α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) have not been well studied in mature brain, although heme oxygenase (HO) activity has been implicated in newborn pigs. In anesthetized rats, 5 min of topical superfusion of 30 and 100 μM AMPA on the cortical surface through a closed cranial window resulted in increases in pial arteriolar diameter. The dilatory response to AMPA was not inhibited by superfusion of an NO synthase inhibitor, a cyclooxygenase-2 inhibitor, or a cytochrome P-450 epoxygenase inhibitor, all of which have been shown to inhibit the cortical blood flow response to sensory activation. However, the 48 ± 13% dilation to 100 μM AMPA was attenuated 56–71% by superfusion of the adenosine A2A receptor antagonist ZM-241385, the A2B receptor antagonist alloxazine, and the HO inhibitor chromium mesoporphyrin. Combination of the latter three inhibitors did not attenuate the dilator response more than the individual inhibitors, whereas an AMPA receptor antagonist fully blocked the vasodilation to AMPA. These results indicate that cortical pial arteriolar dilation to AMPA does not require activation of NO synthase, cyclooxygenase-2, or cytochrome P-450 epoxygenase but does depend on activation of adenosine A2A and A2B receptors. In addition, CO derived from HO appears to play a role in the vascular response to AMPA receptor activation in mature brain by a mechanism that is not additive with that of adenosine receptor activation.
Keywords: carbon monoxide, cerebral circulation, cyclooxygenase, epoxygenase, neurovascular coupling, nitric oxide
INCREASES IN NEURAL ACTIVITY lead to increases in local perfusion. This effect may depend, in part, on activation of ionotropic glutamate receptors, such as N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors (35). Therefore, it is of interest to understand which potential vasodilator mechanisms mediate cerebral vasodilation to pharmacological activation of the various glutamate receptor subtypes.
Application of AMPA to the cortical surface increases local O2 consumption (47), but mediators of the vascular response to AMPA have not been well studied. Pial arterioles have been shown to dilate in response to topical application of NMDA and kainate, and this dilation is attenuated by inhibition of nitric oxide (NO) synthase (14–16, 27). However, the effect of NO synthase inhibition on pial arteriolar dilation to AMPA has not been reported in adult cerebral cortex, although CO generated from heme oxygenase (HO) has been implicated in the pial arteriolar response to AMPA in newborn pigs (45). In adult brain, HO may play a role in cerebral vasodilation to kainate-induced seizures (29).
In the present study, the response of pial arterioles to topical application of AMPA was investigated in vivo in mature rats. The potential role of NO synthase was determined by use of the inhibitor Nω-nitro-L-arginine (L-NNA), and the role of HO was determined by the use of the inhibitor chromium mesoporphyrin (CrMP). Other mediators were also evaluated. Because pial arteriolar dilation to glutamate and to sciatic nerve stimulation was reported to be attenuated by the adenosine A2A receptor antagonist 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5] triazin-5-ylamino]ethyl)phenol (ZM-241385) (21, 28), the contribution of adenosine A2A receptors was assessed. In addition to the high-affinity A2A receptors, low-affinity A2B receptors can also contribute to pial arteriolar dilation to adenosine (31, 46). The A2B receptor antagonist alloxazine was used to discern the potential role of adenosine A2B receptors. Furthermore, cyclooxygenase-2 (COX-2) and cytochrome P-450 epoxygenase metabolites of arachidonic acid have been implicated in the cortical blood flow response to somatosensory activation (33, 40, 41). Moreover, the epoxygenase inhibitor N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (MS-PPOH) blocks the local blood flow response to intrastriatal microdialysis perfusion of NMDA (6). Therefore, the effects of MS-PPOH and the COX-2 inhibitor N-[2-(cyclohexyloxy)-4-nitrophenyl]-methanesulfonamide (NS-398) on the pial arteriolar response to AMPA were also tested.
MATERIALS AND METHODS
Surgical procedures
All procedures were approved by the Johns Hopkins University Animal Care and Use Committee. Anesthesia was induced in male Wistar rats (~300 g) with 4% halothane in O2-enriched air and maintained with 1.5–2% halothane during surgery and 1.5% halothane during the experimental protocol. The lungs were mechanically ventilated through a tracheostomy. The inspired gas contained 30–40% O2. A catheter was inserted through a tail artery for arterial blood pressure monitoring and blood sampling for analysis of pH, PCO2, and PO2.
Pial arteriolar diameter was measured through a closed cranial window by intravital microscopy (4, 18). With the rat in the prone position in a head holder, the skull was exposed, the periosteum was removed, and a 3- to 4-mm-wide craniotomy was made over the left parietal cortex. A plastic ring was secured with dental acrylic to the skull surrounding the craniotomy. The exposed dura mater was kept moist with artificial cerebrospinal fluid (aCSF) containing (in mM): 151 Na+, 3 K+, 1.3 Ca2+, 0.6 Mg2+, 134 Cl−, 24.6 HCO3−, 6 urea, and 3.7 glucose. The dura was incised, gently retracted, and cut to expose the pial surface. The window was filled with warmed aCSF, and a glass coverslip was cemented to the plastic ring. The plastic ring had an inflow and outflow port, a port for monitoring pressure, and a thermistor for monitoring fluid temperature.
Experimental protocol
After ≥45 min from completion of surgery, the window was flushed over a 5-min period with aCSF, and baseline measurements were obtained. In different groups of rats, the window was then superfused with 1 mM L-NNA (n = 7), 100 μM NS-398 (n = 8), or 20 μM MS-PPOH (n = 6). A control group was superfused with 0.1% ethanol vehicle (n = 8). In a second experiment, the window was superfused with 15 μM CrMP (n = 7), 1 μM ZM-241385 (n = 7), 1 μM alloxazine (n = 7), CrMP + ZM-241385 + alloxazine (n = 7), or the AMPA receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(f)quinoxaline (NBQX, n = 5). A control group was superfused with 0.01% DMSO vehicle (n = 7).
L-NNA at 1 mM has been shown to inhibit cortical NO synthase activity and the blood flow response to sensory stimulation within 1 h of administration (22, 41). Therefore, all inhibitors/antagonists were superfused for 1 h before testing of vascular reactivity to ensure sufficient time for penetration into underlying cerebral cortex and a consistent exposure time for all inhibitors/antagonists. The rate of superfusion was 0.1 ml/min for the first 20 min and 0.05 ml/min for the last 40 min of superfusion before testing of the agonist. The height of the outflow catheter tip was adjusted to maintain pressure in the window at 5 mmHg during superfusion. NS-398 at 100 μM has been shown to effectively inhibit the cortical blood flow response to whisker stimulation, but not to hypercapnia, acetylcholine, or brady-kinin (33). The inhibition constant for epoxygenase activity in vitro by MS-PPOH is 13 μM (51). MS-PPOH at 20 μM has been shown to inhibit the blood flow response to neural activation and to NMDA administration without inhibition of NO synthase activity (6, 40, 41). CrMP at 15 μM has been shown to inhibit HO activity and to have little effect on NO synthase or guanylyl cyclase activity (3, 25). Because CrMP is sensitive to light, care was taken to keep the CrMP infusion syringe and catheter wrapped in opaque material and to add carbon black to the acrylic cement of the window. ZM-241385 at 1 μM has been shown to inhibit pial arteriolar dilation to topical adenosine, to a selective A2A receptor agonist, and to glutamate in vivo (31, 46). Alloxazine at 1 μM has been shown to inhibit pial arteriolar dilation to topical adenosine and to a selective A2B receptor agonist in vivo (46).
Measurements of arterial blood gases, blood pressure, and arteriolar diameter were repeated 1 h after the start of superfusion with the corresponding drug inhibitor. Pial arteriolar reactivity to AMPA superfusion was studied with two doses that produced significant, dose-dependent increases in diameter within 5 min of superfusion. AMPA (30 μM) + the same drug inhibitor was superfused at a rate of 0.2 ml/min for 5 min and then washed out for 25 min at a rate of 0.1 ml/min with aCSF containing the respective drug inhibitor. The fluid volume in the window was ~0.15 ml. Arteriolar diameter was measured at 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 7, 10, 15, 20, and 30 min after the start of AMPA superfusion. After the 25-min washout period, arterial blood gases were measured. A higher dose of AMPA (100 μM) + the respective drug inhibitor was superfused for 5 min and then washed out for 25 min with the respective inhibitor/antagonist, and measurements were repeated as described above. At the end of the experiment, vascular reactivity to an NO donor was examined by superfusion of the window with 0.3 μM sodium nitroprusside at a rate of 0.1 ml/min for 10 min.
To contrast the effect of L-NNA on the response to AMPA with the known effect of L-NNA on the response to NMDA, the pial arteriolar response to 100 μM NMDA superfusion was tested before and after application of 1 mM L-NNA in a group of eight rats.
Statistical analysis
For each intervention, the percent change in diameter was calculated for each arteriole ranging in baseline diameter from 20 to 100 μm. Statistical analysis was performed using the average percent change of one to four pial arterioles per rat, such that the sample size is the number of rats. Changes in diameter of pial arterioles after 1 h of superfusion of each drug inhibitor/antagonist were compared with baseline values by paired t-test. For each dose of AMPA, the percent change in diameter over time was compared among inhibitor/antagonist groups by two-way ANOVA with repeated measures. If there were an overall treatment effect, additional two-way ANOVA with repeated measures was performed between the control group and each individual inhibitor/antagonist group. If this ANOVA indicated a significant treatment effect or treatment-time interaction, individual time points were compared with the control group by application of the false discovery rate procedure for multiple t-tests (12). In addition, the maximum percent diameter response was compared among groups by one-way ANOVA, and comparisons were made with the control group by Dunnett’s test. Values are means ± SD. The level of significance was set at P < 0.05.
RESULTS
Superfusion of the cranial window with 30 and 100 μM AMPA produced dose-dependent increases in pial arteriolar diameter (Fig. 1). After superfusion of 1 mM L-NNA, the response to AMPA was not significantly reduced. In contrast, L-NNA decreased the dilator response to 100 μM NMDA.
Fig. 1.
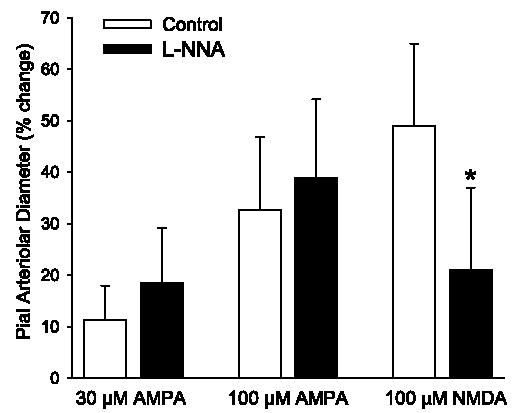
Maximum percent increase in pial arteriolar diameter during superfusion of 30 and 100 μM α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) in control group (n = 8) and a group superfused with Nω-nitro-L-arginine (L-NNA, n = 7). Values are means ± SD. There was no significant effect of L-NNA on the AMPA response. In another group (n = 8), L-NNA reduced the dilator response to 100 μM N-methyl-D-aspartate (NMDA). *P < 0.05 vs. control.
To evaluate the contribution of other possible mediators, the response to 30 and 100 μM AMPA was examined in separate groups of rats after superfusion of the cranial window for 1 h with a single inhibitor. At the end of 1 h of superfusion of these inhibitors, the percent change in pial arteriolar diameter was not significantly different from baseline (Table 1). Mean arterial blood pressure and arterial PCO2 at the time of AMPA superfusion were in the normal range and were not different from the vehicle groups (Table 2). Arterial pH was 7.35–7.49, arterial PO2 was kept above 100 mmHg to maintain oxyhemoglobin saturation, arterial hemoglobin concentration was 11–15 g/dl, and rectal temperature was 36.5–38.0°C.
Table 1.
Baseline diameter and percent change in baseline pial arteriolar diameter 1 h after superfusion of inhibitor/antagonist
| Baseline Diameter
|
||
|---|---|---|
| μm | %Change | |
| Ethanol (0.1%) | 56±14 | −1.9±2.8 |
| L-NNA | 56±8 | −7.3±8.5 |
| NS-398 | 63±14 | 6.4±7.1 |
| MS-PPOH | 50±14 | 2.1±9.2 |
| DMSO (0.01%) | 50±8 | −2.5±6.7 |
| ZM-241385 | 51±18 | 6.9±7.7 |
| Alloxazine | 54±15 | 0.4±4.4 |
| CrMP | 52±10 | −1.4±3.7 |
| Combined | 48±19 | 5.7±8.1 |
| NBQX | 39±6 | 0.2±7.2 |
Values are means ± SD. L-NNA, Nω-nitro-L-arginine; MS-PPOH, N-methonylsulfonyl-6-(2-propargyloxyphenyl) hexanamide; CrMP, chromium mesoporphyrin; NBQX, 6-nitro-7-sulfamoylbenzo (f) quinoxaline.
Table 2.
Mean arterial blood pressure and arterial PCO2 in rats superfused with 30 and 100 μM AMPA and treated with inhibitors
| 30 μM AMPA | 100μM AMPA | |
|---|---|---|
| Mean arterial blood pressure, mmHg | ||
| Ethanol (0.1%) | 92±9 | 91±10 |
| L-NNA | 89±8 | 90±10 |
| NS-398 | 92±11 | 85±7 |
| MS-PPOH | 93±14 | 89±14 |
| DMSO (0.01%) | 96±13 | 99±12 |
| ZM-241385 | 98±6 | 99±5 |
| Alloxazine | 99±3 | 99±5 |
| CrMP | 99±2 | 99±2 |
| Combined | 98±3 | 98±3 |
| NBQX | 99±6 | 99±4 |
| Arterial PCO2, Torr | ||
| Ethanol (0.1%) | 39±3 | 40±3 |
| L-NNA | 40±3 | 40±3 |
| NS-398 | 41±3 | 40±4 |
| MS-PPOH | 42±3 | 40±4 |
| DMSO (0.01%) | 41±3 | 40±3 |
| ZM-241385 | 38±3 | 39±3 |
| Alloxazine | 40±3 | 40±3 |
| CrMP | 41±4 | 42±2 |
| Combined | 39±3 | 38±3 |
| NBQX | 36±2 | 37±2 |
Values are means ± SD. AMPA, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid.
Pial arteriolar diameter was measured at 0.5-min intervals during the 5-min periods of superfusion of 30 and 100 μM AMPA. The maximum increase in diameter during low- and high-dose AMPA superfusion was not significantly reduced by the COX-2 inhibitor NS-398 or by the epoxygenase inhibitor MS-PPOH (Fig. 2).
Fig. 2.
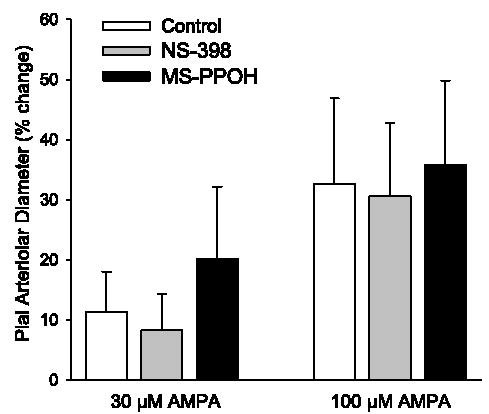
Maximum percent increase in pial arteriolar diameter during superfusion of 30 and 100 μM AMPA in control group (n = 8), a group superfused with 100 μM NS-398 (n = 8), and a group superfused with 20 μM N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (MS-PPOH, n = 6). Values are means ± SD. There was no significant effect of NS-398 or MS-PPOH on the AMPA response.
Treatment with 15 μM CrMP did not significantly affect the response to 30 μM AMPA. However, CrMP substantially blunted the maximum response to 100 μM AMPA (Fig. 3). Two-way repeated-measures ANOVA of the time-course response to 100 μM AMPA between the control and CrMP-treated groups indicated a significant overall treatment effect of CrMP (P < 0.025) and a significant treatment-time interaction (P < 0.001). The response was significantly reduced from 3.5 through 7 min after the start of 100 μM AMPA superfusion (Fig. 4).
Fig. 3.
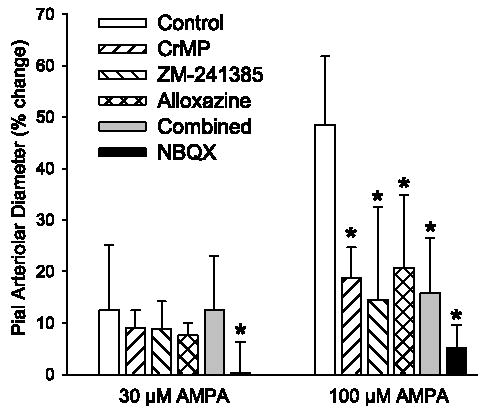
Maximum percent increase in pial arteriolar diameter during superfusion of 30 and 100 μM AMPA in control group (n = 7), a group superfused with 15 μM chromium mesoporphyrin (CrMP, n = 7), a group superfused with 1 μM ZM-241385 (n = 7), a group superfused with 1 μM alloxazine (n = 7), a group superfused with CrMP + ZM-241385 + alloxazine (Combined, n = 7), and a group superfused with 30 μM 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(f)quinoxaline (NBQX, n = 5). Values are means ± SD. *P < 0.05 vs. control.
Fig. 4.
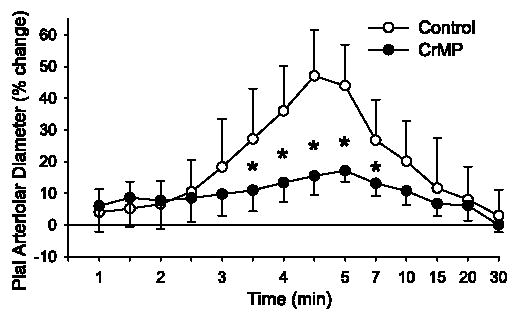
Time course of percent change of pial arteriolar diameter during 5 min of superfusion of 100 μM AMPA in 0.01% DMSO control group and group superfused with 15 μM CrMP. Values are means ±SD (n = 7). *P < 0.05 vs. control at the same time. Superfusion of AMPA started at 0 min at a rate of 0.2 ml/min through a cranial window with a volume of ~0.15 ml. Washout of AMPA with 0.01% DMSO or CrMP in artificial cerebrospinal fluid (aCSF) started at 5 min at a rate of 0.1 ml/min. Note nonlinear time scale during washout.
After superfusion of 1 μM ZM-241385, an adenosine A2A receptor antagonist, two-way repeated-measures ANOVA indicated a significant treatment-time interaction at 30 μM (P < 0.02) and 100 μM (P < 0.001) AMPA and a significant overall treatment effect at 100 μM AMPA (P < 0.005). At 100 μM AMPA, the dilatory response was significantly decreased from 3.5 to 10 min after the start of AMPA superfusion (Fig. 5). The maximum increase in pial arteriolar diameter to 100 μM AMPA was significantly reduced by ZM-241385 (Fig. 3).
Fig. 5.
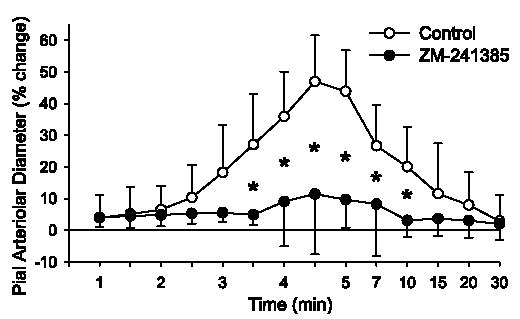
Time course of percent change of pial arteriolar diameter during 5 min of superfusion of 100 μM AMPA in 0.01% DMSO control group and a group superfused with 1 μM ZM-241385. Values are means ± SD (n = 7). *P < 0.05 vs. control at the same time point. Superfusion of AMPA started at 0 min. Washout of AMPA with 0.01% DMSO or ZM-241385 in aCSF started at 5 min. Note nonlinear time scale during washout.
After superfusion of 1 μM alloxazine, the adenosine A2B receptor antagonist, two-way repeated-measures ANOVA did not indicate a significant treatment-time interaction (P < 0.07) at 30 μM AMPA, and the maximal response was not significantly altered. However, at 100 μM AMPA, the treatment-time interaction was significant (P < 0.001), and the dilatory response was significantly attenuated from 3.5 to 5 min after the start of AMPA superfusion (Fig. 6). The maximum increase in pial arteriolar diameter was significantly decreased by alloxazine at 100 μM AMPA (Fig. 3).
Fig. 6.
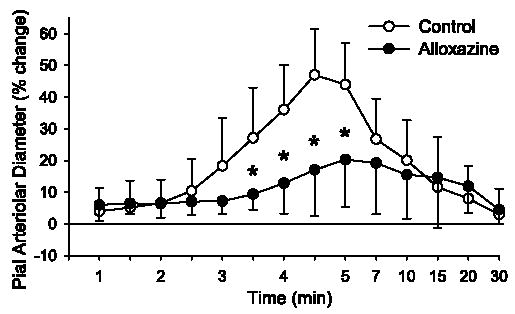
Time course of percent change of pial arteriolar diameter during 5 min of superfusion of 100 μM AMPA in 0.01% DMSO control group and a group superfused with 1 μM alloxazine. Values are means ± SD (n = 7). *P < 0.05 vs. control at the same time. Superfusion of AMPA started at 0 min. Washout of AMPA with 0.01% DMSO or alloxazine in aCSF started at 5 min. Note nonlinear time scale during washout.
Because CrMP, ZM-241385, and alloxazine attenuated, but did not eliminate, the dilatory response to AMPA, these drugs were combined to determine whether they had additive inhibitory effects. Dilation to 100 μM AMPA was attenuated by the combination of these three drugs compared with the control group (Fig. 7). However, the attenuation was similar to that seen with superfusion of the individual drugs (Fig. 3), thereby implying that these drugs do not act in an additive fashion. Moreover, increasing the concentration of ZM-241385 from 1 to 10 μM did not further attenuate the dilator response to 100 μM AMPA (from 14 ± 18% to 18 ± 6%), indicating that the dose of ZM-241385 was not submaximal. Higher doses of CrMP and alloxazine were not tested because of concern that these agents would lose selectivity.
Fig. 7.
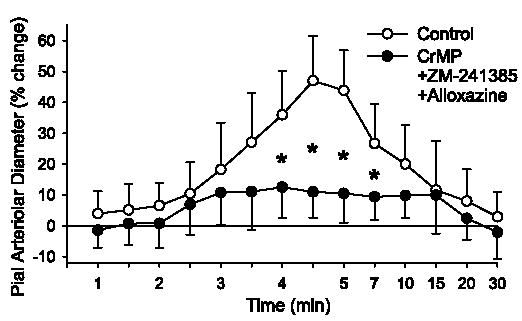
Time course of percent change of pial arteriolar diameter during 5 min of superfusion of 100 μM AMPA in 0.01% DMSO control group and a group superfused with 15 μM CrMP + 1 μM ZM-241385 + 1 μM alloxazine. Values are means ± SD (n = 7). *P < 0.05 vs. control at the same time. Superfusion of AMPA started at 0 min. Washout of AMPA with 0.01% DMSO or drug combination in aCSF started at 5 min. Note nonlinear time scale during washout.
To determine whether the residual response may have been due an effect of AMPA not mediated by AMPA receptors, the effect of the AMPA receptor antagonist NBQX was tested. Superfusion of 30 μM NBQX blocked the dilation to 30 μM (Fig. 3) and 100 μM (Fig. 8) AMPA. The response to 100 μM AMPA after NBQX was significantly different from that after CrMP + ZM-241385 + alloxazine.
Fig. 8.
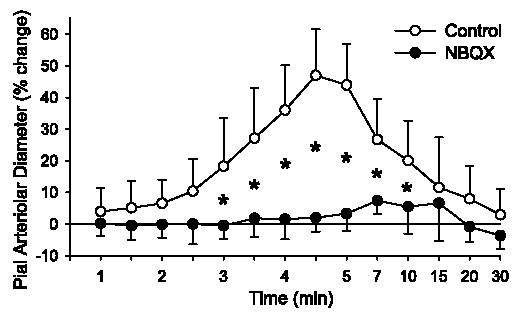
Time course of percent change of pial arteriolar diameter during 5 min of superfusion of 100 μM AMPA in 0.01% DMSO control group (n = 7) and a group superfused with 30 μM NBQX (n = 5). Values are means ± SD. *P < 0.05 vs. control at the same time. Superfusion of AMPA started at 0 min. Washout of AMPA with 0.01% DMSO or NBQX in aCSF started at 5 min. Note nonlinear time scale during washout.
The ability of pial arterioles to dilate to 0.3 μM sodium nitroprusside was tested 25 min after washout of 100 μM AMPA in the continued presence of each inhibitor/antagonist. The percent increase in diameter was not different from the control groups (Fig. 9), thereby demonstrating specificity of the inhibitory effects of CrMP, ZM-241385, and alloxazine on the AMPA response.
Fig. 9.
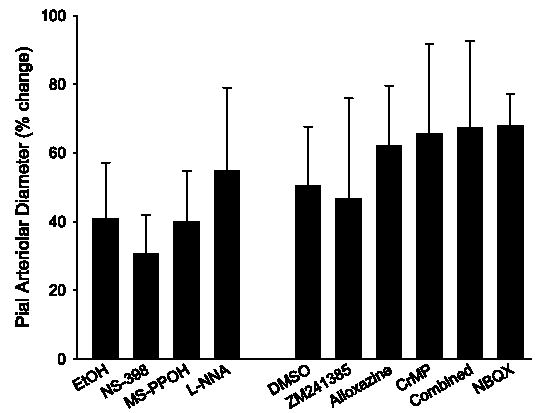
Percent increase in pial arteriolar diameter during superfusion of 0.3 μM sodium nitroprusside in the 0.1% ethanol (EtOH) control group and groups superfused with L-NNA, NS-398, and MS-PPOH in the 1st experiment and in the 0.01% DMSO control group and groups superfused with ZM-241385, alloxazine, CrMP, CrMP + ZM-241385 + alloxazine (Combined), and NBQX in the 2nd experiment. Values are means ± SD. There were no differences from the control groups.
DISCUSSION
The major new findings of this study are as follows: 1) AMPA receptor activation in the cerebral cortex of adult rats leads to dilation of extraparenchymal pial arterioles in vivo, 2) this dilation is partially mediated by activation of adenosine A2A and A2B receptors, 3) HO, rather than NO or arachidonic acid, metabolites of COX-2 or epoxygenases, also contributes to the vasodilatory response, and 4) A2A receptors, A2B receptors, and HO do not act by parallel, independent mechanisms.
The nonselective adenosine receptor antagonist theophylline has been shown to partially inhibit cerebral vasodilation in response to whisker stimulation (13) and topical application of glutamate (21). Superfusion of rat cortical slices with glutamate or AMPA is capable of generating release of adenosine (11, 19). Adenosine dilates pial arterioles and intraparenchymal arterioles primarily through the A2 receptor family (10, 20, 32). Further work by others (31, 46) has demonstrated that ZM-241385 inhibited pial and penetrating arteriolar dilation to low concentrations of adenosine, whereas alloxazine provided greater inhibition than ZM-241385 at high concentrations of adenosine. Availability of highly selective A2B receptor antagonists is limited. Alloxazine is approximately one order of magnitude more selective for A2B than for A2A receptors (8), whereas ZM-241385 is nearly two orders of magnitude more selective for A2A than for A2B receptors (36, 37, 43). In cardiac myocytes, 1 μM alloxazine inhibited by 50% the contractile response to 5′-(N-ethylcarboxamido)-adenosine (NECA), an A2 receptor agonist that appears to have selectivity for A2B receptors, whereas this dose of alloxazine exerted only a slight inhibition of the response to the A2A receptor agonist CGS-21660 (26). In pial arterioles, 1 μM alloxazine completely blocked dilation in vivo to NECA at low doses at which the dilation was not inhibited by 1 μM ZM-241385 (46). Thus, at the doses used in vivo, ZM-241385 and alloxazine are expected to show selectivity for the corresponding receptor subtypes. If the response to AMPA were mediated by A2A, and not by A2B, receptors, the inhibitory effect of alloxazine on the dilatory response would be expected to be much smaller than that of ZM-241385. The similarity of the magnitude of the inhibition of the dilation to AMPA with ZM-241385 and alloxazine suggests involvement of both receptor subtypes. A contribution of both receptor subtypes to the same functional response in the same cell type has precedent in cardiac myocytes (26). However, because the effect of alloxazine on pial arteriolar dilation to an A2A receptor agonist was not tested, an effect of alloxazine on A2A receptors cannot be completely excluded in the present study.
Involvement of A2A receptors in the vascular response to AMPA is consistent with the work of others showing that ZM-241385 inhibits pial arteriolar dilation to sciatic nerve stimulation and glutamate superfusion (21, 28). However, involvement of A2B receptors was not necessarily expected because of their lower affinity for adenosine. Moreover, vasodilation by A2B receptor activation partially depends on NO synthesis (46), whereas NO synthesis inhibition had no effect on AMPA-evoked dilation in the present experiments. An alternative possibility is based on reports that activation of adenosine A2B receptors on astrocytes increases intracellular Ca2+ (2, 24, 42), and increases in astrocyte Ca2+ can be coupled to vasodilation (17, 54). Perhaps astrocyte A2B receptors are part of a signaling pathway coupling AMPA receptor activation to vascular dilation, whereas A2A receptors largely mediate the direct effects of adenosine on pial arteriolar smooth muscle.
Because ZM-241385 and alloxazine did not completely block the dilatory response to AMPA, mediators other than adenosine might be involved. One potential mediator is CO derived from HO activity. HO activity has been implicated in cerebral vasodilation associated with seizures evoked by systemic administration of kainate in rats (29) and bicuculline administration in newborn pigs (44). However, seizure activity releases abundant amounts of glutamate, which can then activate all glutamate receptor subtypes. Our results with the HO inhibitor CrMP indicate that HO activity also participates in pial arteriolar dilation during AMPA receptor activation. At high concentrations, CrMP can inhibit NO synthase and guanylyl cyclase, but 15 μM CrMP (present study) had no significant effect on NO synthase and guanylyl cyclase (3). In addition, NO synthase inhibition in the present study did not have the same effect as CrMP on the vascular response to AMPA. In newborn pigs, glutamate is postulated to act on glutamate receptors in the endothelium and stimulate endothelial HO (38, 39). However, there is little evidence of functional vascular glutamate receptors in adult brain (5, 14, 15, 30). The constitutive isoform of HO, HO-2, is enriched in neurons (50). Thus it is presumed that AMPA acted on neuronal receptors to stimulate HO-2 activity in neurons to generate diffusible CO in the present study on adult rats.
Although CO may cause dilation by increasing cGMP in vascular smooth muscle, it also exerts a primary effect directly on Ca2+ -sensitive K+ channels (23, 52). Adenosine is thought to relax arteriolar smooth muscle by increasing cAMP. The present findings that CrMP, ZM-241385, and alloxazine each inhibit the AMPA response individually by >50% suggest that there may be some interaction in the signaling pathways. Indeed, when the three inhibitors were combined, there was no further inhibition of the response compared with that obtained with each individual drug. The lack of an additive effect with combined inhibition also supports an interaction of adenosine-and CO-signaling pathways. Whether adenosine and CO are regulated sequentially (e.g., CO stimulates release of adenosine or adenosine receptor activation stimulates release of CO) or adenosine receptor activation and CO act cooperatively in permitting the opening of K+ channels is not known.
The finding that none of the inhibitor drugs that were tested alone or in combination completely eliminated the dilatory response to AMPA raised the possibility that AMPA might be partially acting via a non-AMPA receptor mechanism. However, the AMPA receptor antagonist NBQX blocked the response, and the response after NBQX was significantly less than the response to CrMP + ZM-241385 + alloxazine. Thus the vasodilation by AMPA is mediated by AMPA receptors, and an additional mechanism appears to be responsible for the residual dilation after inhibition of HO and adenosine A2A and A2B receptors.
Pial arteriolar vasodilation in response to topical NMDA is dependent on NO synthesis and is presumed to be linked to a coupling of neuronal NO synthase to an NMDA receptor macromolecular complex (9). It is unclear whether pial arteriolar dilation in response to activation of other ionotropic glutamate receptors is also dependent directly on NO generation. Kainate produces pial arteriolar dilation that is attenuated by L-NNA (15). However, it is possible that a high level of activation of kainate receptors can cause release of glutamate, which could exert a secondary effect of NMDA receptor activation (48). The present results demonstrate that a dose of L-NNA that was sufficient to inhibit NMDA-dependent dilation had no effect on the dilation evoked by AMPA. This result was somewhat unexpected, inasmuch as previous work from this laboratory showed that infusion of AMPA into a microdialysis probe in the hippocampus increased the nitroarginine-inhibitable conversion of arginine to citrulline (7). Perhaps differences in the properties of hippocampal and cortical neurons and glia result in a different coupling of AMPA receptors to NO production, or delivery of AMPA by the microdialysis route led to enhanced glutamate release and secondary activation of NMDA receptors. Regional differences are also present between the cerebral cortex and the cerebellum, where NO plays a greater role in AMPA-dependent neurovascular coupling (53). Therefore, the present finding, showing a lack of NO dependence in AMPA-evoked dilation, might be limited to cortical pial arterioles.
Metabolites of COX-2 (33), but not COX-1 (34), have been implicated in cortical vasodilation by whisker stimulation. NS-398 at 100 μM, similar to that presently used, suppressed the response to whisker stimulation ~50%, with no further decrement at 300 μM. However, NS-398 had no significant effect on the pial arteriolar response to AMPA in the present study. Similarly, the cytochrome P-450 epoxygenase inhibitor MS-PPOH at a concentration that suppressed the cortical hyperemia response to whisker and forepaw stimulation (40, 41) had no effect on the response to AMPA. These comparisons imply that stimulation of these two arachidonic acid pathways sufficient to result in vasodilation during functional activation is not mediated by AMPA receptor stimulation. However, our results are restricted to extraparenchymal pial arterioles. Intraparenchymal arterioles may be subjected to greater control by arachidonic acid metabolites. For example, the cytochrome P-450 2C11 epoxygenase is expressed in rat astrocytes (1, 41), and intraparenchymal vessels surrounded by astrocytes may be under greater influence of epoxyeicosatrienoic acid generated by cytochrome P-450 2C11. Moreover, COX-1 activity has recently been implicated in coupling between astrocyte foot processes and intraparenchymal vasodilation (49).
In summary, the present study provides evidence that activation of cortical AMPA receptors produces pial arteriolar dilation that is partially mediated by adenosine A2A and A2B receptors. In addition, CO, rather than NO, appears to participate in the dynamic response to AMPA activation. Adenosine receptor activation and CO act in a nonadditive manner, thereby implying that their signaling is processed in series or in a nonlinear interaction on target proteins such as K+ channels. The lack of effect of NO synthase inhibition on the cortical pial vascular response to AMPA differs from the effect of NMDA in the cortex and from the role of AMPA on vasodilation in the cerebellum. Therefore, activation of specific glutamate receptor subtypes regulates cerebrovascular dilation by distinct pathways in distinct brain regions.
Acknowledgments
The authors thank Tzipora Sofare for editorial assistance.
Footnotes
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-59996.
References
- 1.Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke. 1996;27:971–979. doi: 10.1161/01.str.27.5.971. [DOI] [PubMed] [Google Scholar]
- 2.Alloisio S, Cugnoli C, Ferroni S, Nobile M. Differential modulation of ATP-induced calcium signalling by A1 and A2 adenosine receptors in cultured cortical astrocytes. Br J Pharmacol. 2004;141:935–942. doi: 10.1038/sj.bjp.0705707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appleton SD, Chretien ML, McLaughlin BE, Vreman HJ, Stevenson DK, Brien JF, Nakatsu K, Maurice DH, Marks GS. Selective inhibition of heme oxygenase, without inhibition of nitric oxide synthase or soluble guanylyl cyclase, by metalloporphyrins at low concentrations. Drug Metab Dispos. 1999;27:1214–1219. [PubMed] [Google Scholar]
- 4.Asano Y, Koehler RC, Kawaguchi T, McPherson RW. Pial arteriolar constriction to α2-adrenergic agonist dexmedetomidine in the rat. Am J Physiol Heart Circ Physiol. 1997;272:H2547–H2556. doi: 10.1152/ajpheart.1997.272.6.H2547. [DOI] [PubMed] [Google Scholar]
- 5.Beart PM, Sheehan KAM, Manallack DT. Absence of N-methyl-D-aspartate receptors on ovine cerebral microvessels. J Cereb Blood Flow Metab. 1988;8:879–882. doi: 10.1038/jcbfm.1988.146. [DOI] [PubMed] [Google Scholar]
- 6.Bhardwaj A, Northington FJ, Carhuapoma JR, Falck JR, Harder DR, Traystman RJ, Koehler RC. P-450 epoxygenase and NO synthase inhibitors reduce cerebral blood flow response to N-methyl-D-aspartate. Am J Physiol Heart Circ Physiol. 2000;279:H1616–H1624. doi: 10.1152/ajpheart.2000.279.4.H1616. [DOI] [PubMed] [Google Scholar]
- 7.Bhardwaj A, Northington FJ, Ichord RN, Hanley DF, Traystman RJ, Koehler RC. Characterization of ionotropic glutamate receptor-mediated nitric oxide production in vivo in rats. Stroke. 1997;28:850–857. doi: 10.1161/01.str.28.4.850. [DOI] [PubMed] [Google Scholar]
- 8.Brackett LE, Daly JW. Functional characterization of the A2b adenosine receptor in NIH 3T3 fibroblasts. Biochem Pharmacol. 1994;47:801–814. doi: 10.1016/0006-2952(94)90480-4. [DOI] [PubMed] [Google Scholar]
- 9.Christopherson KS, Hillier BJ, Lim WA, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274:27467–27473. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- 10.Coney AM, Marshall JM. Role of adenosine and its receptors in the vasodilatation induced in the cerebral cortex of the rat by systemic hypoxia. J Physiol. 1998;509:507–518. doi: 10.1111/j.1469-7793.1998.507bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craig CG, White TD. N-methyl-D-aspartate- and non-N-methyl-D-aspartate-evoked adenosine release from rat cortical slices: distinct purinergic sources and mechanisms of release. J Neurochem. 1993;60:1073–1080. doi: 10.1111/j.1471-4159.1993.tb03256.x. [DOI] [PubMed] [Google Scholar]
- 12.Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1–R8. doi: 10.1152/ajpregu.2000.279.1.R1. [DOI] [PubMed] [Google Scholar]
- 13.Dirnagl U, Niwa K, Lindauer U, Villringer A. Coupling of cerebral blood flow to neuronal activation: role of adenosine and nitric oxide. Am J Physiol Heart Circ Physiol. 1994;267:H296–H301. doi: 10.1152/ajpheart.1994.267.1.H296. [DOI] [PubMed] [Google Scholar]
- 14.Faraci FM, Breese KR. Nitric oxide mediates vasodilation in response to activation of N-methyl-D-aspartate receptors in brain. Circ Res. 1993;72:476–480. doi: 10.1161/01.res.72.2.476. [DOI] [PubMed] [Google Scholar]
- 15.Faraci FM, Breese KR, Heistad DD. Responses of cerebral arterioles to kainate. Stroke. 1994;25:2080–2084. doi: 10.1161/01.str.25.10.2080. [DOI] [PubMed] [Google Scholar]
- 16.Faraci FM, Brian JE., Jr 7-Nitroindazole inhibits brain nitric oxide synthase and cerebral vasodilation in response to N-methyl-D-aspartate. Stroke. 1995;26:2172–2176. doi: 10.1161/01.str.26.11.2172. [DOI] [PubMed] [Google Scholar]
- 17.Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res. 2004;95:E73–E81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- 18.Hirata T, Koehler RC, Kawaguchi T, Brusilow SW, Traystman RJ. Impaired pial arteriolar reactivity to hypercapnia during hyperammonemia depends on glutamine synthesis. Stroke. 1996;27:729–736. doi: 10.1161/01.str.27.4.729. [DOI] [PubMed] [Google Scholar]
- 19.Hoehn K, White TD. Role of excitatory amino acid receptors in K+-and glutamate-evoked release of endogenous adenosine from rat cortical slices. J Neurochem. 1990;54:256–265. doi: 10.1111/j.1471-4159.1990.tb13309.x. [DOI] [PubMed] [Google Scholar]
- 20.Ibayashi S, Ngai AC, Meno JR, Winn HR. Effects of topical adenosine analogs and forskolin on rat pial arterioles in vivo. J Cereb Blood Flow Metab. 1991;11:72–76. doi: 10.1038/jcbfm.1991.8. [DOI] [PubMed] [Google Scholar]
- 21.Iliff JJ, D’Ambrosio R, Ngai AC, Winn HR. Adenosine receptors mediate glutamate-evoked arteriolar dilation in the rat cerebral cortex. Am J Physiol Heart Circ Physiol. 2003;284:H1631–H1637. doi: 10.1152/ajpheart.00909.2002. [DOI] [PubMed] [Google Scholar]
- 22.Irikura K, Maynard KI, Moskowitz MA. Importance of nitric oxide synthase inhibition to the attenuated vascular responses induced by topical L-nitroarginine during vibrissal stimulation. J Cereb Blood Flow Metab. 1994;14:45–48. doi: 10.1038/jcbfm.1994.7. [DOI] [PubMed] [Google Scholar]
- 23.Jaggar JH, Li A, Parfenova H, Liu J, Umstot ES, Dopico AM, Leffler CW. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res. 2005;97:805–812. doi: 10.1161/01.RES.0000186180.47148.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jimenez AI, Castro E, Mirabet M, Franco R, Delicado EG, Miras-Portugal MT. Potentiation of ATP calcium responses by A2B receptor stimulation and other signals coupled to Gs proteins in type-1 cerebellar astrocytes. Glia. 1999;26:119–128. [PubMed] [Google Scholar]
- 25.Leffler CW, Nasjletti A, Yu C, Johnson RA, Fedinec AL, Walker N. Carbon monoxide and cerebral microvascular tone in newborn pigs. Am J Physiol Heart Circ Physiol. 1999;276:H1641–H1646. doi: 10.1152/ajpheart.1999.276.5.H1641. [DOI] [PubMed] [Google Scholar]
- 26.Liang BT, Haltiwanger B. Adenosine A2a and A2b receptors in cultured fetal chick heart cells. High- and low-affinity coupling to stimulation of myocyte contractility and cAMP accumulation. Circ Res. 1995;76:242–251. doi: 10.1161/01.res.76.2.242. [DOI] [PubMed] [Google Scholar]
- 27.Meng W, Tobin JR, Busija DW. Glutamate-induced cerebral vasodilation is mediated by nitric oxide through N-methyl-D-aspartate receptors. Stroke. 1995;26:857–863. doi: 10.1161/01.str.26.5.857. [DOI] [PubMed] [Google Scholar]
- 28.Meno JR, Crum AV, Winn HR. Effect of adenosine receptor blockade on pial arteriolar dilation during sciatic nerve stimulation. Am J Physiol Heart Circ Physiol. 2001;281:H2018–H2027. doi: 10.1152/ajpheart.2001.281.5.H2018. [DOI] [PubMed] [Google Scholar]
- 29.Montecot C, Seylaz J, Pinard E. Carbon monoxide regulates cerebral blood flow in epileptic seizures but not in hypercapnia. Neuroreport. 1998;9:2341–2346. doi: 10.1097/00001756-199807130-00035. [DOI] [PubMed] [Google Scholar]
- 30.Morley P, Small DL, Murray CL, Mealing GA, Poulter MO, Durkin JP, Stanimirovic DB. Evidence that functional glutamate receptors are not expressed on rat or human cerebromicrovascular endothelial cells. J Cereb Blood Flow Metab. 1998;18:396–406. doi: 10.1097/00004647-199804000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Ngai AC, Coyne EF, Meno JR, West GA, Winn HR. Receptor subtypes mediating adenosine-induced dilation of cerebral arterioles. Am J Physiol Heart Circ Physiol. 2001;280:H2329–H2335. doi: 10.1152/ajpheart.2001.280.5.H2329. [DOI] [PubMed] [Google Scholar]
- 32.Ngai AC, Winn HR. Effects of adenosine and its analogues on isolated intracerebral arterioles. Extraluminal and intraluminal application. Circ Res. 1993;73:448–457. doi: 10.1161/01.res.73.3.448. [DOI] [PubMed] [Google Scholar]
- 33.Niwa K, Araki E, Morham SG, Ross ME, Iadecola C. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J Neurosci. 2000;20:763–770. doi: 10.1523/JNEUROSCI.20-02-00763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niwa K, Haensel C, Ross ME, Iadecola C. Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ Res. 2001;88:600–608. doi: 10.1161/01.res.88.6.600. [DOI] [PubMed] [Google Scholar]
- 35.Norup Nielsen A, Lauritzen M. Coupling and uncoupling of activity-dependent increases of neuronal activity and blood flow in rat somatosensory cortex. J Physiol. 2001;533:773–785. doi: 10.1111/j.1469-7793.2001.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ongini E, Dionisotti S, Gessi S, Irenius E, Fredholm BB. Comparison of CGS 15943, ZM 241385 and SCH 58261 as antagonists at human adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:7–10. doi: 10.1007/pl00005326. [DOI] [PubMed] [Google Scholar]
- 37.Palmer TM, Poucher SM, Jacobson KA, Stiles GL. 125I-4-(2-[7-amino-2-[2-furyl][1,2,4]triazolo[2,3-a][1,3,5]triazin-5-yl-amino]ethyl) phenol, a high-affinity antagonist radioligand selective for the A2a adenosine receptor. Mol Pharmacol. 1995;48:970–974. [PMC free article] [PubMed] [Google Scholar]
- 38.Parfenova H, Fedinec A, Leffler CW. Ionotropic glutamate receptors in cerebral microvascular endothelium are functionally linked to heme oxygenase. J Cereb Blood Flow Metab. 2003;23:190–197. doi: 10.1097/01.WCB.000004823561824.C4. [DOI] [PubMed] [Google Scholar]
- 39.Parfenova H, Neff RA, III, Alonso JS, Shlopov BV, Jamal CN, Sarkisova SA, Leffler CW. Cerebral vascular endothelial heme oxygenase: expression, localization, and activation by glutamate. Am J Physiol Cell Physiol. 2001;281:C1954–C1963. doi: 10.1152/ajpcell.2001.281.6.C1954. [DOI] [PubMed] [Google Scholar]
- 40.Peng X, Carhuapoma JR, Bhardwaj A, Alkayed NJ, Falck JR, Harder DR, Traystman RJ, Koehler RC. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol. 2002;283:H2029–H2037. doi: 10.1152/ajpheart.01130.2000. [DOI] [PubMed] [Google Scholar]
- 41.Peng X, Zhang C, Alkayed NJ, Harder DR, Koehler RC. Dependency of cortical functional hyperemia to forepaw stimulation on epoxygenase and nitric oxide synthase activities in rats. J Cereb Blood Flow Metab. 2004;24:509–517. doi: 10.1097/00004647-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Pilitsis JG, Kimelberg HK. Adenosine receptor mediated stimulation of intracellular calcium in acutely isolated astrocytes. Brain Res. 1998;798:294–303. doi: 10.1016/s0006-8993(98)00430-2. [DOI] [PubMed] [Google Scholar]
- 43.Poucher SM, Keddie JR, Singh P, Stoggall SM, Caulkett PW, Jones G, Coll MG. The in vitro pharmacology of ZM 241385, a potent, non-xanthine A2a selective adenosine receptor antagonist. Br J Pharmacol. 1995;115:1096–1102. doi: 10.1111/j.1476-5381.1995.tb15923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pourcyrous M, Bada HS, Parfenova H, Daley ML, Korones SB, Leffler CW. Cerebrovasodilatory contribution of endogenous carbon monoxide during seizures in newborn pigs. Pediatr Res. 2002;51:579–585. doi: 10.1203/00006450-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Robinson JS, Fedinec AL, Leffler CW. Role of carbon monoxide in glutamate receptor-induced dilation of newborn pig pial arterioles. Am J Physiol Heart Circ Physiol. 2002;282:H2371–H2376. doi: 10.1152/ajpheart.00911.2001. [DOI] [PubMed] [Google Scholar]
- 46.Shin HK, Shin YW, Hong KW. Role of adenosine A2B receptors in vasodilation of rat pial artery and cerebral blood flow autoregulation. Am J Physiol Heart Circ Physiol. 2000;278:H339–H344. doi: 10.1152/ajpheart.2000.278.2.H339. [DOI] [PubMed] [Google Scholar]
- 47.Sinha AK, Azevedo R, Chi OZ, Weiss HR. Down-regulation of AMPA glutamate receptors reduces cerebrocortical metabolic response to stimulation. Neurochem Res. 2004;29:1425–1430. doi: 10.1023/b:nere.0000026407.36663.e4. [DOI] [PubMed] [Google Scholar]
- 48.Stein-Behrens BA, Elliott EM, Miller CA, Schilling JW, Newcombe R, Sapolsky RM. Glucocorticoids exacerbate kainic acid-induced extracellular accumulation of excitatory amino acids in the rat hippocampus. J Neurochem. 1992;58:1730–1735. doi: 10.1111/j.1471-4159.1992.tb10047.x. [DOI] [PubMed] [Google Scholar]
- 49.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 50.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 51.Wang MH, Brand-Schieber E, Zand BA, Nguyen X, Falck JR, Balu N, Schwartzman ML. Cytochrome P450-derived arachidonic acid metabolism in the rat kidney: characterization of selective inhibitors. J Pharmacol Exp Ther. 1998;284:966–973. [PubMed] [Google Scholar]
- 52.Xi Q, Tcheranova D, Parfenova H, Horowitz B, Leffler CW, Jaggar JH. Carbon monoxide activates KCa channels in newborn arteriole smooth muscle cells by increasing apparent Ca2+ sensitivity of α-subunits. Am J Physiol Heart Circ Physiol. 2004;286:H610–H618. doi: 10.1152/ajpheart.00782.2003. [DOI] [PubMed] [Google Scholar]
- 53.Yang G, Chen G, Ebner TJ, Iadecola C. Nitric oxide is the predominant mediator of cerebellar hyperemia during somatosensory activation in rats. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1760–R1770. doi: 10.1152/ajpregu.1999.277.6.R1760. [DOI] [PubMed] [Google Scholar]
- 54.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]


