Abstract
The various cell types in the vertebrate retina arise from a pool of common progenitors. The way that the cell types are specified has been a long-standing issue. Decades of research have yielded a large body of information regarding the involvement of extrinsic factors, and only recently has the function of intrinsic factors begun to emerge. This article reviews recent studies addressing the role of basic helix–loop–helix (bHLH) factors in specifying retinal cell types, with an emphasis on bHLH hierarchies leading to photoreceptor production. Photoreceptor genesis appears to employ two transcriptional pathways: ngn2→neuroD→raxL and ath5→neuroD→raxL. ngn2 and ath5 function in progenitors, which can potentially develop into different cell types. neuroD represents one of the central steps in photoreceptor specification. Ath5 is also essential for ganglion cell development. It remains to be demonstrated whether a bHLH gene functions as a key player in specifying the other types of retinal cells. Genetic knockout studies have indicated intricate cross-regulation among bHLH genes. Future studies are expected to unveil the mechanism by which bHLH factors network with intrinsic factors and communicate with extrinsic factors to ensure a balanced production of the various types of retinal cells.
Keywords: bHLH genes, neuroD, photoreceptor cells, RPE transdifferentiation, INL cell generation
Introduction
The vertebrate retina contains five major types of neurons (photoreceptor, horizontal, bipolar, amacrine, and ganglion cells) and Müller glia. Each cell type is functionally and morphologically distinct and resides at a stereotyped histological location. After receiving photons, photoreceptors initiate electrophysiological signals that are modulated by and relayed through interneurons (horizontal, bipolar, and amacrine cells) to ganglion cells. Ganglion cells send the information to the brain.
Degeneration of retinal neurons leads to irreversible vision loss, as in glaucoma, retinitis pigmentosa, and age-related macular degeneration, which is the leading cause of blindness in the elderly in the United States (1). Future therapies for these blinding diseases may include stem-cell-based replacement. Understanding the way that each cell type is specified is imperative to such an approach. Over the years, various experimental approaches, including cell lineage tracing with retroviral transduction (2), selective killing of a particular cell type (3,4), and exposing retinal progenitor cells to altered environments (5), have been used to address whether cell lineages play a decisive role in retinal cell fate selection. Results from these and other studies suggest that in the vertebrate retina, cell lineage is not deterministic, and micro-environmental cues surrounding the multipotent progenitors play important roles in specifying retinal cell fate. It is believed that key steps in cell-type specification occur after terminal mitosis (6). More than a decade of investigation has produced literature rich in information about the extrinsic factors contributing to retinal-cell-type specification. These factors include cell–cell interactions (5), growth factors and receptors (7,8), Hedgehog (9,10), and many others (11).
Although appealing and widely accepted, the notion is challenged by two lines of evidence. First, studies by different groups have shown lineage bias of certain retinal cells (12,13). Second, the retinal progenitors at different developmental stages appear intrinsically different (14-17). Additionally, the environmental cue theory has yet to be supported by a direct demonstration of a fate change when a cell from one anatomical location is grafted into another.
Appealing evidence of intrinsic factors playing active roles in retinal-cell-type specification comes from recent studies in which gene expression has been directly manipulated in vivo and in vitro. Gain- and loss-of-function studies have mounted ever-increasing evidence indicating that genetic programming regulated by transcription factors plays an important role in specifying the various cell types in the retina.
The Developing Retina Expresses Several Proneural bHLH Genes
Early studies have shown that during the development of the peripheral nervous system, intrinsic developmental factors play decisive roles in the generation of the diverse cell types. Many of these factors belong to the basic helix–loop–helix (bHLH) family of transcriptional factors and are homologous to the Drosophila proneural genes achaete-scute and atonal. This has generated a surge in studies of proneural bHLH genes in retinal development, including ash1 (18,19), ash3 (20,21), ath3 (22,23), ath5 (24-32), neuroD (33-37), neurogenin1 (22), and neurogenin2 (ngn2; refs. 38 and 39). Additionally, two distantly related genes, NSCL1 (40) and NSCL2 (41), have also been investigated. Of these bHLH genes, ash1, ash3, ngn2, and ath3 are expressed in subpopulations of proliferating progenitor cells. ath3 is also expressed in differentiating bipolar cells. ath5 is expressed in progenitor cells that have withdrawn from the cell cycle and that may develop into various types of retinal neurons. neuroD is expressed mostly in postmitotic cells. NSCL1 is transiently expressed in differentiating ganglion cells and is also expressed in Müller glial cells. NSCL2 is expressed in differentiating amacrine cells and horizontal cells. Bhlhb4, another bHLH gene, is expressed in bipolar cells and involved in their maturation (42).
neuroD Participates in Photoreceptor Specification
Expression of neuroD in Photoreceptors and Their Precursors
neuroD is a vertebrate homolog of Drosophila atonal and plays an important role in the development of numerous neural and non-neural tissues (for a recent review on neuroD; see ref. 43). The retinal expression and function of neuroD have been studied in many vertebrate species. Considerable discrepancies exist in the literature, perhaps because of species variations and differences in the sensitivity and specificity of the detection methods. Overall, each study shows major—if not exclusive—neuroD expression in photoreceptor cells and their precursors, rendering neuroD the only proneural bHLH gene known to be expressed in young photoreceptor cells and their precursors. In situ hybridization under high-stringency conditions (33) and immunohistochemistry with affinity-purified antibody (35) have been used to define neuroD expression in the developing chick retina. As shown in Fig. 1, neuroD messenger RNA (mRNA; Fig. 1B) and NeuroD protein (Fig. 1C) localize to photoreceptor precursors converging at the outer portion of the retinal neuro-epithelium. In a newly stratified retina, NeuroD protein is detected in the developing photoreceptor cells in the newly formed outer nuclear layer (ONL; Fig. 1D). In the teleost retina, in which persistent rod genesis occurs throughout life, neuroD is expressed in newly born cones and in the rod lineage (37).
Fig. 1.
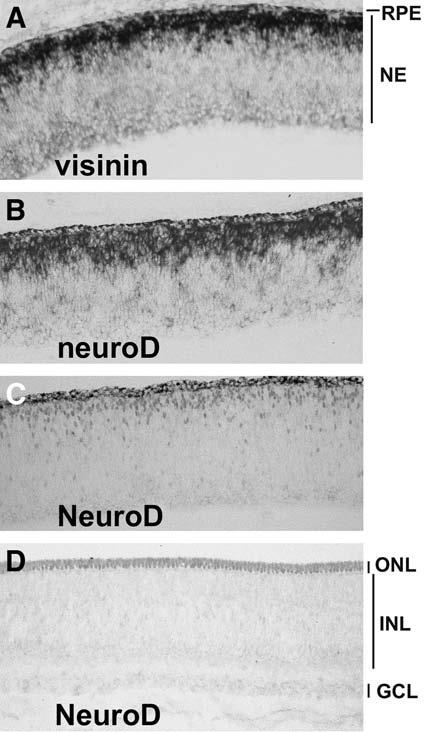
Expression of neuroD in the developing chick retina. (A) Detection of visinin mRNA at E6 with in situ hybridization. (B) Detection of neuroD mRNA at E6 with in situ hybridization. (C,D) Detection of NeuroD protein with antibody at E6 (C) and E9 (D). RPE, retinal pigment epithelium; NE, neuroepithelium; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer.
Slight expression of neuroD has been reported in amacrine cells of mouse retina (34,36,44). In goldfish retina, neuroD is expression in putative amacrine cells (37). Although this expression is consistent with the notion that neuroD specifies amacrine cells fate (refs. 34 and 44; see Roles of bHLH Genes in INL Cell Generation), it remains an interesting possibility that the low level of expression may imply an involvement of neuroD in the production of a subset of the inner retinal photoreceptors (which have recently been reported) (45), which may localize among amacrine cells, horizontal cells (46), and ganglion cells (47). Notably, transient and low levels of expression of photoreceptor-specific genes have been observed in developing amacrine cells in the chick retina. For example, expression of visinin (a gene encoding a photoreceptor-specific calcium-binding protein) has been detected in developing amacrine cells by in situ hybridization (33,35) and by immunostaining with a monoclonal antibody (35). RXRγ, another early photoreceptor gene, is also weakly expressed in some cells in the amacrine cell location (48). These observations imply that developing amacrine cells may transiently express photoreceptor genes (such as neuroD, visinin, and RXRγ), and the expression is suppressed as differentiation proceeds. Rigorous studies are needed to test this possibility.
Overproduction of Photoreceptor Cells On neuroD Misexpression
The first evidence showing that neuroD induces selective overproduction of photoreceptor cells came from a gain-of-function study in the embryonic chick retina (33). Retrovirus RCAS-driven widespread expression (or misexpression) of neuroD in chick retinal neuro-epithelium produced a retina with three, rather than two, layers of photoreceptor cells. The number of cells that expressed visinin increased more than 50% compared with control embryos misexpressing the green fluorescent protein (GFP). No significant changes have been observed in markers for numerous other retinal neurons, including RA4 (ganglion cells), pax6 (ganglion cells and amacrine cells), and chx10 (bipolar cells). Therefore, neuroD alone is sufficient to recruit additional cells into photoreceptors during retinal neurogenesis.
The ability of neuroD to selectively promote photoreceptor genesis has also been observed in mouse retina. Inoue et al. (44) noted that retroviral transduction of neuroD in E17.5 retina selectively promoted the genesis of rods, which account for approx 97% of all photoreceptor cells in the mouse retina.
Photoreceptor Cell Deficiency From Diminished neuroD Expression or Function
The retinal phenotypes of neuroD knockout mice have been examined by different groups. Under the 129/SvEv genetic background, neuroD-null mice develop severe hypoglycemia and die within 5 d. Therefore, Morrow et al. (34) used explant cultures of P0 retina to study the role of neuroD in retinal development. Researchers found that in neuroD-null retina, glial and bipolar cell populations increased, amacrine cell differentiation was delayed, and the photoreceptor number decreased, which was attributed to cell death. Additionally, glial cell bodies were present in the ONL. When crossed into a different genetic background, 60 to 70% of neuroD-null mice survived to adulthood (49). These animals displayed photo-receptor-specific deficits (36). The photoreceptor population was reduced by 50% at 2 to 3 mo, and essentially none were left by 18 mo. This progressive loss indicates slow photoreceptor degeneration in the absence of neuroD. Notably, conspicuous cell death occurs in the developing neuroD-null retina, peaking at P3 and leveling at P5, when the bulk of photoreceptor cells have been produced (50). Although the cause for this early phase of cell death was unclear, it is possible that in the absence of neuroD, certain presumptive photoreceptor cells could not be specified properly and this improper specification resulted in cell death. Despite the pronounced photoreceptor reduction, there were few changes in the inner nuclear layer (INL) and ganglion cell layer in neuroD-null mice. This result is different from those of Morrow et al. (34) and Inoue et al. (44), which showed deficiencies of amacrine cells in neuroD single-knockout and neuroD/ath3 double-knockout, respectively. The discrepancy may arise from the use of explant cultures to allow the developing retina to mature in the latter two studies rather than in vivo conditions used by the former study (43).
Akagi et al. (51) examined double-knockouts of ash1/ngn2, ash1/ath3, ash1/neuroD, ath3/ngn2, ath3/neuroD, and neuroD/ngn2. They noted that although some of them have a reduced number of photoreceptor cells, none has fewer photoreceptor cells than neuroD single-knockout. This suggests that among the bHLH genes tested, only neuroD plays a critical role in photoreceptor cell production.
In the chick retina, the question of whether neuroD is required for photoreceptor cell production has been approached by three strategies: Engrailed (En)-mediated active repression, antisense oligonucleotides, and small interfering RNA (siRNA) to attenuate neuroD expression and function (35). Chick embryos infected with retroviruses expressing an active repression construct, En-NeuroDΔC, exhibited severe photoreceptor deficits. The ONL of the retina is no longer a contiguous structure but becomes fragmented with regions containing fewer or no photoreceptor cells. Photoreceptor deficiency was evident even before the retina became laminated, suggesting that active repression of NeuroD may have affected photoreceptor genesis. No deficiency was observed in other types of retinal cells. The density of AP2α amacrine cells in the infected region was similar to that in the adjacent, uninfected regions. Subjecting the developing retina to antisense oligonucleotides against neuroD yielded fewer photoreceptor cells both in vivo and in vitro. Culturing retinal cells in the presence of siRNA against neuroD resulted in a more than 50% reduction in the number of photoreceptor cells. All three experimental methods produced photoreceptor-cell-specific deficits (35).
In En-NeuroDΔC retina, chx10+ cells were transiently present in the ONL (35), where photoreceptor cells reside. These chx10+ cells in the ONL might be authentic bipolar cells that have migrated into the ONL. They might also be mis-fated, or refated, cells. It is possible that these cells were originally to become photoreceptor cells but switched to chx10+ bipolar cells because of the repression of NeuroD function by Engrailed. This scenario is further supported by the increase in chx10+ cells in the neuroD siRNA experiment (35). Bipolar cell population was reported to be increased in mice lacking neuroD (34). Therefore, it is possible that reducing neuroD activity promotes a fate switch from a photoreceptor cell to a bipolar cell.
RPE Transdifferentiation Toward Photoreceptor Cells Induced by neuroD
Another direct demonstration of neuroD as one of the key players in photoreceptor specification came from retinal pigment epithelium (RPE) transdifferentiation assays (33,52,53). The assay takes advantage of the plasticity of cultured RPE cells, which can adopt a fate other than RPE (i.e., transdifferentiation), and the absence of endogenous expression of proneural genes. Dissociated E6 RPE cells are cultured, and retrovirus RCAS–neuroD is added to the culture. From this culture of non-neural RPE cells emerges de novo a large number of cells expressing visinin (Fig. 2A). No such cells are present in the control culture infected with RCAS–GFP (Fig. 2B). Morphologically, Visinin+ cells resemble bona fide photoreceptor cells developed in culture (Fig. 3A,D). These morphologies include an elon-gated cell body, an axon on the basal side (arrows), elaborate axonal arboration (arrow heads), and an inner segment-like structure (open arrows). Transdifferentiating cells also express the general neural marker mitogen-activated protein 2 and several photoreceptorspecific genes, including raxL (31), which is a homeodomain gene playing an important role in initiating the photoreceptor differentiation program (54), IRBP, cone α-PDE, rhodopsin, and the red, green, and blue pigment genes (52). Because the photopigment genes are normally expressed during the late phases of photoreceptor differentiation in the retina (55), their expression in transdifferentiating cells suggests that neuroD can not only instruct presumptive RPE cells to a photoreceptor fate but can also trigger substantial photoreceptor differentiation.
Fig. 2.
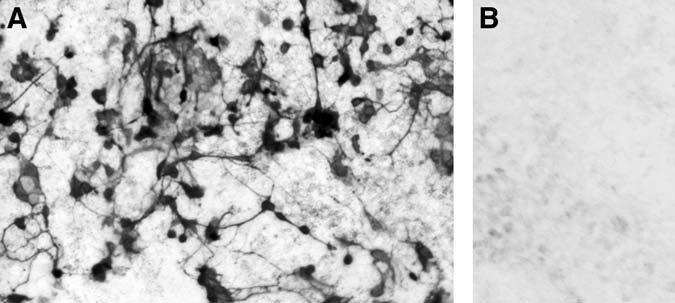
De novo generation of Visinin+ cells from retinal pigment epithelium (RPE) culture under the induction of neuroD. Shown is immunostaining with a monoclonal antibody (7G4). (A) E6 RPE cell culture infected with RCAS–neuroD. (B) The same culture infected with RCAS–GFP.
Fig. 3.
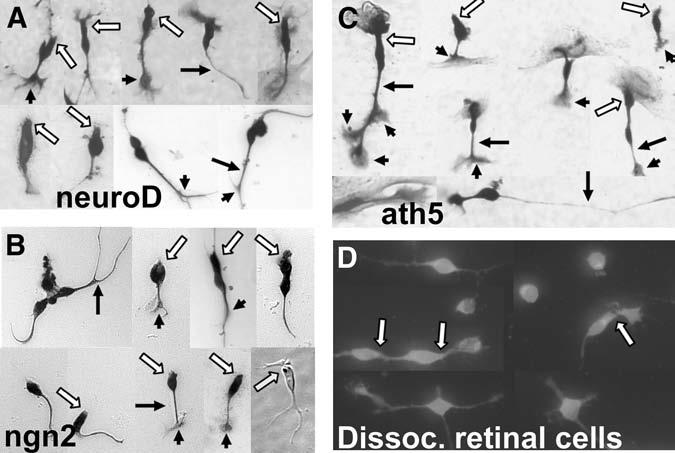
Photoreceptor-like morphologies of transdifferentiating cells after reseeding onto poly-ornithine coated cover slips. Shown are Visinin+ cells with 3-amino-9-ethylcarbzole (A,B) nitroblue tetrazolium (A,C) or fluorescence (D) as the final substrate of immuno-detection. Arrows: axons. Arrowheads: axonal arboration. Open arrows: inner segment-like structures. Yellow arrow in B points to an oil droplet-like structure in a Hoffman image.
Transcription Factors in Photoreceptor Subtype Specification
After the establishment of photoreceptor identity, subsequent subtype specification employs additional transcription factor genes. For example, thyroid hormone receptor-β2 (Thrb; a ligand-activated transcription factor) specifies the identity of M-cones (56). In thrb-null mice, there is a selective loss of M-cones and a concomitant increase in S-opsin immunoreactive cones. nrl, a bZIP transcription factor gene, determines the rod cell fate (57). nrl-null retinas show an extreme transformation of rods into cone-like cells. Nr2e3, a rod photoreceptor-specific nuclear receptor, has been found to repress transcription of multiple cone-specific genes (58).
Two Pathways Leading to Photoreceptor Cell Generation
The ngn2 Pathway
A search for bHLH gene(s) upstream of neuroD in the developing retina led to ngn2 and ath5 (31,38). In the developing chick retina, ngn2 mRNA is detected in cells scattered across the retinal neuro-epithelium and is undetectable in differentiating cells that are accumulating at their prospective anatomical locations within the pseudostratified retinal neuro-epithelium (38). Double-labeling showed that cells expressing ngn2 also incorporated BrdU or expressed proliferating cell nuclear antigen, indicating that proliferating neuroblasts expressed ngn2. Similarly to neuroD, ngn2 induced cultured RPE cells to transdifferentiate along the photoreceptor pathway (38). It induced neuroD and raxL, but not vise versa, indicating that its photoreceptor-promoting activity is mediated by neuroD, which, in turn, induces raxL (31). The neural transdifferentiation of RPE cells induced by ngn2 is extensive, as assessed by the expression of several neural-specific genes and the development of elaborate morphologies, such as an oil-droplet-like structure (Fig. 3B) that is characteristics of chick photoreceptors. In mouse retina, regions lacking ngn2 expression contain no photoreceptor cells (39). Therefore, photoreceptor formation likely employs a transcriptional hierarchy of ngn2→neuroD→raxL→photoreceptor differentiation.
Conversely to neuroD (which induces RPE to transdifferentiate toward photoreceptor cells selectively), ngn2 induces cultured RPE cells to transdifferentiate along various retinal pathways, including photoreceptor and ganglion cell pathways (38). This suggests that ngn2 is not present during the key step in specifying the photoreceptor fate or the ganglion cell fate. This is further supported by our recent studies (Ma et al., manuscript submitted) of the final fates of cells that transiently express ngn2 during development using the conditional, binary CreER™ –LacZ system with the ngn2-CreER™ mice (59). In these mice, LacZ+ cells were detected in all three nuclear layers of the retina and included all major types of retinal cells, even Müller glia. Furthermore, the temporal window in which a particular cell type was marked appeared nonrandom but was similar to its birth date, and the overall order of LacZ labeling of major cell types closely resembled their birth sequence. Therefore, ngn2 is likely to be involved in a developmental step prior to key events in retinal-cell-type specification (Fig. 4).
Fig. 4.
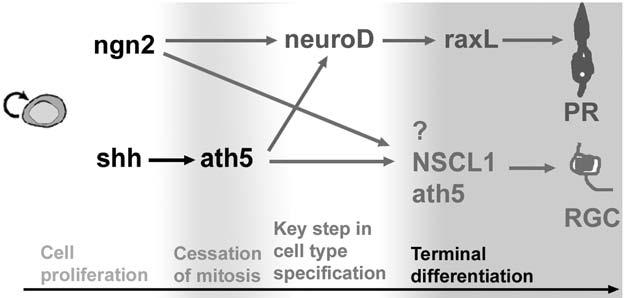
A schematic diagram of the molecular pathways leading to photoreceptor cells and retinal ganglion cells. The life history of a retinal cell is divided into four developmental phases: proliferation, cessation of mitosis, key step in cell-type specification, and terminal differentiation. ngn2 functions in proliferating progenitor cells, whereas ath5 is involved in postmitotic cells. Both can participate in the genesis of multiple types of cells, including photoreceptor and ganglion cells. ath5 and NSCL1 promote retinal ganglion cell differentiation. The developmental transition from cell proliferation to cell differentiation is a critical window for retinal-cell fate specification, and neuroD may act at this step in specifying the photoreceptor fate. Induction of zebrafish ath5 by shh was proposed by Masai et al. (25) and Stenkamp and Frey (30).
The ath5 Pathway
The observation that ath5 can lead to the photoreceptor pathway was surprising, because ath5 had been known for its role in retinal ganglion cell development (see the next section). In the developing chick retina, ath5 is expressed in two zones of cells. One zone coincides with the anatomical location of differentiating retinal ganglion cells, and the other is adjacent to young photoreceptor cells concentrating at the outer portion of the retinal neuro-epithelium. Co-expression with neuroD is observed in the zone adjacent to young photoreceptor cells (31). This is consistent with ath5 being involved in photoreceptor production. The two-zone pattern of ath5 expression was also observed in mouse retina expressing an ath5–LacZ knock-in (27).
A direct indication of ath5 participating in photoreceptor genesis came from experiments using the developing retina and retinal cells (31). Misexpression of ath5 increases the number of photoreceptor cells. Notably, the increase is developmentally stage-dependent and is observed at times coinciding with photoreceptor genesis. At earlier stages, ath5 misexpression leads to an increase in ganglion cells, which are born before photoreceptor cells. This time dependency implies that ath5 participates in retinal neurogenesis by interacting with temporally regulated factors or cues. Therefore, ath5 participates in the production of not only ganglion cells but also photoreceptor cells and, consequently, may not specify a particular cell type in the retina. This proposition is consistent with the results of the cell-tracing study by Yang et al. (60), which showed that in mouse retinal cells, expressing ath5 may differentiate into various types of cells, including photoreceptor cells, amacrine cells, and ganglion cells.
ath5 leading to the photoreceptor pathway was also shown by RPE transdifferentiation assay (31). Retroviral-driven ectopic expression of ath5 induces cultured RPE cells to transdifferentiate into cells that express photoreceptor-specific genes, including visinin, IRBP, red opsin, and rhodopsin. The expression of the red opsin and rhodopsin indicates that ath5 can lead to the genesis of both cones and rods. Transdifferentiating cells develop highly structured morphologies (Fig. 3C) resembling young photoreceptor cells derived from the developing retina (Fig. 3D). These results suggest that ath5 can trigger a spectrum of genes associated with photoreceptor development and differentiation of both cones and rods. The repertoire of genes includes neuroD and raxL (31). Therefore, it appears that a pathway of ath5→neuroD→raxL and other photoreceptor genes might underlie the de novo generation of photoreceptor-like cells in the RPE cell cultures ectopically expressing ath5.
The RPE transdifferentiation assay was used to address the possibility of whether ath5 is genetically downstream of ngn2. Reverse transcriptase-polymerase chain reaction showed no induction of ath5 by ngn2 or by neuroD, and there was no induction of ngn2 by ath5 (31). Studies using the zebrafish retina have indicated that sonic hedgehog may induce ath5 expression (25,30). Because of these findings, ngn2 and ath5 may belong to separate pathways that interconnect at neuroD leading to photoreceptor genesis. Note that in this hypothesis, neuroD expression constitutes one branch of the bHLH hierarchies represented by ngn2 and ath5, which can also lead to other types of retinal neurons (Fig. 4).
The presence of two pathways independently inducing neuroD implies that photoreceptor genesis deploys a central step(s) that involves neuroD. This central step ties into, or receives inputs from, other bHLH networks and, perhaps, other regulatory networks to ensure that photoreceptor genesis keeps pace with the production of other retinal cell types for the formation of a functional retina. However, the current information does not rule out the possible presence of a neuroD-independent pathway(s) leading to photoreceptor genesis. The neuroD-independent pathway may operate during normal development or may be set in motion only under abnormal conditions, such as genetic alterations.
Requirement of Intrinsic and Extrinsic Factors in Ganglion Cell Generation
Retinal ganglion cells are the first cell type to be generated during retinal neurogenesis. One of the most well-studied bHLH genes in ganglion cell production is ath5. Misexpression of ath5 in the retina of various species results in an increase in ganglion cell number (21,28,31,32,61), implying that ath5 is sufficient to guide a retinal progenitor cell to differentiate as a ganglion cell. Mouse retinas lacking ath5 exhibit profound deficits in ganglion cell population (26,27). Mutation in zebrafish ath5 results in complete elimination of ganglion cells (29). These gain- and loss-of-function studies are consistent with ath5 specifying the retinal ganglion cell type. However, cell tracing showed that ath5-expressing cells may adopt various fates (60), and functional analyses with retinal and RPE cells show that ath5 can lead to photoreceptor cells, as discussed in the previous section. Additionally, an RPE transdifferentiation assay showed that ath5 alone was unable to induce de novo expression of ganglion cell markers such as RA4 (62). Rather, ath5 was shown to enhance basic fibroblast growth factor (bFGF)-initiated RPE transdifferentiation toward ganglion cells. Together, current information indicates that ath5 may not specify a particular cell type; rather, it is involved in multiple steps of retinal neurogenesis, including the differentiation of ganglion cells and the development of progenitor cells, which are subsequently specified into different types of retinal cells.
Differentiating ganglion cells or their precursors express another bHLH gene: NSCL1 (40). Misexpression of NSCL1 in the developing retina results in a moderate increase in the ganglion cell population (32). In an RPE transdifferentiation assay, NSCL1 alone is insufficient to induce transdifferentiation toward ganglion cells but can enhance such transdifferentiation initiated by bFGF. Therefore, similarly to ath5, NSCL1 is unlikely to play a key role in ganglion cell fate specification but, rather, plays a role in ganglion cell differentiation. Co-expression of ath5 and NSCL1 promotes retinal ganglion cell differentiation to a greater extent than either gene alone, both in vivo in the developing chick retina and in vitro in RPE cell cultures (32), implying a combinational effect of the two bHLH genes.
ngn2, which is expressed in progenitor cells still in the cell cycle, may lead to the ganglion pathway, in addition to the photoreceptor pathway (as discussed in the previous section). ngn2 alone is sufficient to guide RPE transdifferentiation toward ganglion cells, including the expression of NSCL1 and the development of cellular processes typical of long-projecting neurons (38). Both functional assays and cell-fate-tracing studies have indicated that ngn2 does not specify a ganglion cell fate.
Extrinsic factor bFGF has been shown to potentiate a ganglion cell fate (63,64). In the RPE transdifferentiation assay, bFGF elicits the expression of ganglion cell marker RA4. However, the extent of differentiation is very limited (53,62), because those cells do not express many other ganglion cell genes. Expression of these markers was detected in bFGF-primed RPE cultures infected with RCAS–ath5 or RCAS–NSCL1 (62), suggesting that the bHLH hierarchy may incorporate input from extrinsic factor bFGF to promote retinal ganglion cell genesis and development.
Hedgehog is another extrinsic factor shown to regulate ganglion cell generation and differentiation (25,30,65-67). It has been proposed that Hedgehog signaling participates in propagating the expression of ath5 in zebrafish (25,30). Current information indicates that specification of retinal ganglion cells may be a stepwise process involving both extrinsic and intrinsic factors of a hierarchical gene regulatory network (for a resent review, see ref. 68).
Roles of bHLH Genes in INL Cell Generation
Bipolar Cells
Genetic knockouts have been instrumental in elucidating the role of bHLH genes in INL neuron production. Mice lacking ash1 do not exhibit any obvious abnormalities in eye development during embryogenesis or at birth, the time when the mutant mice die. However, explant cultures of ash1-null retinas showed a delay in differentiation of rod photoreceptors, horizontal cells, and bipolar cells as well as a decrease in the number of bipolar cells (18). In ash1/ath3 double-knockouts, bipolar cells were virtually abolished (69). These results indicate a role of ash1 and ath3 in bipolar cell generation. Misexpression of ash1 or ath3 alone does not promote bipolar cell genesis, but co-misexpression of ash1, ath3, and chx10 results in an increase in bipolar cells (23). A role of ngn2 in bipolar cell genesis has been reported (39). Therefore, bipolar genesis likely involves multiple genes, and a bHLH gene with a key role in bipolar cell specification, should it exist, remains to be identified.
Horizontal Cells
Aiming to find the regulator of horizontal cell specification, Akagi et al. (51) examined the retinas from double- and triple-knockouts of various bHLH genes. In retinas lacking ash1/ath3/ngn2 or lacking ath3/neuroD/ngn2, the number of horizontal and other neurons was much lower. The number of horizontal cells was not affected by a lack of ash1/ath3/neuroD. These results suggest that ngn2 may play an important role in horizontal cell genesis. However, horizontal cells developed normally in double-knockouts of ash1/ngn2, ath3/ngn2, and neuroD/ngn2. These phenotypes underscore the complexity of bHLH genes in retinal development. Detailed analyses of an array of triple-knockouts of bHLH genes have revealed altered expression of remaining bHLH genes, which are believed to at least partly contribute to the somewhat perplexing phenotypes (51).
Amacrine Cells
neuroD has been reported to be a key player in amacrine cell specification; however, the issue remains controversial. It has been reported that a neuroD knockout reduces amacrine cell number (34), and an ath3/neuroD double-knockout abolishes amacrine cells (44). However, amacrine cells developed normally in another neuroD single-knockout study (36) and in an ath3/ash/neuroD triple-knockout (51). In gain-of-function studies, retroviral-driven overexpression of neuroD in rodent retina was found to promote amacrine cells (34) but was found to promote rod photoreceptor cells in another study (44). Although the underlying cause of the discrepancy in the same species (mouse) is unclear, the inconsistent observations further highlight the intricacy in dissecting the role of bHLH. In the chick embryos, although profound photoreceptor deficits were observed (35), Engrailed-mediated active repression of NeuroD protein did not reduce the number of amacrine cells, and retroviral misexpression of neuroD did not increase the production of amacrine cells (33).
Cell-Type Specification by Other Transcription Factors
Cell-type specification of INL neurons involves other types of transcription factors. Homeobox gene chx10 has been shown to play an important role in bipolar cell production. chx10 is initially expressed in retinal progenitor cells and is expressed later in differentiated bipolar cells (70). Mutations in chx10 result in microphthalmia and a complete loss of bipolar cells (71). However, chx10 is not believed to be the factor for bipolar cell fate decision, although it is required for bipolar cell development (23). Another homeobox gene, prox1, is both necessary and sufficient for cell fate specification of horizontal cells. Dyer et al. (72) reported that the prox1-null retina was deficient in horizontal cells, and misexpression of prox1 in progenitor cells promoted horizontal cell formation. Recently, Li et al. (73) showed that foxn4, a gene upstream of prox1 and encoding a winged helix/forkhead transcription factor, is both necessary and sufficient for amacrine cell genesis and is required for horizontal cell genesis. Targeted disruption of foxn4 largely eliminates amacrine neurons and completely abolishes horizontal cells, whereas overexpression of foxn4 strongly promotes an amacrine cell fate.
The Linage Factor
Experimental evidence demonstrates that certain types of retinal cells exhibit lineage bias. Alexiades and Cepko (13) showed that amacrine cells, horizontal cells, and rods—but not cones— are descendents of one subpopulation (VC1.1+) of progenitors, whereas VC1.1− progenitors give rise to cones. Moody and colleagues (74) used the highly stereotypic blastomeres of Xenopus cleavage embryos to create reproducible, quantitative fate maps. Such maps have revealed lineage bias toward subsets of amacrine cells (12,75). Further analyses showed that the acquisition of the bias occurs at different developmental steps, from the initial cleavage to the optic vesicle stage. Interestingly, at each of these steps, a fraction of the progenitor pool is biased, and the remaining pool maintains multipotency (76).
Importance of Tight Regulation of bHLH Genes
All the proneural bHLH genes display spatially and/or temporally restricted expression in the developing retina, suggesting that their regulated expression could be important for normal retinal development. The importance of restricted expression is illustrated by the gross abnormality in retinal development caused by experimental misexpression of bHLH genes. Small eyes were produced when NSCL1 was misexpressed in the retinal neuroepithelium through viral transduction (40). Pulse-labeling with BrdU and [3H]thymidine revealed a significant decrease in cell-proliferation activity with NSCL1 misexpression. Massive cell death occurred, but only after cell proliferation activity had subsided, resulting in major distortions of retinal structure. Presently, it is unclear how NSCL1 modulates cell proliferation and cell death. Nonetheless, it could act through reducing bmp4 expression (77), which is hypothesized to play important roles in cell proliferation and cell death (78-80).
NSCL2 is expressed in amacrine and horizontal cells, and its expression is maintained in the mature retina (41). Retroviral-driven misexpression of NSCL2 in the developing chick retina resulted in missing photoreceptor cells and gross deficits in the ONL. These deficits did not result from decreased photoreceptor production, because the ONL appeared normal in early developmental stages. TUNEL+ cells were detected in the ONL, indicating that photoreceptor cells underwent apoptosis in retinas mis-expressing NSCL2. NSCL2 also caused atrophy of Müller glia, because Müller glial cells were far fewer in the experimental retina than in the control. The onset of the disappearance of the Müller glia preceded photoreceptor degeneration, indicating that without Müller glia, photoreceptors cannot survive for long. Although the mechanisms underlying Müller glial atrophy and photoreceptor atrophy by misexpression of NSCL2 are unknown, one may speculate that NSCL2 interfered with NeuroD function in photoreceptor cells or NSCL1 protein in Müller glia (40). Alternatively, misexpression of NSCL2 caused atrophy of Müller glia, which subsequently triggered the death of photoreceptors.
Conclusions
Studies employing diverse experimental systems in various species have shown that proneural bHLH genes make important contributions to the development of the retinal. Current information indicates that only a small fraction of these bHLH genes directly participates in key steps in the retinal-cell-type-specification process, whereas the others are involved in the development (or restricting the potential) of common progenitors and/or in cellular differentiation (Table 1). Gain- and loss-of-functions studies using various systems have illustrated the enormous complexity of bHLH gene networks in retinal neurogenesis. Future studies are expected to shed light on (a) the hierarchies and networks of bHLH genes, (b) the interaction of bHLH genes with other transcription factor genes, and (c) how intrinsic factors sense and respond to environmental cues in orchestrating the genesis of the diverse cell types in the vertebrate retina.
Table 1.
Participation of Proneural bHLH Genes in Various Steps of Retinal Development
| ash1 | Multipotent progenitor cells at late stages, generation of bipolar cells and rod photoreceptors, neuron vs glia (18,23,69) |
| ash3 | Progenitor cells (20,21) |
| ath3 | Generation of bipolar cells and amacrine cells (23,44), neuron vs glia (23) |
| ath5 | Progenitor cells leading to multiple cell types, including photoreceptor cells and ganglion cells (31,60), ganglion cell generation (25,27-29,32), and ganglion cell differentiation (26,30,31,32) |
| neuroD | Photoreceptor fate specification (33,35,44), photoreceptor survival (36), amacrine cell fate specification (34,44), neural vs glia (34) |
| ngn2 | Multipotent progenitor cells leading to multiple cell types (refs. 38 and 39; Ma et al., submitted) |
| NSCL1 | Cell proliferation and cell death (40), ganglion cell production when coexpressed with ath5 (32), ganglion cell differentiation (31,32) |
| NSCL2 | Horizontal and amacrine neuron differentiation (ref. 41; our unpublished data 2004–2005) |
Acknowledgments
This study was supported by National Institutes of Health/National Eye Institute grant EY11640, EyeSight Foundation of Alabama grant 01-7, and an unrestricted grant to University of Alabama at Birmingham Department of Ophthalmology from Research to Prevent Blindness. S. Z. Wang is a Research to Prevent Blindness Dolly Green Scholar.
References
- 1.Curcio CA. Photoreceptor topography in ageing and age-related maculopathy. Eye. 2001;15:376–383. doi: 10.1038/eye.2001.140. [DOI] [PubMed] [Google Scholar]
- 2.Turner DL, Cepko CL. A common progenitor for neurons and glial persists in rat retina late in development. Nature. 1987;328:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- 3.Raymond PA. Cell determination and positional cues in the teleost retina: development of photoreceptors and horizontal cells. In: Lam D-K, Shatz CJ, editors. Development of the Visual System. The MIT Press; Cambridge, MA: 1991. pp. 59–78. [Google Scholar]
- 4.Reh TA. Determination of cell fate during retinal histogenesis: Intrinsic and extrinsic mechanisms. In: Lam D-K, Shatz CJ, editors. Development of the Visual System. The MIT Press; Cambridge, MA: 1991. pp. 79–94. [Google Scholar]
- 5.Adler R, Hatlee M. Plasticity and differentiation of embryonic retinal cells after terminal mitosis. Science. 1989;243:391–393. doi: 10.1126/science.2911751. [DOI] [PubMed] [Google Scholar]
- 6.Adler R. A model of retinal cell differentiation in the chick embryo. Prog. Retina Eye Res. 2000;19:529–557. doi: 10.1016/s1350-9462(00)00008-2. [DOI] [PubMed] [Google Scholar]
- 7.Lillien L. Changes in retinal cell fate induced by overexpression of EGF receptor. Nature. 1995;377:158–162. doi: 10.1038/377158a0. [DOI] [PubMed] [Google Scholar]
- 8.McFarlane S, Zuber ME, Holt CE. A role for the fibroblast growth factor receptor in cell fate decisions in the developing vertebrate retina. Development. 1998;125:3967–3975. doi: 10.1242/dev.125.20.3967. [DOI] [PubMed] [Google Scholar]
- 9.Levine EM, Roelink H, Turner J, Reh TA. Sonic hedgehog promotes rod photoreceptor differentiation in mammalian retinal cells in vitro. J. Neurosci. 1997;17:6277–6288. doi: 10.1523/JNEUROSCI.17-16-06277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stenkamp DL, Frey RA, Prabhudesai SN, Raymond PA. Function for Hedgehog genes in zebrafish retinal development. Dev. Biol. 2000;220:238–252. doi: 10.1006/dbio.2000.9629. [DOI] [PubMed] [Google Scholar]
- 11.Levine EM, Fuhrmann S, Reh TA. Soluble factors and the development of rod photoreceptors. Cell Mol. Life Sci. 2000;57:224–234. doi: 10.1007/PL00000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang S, Moody SA. Three types of serotonin-containing amacrine cells in tadpole retina have distinct clonal origins. J. Comp. Neurol. 1997;387:42–52. [PubMed] [Google Scholar]
- 13.Alexiades MR, Cepko CL. Subsets of retinal progenitors display temporally regulated and distinct biases in the fates of their progeny. Development. 1997;124:1119–1131. doi: 10.1242/dev.124.6.1119. [DOI] [PubMed] [Google Scholar]
- 14.Braisted JE, Essman TF, Raymond PA. Selective regeneration of photoreceptors in goldfish retina. Development. 1994;120:2409–2419. doi: 10.1242/dev.120.9.2409. [DOI] [PubMed] [Google Scholar]
- 15.Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc. Natl. Acad. Sci. USA. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belliveau MJ, Young TL, Cepko CL. Late retinal progenitor cells show intrinsic limitations in the production of cell types and the kinetics of opsin synthesis. J. Neurosci. 2000;20:2247–2254. doi: 10.1523/JNEUROSCI.20-06-02247.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cayouette M, Barres BA, Raff M. Importance of intrinsic mechanisms in cell fate decisions in the developing rat retina. Neuron. 2003;40:897–904. doi: 10.1016/s0896-6273(03)00756-6. [DOI] [PubMed] [Google Scholar]
- 18.Tomita K, Nakanishi S, Guillemot F, Kageyama R. Mash1 promotes neuronal differentiation in the retina. Genes Cells. 1996;1:765–774. doi: 10.1111/j.1365-2443.1996.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 19.Jasoni CL, Walker MB, Morris MD, Reh TA. A chicken achaete-scute homolog (CASH-1) is expressed in a temporally and spatially discrete manner in the developing nervous system. Development. 1994;120:769–783. doi: 10.1242/dev.120.4.769. [DOI] [PubMed] [Google Scholar]
- 20.Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- 21.Kanekar S, Perron M, Dorsky R, et al. Xath5 participates in a network of bHLH genes in the developing Xenopus retina. Neuron. 1997;19:981–994. doi: 10.1016/s0896-6273(00)80391-8. [DOI] [PubMed] [Google Scholar]
- 22.Perron M, Opdecamp K, Butler K, Harris WA, Bellefroid EJ. X-ngnr-1 and Xath3 promote ectopic expression of sensory neuron markers in the neurula ectoderm and have distinct inducing properties in the retina. Proc. Natl. Acad. Sci. USA. 1999;96:14,996–15,001. doi: 10.1073/pnas.96.26.14996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatakeyama J, Tomita K, Inoue T, Kageyama R. Roles of homeobox and bHLH genes in specification of a retinal cell type. Development. 2001;128:1313–1322. doi: 10.1242/dev.128.8.1313. [DOI] [PubMed] [Google Scholar]
- 24.Brown NL, Kanekar S, Vetter ML, Tucker PK, Gemza DL, Glaser T. Math5 encodes a murine basic helix-loop-helix transcription factor expressed during early stages of retinal neurogenesis. Development. 1998;125:4821–4833. doi: 10.1242/dev.125.23.4821. [DOI] [PubMed] [Google Scholar]
- 25.Masai I, Stemple DL, Okamoto H, Wilson SW. Midline signals regulate retinal neurogenesis in zebrafish. Neuron. 2000;27:251–263. doi: 10.1016/s0896-6273(00)00034-9. [DOI] [PubMed] [Google Scholar]
- 26.Wang SW, Kim BS, Ding K, et al. Requirement for math5 in the development of RGCs. Genes Dev. 2001;15:24–29. doi: 10.1101/gad.855301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown NL, Patel S, Brzezinski J, Glaser T. Math5 is required for retinal ganglion cell and optic nerve formation. Development. 2001;128:2497–2508. doi: 10.1242/dev.128.13.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W, Mo Z, Xiang M. The Ath5 proneural genes function upstream of Brn3 POU domain transcription factor genes to promote retinal ganglion cell development. Proc. Natl. Acad. Sci. USA. 2001;98:1649–1654. doi: 10.1073/pnas.98.4.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kay JN, Finger-Baier KC, Roeser T, Staub W, Baier H. Retinal ganglion cell genesis requires lakritz, a Zebrafish atonal Homolog. Neuron. 2001;30:725–736. doi: 10.1016/s0896-6273(01)00312-9. [DOI] [PubMed] [Google Scholar]
- 30.Stenkamp DL, Frey RA. Extraretinal and retinal hedgehog signaling sequentially regulate retinal differentiation in zebrafish. Dev Biol. 2003;258:349–363. doi: 10.1016/s0012-1606(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 31.Ma W, Yan R-T, Xie W, Wang S-Z. A role of ath5 in inducing neuroD and the photoreceptor pathway. J. Neurosci. 2004;24:7150–7158. doi: 10.1523/JNEUROSCI.2266-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie W, Yan R-T, Ma W, Wang S-Z. Enhanced retinal ganglion cell differentiation by ath5 and NSCL1 coexpression. Invest. Ophthalmol. Vis. Sci. 2004;45:2922–2928. doi: 10.1167/iovs.04-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan R-T, Wang S-Z. neuroD induces photoreceptor cell overproduction in vivo and de novo generation in vitro. J. Neurobiol. 1998;36:485–496. [PMC free article] [PubMed] [Google Scholar]
- 34.Morrow EM, Furukawa T, Lee JE, Cepko CL. NeuroD regulates multiple functions in the developing neural retina in rodent. Development. 1999;126:23–36. doi: 10.1242/dev.126.1.23. [DOI] [PubMed] [Google Scholar]
- 35.Yan R-T, Wang S-Z. Requirement of neuroD for photoreceptor formation in the chick retina. Invest. Ophthalmol. Vis. Sci. 2004;45:48–58. doi: 10.1167/iovs.03-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pennesi ME, Cho JH, Yang Z, et al. BETA2/NeuroD1 null mice: a new model for transcription factor-dependent photoreceptor degeneration. J. Neurosci. 2003;23:453–461. doi: 10.1523/JNEUROSCI.23-02-00453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hitchcock P, Kakuk-Atkins L. The basic helix-loop-helix transcription factor neuroD is expressed in the rod lineage of the teleost retina. J. Comp. Neurol. 2004;477:108–117. doi: 10.1002/cne.20244. [DOI] [PubMed] [Google Scholar]
- 38.Yan R-T, Ma W-X, Wang S-Z. neurogenin2 elicits the genesis of retinal neurons from cultures of non-neural cells. Proc. Natl. Acad. Sci. USA. 2001;98:15,014–15,019. doi: 10.1073/pnas.261455698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 40.Li C-M, Yan R-T, Wang S-Z. Misexpression of cNSCL1 disrupts retinal development. Mol. Cell. Neurosci. 1999;14:17–27. doi: 10.1006/mcne.1999.0765. [DOI] [PubMed] [Google Scholar]
- 41.Li C-M, Yan R-T, Wang S-Z. Misexpression of chick NSCL2 causes atrophy of Müller glia and photoreceptor cells. Invest. Ophthalmol. Vis. Sci. 2001;42:3103–3109. [PMC free article] [PubMed] [Google Scholar]
- 42.Bramblett DE, Pennesi ME, Wu SM, Tsai MJ. The transcription factor Bhlhb4 is required for rod bipolar cell maturation. Neuron. 2004;43:779–793. doi: 10.1016/j.neuron.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 43.Chae JH, Stein GH, Lee JE. NeuroD: The predicted and the surprising. Mol. Cells. 2004;18:271–288. [PubMed] [Google Scholar]
- 44.Inoue T, Hojo M, Bessho Y, Tano Y, Lee JE, Kageyama R. Math3 and NeuroD regulate amacrine cell fate specification in the retina. Development. 2002;129:831–842. doi: 10.1242/dev.129.4.831. [DOI] [PubMed] [Google Scholar]
- 45.Foster RG, Bellingham J. Inner retinal photoreceptors (IRPs) in mammals and teleost fish. Photochem. Photobiol. Sci. 2004;3:617–627. doi: 10.1039/b400092g. [DOI] [PubMed] [Google Scholar]
- 46.Soni BG, Philp AR, Knox BE, Foster RG. Novel retinal photoreceptors. Nature. 1998;394:27–28. doi: 10.1038/27794. [DOI] [PubMed] [Google Scholar]
- 47.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 48.Hoover F, Seleiro EA, Kielland A, Brickell PM, Glover JC. Retinoid X receptor gamma gene transcripts are expressed by a subset of early generated retinal cells and eventually restricted to photoreceptors. J. Comp. Neurol. 1998;391:204–213. [PubMed] [Google Scholar]
- 49.Liu M, Pleasure SJ, Collins AE, et al. Loss of BETA2/NeuroD leads to malformation of the dentate gyrus and epilepsy. Proc. Natl. Acad. Sci. USA. 2000;97:865–870. doi: 10.1073/pnas.97.2.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- 51.Akagi T, Inoue T, Miyoshi G, et al. Requirement of multiple basic helix-loop-helix genes for retinal neuronal subtype specification. J. Biol. Chem. 2004;279:28,492–28,498. doi: 10.1074/jbc.M400871200. [DOI] [PubMed] [Google Scholar]
- 52.Yan R-T, Wang S-Z. Expression of an array of photoreceptor genes in chick embryonic RPE cell cultures under the induction of neuroD. Neurosci. Lett. 2000;280:83–86. doi: 10.1016/s0304-3940(99)01003-4. [DOI] [PubMed] [Google Scholar]
- 53.Yan R-T, Wang S-Z. Differential induction of gene expression by basic fibroblast growth factor and neuroD in cultured retinal pigment epithelial cells. Vis. Neurosci. 2000;17:157–164. doi: 10.1017/s0952523800171172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen CM, Cepko CL. The chicken RaxL gene plays a role in the initiation of photoreceptor differentiation. Development. 2002;129:5363–5375. doi: 10.1242/dev.00114. [DOI] [PubMed] [Google Scholar]
- 55.Bruhn SL, Cepko CL. Development of the pattern of photoreceptors in the chick retina. J. Neurosci. 1996;16:1430–1439. doi: 10.1523/JNEUROSCI.16-04-01430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ng L, Hurley JB, Dierks B, et al. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat. Genet. 2001;27:94–98. doi: 10.1038/83829. [DOI] [PubMed] [Google Scholar]
- 57.Mears AJ, Kondo M, Swain PK, et al. Nrl is required for rod photoreceptor development. Nat. Genet. 2001;29:447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- 58.Chen J, Rattner A, Nathans J. The rod photoreceptor-specific nuclear receptor Nr2e3 represses transcription of multiple cone-specific genes. J Neurosci. 2005;25:118–129. doi: 10.1523/JNEUROSCI.3571-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zirlinger M, Lo L, McMahon J, McMahon AP, Anderson DJ. Transient expression of the bHLH factor neurogenin-2 marks a subpopulation of neural crest cells biased for a sensory but not a neuronal fate. Proc. Natl. Acad. Sci. USA. 2002;99:8084–8089. doi: 10.1073/pnas.122231199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang Z, Ding K, Pan L, Deng M, Gan L. Math5 determines the competence state of retinal ganglion cell progenitors. Dev Biol. 2003;264:240–254. doi: 10.1016/j.ydbio.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Hutcheson DA, Vetter ML. The bHLH factors Xath5 and XNeuroD can upregu-late the expression of XBrn3d, a POU-home-odomain transcription factor. Dev. Biol. 2001;232:327–338. doi: 10.1006/dbio.2001.0178. [DOI] [PubMed] [Google Scholar]
- 62.Ma W, Yan R-T, Xie W, Wang S-Z. bHLH genes cath5 and cNSCL1 promote bFGF-stimulated RPE cells to transdifferentiate towards RGCs. Dev. Biol. 2004;265:320–328. doi: 10.1016/j.ydbio.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 63.Guillemot F, Cepko CL. Retinal fate and ganglion cell differentiation are potentiated by acidic FGF in an in vitro assay of early retinal development. Development. 1992;114:743–754. doi: 10.1242/dev.114.3.743. [DOI] [PubMed] [Google Scholar]
- 64.Fischer AJ, Dierks BD, Reh TA. Exogenous growth factors induce the production of ganglion cells at the retinal margin. Development. 2002;129:2283–2291. doi: 10.1242/dev.129.9.2283. [DOI] [PubMed] [Google Scholar]
- 65.Neumann CJ, Nuesslein-Volhard C. Patterning of the zebrafish retina by a wave of sonic hedgehog activity. Science. 2000;289:2137–2139. doi: 10.1126/science.289.5487.2137. [DOI] [PubMed] [Google Scholar]
- 66.Zhang XM, Yang XJ. Regulation of retinal ganglion cell production by Sonic hedgehog. Development. 2001;128:943–957. doi: 10.1242/dev.128.6.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spence JR, Madhavan M, Ewing JD, Jones DK, Lehman BM, Del Rio-Tsonis K. The hedgehog pathway is a modulator of retina regeneration. Development. 2004;131:4607–4621. doi: 10.1242/dev.01298. [DOI] [PubMed] [Google Scholar]
- 68.Mu X, Klein WH. A gene regulatory hierarchy for retinal ganglion cell specification and differentiation. Semin. Cell Dev. Biol. 2004;15:115–123. doi: 10.1016/j.semcdb.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 69.Tomita K, Moriyoshi K, Nakanishi S, Guillemot F, Kageyama R. Mammalian achaete-scute and atonal homologs regulate neuronal versus glial fate determination in the central nervous system. EMBO J. 2000;19:5460–5472. doi: 10.1093/emboj/19.20.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu IS, Chen JD, Ploder L, et al. Developmental expression of a novel murine homeobox gene (Chx10): evidence for roles in determination of the neuroretina and inner nuclear layer. Neuron. 1994;13:377–393. doi: 10.1016/0896-6273(94)90354-9. [DOI] [PubMed] [Google Scholar]
- 71.Burmeister M, Novak J, Liang MY, et al. Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nat. Genet. 1996;12:376–384. doi: 10.1038/ng0496-376. [DOI] [PubMed] [Google Scholar]
- 72.Dyer MA, Livesey FJ, Cepko CL, Oliver G. Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat. Genet. 2003;34:53–58. doi: 10.1038/ng1144. [DOI] [PubMed] [Google Scholar]
- 73.Li S, Mo Z, Yang X, Price SM, Shen MM, Xiang M. Foxn4 controls the genesis of amacrine and horizontal cells by retinal progenitors. Neuron. 2004;43:795–807. doi: 10.1016/j.neuron.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 74.Moody SA. Cell lineage analysis in Xenopus embryos. Methods Mol Biol. 2000;135:331–347. doi: 10.1385/1-59259-685-1:331. [DOI] [PubMed] [Google Scholar]
- 75.Huang S, Moody SA. Asymmetrical blastomere origin and spatial domains of dopamine and neuropeptide Y amacrine subtypes in Xenopus tadpole retina. J. Comp. Neurol. 1995;360:442–453. doi: 10.1002/cne.903600306. [DOI] [PubMed] [Google Scholar]
- 76.Moody SA, Chow I, Huang S. Intrinsic bias and lineage restriction in the phenotype determination of dopamine and neuropeptide Y amacrine cells. J. Neurosci. 2000;20:3244–3253. doi: 10.1523/JNEUROSCI.20-09-03244.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yan R-T, Wang S-Z. Embryonic abnormalities from misexpression of cNSCL1. Biochem. Biophys. Res. Cummun. 2001;287:949–955. doi: 10.1006/bbrc.2001.5690. [DOI] [PubMed] [Google Scholar]
- 78.Trousse F, Esteve P, Bovolenta P. Bmp4 mediates apoptotic cell death in the developing chick eye. J. Neurosci. 2001;21:1292–1301. doi: 10.1523/JNEUROSCI.21-04-01292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu J, He S, Friedman JS, et al. Altered expression of genes of the Bmp/Smad and Wnt/calcium signaling pathways in the cone-only Nrl−/− mouse retina, revealed by gene profiling using custom cDNA microarrays. J. Biol. Chem. 2004;279:42,211–42,220. doi: 10.1074/jbc.M408223200. [DOI] [PubMed] [Google Scholar]
- 80.Fischer AJ, Schmidt M, Omar G, Reh TA. BMP4 and CNTF are neuroprotective and suppress damage-induced proliferation of Muller glia in the retina. Mol. Cell. Neurosci. 2004;27:531–542. doi: 10.1016/j.mcn.2004.08.007. [DOI] [PubMed] [Google Scholar]


