Abstract
The expression of unique surface structures on tumors that allow for recognition and activation of host immunocompetent cells plays an important role in determining tumor growth and/or metastasis. Recent studies have identified an important role for heat shock proteins (Hsp) in antitumor surveillance; however, the exact role of Hsp expressed on the surface of tumors has not been fully addressed. In this study, we show that 4T1 mammary adenocarcinoma cells sorted for high Hsp25 surface expression (Hsp25high) grow significantly faster than cells sorted for intermediate Hsp25 surface expression (Hsp25intermediate) or wild-type 4T1 cells implanted into the abdominal breast gland of female BALB/c mice (p < 0.05). In addition, histological examination of lung tissues revealed that Hsp25high 4T1 cells metastasized to the lungs more aggressively than either Hsp25intermediate or wild-type 4T1 cells (p < 0.05). Exposure of 4T1 cells to nonlethal heat shock (43°C, 30 min) induced the surface expression of Hsp72 and a concomitant reduction in Hsp25 surface expression. The growth and metastastic potential of Hsp72+ 4T1 cells was significantly less than that of Hsp25high, Hsp25intermediate or wild-type 4T1 cells (p < 0.05). Taken together, these studies identify an important role for expression of Hsp25 and Hsp72 during tumor growth and metastatic spread which might be helpful in the design of antimetastatic therapies.
Keywords: Breast carcinoma, Chaperokine, Cytokine, Heat shock proteins, Metastasis
Introduction
Tumor-host interactions play an important role in determining tumor progression, especially in cases that involve metastasis. Biological response modifiers including cytokines, chemokines, growth factors and heat shock proteins (Hsp) have been shown to orchestrate some of these events. Hsp are highly conserved proteins found in all prokaryotes and eukaryotes. Under normal physiological conditions, Hsp are expressed at low levels [1]. However, a wide variety of stressful stimuli, including environmental stimuli (UV radiation, heat shock, heavy metals and amino acids), pathological stimuli (viral, bacterial and parasitic infections or fever, inflammation, malignancy or autoimmunity) and physiological stimuli (growth factors, cell differentiation, hormonal stimulation or tissue development), induce a marked increase in intracellular Hsp synthesis, a process known as the stress response [2]. The heat shock response is an evolutionarily conserved process which is geared to result in an increase in Hsp. This process is regulated at the transcriptional and translational level. Hsp genes are under the control of specific regulatory sequences, known as heat shock elements, which are localized upstream of the Hsp genes. In response to a variety of stressful stimuli, mentioned above, a specific transcription factor, heat shock factor-1, is activated through a trimerization process which triggers its binding to heat shock elements and leads to the transcriptional activation of heat shock genes [3].
Based on their apparent molecular mass, Hsp are subdivided into two main groups, the small and large Hsp. Hsp25, the murine homologue of human Hsp27, is a ubiquitously expressed member of the small Hsp family that has been implicated in various biological functions. In contrast to large Hsp, Hsp25/27 act through ATP-independent mechanisms, and in vivo they act in concert with other chaperones by creating a reservoir of folding intermediates. Hsp25/Hsp27 are associated with estrogen-responsive malignancies and are expressed at high levels in biopsies as well as circulating in the serum of breast cancer patients [4–6]. Activation of the heat shock-responsive element on the Hsp25/27 gene results in biological functions associated with cytoprotection from a variety of stressful stimuli as mentioned above. The upregulation of intracellular Hsp25/27 protects cells from tumor necrosis factor-alpha (TNF-α)-mediated apoptosis by a mechanism that involves the downregulation of reactive oxygen species [7–9].
The Hsp70 family, known as large Hsp, constitutes the most conserved and best studied class of Hsp, which are categorized on the basis of their cellular location and molecular weight. The Hsp70 family includes the constitutively expressed Hsp70 (Hsc70, 73 kDa), the stress-inducible Hsp70 (Hsp72, 72 kDa), which is principally located in the cytosol, the Hsp70 located in the mitochondria (Hsp75, 75 kDa) and the Hsp70 resident in the endoplasmic reticulum [glucose-regulated protein 78 (Grp78), 78 kDa]. In response to the various kinds of stressful stimuli mentioned above, there is an increased synthesis of Hsp72, which enhances the ability of the cell to cope with concentrations of unfolded or denatured proteins [10]. Recent studies have begun to define other functions associated with Hsp72, which seem to depend on its location within the host. When expressed at high levels within the cell, Hsp72 interferes with apoptosis and provides cytoprotection against the variety of stresses mentioned above [11]. When expressed on the cell surface, Hsp72 directly activates natural killer (NK) cell lytic machinery against Hsp72-expressing tumors [12, 13]. When Hsp72 is released into the extracellular milieu, including areas of inflammation and/or found freely circulating in the serum, extracellular Hsp72 stimulates the secretion of proinflammatory cytokines by antigen-presenting cells (APC), referred to as the chaperokine activity of Hsp72 [14–16].
In this study, we answer the question as to what role the two Hsp Hsp25 and Hsp72 have on tumor growth and metastatic spread of tumors when expressed on the surface of 4T1 tumor cells. By sorting 4T1 mammary adenocarcinoma cells based on the surface expression of either Hsp25 or Hsp72, we show that these proteins greatly affect tumor growth and metastatic spread of tumors from the abdominal breast gland to the lungs and ultimately affect survival.
Materials and Methods
Cell Lines and Culture Conditions
Murine breast carcinoma 4T1 cells (kind gift from Dr. Christopher Nicchitta, Duke University Medical Center, Durham, N.C., USA) are a 6-thioguanine-resistant cell line selected from 410.4 tumor without mutagen treatment. When injected into the abdominal breast gland of female BALB/c mice (8–12 weeks old), 4T1 spontaneously produce highly metastatic tumors that can metastasize to the lung, liver, lymph nodes and brain while the primary tumor is growing in situ. The primary tumor does not have to be removed to induce metastatic growth. The tumor growth and metastatic spread of 4T1 cells in BALB/c mice very closely mimic human breast cancer.
For the present study, 4T1 cells were maintained in Dulbecco’s modified Eagle medium (Invitrogen, Carlsbad, Calif., USA) containing 2 mM L-glutamine and adjusted to contain 1.5 g/l sodium bicarbonate, 4.5 g/l glucose, 10 mM HEPES, 1.0 mM sodium pyruvate and 10% fetal bovine serum at 37°C in a humidified incubator with a 5% CO2 atmosphere.
Animals and Tumor Challenges
BALB/c mice purchased from Jackson Laboratories (Bar Harbor, Me., USA) were challenged by injection of 4T1 cells into the abdominal mammary gland, and tumor volume was measured at regular intervals using an electronic caliper until tumor size reached 1,000 mm3. The tumor volume was estimated using the formula for the volume of an ellipsoid (length × width × height × 0.5236). All animals were treated humanely and in accordance with the guidelines of the Committee on the Care and Use of Laboratory Animals of the Institute of Animal Resources, National Research Council and Boston University School of Medicine.
Clonogenicity (Tumor Cell Survival) Assay
The ability of tumor cells to divide following various treatment protocols was assessed using the clonogenicity assay as previously described [17, 18]. Briefly, lung tissue was aseptically removed, minced with trypsin, seeded in triplicate at 1,000 cells/60-mm3 Petri dish and incubated at 37°C in a 5% CO2 air atmosphere. Ten to twelve days later, the plates were washed twice with PBS and colonies were stained with crystal violet and counted. The colonizing efficiency was scored, and results were compared to those in vehicle-treated cells.
Flow Cytometry and Cell Sorting
Exponentially growing cells were harvested and incubated with either anti-murine Hsp25 (StressGen Biotechnologies, Victoria, Canada) or anti-Hsp72 (StressGen Biotechnologies) or both, and the relative intensity was measured using a FACSCAN flow cytometer and sorting was performed on a MoFlow cytometer. Anti-rabbit Alexa Fluor-labeled and anti-mouse PE-labeled secondary antibodies were isotype matched and used as negative controls. The viability of cells was tested by propidium iodine exclusion test, and only cultures with <5% dead cells were used (data not shown). Briefly, flow cytometric analysis was performed on a FACScan with a CellQuest software program (Becton Dickinson, Mountain View, Calif., USA). Individual cells were gated on the basis of forward (FSC) and orthogonal scatter (SSC). The photomultiplier (PMT) for FITC (FL1 – height) or PE (FL2 – height) was set on a logarithmic scale. Cell debris was excluded by raising the FSC – height PMT threshold. The flow rate was adjusted to < 200 cells/s, and at least 30,000 cells were analyzed for each sample.
Western Blot Analysis
Following various treatment protocols, cells were lyzed using RIPA buffer containing appropriate protease inhibitors, and the protein concentration was determined using the Bradford method (Bio-Rad, Hercules, Calif., USA) with a DU-650 Spectrophotometer (Beckman Coulter). Samples were run in a 12% SDS-PAGE gel and transferred onto a nitrocellulose membrane. The membrane was blocked for 1 h at 4°C with Tween 20-Tris-buffered saline (T-TBS) containing 5% milk. After rinsing, the membrane was probed with a primary antibody against Hsp72 (StressGen Biotechnologies) in a dilution ratio of 1:2,000 or Hsp25 (StressGen Biotechnologies) in a dilution ratio of 1:1,000. Antibodies were diluted in T-TBS containing 5% milk. After 1 h of incubation at room temperature, the membrane was washed in T-TBS three times. Corresponding HRP-conjugated IgG secondary antibodies (Sigma-Aldrich, St. Louis, Mo., USA) were added and the membrane was incubated for 30 min at room temperature. After additional washes, bands were visualized using enhanced chemiluminescence (Amersham, Little Chalfont, UK).
Statistical Analysis
The data were analyzed using a two-tailed t test after applying ANOVA. Differences were considered significant when p < 0.05.
Results
Expression of Hsp25 and/or Hsp72 on 4T1 Breast Adenocarcinoma Cells
Although Hsp25 has been shown to be expressed at high levels within the cytosol of breast tumors, little is known about the role of Hsp25 surface expression during tumor growth and metastasis. To give insight into a possible answer to this question, we used the 4T1 breast adenocarcinoma model. 4T1 cells spontaneously produce highly metastatic tumors that can metastasize to the lung, liver, lymph nodes and brain while the primary tumor is growing in situ. Unlike other metastatic tumor models, the primary tumor does not have to be removed to induce metastatic growth. Importantly, the tumor growth and metastatic spread of 4T1 cells in syngenic BALB/c mice very closely mimic human breast cancer. Indeed, injection of as few as 7 × 103 4T1 cells into the abdominal breast gland of female BALB/c mice resulted in visible lung metastasis 21 days after tumor cell inoculation (fig. 1). Phenotypic analysis of cultured 4T1 cells showed that between 15 and 20% of the cultured cells express Hsp25 and little to no Hsp72 on the cell surface (fig. 2, middle panel). Flow cytometric analysis revealed that there were two subpopulations of Hsp25-expressing 4T1 cells, i.e. cells with intermediate- (Hsp25intermediate) and high (Hsp25high)-intensity expression (fig. 2, middle panel). Exposure of 4T1 cells to nonlethal heat shock (43°C, 30 min) treatment upregulated Hsp72 surface expression to approximately 25% and concomitantly suppressed Hsp25 surface expression to approximately 10% (fig. 2, bottom panel). Studies performed to examine the total expression of Hsp25 and Hsp72 by 4T1 mammary adenocarcinoma cells following nonlethal heat shock (43°C, 30 min) treatment revealed that 4T1 cells exhibit a normal stress response in reaction to exposure to heat shock by upregulating the expression of both Hsp72 and Hsp25 (fig. 3).
Fig. 1.
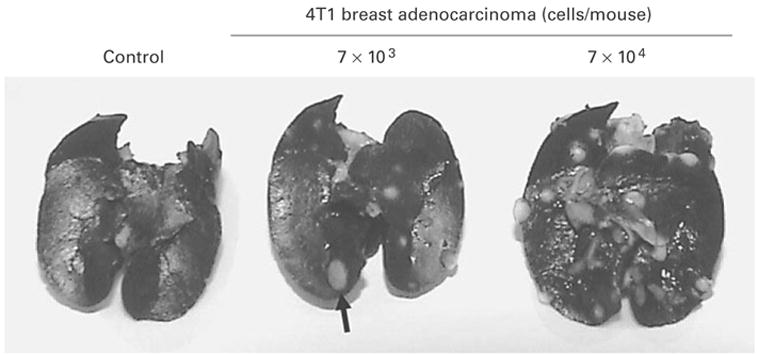
Injection of 4T1 breast adenocarcinoma cells into the abdominal breast gland results in the development of lung metastasis. Female BALB/c mice (6–8 weeks old) were injected with 103 (middle panel) or 7 × 104 (right panel) 4T1 cells, or with culture medium alone (left panel) subcutaneously into the abdominal breast gland. Twenty-one days after treatment, the animals were euthanized and the lungs were filled with a solution of India ink (1:6 in PBS) via the intratracheal route. The figures show lung metastasis (represented by a solid arrow) and are representative of three independently performed experiments with similar results.
Fig. 2.
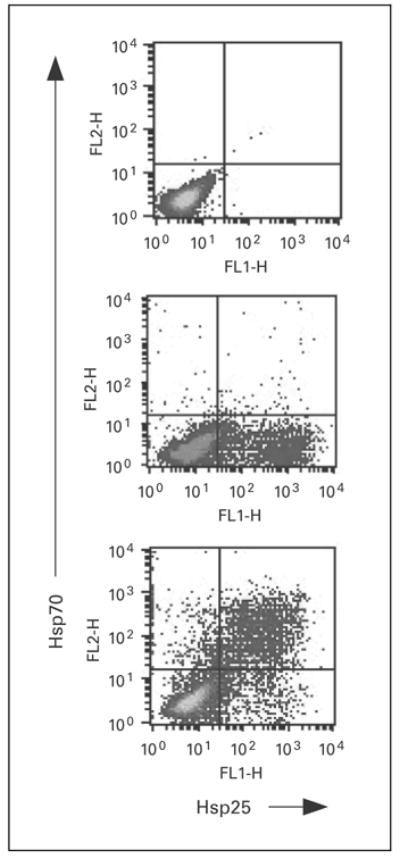
Surface expression of Hsp is modulated by exposure of tumors to heat shock treatment. 4T1 breast adenocarcinoma cells (2 × 106) were exposed to nonlethal heat shock treatment (43°C, 30 min; lower panel) or maintained at 37°C (middle panel) and allowed to recover at 37°C overnight. Cells were simultaneously stained with anti-Hsp72-PE, anti-Hsp25-Alexa Fluor or isotype control (top panel), and relative surface expression was analyzed by flow cytometry. Dot plots represent the relative percentage of cells expressing Hsp27 or Hsp72 from the gated population of cells and are representative of six independently performed experiments with similar results.
Fig. 3.
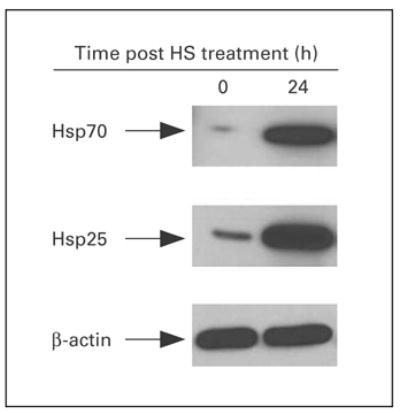
Kinetics of total expression of Hsp25 and Hsp72. Exponentially growing 4T1 cells were either exposed to nonlethal heat shock (HS) treatment (43°C, 30 min) and immediately lyzed (0 h) or allowed to recover for 24 h and lyzed (24 h). The extracted proteins were analyzed by 12% SDS-PAGE and immunoblot with antibodies specific for Hsp72 (upper panel) or Hsp25 (middle panel) and visualized with a coupled secondary antibody-chemiluminescence method. β-actin (bottom panel) was used as an internal control. Results are representative of two independently performed experiments with similar results.
Surface Expression of Hsp25 and/or Hsp72 during Tumor Growth of 4T1 Breast Adenocarcinoma
Pioneering work from the Multhoff laboratory clearly demonstrates that the expression of Hsp72 on the surface of tumors is a target for NK cell-mediated cytolytic machinery [12, 13, 19, 20]. In agreement with these studies, nonlethal heat shock treatment of 4T1 cells injected into the abdominal breast gland of female BALB/c mice resulted in a slower growth of tumors as compared to non-heat-shocked 4T1 tumors (data not shown). Since nonlethal heat shock treatment induces 4T1 cells to express Hsp72 on the plasma surface, this would make the 4T1 cells more susceptible to NK cell-mediated lysis and result in slower growth of 4T1 tumors that have been exposed to nonlethal heat shock prior to implantation (table 1). We further hypothesized that the expression of Hsp25 on the surface of the 4T1 cells enhanced their ability to grow at the site of inoculation and metastasize to the lungs. As expected, by day 5 after tumor cell inoculation, mice injected with Hsp25high/Hsp72− 4T1 tumors had tumors of approximately 70–90 mm3, whereas mice injected with either Hsp25intermediate/Hsp72−-expressing or unsorted or Hsp25−/Hsp72+-expressing 4T1 cells exhibited tumors of below 10 mm3 (fig. 4). By day 20 after tumor cell inoculation, tumors of mice injected with Hsp25high/Hsp72− 4T1 cells ranged from 425 to 475 mm3, as compared to tumors of mice injected with Hsp25intermediate/Hsp72−-expressing (225–280 mm3) or unsorted (125–175 mm3) or Hsp25−/ Hsp72+-expressing 4T1 cells (fig. 4). Taken together, these data suggest that the high expression of Hsp25 or Hsp72 on the surface of injected tumors correlates with enhanced or inferior growth of primary tumors, respectively.
Table 1.
Phenotypic characterization of 4T1 mammary adenocarcinoma cells in vivo
| Treatment1 | Percentage of 4T1 cells expressing Hsp25 or Hsp70 on the cell surface recovered from2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| abdominal breast gland
|
lungs
|
|||||||
| day 0
|
day 21
|
day 0
|
day 21
|
|||||
| Hsp25+ | Hsp70+ | Hsp25+ | Hsp70+ | Hsp25+ | Hsp70+ | Hsp25+ | Hsp70+ | |
| Unsorted | 45 | 1 | 78 | 12 | 0 | 0 | 98 | 2 |
| Hsp25+/Hsp70− | 98 | 2 | 82 | 15 | 0 | 0 | 97 | 3 |
| Hsp25−/Hsp70+ | 5 | 97 | 75 | 15 | 0 | 0 | 98 | 2 |
4T1 mammary adenocarcinoma cells were either sorted for Hsp25+/Hsp70−- or Hsp25−/Hsp70+-expressing cells by flow cytometry or not sorted. 4T1 cells (7 × 103) were then injected subcutaneously into the abdominal breast gland of female BALB/c mice.
The percentages of cells expressing Hsp25/Hsp70 from either the abdominal breast gland or lungs were analyzed by flow cytometry 0 or 21 days after tumor cell inoculation. Day 0 represents the phenotype of 4T1 cells directly after sorting and immediately before tumor cell inoculation. Data are representative of three experiments with similar results.
Fig. 4.
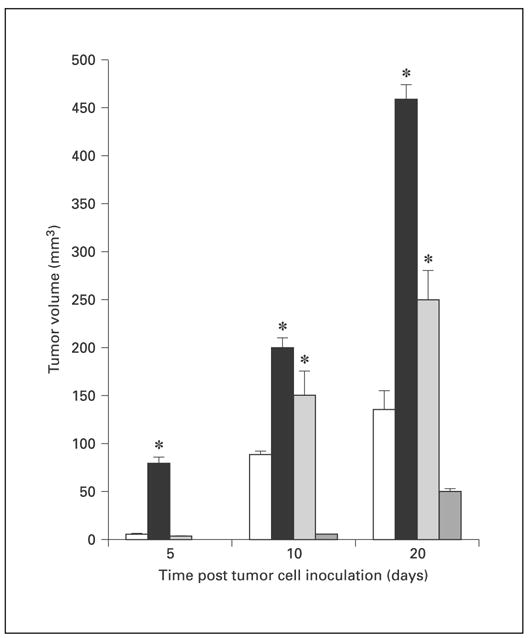
Surface expression of Hsp25 and Hsp72 on 4T1 cells correlates with enhanced and suppressed tumor growth, respectively. Female BALB/c mice (6–8 weeks old) received an injection into the abdominal breast gland of 7 × 104 4T1 breast adenocarcinoma cells that were either unsorted (white bars) or sorted for Hsp25high/Hsp72−-expressing (black bars), Hsp25intermediate/Hsp72−-expressing (light-gray bars) or Hsp25−/Hsp72+-expressing (dark-gray bars) cells, and tumor growth was measured using an electronic caliper. Data are mean tumor volume ± SE and represent the sum of two independently performed experiments with 5 mice per treatment group. tci = Tumor cell inoculation. * p < 0.05 versus unsorted cells.
Surface Expression of Hsp25 and/or Hsp72 during Metastatic Spread of 4T1 Breast Adenocarcinoma
To determine the relative importance of the surface expression of Hsp25 for the metastatic spread of 4T1 cells from the abdominal breast gland to the lungs, we performed a clonogenicity assay on lungs of mice that had undergone injection of 4T1 cells in the abdominal breast gland 21 days previously. Mice injected with 4T1 cells that had been exposed to nonlethal heat shock treatment expressed significantly fewer colonies than those injected with non-heat-shocked tumors (fig. 5). When sorted on the basis of Hsp25 or Hsp72 surface expression, Hsp25+/ Hsp72−-expressing 4T1 tumors exhibited a far greater potential to metastasize to the lungs than Hsp25−/ Hsp72+-expressing tumors, which exhibited a far weaker ability to metastasize and colonize the lungs (fig. 5). Phenotypic characterization of the 4T1 cells that metastasized to the lungs revealed a disproportionately high expression of Hsp25 on the plasma surface (table 1). Interestingly, 4T1 cells that were sorted for their specific lack of Hsp25 surface expression, i.e. Hsp25−/Hsp72+ cells, although they metastasized poorly to the lungs (fig. 5), when analyzed for surface expression, they showed a reappearance of Hsp25 and a lack of Hsp72 expression (table 1). These results suggest that after tumor cell inoculation in the abdominal breast gland, survival, growth and eventual metastasis to the lungs favor Hsp25- but not Hsp72-expressing cells.
Fig. 5.
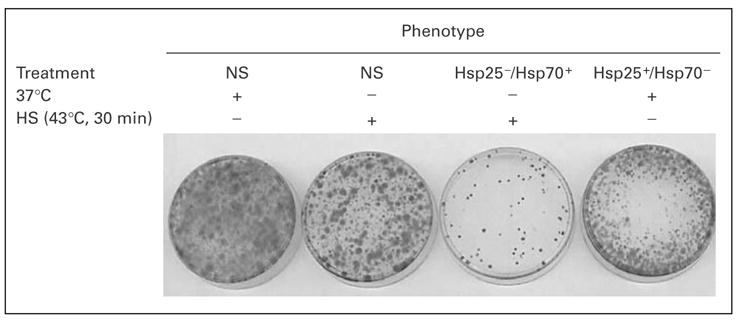
Clonogenicity assay to measure the metastatic potential of 4T1 breast adenocarcinoma cells correlates with high Hsp25 surface expression. 4T1 breast adenocarcinoma cells (7 × 104) that were either not sorted (NS) or sorted for Hsp25−/Hsp72+ or Hsp25+/ Hsp72−cells were injected into the abdominal breast gland of female BALB/c mice (6–8 weeks old). After 21 days, lungs were excised, digested and made into a single-cell suspension, then seeded in triplicate at 1,000 cells/60-mm3 petri dish and incubated at 37°C in a 5% CO2 air atmosphere. Ten to twelve days later, the plates were washed twice with PBS and colonies were stained with crystal violet. Results represent the cloning efficiencies following various treatment protocols and are representative of two independently performed experiments with similar results. HS = Heat shock.
Expression of Hsp25 and/or Hsp72 and Survival of Animals after 4T1 Tumor Challenge
We next performed survival experiments to determine the importance of Hsp25 and Hsp72 in the ultimate survival of the animals with 4T1 tumors. Mice were injected with 4T1 cells into the abdominal breast gland and survival was followed over 40 days. The expression of Hsp72, induced by nonlethal heat shock treatment, greatly enhanced the survival of animals after challenge with 4T1 tumors (fig. 6). By day 25 after tumor cell inoculation, while 100% of animals injected with heat-shocked 4T1 cells were still alive, only 50% of mice injected with non-heat-shocked 4T1 cells were still alive (fig. 6). At the end of the experiment, 50% of mice injected with heat-shocked 4T1 cells were still alive, as compared to no survivors in the non-heat shock-treated controls (fig. 6). Taken together, these results suggest that the upregulation of surface-expressed Hsp72 and concomitant suppression of Hsp25 induced by nonlethal heat shock treatment play an important role in the survival of animals after tumor challenge.
Fig. 6.
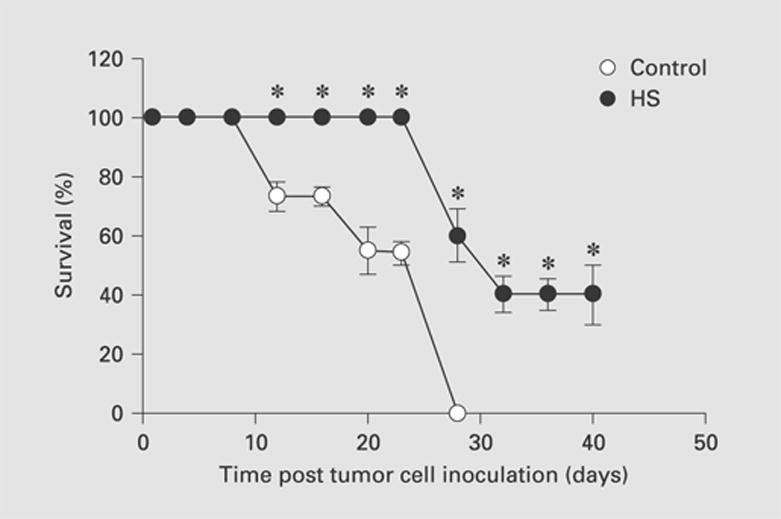
Exposure of 4T1 breast adenocarcinoma cells to nonlethal heat shock treatment increased the survival of animals after tumor challenge. 4T1 breast adenocarcinoma cells (7 × 104) that were either maintained at 37°C (open circles) or exposed to nonlethal heat shock treatment (HS; 43°C, 30 min) and allowed to recover for 24 h at 37°C (filled circles) were injected into the abdominal breast gland of female BALB/c mice (6–8 weeks old). Data are the mean percentage survival ± SEM and represent the sum of three independently performed experiments. * p < 0.05 versus control.
Discussion
Our studies were designed to examine the role of Hsp72 and Hsp25 surface expression during tumor growth and metastatic spread of 4T1 mammary adenocarcinoma tumors from the abdominal breast gland to the lungs. To address this question, we used the 4T1 breast adenocarcinoma tumor model; when injected into the abdominal breast gland of BALB/c mice, 4T1 tumors spontaneously metastasize to the lung, liver, lymph nodes and brain without the need to remove the primary tumor [21]. This makes the model uniquely similar to human breast cancer with respect to growth and metastasis. Several studies have been performed to determine the role of Hsp25/27 and Hsp70 during tumor progression [5, 22] and in response to current anticancer drugs [4, 23]. Most studies have attributed a modest prognostic value to Hsp27 in breast cancer, and it has been implicated in drug resistance [23]. Several studies report that enhanced or maintained expression of Hsp27 is an independent and accurate predictor of poor clinical outcome for individuals with breast [5] or prostate cancer [24]. Our findings support these studies; however, these studies measured total Hsp27 and Hsp70 expression, whereas our studies specifically identified Hsp25 and Hsp72 expressed on the surface of 4T1 tumors. This is a very important distinction because of the dichotomy that has been revealed between intracellularly and extracellularly expressed Hsp [for a review, see ref. 25]. Enhanced expression of intracellular Hsp72 has clearly been shown to be anti-inflammatory [17] and antiapoptotic [26], to induce cell cycle arrest [26] and to protect cells from a variety of stressful stimuli [for reviews, see ref. 11, 27]. In contrast, enhanced extracellular expression of Hsp72 either on the surface of tumors or in the extracellular milieu either enhances NK cell-mediated lysis [28] or upregulates APC-mediated acute-phase responses [14–16, 29], respectively.
With this background, it might be expected that Hsp25 would exhibit a similar differential pattern of activation depending on its cellular location. In this study, we showed that high surface expression of Hsp25 on 4T1 tumors significantly increases their ability to grow within the abdominal breast gland (fig. 4) and to successfully metastasize and colonize the lungs (fig. 5). Indeed, when tumors were further sorted according to the quality of the Hsp25 expressed on the surface, Hsp25high-expressing tumors grew more aggressively at the site of inoculation than the Hsp25intermediate-expressing tumors or unsorted controls (fig. 4). These results suggest that the interaction between host effector cells and tumors expressing high levels of surface-bound Hsp25 results in abrogation and/ or deactivation of host antitumor responses. This is intriguing, since this study and the work of others demonstrate that the interaction between host effector cells and Hsp72 expressed on the surface of tumors activates specific host antitumor responses [19, 28, 30, 31]. Here, we showed that the expression of Hsp72 on 4T1 tumors implanted into the breast gland of female BALB/c mice results in significantly prolonged survival as compared to 4T1 tumors expressing high levels of Hsp25 on their surface (p < 0.05) (fig. 6).
Our studies have not conclusively determined the exact molecular mechanism by which Hsp25 expressed on the surface of tumors deactivates host antitumor responses; however, our findings show a correlation between high Hsp25 surface expression and enhanced tumor growth and metastasis. We predict that in the 4T1 tumor model, high Hsp25 surface expression is not the only factor that causes tumors to survive and grow within the abdominal mammary gland and eventually metastasize to the lungs. We hypothesize that anti-inflammatory mediators, including IL-4, IL-5, IL-10 and TGF-β, play an immunosuppressive role and promote tumor growth and metastasis. Studies are currently under way to address this question [Bausero et al., in preparation]. In line with this hypothesis, studies by De et al. [32] demonstrated that exogenous Hsp27 admixed with human monocytes induced the release of IL-10 and TNF-α. It was shown that the Hsp27-induced IL-10 production occurred via the p38-dependent pathway, which proved to be independent of TNF-α activation, thereby suggesting that this signals a predominantly anti-inflammatory signal within the tumor microenvironment [32]. Although in our studies we did not admix exogenous Hsp25 with immunocompetent cells, it is plausible that ‘tumor-cell’ contact between 4T1 cells that express high levels of surface Hsp25 and immunocompetent cells might have a similar effect, consequently inducing IL-10 secretion and minimal TNF-α release by APC that have migrated into the tumor microenvironment.
Studies from the Multhoff laboratory on the expression of Hsp72 on the surface of tumors have contributed greatly to furthering our knowledge of NK cell-susceptible tumor targets. In comparison with immunocompetent cells, malignant tumor cells, including biopsies from colorectal, lung, neuronal and pancreas carcinomas, liver metastases and leukemic blasts of patients with acute myelogenous leukemia, express high levels of surface Hsp72 [20, 33–36]. The Hsp72 expression on tumors correlates with an increased sensitivity to NK cell-mediated cytolysis following cytokine stimulation [37–39]. Indeed, Hsp72-selective NK cell activity was reported to be stimulated in a clinical phase I trial of patients with advanced metastasized colorectal and lung carcinoma when treated with the Hsp72 peptide TKD plus low-dose IL-2 [31]. These studies help explain the remarkable ability of non-lethal heat shock treatment, which induces surface expression of Hsp72, to suppress the potent metastatic potential of Hsp25-expressing 4T1 tumors exhibited in this study. The possibility also exists that an additional mechanism by which nonlethal heat shock treatment results in lower tumor growth and reduced ability of 4T1 cells to colonize the lungs is due to the chaperokine activity of Hsp72 [14–16, 25, 40]. It is possible that exposure of 4T1 tumors to nonlethal heat shock treatment results in the active release of Hsp72 from tumors. The released Hsp72 chaperones tumor-derived peptides that are taken up by APC [14, 41, 42], and presents tumor-associated antigens to specific cytotoxic T lymphocytes to mount a potent anti-tumor response. This is currently the working hypothesis for Hsp-based immunotherapies [for a review, see ref. 43].
In summary, our studies suggest that in certain cancers, tumor development and metastatic spread favors tumors that overexpress high levels of Hsp25 on their plasma surface, while tumors that express Hsp72 on their plasma surface are sensitive to antitumor effector cells. This implies that therapies that can upregulate Hsp72 surface expression and concomitantly suppress Hsp25/27 surface expression on tumors might result in the suppression of tumor growth and abrogate the tumors’ metastatic potential.
Acknowledgments
The authors thank Dr. Ajit Bharti (Boston University School of Medicine, Boston, Mass., USA) and Dr. Susana Fiorentino (IN-SERM U462, Saint-Louis Hospital, Paris, France) for helpful discussions. This work was supported in part by the National Institute of Health grant RO1CA91889, a Joint Center for Radiation Therapy Foundation Grant, Harvard Medical School and Institutional support from the Department of Medicine, Boston University School of Medicine (to A.A.).
References
- 1.Craig EA, Gross CA. Is hsp70 the cellular thermometer? Trends Biochem Sci. 1991;16:135–140. doi: 10.1016/0968-0004(91)90055-z. [DOI] [PubMed] [Google Scholar]
- 2.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 3.Morimoto RI, Tissières A, Georgopoulos C. The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 4.Ciocca DR, Green S, Elledge RM, Clark GM, Pugh R, Ravdin P, Lew D, Martino S, Osborne CK. Heat shock proteins hsp27 and hsp70: Lack of correlation with response to tamoxifen and clinical course of disease in estrogen receptor-positive metastatic breast cancer (a Southwest Oncology Group Study) Clin Cancer Res. 1998;4:1263–1266. [PubMed] [Google Scholar]
- 5.Ciocca DR, Vargas-Roig LM. Hsp27 as a prognostic and predictive factor in cancer. Prog Mol Subcell Biol. 2002;28:205–218. doi: 10.1007/978-3-642-56348-5_11. [DOI] [PubMed] [Google Scholar]
- 6.Ciocca DR, Roig LM. Estrogen receptors in human nontarget tissues: Biological and clinical implications. Endocr Rev. 1995;16:35–62. doi: 10.1210/edrv-16-1-35. [DOI] [PubMed] [Google Scholar]
- 7.Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, Paul C, Wieske M, Arrigo AP, Buchner J, Gaestel M. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/ tumor necrosis factor alpha by phosphorylation. J Biol Chem. 1999;274:18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- 8.Mehlen P, Kretz-Remy C, Preville X, Arrigo AP. Human hsp27, Drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFalpha-induced cell death. EMBO J. 1996;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- 9.Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nollen EA, Brunsting JF, Roelofsen H, Weber LA, Kampinga HH. In vivo chaperone activity of heat shock protein 70 and thermotolerance. Mol Cell Biol. 1999;19:2069–2079. doi: 10.1128/mcb.19.3.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaattela M. Programmed cell death: Many ways for cells to die decently. Ann Med. 2002;34:480–488. doi: 10.1080/078538902321012423. [DOI] [PubMed] [Google Scholar]
- 12.Gross C, Hansch D, Gastpar R, Multhoff G. Interaction of heat shock protein 70 peptide with NK cells involves the NK receptor CD94. Biol Chem. 2003;384:267–279. doi: 10.1515/BC.2003.030. [DOI] [PubMed] [Google Scholar]
- 13.Gross C, Koelch W, DeMaio A, Arispe N, Multhoff G. Cell surface-bound heat shock protein 70 (Hsp70) mediates perforin-independent apoptosis by specific binding and uptake of granzyme B. J Biol Chem. 2003;278:41173–41181. doi: 10.1074/jbc.M302644200. [DOI] [PubMed] [Google Scholar]
- 14.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70:Role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 15.Asea A, Kabingu E, Stevenson MA, Calderwood SK. HSP70 peptide-bearing and peptide-negative preparations function as chaperokines. Cell Stress Chaperones. 2000;5:425–431. doi: 10.1379/1466-1268(2000)005<0425:hpbapn>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 17.Asea A, Ara G, Teicher BA, Stevenson MA, Calderwood SK. Effects of the flavonoid drug quercetin on the response of human prostate tumours to hyperthermia in vitro and in vivo. Int J Hyperthermia. 2001;17:347–356. doi: 10.1080/02656730110053146. [DOI] [PubMed] [Google Scholar]
- 18.Asea A, Mallick R, Lechpammer S, Gulshan A, Teicher BA, Fiorentino S, Stevenson MA, Calderwood SK. Cyclooxygenase inhibitors are potent sensitizers of prostate tumors to hyperthermia and radiation in vivo. Int J Hyperthermia. 2001;17:401–414. doi: 10.1080/02656730110058600. [DOI] [PubMed] [Google Scholar]
- 19.Multhoff G. Activation of natural killer cells by heat shock protein 70. Int J Hyperthermia. 2002;18:576–585. doi: 10.1080/0265673021000017109. [DOI] [PubMed] [Google Scholar]
- 20.Multhoff G, Botzler C, Jennen L, Schmidt J, Ellwart J, Issels R. Heat shock protein 72 on tumor cells: A recognition structure for natural killer cells. J Immunol. 1997;158:4341–4350. [PubMed] [Google Scholar]
- 21.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–1405. [PubMed] [Google Scholar]
- 22.Oesterreich S, Hilsenbeck SG, Ciocca DR, Allred DC, Clark GM, Chamness GC, Osborne CK, Fuqua SA. The small heat shock protein HSP27 is not an independent prognostic marker in axillary lymph node-negative breast cancer patients. Clin Cancer Res. 1996;2:1199–1206. [PubMed] [Google Scholar]
- 23.Ciocca DR, Rozados VR, Cuello Carrion FD, Gervasoni SI, Matar P, Scharovsky OG. Hsp25 and Hsp70 in rodent tumors treated with doxorubicin and lovastatin. Cell Stress Chaperones. 2003;8:26–36. doi: 10.1379/1466-1268(2003)8<26:hahirt>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornford PA, Dodson AR, Parsons KF, Desmond AD, Woolfenden A, Fordham M, Neoptolemos JP, Ke Y, Foster CS. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000;60:7099–7105. [PubMed] [Google Scholar]
- 25.Asea A. Exogenous Hsp70:Principles and application of the chaperokine activity of Hsp70; In: Henderson B, Pockley AG, editors. The Extracellular Biology of Molecular Chaperones. London: Cambridge University Press; in press. [Google Scholar]
- 26.Jaattela M, Wissing D, Kokholm K, Kallunki T, Egeblad M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 1998;17:6124–6134. doi: 10.1093/emboj/17.21.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calderwood SK, Asea A. Targeting HSP70-induced thermotolerance for design of thermal sensitizers. Int J Hyperthermia. 2002;18:597–608. doi: 10.1080/0265673021000019666. [DOI] [PubMed] [Google Scholar]
- 28.Farkas B, Hantschel M, Magyarlaki M, Becker B, Scherer K, Landthaler M, Pfister K, Gehrmann M, Gross C, Mackensen A, Multhoff G. Heat shock protein 70 membrane expression and melanoma-associated marker phenotype in primary and metastatic melanoma. Melanoma Res. 2003;13:147–152. doi: 10.1097/00008390-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Basu S, Suto R, Binder RJ, Srivastava PK. Heat shock proteins as novel mediators of cytokine secretion by macrophages. Cell Stress Chaperones. 1998;3:11–16. [Google Scholar]
- 30.Kleinjung T, Arndt O, Feldmann HJ, Bockmuhl U, Gehrmann M, Zilch T, Pfister K, Schonberger J, Marienhagen J, Eilles C, Rossbacher L, Multhoff G. Heat shock protein 70 (Hsp70) membrane expression on head-and-neck cancer biopsy – a target for natural killer (NK) cells. Int J Radiat Oncol Biol Phys. 2003;57:820–826. doi: 10.1016/s0360-3016(03)00629-1. [DOI] [PubMed] [Google Scholar]
- 31.Gehrmann M, Schmetzer H, Eissner G, Haferlach T, Hiddemann W, Multhoff G. Membrane-bound heat shock protein 70 (Hsp70) in acute myeloid leukemia: A tumor specific recognition structure for the cytolytic activity of autologous NK cells. Haematologica. 2003;88:474–476. [PubMed] [Google Scholar]
- 32.De AK, Kodys KM, Yeh BS, Miller-Graziano C. Exaggerated human monocyte IL-10 concomitant to minimal TNF-alpha induction by heat-shock protein 27 (Hsp27) suggests Hsp27 is primarily an antiinflammatory stimulus. J Immunol. 2000;165:3951–3958. doi: 10.4049/jimmunol.165.7.3951. [DOI] [PubMed] [Google Scholar]
- 33.Hantschel M, Pfister K, Jordan A, Scholz R, Andreesen R, Schmitz G, Schmetzer H, Hiddemann W, Multhoff G. Hsp70 plasma membrane expression on primary tumor biopsy material and bone marrow of leukemic patients. Cell Stress Chaperones. 2000;5:438–442. doi: 10.1379/1466-1268(2000)005<0438:hpmeop>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Botzler C, Schmidt J, Luz A, Jennen L, Issels R, Multhoff G. Differential Hsp70 plasma-membrane expression on primary human tumors and metastases in mice with severe combined immunodeficiency. Int J Cancer. 1998;77:942–948. doi: 10.1002/(sici)1097-0215(19980911)77:6<942::aid-ijc25>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 35.Botzler C, Issels R, Multhoff G. Heat-shock protein 72 cell-surface expression on human lung carcinoma cells in associated with an increased sensitivity to lysis mediated by adherent natural killer cells. Cancer Immunol Immunother. 1996;43:226–230. doi: 10.1007/s002620050326. [DOI] [PubMed] [Google Scholar]
- 36.Multhoff G, Hightower LE. Cell surface expression of heat shock proteins and the immune response. Cell Stress Chaperones. 1996;1:167–176. doi: 10.1379/1466-1268(1996)001<0167:cseohs>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Botzler C, Li G, Issels RD, Multhoff G. Definition of extracellular localized epitopes of Hsp70 involved in an NK immune response. Cell Stress Chaperones. 1998;3:6–11. doi: 10.1379/1466-1268(1998)003<0006:doeleo>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Multhoff G, Mizzen L, Winchester CC, Milner CM, Wenk S, Eissner G, Kampinga HH, Laumbacher B, Johnson J. Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of natural killer cells. Exp Hematol. 1999;27:1627–1636. doi: 10.1016/s0301-472x(99)00104-6. [DOI] [PubMed] [Google Scholar]
- 39.Multhoff G, Pfister K, Gehrmann M, Hantschel M, Gross C, Hafner M, Hiddemann W. A 14-mer Hsp70 peptide stimulates natural killer (NK) cell activity. Cell Stress Chaperones. 2001;6:337–344. doi: 10.1379/1466-1268(2001)006<0337:amhpsn>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asea A. Chaperokine-induced signal transduction pathways. Exerc Immunol Rev. 2003;9:25–33. [PMC free article] [PubMed] [Google Scholar]
- 41.Binder RJ, Han DK, Srivastava PK. CD91:A receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 42.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 43.Srivastava PK. Heat shock protein-based novel immunotherapies. Drug News Perspect. 2000;13:517–522. doi: 10.1358/dnp.2000.13.9.858479. [DOI] [PubMed] [Google Scholar]


