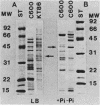
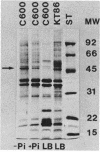
Abstract
Tellurite (TeO3(2-)) is highly toxic toward Escherichia coli (MIC, approximately 1 microgram ml-1). Mutants (Tel) that were resistant to low levels of TeO3(2-) (MIC, approximately 10 micrograms ml-1) and collaterally resistant to arsenate were isolated. These Tel mutants were unable to grow on media containing low levels of Pi, which supported growth of the parent strain. When grown at much higher Pi levels they exhibited depressed levels of the outer membrane phoE protein and the periplasmic phoS protein, as well as several other proteins indicative of Pi starvation. Tel mutants were markedly defective in 32Pi transport, and TeO3(2-) was shown to be a potent competitive inhibitor of 32Pi transport in the parent strain. The Tel phenotype could be complemented by an F' plasmid harboring the phoR, phoB, and phoA loci, and curing of the F' plasmid completely restored TeO3(2-) resistance. Of a variety of well-characterized Pi transport mutants, only phoB mutants were equally resistant to TeO3(2-), and susceptibility could also be restored in strains carrying an F' plasmid for the phoB region and lost once more after F' curing. The tel and phoB loci were equally cotransducible with lac. Tel mutants still synthesized alkaline phosphatase, the phoA gene product, after Pi starvation, suggesting that the phoB locus per se was not involved because phoB is a positive regulatory gene for phoA expression. The results indicate that TeO3(2-) is transported into E. coli by a phosphate transport system and that resistance to TeO3(2-) specifically selects for as yet uncharacterized mutants in the phoB-phoA region of the chromosome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amemura M., Makino K., Shinagawa H., Kobayashi A., Nakata A. Nucleotide sequence of the genes involved in phosphate transport and regulation of the phosphate regulon in Escherichia coli. J Mol Biol. 1985 Jul 20;184(2):241–250. doi: 10.1016/0022-2836(85)90377-8. [DOI] [PubMed] [Google Scholar]
- Amemura M., Shinagawa H., Makino K., Otsuji N., Nakata A. Cloning of and complementation tests with alkaline phosphatase regulatory genes (phoS and phoT) of Escherichia coli. J Bacteriol. 1982 Nov;152(2):692–701. doi: 10.1128/jb.152.2.692-701.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Benz R., Darveau R. P., Hancock R. E. Outer-membrane protein PhoE from Escherichia coli forms anion-selective pores in lipid-bilayer membranes. Eur J Biochem. 1984 Apr 16;140(2):319–324. doi: 10.1111/j.1432-1033.1984.tb08104.x. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Rosenberg H., Downie J. A., Silver S. Genetic analysis of mutants affected in the Pst inorganic phosphate transport system. J Bacteriol. 1981 Oct;148(1):1–9. doi: 10.1128/jb.148.1.1-9.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J. Plasmid-determined resistance to antimicrobial drugs and toxic metal ions in bacteria. Microbiol Rev. 1983 Sep;47(3):361–409. doi: 10.1128/mr.47.3.361-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan C. D., Wanner B., Inouye H. Analysis of regulation of phoB expression using a phoB-cat fusion. J Bacteriol. 1983 Nov;156(2):710–717. doi: 10.1128/jb.156.2.710-717.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONDO E., MITSUHASHI S. DRUG RESISTANCE OF ENTERIC BACTERIA. IV. ACTIVE TRANSDUCING BACTERIOPHAGE P1 CM PRODUCED BY THE COMBINATION OF R FACTOR WITH BACTERIOPHAGE P1. J Bacteriol. 1964 Nov;88:1266–1276. doi: 10.1128/jb.88.5.1266-1276.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Makino K., Shinagawa H., Nakata A. Regulation of the phosphate regulon of Escherichia coli K-12: regulation and role of the regulatory gene phoR. J Mol Biol. 1985 Jul 20;184(2):231–240. doi: 10.1016/0022-2836(85)90376-6. [DOI] [PubMed] [Google Scholar]
- Marques A. M., Congregado F., Simon-Pujol D. M. Antibiotic and heavy metal resistance of Pseudomonas aeruginosa isolated from soils. J Appl Bacteriol. 1979 Oct;47(2):347–350. doi: 10.1111/j.1365-2672.1979.tb01765.x. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Rosen B. P. Energetics of plasmid-mediated arsenate resistance in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6119–6122. doi: 10.1073/pnas.79.20.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt C., Torriani A. Complementation test between alkaline phosphatase regulatory mutations phoB and phoRc in Escherichia coli. Genetics. 1977 Feb;85(2):203–208. doi: 10.1093/genetics/85.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H., Gerdes R. G., Chegwidden K. Two systems for the uptake of phosphate in Escherichia coli. J Bacteriol. 1977 Aug;131(2):505–511. doi: 10.1128/jb.131.2.505-511.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa H., Makino K., Nakata A. Regulation of the pho regulon in Escherichia coli K-12. Genetic and physiological regulation of the positive regulatory gene phoB. J Mol Biol. 1983 Aug 15;168(3):477–488. doi: 10.1016/s0022-2836(83)80297-6. [DOI] [PubMed] [Google Scholar]
- Silver S., Keach D. Energy-dependent arsenate efflux: the mechanism of plasmid-mediated resistance. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6114–6118. doi: 10.1073/pnas.79.20.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Jacoby G. A. Plasmid-determined resistance to tellurium compounds. J Bacteriol. 1977 Jan;129(1):276–281. doi: 10.1128/jb.129.1.276-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERAI T., KAMAHORA T., YAMAMURA Y. Tellurite reductase from Mycobacterium avium. J Bacteriol. 1958 May;75(5):535–539. doi: 10.1128/jb.75.5.535-539.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Summers A. O. Association of tellurium resistance and bacteriophage inhibition conferred by R plasmids. J Bacteriol. 1979 Mar;137(3):1430–1433. doi: 10.1128/jb.137.3.1430-1433.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., de Geus P., Lugtenberg B., Hackett J., Reeves P. Regulation of the pho regulon of Escherichia coli K-12. Cloning of the regulatory genes phoB and phoR and identification of their gene products. J Mol Biol. 1982 May 15;157(2):265–274. doi: 10.1016/0022-2836(82)90233-9. [DOI] [PubMed] [Google Scholar]
- Willsky G. R., Bennett R. L., Malamy M. H. Inorganic phosphate transport in Escherichia coli: involvement of two genes which play a role in alkaline phosphatase regulation. J Bacteriol. 1973 Feb;113(2):529–539. doi: 10.1128/jb.113.2.529-539.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]