Abstract
Immaturity of the immune system has been suggested as an underlying factor for the high rate of morbidity and mortality from infections in newborns. Functional impairment of neonatal T cells is frequently quoted as the main underlying mechanism for such immaturity. However, recent studies suggest that neonatal antigen-presenting cells (APCs) also exhibit functional alterations, which could lead to secondary defects of adaptive T cell responses. In this review, we summarize what is known on the functionality of APC at birth and during early childhood. Compared to adults, neonatal APCs display markers of immaturity and produce low levels of cytokines. Multiple factors could be involved in neonatal APC alteration, such as intrinsic immaturity, defective interaction between APCs and T cells, and regulatory T cell-mediated inhibition. Characterization of the relative contribution of each mechanism is clearly needed to better understand the functional capability of the neonatal immune system.
Keywords: Neonate, Immune System, Antigen-presenting cells, Dendritic cells, Regulatory T cells, Macrophages, Monocytes, Childhood, Costimulatory molecules, Cytokines
Introduction
The world Health Organization estimates that approximately 7.1 million infants between 1 and 12 months of age die annually of infections [1]. The most common causes of early death are acute respiratory and diarrheal diseases, caused by bacterial and viral pathogens [1-3]. Such infections are rarely fatal in older children and adults. The immaturity of the neonatal immune system has been suggested as an underlying factor for such high rate of morbidity and mortality [2]. Defects in the immune system during early life also influence the responses to neonatal immunizations, frequently resulting in poor antibody responses [4,5]. Indeed, with the exception of BCG, most vaccines administered during the first six months of life require several doses to induce protection, and the ability of vaccines to induce protection is directly correlated with the progressive maturation of the infant's immune system [5].
It has been speculated that the immaturity of the immune system in early life is due to a functional impairment of T cells. Neonatal T cells exhibit intrinsic deficiencies that prevent them from becoming fully activated [6,7], which are summarized in Table 1. However, recent studies suggest that the immaturity of the neonate's immune system implies more than alterations in T cell activation. In particular, neonatal antigen-presenting cells (APCs) also exhibit functional alterations (summarized in Table 1), which could lead to secondary defects of adaptive T cell responses. The immaturity of APC system in murine neonates has been extensively documented (for review, see [8,9]. However, due to the well-established differences in development between human and murine neonates [5], we have focused this review on the findings obtained with human APCs.
Table 1.
Findings supporting defective function of cord blood (CB) T cells and neonatal APCs
| Findings that support defective function of CB T cells |
|
|
Findings that support defective function of CB monocytes and macrophages |
|
|
Findings that support defective function of CB dendritic cells |
|
Antigen-presenting cells
APCs are key players during innate immune responses, and are responsible for the induction of adaptive immune responses. The immunological outcome after an antigenic challenge depends on several factors, including the type of APC. Naïve T cell activation requires at least two signals provided by APCs: one is antigen-dependent, TCR-mediated (signal 1) and the other one is antigen-independent and depends on co-stimulation (signal 2). Recently, antigen-independent signals have themselves been divided into a signal 2, which corresponds to cognate interaction with co-stimulatory molecules, and a signal 3 constituted by the action of soluble mediators such as cytokines. It is also known that the interactions between APC and activated T cells constitute a two-way dialog that leads to APC maturation on one hand, and the generation of fully-activated effector T cells on the other hand [10,11]. Both quantitative and qualitative alterations in such interactions promote the development of tolerance in effector T cells [11-14].
Neonatal monocytes and macrophages
Functional defects of neonatal mononuclear phagocytes have been documented, compared to their adult counterparts. Such defects include evidence of immaturity, decreased production of cytokines and altered phagocytosis (see Table 1). In particular, neonatal monocytes exhibit low baseline expression of the costimulatory molecules CD86 and CD40. Expression of these molecules is not up-regulated by potent activators, such as the combination of IFN-γ and CD40 Ligand (CD40L) [15]. In addition, neonatal monocytes and macrophages produce low levels of proinflammatory cytokines such as TNF-α, IL-1β or IL-12, in response to bacterial lipopolysaccharide (LPS, a TLR4 ligand) [16-18], and other TLR ligands such as triacylated BLP (TLR1/2 ligand), mycoplasma-associated lipopeptide (TLR2/6 ligand) and imiquimod (TLR7 ligand) [19]. These alterations probably result from defects in the signaling pathways downstream of TLR engagement, but the exact nature of such defect(s) is not yet elucidated. Although neonatal monocytes express similar levels of mRNA for different TLRs than adult monocytes [20,19], these data do not exclude possible differences in the protein expression of such receptors. Some data also support a defect in TLR signaling: (1) the up-regulation of TLR4 and CD14 expression upon LPS stimulation is not observed in neonatal monocytes [19,21]; and (2) the protein expression of MyD88, an important adaptor molecule involved in TLR-mediated signaling, is decreased in neonatal monocytes [21], although MyD88 mRNA levels are similar in neonatal and adult monocytes [19]. It should be noted that the expression of several key molecules involved in TLR signaling, such as TIRAP and IRAK-4, has not been carefully examined. Similar levels of mRNA have been reported for those 2 molecules, but protein expression has not been determined [19]. Finally, interestingly, TLR4 protein expression is lower on monocytes from premature babies compared to those from full-term babies [20]. This difference could constitute an underlying mechanism for the exacerbated susceptibility to infections exhibited by premature babies, and suggest a regulation of TLR expression in relation to fetal development.
Adding to the complexity, differential defects were reported depending on the TLR ligand used to stimulate neonatal monocytes. In particular, phosphorylation of p38 MAPK and TNF-α production was decreased in neonatal monocytes upon LPS stimulation, while it was normal after R-848 stimulation, a TLR 7/8 ligand [19]. This observation may reflect either the presence in neonate monocytes of factors interfering specifically with TLR4 signaling, or the existence of alternative pathways to activate p38 MAPK.
Neonatal macrophages also exhibit decreased responsiveness to IFN-γ, which is linked to a marked alteration in STAT-1 phosphorylation [22,23]. Importantly, this latter finding is associated with deficient killing of intra-cellular pathogens by IFN-γ-stimulated neonatal macrophages [24], which could have broad implications in vivo.
Decreased production of IL-12 by CBMC stimulated by Staphylococcus aureus Cowan (SAC) has also been described [25,17]. Since monocytes are the main producers of IL-12 in SAC-stimulated PBMC, these results also point towards defective activation of neonatal monocytes.
Phagocytosis constitutes a major function of monocytes. In this regard, neonatal monocytes exhibit a trend towards lower phagocytosis of E. coli compared to adult monocytes, this alteration being even more pronounced in fetuses younger than 30 weeks of gestation [26]. However, pinocytosis appears functional in neonatal monocytes, since an intact ability to take up proteins such as bovine serum albumin has been reported [27]. Finally, the capacity of monocytes to differentiate into DCs is also altered, as suggested by differences in the morphology of neonatal monocyte-derived dendritic cells (MDDCs) compared to MDDCs from adults [28].
In summary, although defects in TLR activation cascade are clearly present in neonatal monocytes/macrophages, a better definition of both the extent of such defects and their in vivo relevance has not yet been achieved. Those signaling pathways converge in the activation of NF-kB and MAP-Kinases. Alternative pathways leading to the activation of these key molecules also appear to exist, but their regulation and function in neonatal monocytes/macrophages are not yet understood. A better molecular understanding of those pathways is clearly required to better delineate the exact nature of the functional defects of neonatal monocytes.
Cord blood monocyte-derived dendritic cells
Due to the low frequency of DCs in peripheral blood, most studies of neonatal DCs have been carried out using in vitro monocyte-derived dendritic cells (MDDCs). Defective functions of cord blood MDDCs (CB-MDDC) compared to adult MDDCs have been reported (see Table 1). In particular, CB-MDDCs exhibit signs of immaturity, including a low basal expression of MHC Class II and costimulatory molecules (CD80, CD40). Expression of CD1a molecules, which are important for the presentation of lipopeptidic antigens, is also lower on CB-MDDC [28,29]. Following LPS or necrotic cell stimulation, CB-MDDCs retain an immature phenotype, demonstrated by their failure to up-regulate HLA-DR, CD86 and CD83 [30,31]. As discussed earlier for neonatal monocytes, this incomplete DC maturation may be a consequence of defective responses to TLR signaling. Of interest, mRNA levels of molecules downstream with TLR4 signaling such as MAPKKK, NF-KB and TANK, are markedly decreased in LPS-stimulated CBMDDCs compared to adult MDDCs [32]. However, it is important to underline that the response to other TLRs expressed in this cellular population, TLR1, 2, 3, 6, and 8 and possibly TLR5 [33], has not yet been reported in neonate cells.
CB-MDDC exhibit additional evidence of functional immaturity, including defective endocytosis, which could result from their decreased expression of the mannose receptor [28,31], and decreased IL-12 production in response to both LPS and CD40L [29]. This IL-12 defect appears to mainly stem from alterations in the expression of the p35 chain, following transcriptional repression at the chromatin level [34], and it can be restored by addition of recombinant IFN-γ [29].
The weak co-stimulation delivered by neonatal APCs may be responsible for altered function of neonatal T cells. Consistent with this hypothesis, CB-MDDCs are poor inducers of proliferation or production of IFN-γ by allogeneic CB T cells, compared to adult MDDCs [28,29,35]. In contrast, mature CB-MDDCs can efficiently prime antigen-specific CD8+ T cells [36].
Considered together, those data suggest that neonatal MDDCs require a higher level of activation than adult DCs but, once activated, they are fully competent for the induction of adaptive effector responses. However, the detailed molecular analysis of activation pathways in these cells remains too sketchy to draw definitive conclusions on the functionality of those cells.
Cord blood dendritic cells
Early studies isolated cord blood DCs (CB-DCs) by fractionation of CBMCs by T-cell rosetting, plate adherence and metrizamide density gradient, and identified them by morphology. Such CB-DCs were poor stimulators of both neonatal and adult allogeneic T cells [37,38], potentially as a consequence of decreased expression of MHC Class II, Class I and ICAM-1 molecules on CB-DCs [37]. Following the discovery of markers that allow a better identification of DC subsets, it was recently shown that CB-DCs (defined as Lineage−/HLA-DR+) represent 0.3% of the CB mononuclear cells [27]. Interestingly, the majority of the CB-DCs in this study showed no expression of CD11c, the classical marker of myeloid DC (mDCs), but did express CD123, a marker widely used to characterize plasmacytoid DCs (pDCs) [27]. In general, an increased number and proportion of pDCs was reported in CB compared to adult peripheral blood. Widely-different pDC:mDC ratios in CB have been reported by different investigators, ranging from 1:1 to 3:1 [39-43]. Nevertheless, since the usual pDC:mDC ratio in adults is 1:2, a consensus towards increased pDC:mDC ratio in CB is emerging from these studies. However, it should be noted that the use of different combinations of markers to identify pDC and mDC among the studies makes it difficult to directly compare the published data.
CB-DCs appear to be immature, as they exhibit low or no basal expression of CD40, CD80 or CD86 [27,39]. In the latter study, DCs were identified as Lin−/HLA-DR+, and differential evaluation of costimulatory molecules on pDCs vs mDCs was not done. In another study, neonatal pDCs (Lin−/HLA-DR+/CD11c−/CD123+) exhibited incomplete maturation after stimulation with the TLR9 agonist CpG, as demonstrated by the lower expression of CD80, CD83, CD86, and CD40 in comparison to stimulated adult pDCs [44]. Similarly, upregulation of CD40 and CD80 on CB-mDCs in response to LPS and the TLR3 agonist poly (I:C) was significantly lower compared to that of adult mDCs [45]. Of note, in contrast to their lower level on neonatal monocytes, protein expression of TLR2 and TLR4 is normal in neonatal mDCs [41]. However, the regulation of critical molecules involved in TLR signaling has not yet been evaluated in neonatal DCs, and could account for the relative unresponsiveness of neonatal DCs to TLR stimulation.
Neonatal pDCs fail to upregulate IFN-α mRNA expression in response to CpG stimulation [44]. Interestingly, defective production of virus-stimulated IFN-α by unfractionated CB mononuclear cells had been reported in earlier studies [46,47]. Because pDCs were subsequently identified to be the major cellular source of IFN-α in virus-stimulated mononuclear cells, decreased IFN-α production by unfractionated CB mononuclear cells is likely the reflection of defective production by CB-pDCs. Lower levels of cytokine production by CB-mDCs in response to LPS, combined or not with IFN-γ, have been observed and affect TNF-α, IL-1, IL-6, and IL-12 production [40,41]. Interestingly, the latter study showed that the number of expressing cells was equivalent, but the level of expression per individual cell was lower. In addition, IFN-α secretion by unseparated CB cells is decreased in response to the TLR3 agonist poly (I:C) [45]. Since the expression of TLR3 is limited to mDCs, this result suggests again a defect in the response of these cells to viral products.
When the capacity of CB-DCs to stimulate allogeneic CB T cells was analyzed, weak proliferation was observed in pDC-stimulated cultures compared to total CB-DC-stimulated ones [39], which was linked to an increased proportion of apoptotic T cells in the former cultures. Mechanisms underlying such data have not been elucidated and could involve either a direct apoptotic signal given by CB-pDCs or a failure in the induction of rescuing signals. The ability of CB-DCs to capture antigenic protein is also altered, suggesting that those cells may in fact not function properly as APCs [27]. However, this hypothesis is challenged by the fact that unfractionated CB-DCs induce strong allogeneic responses [39], suggesting a complex picture in terms of the functionality of neonatal DCs. In that regard, data obtained in babies who have been exposed in utero to viruses bring some important information.
Several cohorts of babies exposed to Human Immunodeficiency Virus-1 (HIV) and human cytomegalovirus (CMV) have been analyzed. Although congenitally HIV-infected infants present both a more severe course of disease and lower T cell reactivity than infected adults [48], it has also been shown that CB T cells from some uninfected neonates born to HIV-infected mothers are strongly responsive to peptides derived from the HIV envelop glycoprotein (env), as detected by IL-2 production after in vitro restimulation [49]. In that study, cells from 8 out of 23 neonates responded to two or more env peptides. Also, the proportion of both activated and memory CD4+ T cells (defined as HLA-DR+CD38+ and CD45RO+, respectively) was increased in these neonates compared to neonates born to HIV-uninfected mothers [50]. Moreover, HIV-1-specific CTL precursors have been detected in CB from both uninfected (2/22) and infected (1/3) neonates born to HIV-infected mothers [48,51]. Similarly, during congenital CMV infection, expansion and differentiation of CMV-specific CD8+ T cells have been observed [52]. These data suggest that priming of both CD4+ and CD8+ T cells can occur in utero, arguing against the hypothesis that neonatal DC are completely defective. However, it should be noted that both HIV and CMV can infect DCs. Since it has been shown in murine models that viral infection of DCs can override the need for CD4+ T cell costimulation to induce CD8+ responses [53], a similar mechanism could be at play for in utero infection by HIV and CMV. Consequently, activation of specific T cell responses may have been induced despite the partial immaturity of the neonatal APC system.
Mechanisms underlying APC dysfunction
The following mechanisms can be proposed to explain the dysfunction of neonatal APCs (Fig. 1):
Intrinsic immaturity of these cells. As mentioned above, neonatal APCs appear to require a higher level of activation to reach functional status than adult APCs do. Experimental evidence suggest that the signaling cascades downstream of TLR engagement are affected in several neonatal APC populations.
Defective interaction between APCs and T cells. Since optimal activation of APCs involves crosstalk with activated CD4+ T cells [54,55], defective neonatal T cell activation will induce defective function of neonatal APCs.
Inhibition by regulatory T cells. The direct inhibitory effect exerted by natural regulatory T cells on DC function has recently been demonstrated in several murine models [56,57]. In addition, nTreg could indirectly inhibit APC function as a result of their inhibition of effector T cells and/or through secretion of anti-inflammatory cytokines such IL-10 and TGF-β [58-60]. Since nTreg are prevalent and functionally active in human CB (see below), Treg-mediated inhibition could constitute an additional mechanism for APC dysfunction.
Figure 1.
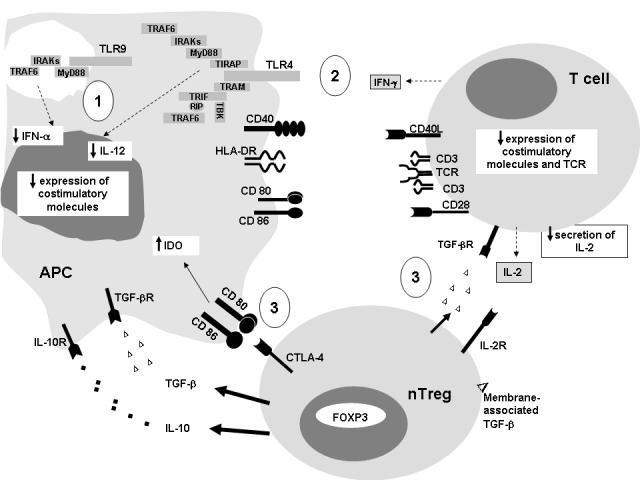
- Intrinsic immaturity of neonatal DCs. Basal expression of costimulatory molecules is decreased in neonatal APCs. In addition, alterations in the TLR complex, affecting principally TLR4 and TLR9, have been described in neonatal APCs. This could lead to defects in up-regulation of costimulatory molecule expression, as well as cytokine secretion (IL-12, IFN-α) following stimulation with bacterial and/or viral products.
- Defective interaction between neonatal APCs and T cells. Defects in activation levels of neonatal T cells could lead to alterations in APC functions, since T cell-mediated signals play a major role in APC maturation/activation. Among the described T cell defects are alterations in IFN-γ and IL-2 secretion in response to TCR-dependent stimulation, decreased expression of CD40L at basal condition and after stimulation, and decreased expression of the TCR complex.
- Inhibition by nTreg of both neonatal APCs and T cells. Neonatal nTreg can down-modulate the function of both APCs and T cells through direct and indirect mechanisms. Among these mechanisms are interaction between the CTLA-4 molecule expressed by Treg and the CD80/CD86 molecules expressed by APCs, leading to increased IDO expression; secretion of immunosuppressive cytokines, IL-10 and TGF-β, and expression of membrane-associated TGF-β; consumption of IL-2 by the high-affinity IL-2R expressed by nTreg; and finally, FOXP3-mediated mechanisms that have not yet been identified.
Natural Treg in neonates
Natural regulatory T cells (nTreg) have a thymic origin [61] and have proved essential in the establishment and maintenance of immunological tolerance [58]. These cells are characterized by the constitutional expression of CTL-associated antigen 4 (CTLA-4) and of the high affinity IL-2 receptor chain, CD25. They also express high levels of the transcription factor from the forkhead family, FOXP3 [58]. The mechanisms by which nTreg exert their immunosuppressive function are not clearly established, but APCs are one of their cellular targets. An interesting piece of evidence is the nTreg -induced up-regulation of the tryptophancatabolizing enzyme indoleamine 2,3-dioxigenase (IDO) in APCs, mainly DCs [62,63]. Since tryptophan is required for T cell function, its increased catabolism would induce T cell suppression. Increased IDO expression by APCs is mediated through the interactions of CTLA-4, constitutively expressed by nTreg, with B7 molecules on DCs. These data have led to propose the concept of IDO-expressing,“tolerogenic” APCs. It has also been observed that the interactions of nTreg and DCs result in the down-regulation of the expression of CD80, CD86 and MHC Class II molecules on DC, which also converts these DCs into “tolerogenic” APCs [57,64]. Finally, nTreg directly interact with DCs during in vivo suppression, further suggesting a role for these cells in the down-regulation of DC function [65]. However, results from in vitro and in vivo studies indicate that nTreg also directly inhibit effector T cell function, something that would indirectly affect DC function. In addition, the role played by the Treg secretion of immunosuppressive cytokines such IL-10 and TGF-β is still debated, since in vivo and in vitro models provide somewhat discrepant results [58-60]. There is also evidence supporting the effect of membrane-associated TGF-β as a mechanism underlying nTreg activity [66]. Finally, it has been postulated that Treg could function as an IL-2 “sink”, since they are the only cells expressing at homeostasis the high-affinity IL-2 receptor. Interestingly, FOXP3 has been shown to upregulate both CTLA-4 and CD25 genes [67], which could explain why ectopic expression of FOXP3 transforms non-Treg into functional inhibitory Treg [68].
In the neonatal context, nTreg play an important role during pregnancy, maintaining maternal tolerance to the fetus [69]. CTLA-4-expressing nTreg are present in high numbers in the decidua [70,71]. They may be crucial in the high IDO level detected at the fetal-maternal interface, which has been proposed as a major factor in the lower immune reactivity of this compartment [71-75].
At birth, Hara group's recently reported a high proportion of CD4+CD25+ nTreg in CB, which is particularly marked in premature babies compared to full-term babies [76]. These cells express the Treg markers CTLA-4 and FOXP3, but, unlike the adult nTreg, they exhibit a naïve phenotype evidenced by CD45RA expression and high levels of T cell receptor recombination excision circles (TREC) [76]. Neonatal nTreg exert potent immunosuppressive activity, and suppress T effector proliferation and cytokine production following stimulation with either mitogens, alloantigens or specific antigens [76-79]. The mechanism(s) by which these nTreg cells exert their suppressive activity in neonates is not fully established but they appear similar to those exerted by adult nTreg, since neonatal nTreg act in vitro through contact-dependent, but IL-10- and TGF-β-independent mechanism(s) [76,77]. It has also been observed that CB-MDDCs induce neonatal and adult T cells to produce low levels of IFN-γ but high levels of IL-10, which is reminiscent of a regulatory T cell-type 1 (Tr1) profile [35]. However, the existence of additional inhibitory mechanisms mediated by neonatal nTreg should be further evaluated.
Together, these findings suggest a model whereby elevated numbers of Treg (nTreg or induced Tr1) in neonates are critical in maintaining homeostasis and preventing autoimmunity, as suggested by the low incidence of graft-versus-host disease observed after CB transplantation [80,81]. Conversely, functional neonatal Tregs could play a detrimental role, by impairing the capacity of the neonatal immune system to control infections, in particular through their down-modulation of APC function.
The immune response in early childhood years
Despite the fact that the immune competence of neonates increases with age, several alterations in the number and function of APCs are present during early childhood. Percentages of circulating mDCs are decreased in 12 month-old children compared to adults [82]. A progressive decrease in the absolute number of pDCs occurs in the first year of life, but adult levels are reached only around 5 years of age [83]. An age-related increase in the capacity of PBMC to synthesize IL-12 in response to LPS or SAC stimulation has also been reported, but levels of production of such cytokine in 12 month-old children remain low [17,25]. A complex picture is emerging from different studies concerning the capacity of APC from young children to stimulate T cells, depending on the functional readout. Indeed, studying responses in children older than a year, Clerici et al. reported adult-levels of T cell proliferation after stimulation with alloantigens or vaccine antigens [84]. In contrast, decreased production of cytokines in response to vaccine antigens was described for T cells from 12-month-old infants [82]. These apparently discrepant results could come from the differential activation signals involved in different T cell functions.
Most of the studies of APC functionality during early childhood have been done in relation to the development of asthma/atopy, and have focused on Th2 versus Th1 pattern. Interestingly, neonates appear to exhibit a predisposition toward production of Th2 cytokines after exposure to environmental allergens [85,2], followed by an age-dependent change toward Th-1-like responses [86,87]. This Th2 bias is usually considered to be a major underlying mechanism for the development of atopy in early life, and could again be a consequence of APC defects. Supporting this concept, low numbers of IL-12-producing cells have been observed in neonates with inherited atopic disease [88,89], as well as low LPS-induced production of IL-12 in children who develop atopic diseases compared to children who do not [90]. The baseline expression of HLA Class II on CB monocytes is lower in children in whom allergic diseases developed within the first two years of life [91]. Children with allergic asthma have lower pDC numbers compared to healthy children. Interestingly, children with atopic dermatitis exhibited increased numbers of another DC population (Lin−/HLA-DR+/CD11c−/CD123dim), which are considered to be less differentiated DCs and are also present in high numbers in cord blood [92]. Considered together, these results suggest a role of DC immaturity/dysfunction in the susceptibility to atopic diseases during early life. However, further studies that evaluate the phenotype and function of APCs are clearly required to define the extent of APC defects during early childhood, as well as to better characterize the kinetics of maturation of these cells.
Conclusions
As reviewed herein, strong experimental evidence suggest functional defects in APC from neonates and young infants. Such defects are expected to contribute to the functional immaturity of the immune system of human newborns, in addition to the intrinsic defects on neonatal T cells. However, the use of different experimental settings has made difficult to compare, and thus validate, the results obtained in different studies. The few studies carried-out with primary DCs, and not in vitro monocyte-derived cells, probably best reflect the in vivo phenotype and function of neonatal DCs. However, due to the possibility that maternal factors present in cord blood may influence the characterization of neonatal DCs, the ideal setting would be to study peripheral blood neonatal DCs. Unfortunately, this approach is very difficult, due to the amount of blood required to study such rare cellular populations. Despite such limitations, the available data strongly suggest a defect of APCs in delivering costimulatory signals to T cells, as a consequence of their incomplete activation and/or maturation. Considering that TLR engagement is one of the major pathways for DC activation, the study of molecules involved in the signaling cascades downstream of TLRs should be a priority for research.
Another mechanism of APC dysfunction in neonates could arise from the activity of nTreg. The role played by such cells in the failure of the immune system to control infections on one hand, and in contrast, in controlling autoimmune pathological processes, has emerged only recently. It is expected that the role played by nTreg number and/or activity in many childhood diseases will be recognized in the future. Of great interest as well, several alternative mechanisms mediating nTreg suppressive activity have been postulated, in adults and neonates, including a direct effect on the level of activation of APC. However, the real contribution of each of these mechanisms to neonatal immune dysfunction is currently unknown, and their elucidation will without doubt become a vibrant field of research for immunologists interested in pediatric disorders.
Finally, a better understanding of the kinetics of maturation of APCs during early childhood is clearly necessary to improve the design of vaccination strategies as well as to prevent the development of autoimmune diseases and atopic disorders.
Acknowledgements
The authors thank Drs Lisa Filipovitch and Gene M. Shearer for their critical reading of this manuscript. This work was supported by a grant from Colciencias, Colombia (111504-12949 to MTR) and division funds from Cincinnati Children's Hospital Research Foundation (to CC). PAV is supported by a fellowship from Colciencias.
References
- 1.WHO Prevention and Care of Illness. Neonates and Infants NEWBORN HEALTH AND SURVIVAL. 2004 A CALL TO ACTIION. [Google Scholar]
- 2.Kovarik J, Siegrist CA. Immunity in early life. Immunol Today. 1998;19:150–152. doi: 10.1016/s0167-5699(97)01230-9. [DOI] [PubMed] [Google Scholar]
- 3.Wright PF. Infectious diseases in early life in industrialized countries. Vaccine. 1998;16:1355–1359. doi: 10.1016/s0264-410x(98)00091-7. [DOI] [PubMed] [Google Scholar]
- 4.Marchant A, Newport M. Prevention of infectious diseases by neonatal and early infantile immunization: prospects for the new millennium. Curr Opin Infect Dis. 2000;13:241–246. doi: 10.1097/00001432-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–3346. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 6.Harris DT, Schumacher MJ, Locascio J, Besencon FJ, Olson GB, DeLuca D, Shenker L, Bard J, Boyse EA. Phenotypic and functional immaturity of human umbilical cord blood T lymphocytes. Proc Natl Acad Sci U S A. 1992;89:10006–10010. doi: 10.1073/pnas.89.21.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durandy A, De Saint Basile G, Lisowska-Grospierre B, Gauchat JF, Forveille M, Kroczek RA, Bonnefoy JY, Fischer A. Undetectable CD40 ligand expression on T cells and low B cell responses to CD40 binding agonists in human newborns. J Immunol. 1995;154:1560–1568. [PubMed] [Google Scholar]
- 8.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 9.Dakic A, Shao QX, D'Amico A, O'Keeffe M, Chen WF, Shortman K, Wu L. Development of the dendritic cell system during mouse ontogeny. J Immunol. 2004;172:1018–1027. doi: 10.4049/jimmunol.172.2.1018. [DOI] [PubMed] [Google Scholar]
- 10.Wallet MA, Sen P, Tisch R. Immunoregulation of dendritic cells. Clin Med Res. 2005;3:166–175. doi: 10.3121/cmr.3.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shreedhar V, Moodycliffe AM, Ullrich SE, Bucana C, Kripke ML, Flores-Romo L. Dendritic cells require T cells for functional maturation in vivo. Immunity. 1999;11:625–636. doi: 10.1016/s1074-7613(00)80137-5. [DOI] [PubMed] [Google Scholar]
- 12.Kuwana M. Induction of anergic and regulatory T cells by plasmacytoid dendritic cells and other dendritic cell subsets. Hum Immunol. 2002;63:1156–1163. doi: 10.1016/s0198-8859(02)00754-1. [DOI] [PubMed] [Google Scholar]
- 13.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 14.Roncarolo MG, Levings MK, Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J Exp Med. 2001;193:F5–9. doi: 10.1084/jem.193.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han P, McDonald T, Hodge G. Potential immaturity of the T-cell and antigen-presenting cell interaction in cord blood with particular emphasis on the CD40-CD40 ligand costimulatory pathway. Immunology. 2004;113:26–34. doi: 10.1111/j.1365-2567.2004.01933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodge S, Hodge G, Flower R, Han P. Cord blood leucocyte expression of functionally significant molecules involved in the regulation of cellular immunity. Scand J Immunol. 2001;53:72–78. doi: 10.1046/j.1365-3083.2001.00845.x. [DOI] [PubMed] [Google Scholar]
- 17.Upham JW, Lee PT, Holt BJ, Heaton T, Prescott SL, Sharp MJ, Sly PD, Holt PG. Development of interleukin-12-producing capacity throughout childhood. Infect Immun. 2002;70:6583–8. doi: 10.1128/IAI.70.12.6583-6588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chelvarajan RL, Collins SM, Doubinskaia IE, Goes S, Van Willigen J, Flanagan D, De Villiers WJ, Bryson JS, Bondada S. Defective macrophage function in neonates and its impact on unresponsiveness of neonates to polysaccharide antigens. J Leukoc Biol. 2004;75:982–994. doi: 10.1189/jlb.0403179. [DOI] [PubMed] [Google Scholar]
- 19.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004;173:4627–4634. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 20.Forster-Waldl E, Sadeghi K, Tamandl D, Gerhold B, Hallwirth U, Rohrmeister K, Hayde M, Prusa AR, Herkner K, Boltz-Nitulescu G, Pollak A, Spittler A. Monocyte toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr Res. 2005;58:121–124. doi: 10.1203/01.PDR.0000163397.53466.0F. [DOI] [PubMed] [Google Scholar]
- 21.Yan SR, Qing G, Byers DM, Stadnyk AW, Al-Hertani W, Bortolussi R. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect Immun. 2004;72:1223–1229. doi: 10.1128/IAI.72.3.1223-1229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marodi L, Goda K, Palicz A, Szabo G. Cytokine receptor signalling in neonatal macrophages: defective STAT-1 phosphorylation in response to stimulation with IFN-gamma. Clin Exp Immunol. 2001;126:456–460. doi: 10.1046/j.1365-2249.2001.01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marodi L. Deficient interferon-gamma receptor-mediated signaling in neonatal macrophages. Acta Paediatr Suppl. 2002;91:117–119. doi: 10.1111/j.1651-2227.2002.tb02915.x. [DOI] [PubMed] [Google Scholar]
- 24.Marodi L, Kaposzta R, Nemes E. Survival of group B streptococcus type III in mononuclear phagocytes: differential regulation of bacterial killing in cord macrophages by human recombinant gamma interferon and granulocyte-macrophage colony-stimulating factor. Infect Immun. 2000;68:2167–2170. doi: 10.1128/iai.68.4.2167-2170.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chougnet C, Kovacs A, Baker R, Mueller BU, Luban NL, Liewehr DJ, Steinberg SM, Thomas EK, Shearer GM. Influence of human immunodeficiency virus-infected maternal environment on development of infant interleukin-12 production. J Infect Dis. 2000;181:1590–1597. doi: 10.1086/315458. [DOI] [PubMed] [Google Scholar]
- 26.Strunk T, Temming P, Gembruch U, Reiss I, Bucsky P, Schultz C. Differential maturation of the innate immune response in human fetuses. Pediatr Res. 2004;56:219–226. doi: 10.1203/01.PDR.0000132664.66975.79. [DOI] [PubMed] [Google Scholar]
- 27.Sorg RV, Kogler G, Wernet P. Identification of cord blood dendritic cells as an immature CD11c- population. Blood. 1999;93:2302–2307. [PubMed] [Google Scholar]
- 28.Liu E, Tu W, Law HK, Lau YL. Decreased yield, phenotypic expression and function of immature monocyte-derived dendritic cells in cord blood. Br J Haematol. 2001;113:240–246. doi: 10.1046/j.1365-2141.2001.02720.x. [DOI] [PubMed] [Google Scholar]
- 29.Goriely S, Vincart B, Stordeur P, Vekemans J, Willems F, Goldman M, De Wit D. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J Immunol. 2001;166:2141–2146. doi: 10.4049/jimmunol.166.3.2141. [DOI] [PubMed] [Google Scholar]
- 30.Langrish CL, Buddle JC, Thrasher AJ, Goldblatt D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin Exp Immunol. 2002;128:118–123. doi: 10.1046/j.1365-2249.2002.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong OH, Huang FP, Chiang AK. Differential responses of cord and adult blood-derived dendritic cells to dying cells. Immunology. 2005;116:13–20. doi: 10.1111/j.1365-2567.2005.02191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satwani P, Morris E, van de Ven C, Cairo MS. Dysregulation of expression of immunoregulatory and cytokine genes and its association with the immaturity in neonatal phagocytic and cellular immunity. Biol Neonate. 2005;88:214–227. doi: 10.1159/000087585. [DOI] [PubMed] [Google Scholar]
- 33.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 34.Goriely S, Van Lint C, Dadkhah R, Libin M, De Wit D, Demonte D, Willems F, Goldman M. A defect in nucleosome remodeling prevents IL-12(p35) gene transcription in neonatal dendritic cells. J Exp Med. 2004;199:1011–1016. doi: 10.1084/jem.20031272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu E, Law HK, Lau YL. Tolerance associated with cord blood transplantation may depend on the state of host dendritic cells. Br J Haematol. 2004;126:517–526. doi: 10.1111/j.1365-2141.2004.05061.x. [DOI] [PubMed] [Google Scholar]
- 36.Salio M, Dulphy N, Renneson J, Herbert M, McMichael A, Marchant A, Cerundolo V. Efficient priming of antigen-specific cytotoxic T lymphocytes by human cord blood dendritic cells. Int Immunol. 2003;15:1265–1273. doi: 10.1093/intimm/dxg123. [DOI] [PubMed] [Google Scholar]
- 37.Hunt DW, Huppertz HI, Jiang HJ, Petty RE. Studies of human cord blood dendritic cells: evidence for functional immaturity. Blood. 1994;84:4333–4343. [PubMed] [Google Scholar]
- 38.Petty RE, Hunt DW. Neonatal dendritic cells. Vaccine. 1998;16:1378–1382. doi: 10.1016/s0264-410x(98)00095-4. [DOI] [PubMed] [Google Scholar]
- 39.Borras FE, Matthews NC, Lowdell MW, Navarrete CV. Identification of both myeloid CD11c+ and lymphoid CD11c− dendritic cell subsets in cord blood. Br J Haematol. 2001;113:925–931. doi: 10.1046/j.1365-2141.2001.02840.x. [DOI] [PubMed] [Google Scholar]
- 40.Crespo I, Paiva A, Couceiro A, Pimentel P, Orfao A, Regateiro F. Immunophenotypic and functional characterization of cord blood dendritic cells. Stem Cells Dev. 2004;13:63–70. doi: 10.1089/154732804773099263. [DOI] [PubMed] [Google Scholar]
- 41.Drohan L, Harding JJ, Holm B, Cordoba-Tongson E, Dekker CL, Holmes T, Maecker H, Mellins ED. Selective developmental defects of cord blood antigen-presenting cell subsets. Hum Immunol. 2004;65:1356–1369. doi: 10.1016/j.humimm.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Ueda Y, Hagihara M, Okamoto A, Higuchi A, Tanabe A, Hirabayashi K, Izumi S, Makino T, Kato S, Hotta T. Frequencies of dendritic cells (myeloid DC and plasmacytoid DC) and their ratio reduced in pregnant women: comparison with umbilical cord blood and normal healthy adults. Hum Immunol. 2003;64:1144–1151. doi: 10.1016/j.humimm.2003.08.342. [DOI] [PubMed] [Google Scholar]
- 43.Szabolcs P, Park KD, Reese M, Marti L, Broadwater G, Kurtzberg J. Absolute values of dendritic cell subsets in bone marrow, cord blood, and peripheral blood enumerated by a novel method. Stem Cells. 2003;21:296–303. doi: 10.1634/stemcells.21-3-296. [DOI] [PubMed] [Google Scholar]
- 44.De Wit D, Olislagers V, Goriely S, Vermeulen F, Wagner H, Goldman M, Willems F. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood. 2004;103:1030–1032. doi: 10.1182/blood-2003-04-1216. [DOI] [PubMed] [Google Scholar]
- 45.De Wit D, Tonon S, Olislagers V, Goriely S, Boutriaux M, Goldman M, Willems F. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J Autoimmun. 2003;21:277–281. doi: 10.1016/j.jaut.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Cederblad B, Riesenfeld T, Alm GV. Deficient herpes simplex virus-induced interferon-alpha production by blood leukocytes of preterm and term newborn infants. Pediatr Res. 1990;27:7–10. doi: 10.1203/00006450-199001000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Neustock P, Kruse A, Bock S, Pierre B, Kirchner H. Deficient interferon-alpha response of newborns in comparison to adults. Lymphokine Cytokine Res. 1993;12:109–114. [PubMed] [Google Scholar]
- 48.Luzuriaga K, Holmes D, Hereema A, Wong J, Panicali DL, Sullivan JL. HIV-1-specific cytotoxic T lymphocyte responses in the first year of life. J Immunol. 1995;154:433–443. [PubMed] [Google Scholar]
- 49.Clerici M, Sison AV, Berzofsky JA, Rakusan TA, Brandt CD, Ellaurie M, Villa M, Colie C, Venzon DJ, Sever JL, et al. Cellular immune factors associated with mother-to-infant transmission of HIV. AIDS. 1993;7:1427–1433. doi: 10.1097/00002030-199311000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Rich KC, Siegel JN, Jennings C, Rydman RJ, Landay AL. Function and phenotype of immature CD4+ lymphocytes in healthy infants and early lymphocyte activation in uninfected infants of human immunodeficiency virus-infected mothers. Clin Diagn Lab Immunol. 1997;4:358–361. doi: 10.1128/cdli.4.3.358-361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wasik TJ, Bratosiewicz J, Wierzbicki A, Whiteman VE, Rutstein RR, Starr SE, Douglas SD, Kaufman D, Sison AV, Polansky M, Lischner HW, Kozbor D. Protective role of beta-chemokines associated with HIV-specific Th responses against perinatal HIV transmission. J Immunol. 1999;162:4355–4364. [PubMed] [Google Scholar]
- 52.Marchant A, Appay V, Van Der Sande M, Dulphy N, Liesnard C, Kidd M, Kaye S, Ojuola O, Gillespie GM, Vargas Cuero AL, Cerundolo V, Callan M, McAdam KP, Rowland-Jones SL, Donner C, McMichael AJ, Whittle H. Mature CD8(+) T lymphocyte response to viral infection during fetal life. J Clin Invest. 2003;111:1747–1755. doi: 10.1172/JCI17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 54.Friedl P, den Boer AT, Gunzer M. Tuning immune responses: diversity and adaptation of the immunological synapse. Nat Rev Immunol. 2005;5:532–545. doi: 10.1038/nri1647. [DOI] [PubMed] [Google Scholar]
- 55.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serra P, Amrani A, Yamanouchi J, Han B, Thiessen S, Utsugi T, Verdaguer J, Santamaria P. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity. 2003;19:877–889. doi: 10.1016/s1074-7613(03)00327-3. [DOI] [PubMed] [Google Scholar]
- 57.Vendetti S, Chai JG, Dyson J, Simpson E, Lombardi G, Lechler R. Anergic T cells inhibit the antigen-presenting function of dendritic cells. J Immunol. 2000;165:1175–1181. doi: 10.4049/jimmunol.165.3.1175. [DOI] [PubMed] [Google Scholar]
- 58.Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004;114:1209–1217. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gavin M, Rudensky A. Control of immune homeostasis by naturally arising regulatory CD4+ T cells. Curr Opin Immunol. 2003;15:690–696. doi: 10.1016/j.coi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 60.Horwitz DA, Zheng SG, Gray JD. The role of the combination of IL-2 and TGF-beta or IL-10 in the generation and function of CD4+ CD25+ and CD8+ regulatory T cell subsets. J Leukoc Biol. 2003;74:471–478. doi: 10.1189/jlb.0503228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 62.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 63.Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 64.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 65.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, Rao A. FOXP3 Controls Regulatory T Cell Function through Cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 68.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 69.Saito S, Sasaki Y, Sakai M. CD4(+)CD25high regulatory T cells in human pregnancy. J Reprod Immunol. 2005;65:111–120. doi: 10.1016/j.jri.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 70.Saito S, Nishikawa K, Morii T, Narita N, Enomoto M, Ichijo M. Expression of activation antigens CD69, HLA-DR, interleukin-2 receptor-alpha (IL-2R alpha) and IL-2R beta on T cells of human decidua at an early stage of pregnancy. Immunology. 1992;75:710–712. [PMC free article] [PubMed] [Google Scholar]
- 71.Heikkinen J, Mottonen M, Komi J, Alanen A, Lassila O. Phenotypic characterization of human decidual macrophages. Clin Exp Immunol. 2003;131:498–505. doi: 10.1046/j.1365-2249.2003.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 73.Mellor AL, Munn DH. Tryptophan catabolism prevents maternal T cells from activating lethal anti-fetal immune responses. J Reprod Immunol. 2001;52:5–13. doi: 10.1016/s0165-0378(01)00118-8. [DOI] [PubMed] [Google Scholar]
- 74.Santoso DI, Rogers P, Wallace EM, Manuelpillai U, Walker D, Subakir SB. Localization of indoleamine 2,3-dioxygenase and 4-hydroxynonenal in normal and pre-eclamptic placentae. Placenta. 2002;23:373–379. doi: 10.1053/plac.2002.0818. [DOI] [PubMed] [Google Scholar]
- 75.Honig A, Rieger L, Kapp M, Sutterlin M, Dietl J, Kammerer U. Indoleamine 2,3-dioxygenase (IDO) expression in invasive extravillous trophoblast supports role of the enzyme for materno-fetal tolerance. J Reprod Immunol. 2004;61:79–86. doi: 10.1016/j.jri.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 76.Takahata Y, Nomura A, Takada H, Ohga S, Furuno K, Hikino S, Nakayama H, Sakaguchi S, Hara T. CD25+CD4+ T cells in human cord blood: an immunoregulatory subset with naive phenotype and specific expression of forkhead box p3 (Foxp3) gene. Exp Hematol. 2004;32:622–629. doi: 10.1016/j.exphem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 77.Godfrey WR, Spoden DJ, Ge YG, Baker SR, Liu B, Levine BL, June CH, Blazar BR, Porter SB. Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 2005;105:750–758. doi: 10.1182/blood-2004-06-2467. [DOI] [PubMed] [Google Scholar]
- 78.Wing K, Larsson P, Sandstrom K, Lundin SB, Suri-Payer E, Rudin A. CD4+ CD25+ FOXP3+ regulatory T cells from human thymus and cord blood suppress antigen-specific T cell responses. Immunology. 2005;115:516–525. doi: 10.1111/j.1365-2567.2005.02186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thornton CA, Upham JW, Wikstrom ME, Holt BJ, White GP, Sharp MJ, Sly PD, Holt PG. Functional maturation of CD4+CD25+CTLA4+CD45RA+ T regulatory cells in human neonatal T cell responses to environmental antigens/allergens. J Immunol. 2004;173:3084–3092. doi: 10.4049/jimmunol.173.5.3084. [DOI] [PubMed] [Google Scholar]
- 80.Han P, Hodge G, Story C, Xu X. Phenotypic analysis of functional T-lymphocyte subtypes and natural killer cells in human cord blood: relevance to umbilical cord blood transplantation. Br J Haematol. 1995;89:733–740. doi: 10.1111/j.1365-2141.1995.tb08409.x. [DOI] [PubMed] [Google Scholar]
- 81.Chang CC, Satwani P, Oberfield N, Vlad G, Simpson LL, Cairo MS. Increased induction of allogeneic-specific cord blood CD4+CD25+ regulatory T (Treg) cells: a comparative study of naive and antigenic-specific cord blood Treg cells. Exp Hematol. 2005;33:1508–1520. doi: 10.1016/j.exphem.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 82.Upham JW, Rate A, Rowe J, Kusel M, Sly PD, Holt PG. Dendritic cell immaturity during infancy restricts the capacity to express vaccine-specific T-cell memory. Infect Immun. 2006;74:1106–1112. doi: 10.1128/IAI.74.2.1106-1112.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Teig N, Moses D, Gieseler S, Schauer U. Age-related changes in human blood dendritic cell subpopulations. Scand J Immunol. 2002;55:453–457. doi: 10.1046/j.1365-3083.2002.01068.x. [DOI] [PubMed] [Google Scholar]
- 84.Clerici M, DePalma L, Roilides E, Baker R, Shearer GM. Analysis of T helper and antigen-presenting cell functions in cord blood and peripheral blood leukocytes from healthy children of different ages. J Clin Invest. 1993;91:2829–2836. doi: 10.1172/JCI116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prescott SL, Macaubas C, Holt BJ, Smallacombe TB, Loh R, Sly PD, Holt PG. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. J Immunol. 1998;160:4730–4737. [PubMed] [Google Scholar]
- 86.Krampera M, Vinante F, Tavecchia L, Morosato L, Chilosi M, Romagnani S, Zanolin ME, Pizzolo G. Progressive polarization towards a T helper/cytotoxic type-1 cytokine pattern during age-dependent maturation of the immune response inversely correlates with CD30 cell expression and serum concentration. Clin Exp Immunol. 1999;117:291–297. doi: 10.1046/j.1365-2249.1999.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hartel C, Adam N, Strunk T, Temming P, Muller-Steinhardt M, Schultz C. Cytokine responses correlate differentially with age in infancy and early childhood. Clin Exp Immunol. 2005;142:446–453. doi: 10.1111/j.1365-2249.2005.02928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gabrielsson S, Soderlund A, Nilsson C, Lilja G, Nordlund M, Troye-Blomberg M. Influence of atopic heredity on IL-4-, IL-12- and IFN-gamma-producing cells in in vitro activated cord blood mononuclear cells. Clin Exp Immunol. 2001;126:390–396. doi: 10.1046/j.1365-2249.2001.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nilsson C, Larsson AK, Hoglind A, Gabrielsson S, Troye Blomberg M, Lilja G. Low numbers of interleukin-12-producing cord blood mononuclear cells and immunoglobulin E sensitization in early childhood. Clin Exp Allergy. 2004;34:373–380. doi: 10.1111/j.1365-2222.2004.01896.x. [DOI] [PubMed] [Google Scholar]
- 90.Prescott SL, Taylor A, King B, Dunstan J, Upham JW, Thornton CA, Holt PG. Neonatal interleukin-12 capacity is associated with variations in allergen-specific immune responses in the neonatal and postnatal periods. Clin Exp Allergy. 2003;33:566–72. doi: 10.1046/j.1365-2222.2003.01659.x. [DOI] [PubMed] [Google Scholar]
- 91.Upham JW, Holt PG, Taylor A, Thornton CA, Prescott SL. HLA-DR expression on neonatal monocytes is associated with allergen-specific immune responses. J Allergy Clin Immunol. 2004;114:1202–1208. doi: 10.1016/j.jaci.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 92.Hagendorens MM, Ebo DG, Schuerwegh AJ, Huybrechs A, Van Bever HP, Bridts CH, De Clerck LS, Stevens WJ. Differences in circulating dendritic cell subtypes in cord blood and peripheral blood of healthy and allergic children. Clin Exp Allergy. 2003;33:633–639. doi: 10.1046/j.1365-2222.2003.01649.x. [DOI] [PubMed] [Google Scholar]


