Abstract
To clarify the mechanism of the effects of angiotensin II AT1 receptor antagonists on adipose tissue, we treated 8 week-old male Wistar Kyoto rats with the angiotensin II AT1 receptor antagonist Candesartan cilexetil (10 mg/kg/day) for 18 weeks. Candesartan cilexetil reduced body weight gain, decreased fat tissue mass due to hypotrophy of epididymal and retroperitoneal adipose tissue and decreased adipocyte size without changing the number of adipocytes. Candesartan cilexetil decreased serum leptin levels and epididymal leptin mRNA, increased serum adiponectin levels and epididymal adiponectin mRNA, decreased epididymal tumor-necrosis factor alpha (TNFα) mRNA, and increased fatty acid synthase mRNA. Considered free of peroxisome proliferator-activated receptor γ (PPARγ) agonist activity, Candesartan cilexetil increased epididymal expression of PPARγ mRNA. The effects of Candesartan cilexetil on adipokine production and release may be attributable to PPARγ activation and/or decrease in adipocyte cell size. In addition, Candesartan cilexetil treatment increased the expression of epididymal angiotensin II AT2 receptor mRNA and protein and decreased the expression of renin receptor mRNA. These results suggest that Candesartan cilexetil influences lipid metabolism in adipose tissue by promoting adipose tissue rearrangement and modulating adipokine expression and release. These effects are probably consequences of local angiotensin II AT1 receptor inhibition, angiotensin II AT2 receptor stimulation, and perhaps additional angiotensin II -independent mechanisms. Our results indicate that the activity of local renin-angiotensin system plays an important role in adipose tissue metabolism The decrease in the pro-inflammatory cytokine TNFα and the increase in the anti-inflammatory adipokine adiponectin indicate that Candesartan cilexetil may exert significant anti-inflammatory properties.
Keywords: Renin-Angiotensin-System, Candesartan cilexetil, adipokines, adipocytes
1. Introduction
Circulating angiotensin II, the active product of the renin-angiotensin system, is a hormonal regulator of cardiovascular function and electrolyte metabolism (Peach, 1977). Angiotensin II is also produced by local renin-angiotensin systems in many organs including adipose tissue (Engeli et al., 2003), which is in turn an important source of the angiotensin II precursor angiotensinogen (Massiera et al., 2001).
Angiotensin II was proposed as a trophic factor in white adipose tissue growth and development, since renin-angiotensin system components are regulated according to the nutritional state and adipose tissue mass (Boustany et al., 2004; Hainault et al., 2002; Saint-Marc et al., 2001). Both circulating and locally formed angiotensin II contribute to the regulation of adipose tissue metabolism through angiotensin II AT1 receptors located in adipocytes (Crandall et al., 1994) but may affect adipose tissue differently, since results from in vitro and in vivo experiments using different models are not always in agreement. In particular, there are results indicating that angiotensin II both inhibits (Janke et al., 2002; Massiera et al., 2001) and stimulates (Darimont et al., 1994a, b; Saint-Marc et al., 2001) adipocyte differentiation.
In addition, angiotensin II regulates the production of adipokines. These bioactive substances are released into the bloodstream from adipose tissue and regulate the cardiovascular system and insulin sensitivity (Guerre-Millo, 2004). Angiotensin II increases leptin ob gene expression and secretion (Cassis et al., 2004; Kim et al., 2002), increases the expression and the release of pro-inflammatory cytokines (Skurk et al., 2004) and reduces plasma levels and gene expression of adiponectin, an insulin-sensitizing, anti-inflammatory adipokine (Hattori et al., 2005).
Overall, the trophic effects of angiotensin II overstimulation appear to promote the development of insulin resistance and type 2 diabetes, because hypertrophic adipocytes are insulin resistant (Weyer et al., 2000; Zorad et al., 1997) and increased numbers of large adipocytes decrease insulin sensitivity and promote ectopic deposition of lipids. In turn, blockade of the renin-angiotensin system with inhibitors of angiotensin II formation (Angiotensin Converting Enzyme inhibitors) or angiotensin II AT1 receptor blockers decreases body weight, improves insulin sensitivity and prevents development of insulin resistance (Goossens et al., 2003; Jandeleit-Dahm et al., 2005). For these reasons, blockade of the renin-angiotensin system has been proposed as beneficial for the prevention and treatment of type 2 diabetes.
To clarify the effect of angiotensin II and renin-angiotensin system blockade on adipose tissue growth and adipokine formation and release, we studied the effect of long-term treatment with Candesartan cilexetil, a selective, insurmountable angiotensin II AT1 receptor blocker (Morsing et al., 1999), administered orally. To minimize the influence of hemodynamic changes, we did not study hypertensive animals. We selected a group of non-obese normotensive Wistar Kyoto (WKY) rats to determine adipose tissue mass and cellularity and the expression and release of selected adipokines. We chose to study animals without obesity, diabetes or hypertension to clarify our observations of reduction of weight gain in normal rats and as a preliminary study to later address the role of the renin-angiotensin system in animal models of obesity and diabetes.
2. Materials and Methods
2.1. Animals
Eight week-old male inbred Wistar Kyoto rats were purchased from Taconic Farms (Germantown, NY), housed two per cage at 22°C under a 12:12 h light:dark cycle, and given free access to a normal diet and tap water. The National Institute of Mental Health Animal Care and Use Committee (Bethesda, Maryland, USA) approved all procedures.
2.2. Treatment
Groups of 10 animals received either Candesartan cilexetil (TCV-116, Astra-Zeneca, Mölndal, Sweden, 10 mg/kg per day for 18 weeks) or vehicle, both dissolved in their drinking water. Candesartan cilexetil was first dissolved as a 1mg/ml stock solution in polyethylene glycol 400/ethanol/Cremophor EL (Sigma, St. Louis, MO)/water (10/5/2/83%) adjusted to pH 9.0 with 1M Na2CO3. The stock solution was diluted in drinking water to a final concentration below 1/0.5/0.2% polyethylene glycol 400/ethanol/Cremophor EL.
The body weight was evaluated once a week for each individual animal (n=10). Food and water intake were evaluated once a week for each cage housing two animals (n= 5). After 18 weeks of treatment, animals were fasted for 15 h before being sacrificed by decapitation. Trunk blood was collected for the preparation of serum. Epididymal and retroperitoneal fat tissues were removed and weighed immediately. Part of the epididymal adipose tissue was used for adipocyte isolation and the rest was frozen and stored at −80°C until further processing.
Separate groups of age- and treatment-matched animals (n=6) were used for blood pressure analysis. Blood pressures were determined in conscious animals with the tail-cuff method (Bunag and Butterfield, 1982) using a SC1000 blood pressure analysis sytem (Hatteras Instruments, Cary, NC) after 18 weeks of treatment. The results of three consecutive measurements were averaged.
2.3. Preparation of adipocytes
Rat adipocytes from epididymal adipose tissue were isolated by collagenase digestion as previously described (Pinterova et al., 2001). Briefly, tissue was minced in Krebs-Ringer bicarbonate-HEPES (KRBH) buffer, pH 7.4 supplemented with 1.5% bovine serum albumin (BSA), 5mM glucose and 1mg/ml collagenase II (Sigma, St. Louis, MO). The tissue was then digested for 60 min at 37°C under an O2:CO2 (95:5) atmosphere. The digested tissue was filtered through 150 μm nylon mesh to remove the vascular tissue fraction. Adipocytes were washed twice in KRBH buffer. The diameter of at least 100 cells from each adipocyte suspension was determined optically by light microscopy.
2.4. Plasma membrane preparation and Western blotting
Epididymal adipose tissue was homogenized with a Polytron homogenizer in Tris-HCl buffer (5mM Tris-HCl, 250mM sucrose, pH 7.4) containing 1% protease-inhibitor cocktail (Sigma, St. Louis, MO). The homogenate was filtered through surgical gauze to minimize contamination from the vascular fraction and centrifuged at 1,000g for 10 min to remove nuclei and cell debris. The supernatant was centrifuged at 15,000g for 15 min. The pellet of crude plasma membranes was resuspended in Tris-HCl buffer to a protein concentration of 2.5 μg/μl. The membranes were solubilized by adding 5x protein gel loading buffer (62.5 mM Tris, 2% sodium dodecyl sulfate, 0.02% bromophenol blue, 10% glycerol, pH 6.8) and boiling at 100°C for 8 min.
For Western blotting 40 μg of membrane proteins were separated by electrophoresis on 10% Tris-Glycine gel (Invitrogen Life Technologies, Carlsbad, CA) and transferred to polyvinylidene difluoride membrane (Invitrogen Life Technologies, Carlsbad, CA). The membranes were blocked for 60 min in blocking buffer (Sigma, St. Louis, MO) and incubated at 4°C overnight with rabbit polyclonal antibodies against rat angiotensin II AT1 and AT2 receptors (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA). In order to control sample loading variations, the level of β-actin was measured in each blot by an anti β-actin antibody (1:500, Santa Cruz Biotechnology, Santa Cruz, CA), and used as a reference protein for normalization. The membranes were washed in washing buffer (10mM Tris-HCl, 100mM NaCl, 0.1% Tween 20, pH 7.4) and incubated for 60 min at room temperature with a horse-radish peroxidase (HRP)-conjugated secondary antibody (donkey ECL anti-rabbit IgG, 1:2500, Amersham Biosciences, Buckinghamshire, England). After subsequent washing, the membranes were subjected to a chemiluminescent reagent (Luminol Reagent, Santa Cruz Biotechnology, Santa Cruz, CA) and exposed to X-ray film. The chemiluminescent signal was acquired by densitometric scanning and evaluated with Scion Image software (Scion Corporation, Frederick, MD).
2.5. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA from 100 mg of adipose tissue was isolated with Trizol Reagent (Invitrogen Life Technologies, Carlsbad, CA) and purified with the RNeasyLipid Tissue Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s directions. Four μg of total RNA was used for reverse transcription (RT) reaction using the Ready-To-Go You-Prime First-Strand Beads Kit (Amersham Biosciences, Piscataway, NJ) according to manufacturer’s instructions in a final volume of 33 μl.
PCR was performed in a final volume of 50 μl containing 2.5 μl of the cDNA, 0.2 μmol/l of each forward and reverse primer, and 45 μl of PCR SuperMix (Invitrogen Life Technologies, Carlsbad, CA). Preliminary experiments were carried out varying the number of PCR cycles to determine the non-saturating conditions for all the genes studied (data not shown). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a constitutively expressed internal control, because it has been reported to be the best suited for gene expression studies in adipose cells (Gorzelniak et al., 2001). The PCR cycle numbers were 28 for leptin, 30 for GAPDH, adiponectin and angiotensin IIAT1 receptor, 34 for angiotensin-converting enzyme (ACE), 35 for adipocyte fatty acid-binding protein (aP2), glucose transporter 4 (GLUT4) and angiotensinogen, 36 for fatty acid synthase (FAS) and hormone-sensitive lipase (HSL), 38 for tumor necrosis factor alpha (TNFα), peroxisome proliferator-activated receptor γ (PPARγ), angiotensin II AT2 receptor, and preadipocyte factor 1 (Pref-1). The PCR reaction mixture was denatured at 94°C for 2 min and allowed to proceed for amplification. Each cycle consisted of denaturation at 94°C for 30s, primer annealing at 60°C for leptin and 55°C for all other primers, and primer extension at 72°C for 1min. A final extension step for 10 min at 72°C was performed in all the reactions. The products were separated on 2% agarose gels and stained with ethidium bromide. The relative density of the bands was evaluated using the Kodak Image Station 440CF system and 1D Image Analysis Software (Eastman Kodak Company Rochester, NY). All the measured PCR products were normalized to the amount of GAPDH mRNA in each sample.
All primers used in the study are listed in Table 1. The primers were designed using Primer3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) as described (Rozen and Skaletsky, 2000) or taken from previously published data as indicated in Table 1.
TABLE 1.
Primers used for RT-PCR and qPCR
| Gene | Primer sequencea | Referenceb |
|---|---|---|
| ACE |
F: ATGTACCTGGAACCACAGCAG
R: GCTTGGGTATGTTCCACTCAA |
U03734 |
| Adiponectin |
F: CTCCACCCAAGGAAACTTGT
R: CTGGTCCACATTTTTTTCCT |
(Tsuda et al., 2004) |
| Angiotensinogen |
F: TTGTTGAGAGCTTGGGTCCCTTCA
R: CAGACACTGAGGTGCTGTTGTCCA |
(Shyu et al., 1995) |
| aP2 |
F: AGCGTAGAAGGGGACTTGGT
R: ATGGTGGTCGACTTTCCATC |
U75581 |
| AT1 |
F: GCACAATCGCCATAATTATCC
R: CACCTATGTAAGATCGCTTC |
(Llorens-Cortes et al., 1994) |
| AT2 |
F: TTATGGCTTTCCCACCTGAG
R: AAGGAAGTGCCAGGTCAATG |
U01908 |
| FAS |
F: GAGTCTGTCTCCCGCTTGAC
R: TGGAAATGAGGGCCATAGTC |
NM 017332 |
| GAPDH |
F: GTCGGTGTCAACGGATTTG
R: GGGTTTCCCATTGATGACC |
NM 017008 |
| GAPDH (qPCR) |
F: ATGACTCTACCCACGGCAAG
R: TGGAAGATGGTGATGGGTTT |
NM 017008 |
| GLUT4 |
F: TTTCCAGTATGTTGCGGATG
R: TCAGTCATTCTCATCTGGCC |
(Hosaka et al., 1992) |
| HSL |
F: ATCATTCCCAAGCCACAAAG
R: GTCCCCACGTGTTCATCTCT |
NM 012859 |
| Leptin |
F: CCAGGATGACACCAAAACCCTC
R: ATCCAGGCTCTCTGGCTTCTGC |
NM 013076 |
| Pref-1 |
F: ATCGTGACCAACAGCTGTACC
R: CTTTCATGGACACCTTCAGGA |
U25680 |
| PPAR γ |
F: CATTTCTGCTCCACACTATGAA
R: CGGGAAGGACTTTATGTATGAG |
(Tsuda et al., 2004) |
| Renin |
F: CTTGTTGCTCTGGACCTCTTG
R: TGACTTTGAAGGTCTGGGATG |
J02941 |
| Renin (qPCR) |
F: CTGTGCATACTGGCTCTCCA
R: GAACCCGATGCGATTGTTAT |
J022941 |
| Renin receptor (qPCR) |
F: TGGCCTATACCAGGAGATCG
R: AATAGGTTGCCCACAGCAAG |
AB188298 |
| TNF α |
F: CCACCACGCTCTTCTGTCTAC
R: ACCACCAGTTGGTTGTCTTTG |
X66539 |
F: Forward primer 5′-3′, R: Reverse primer 5′-3′
Reference shows GenBank accession number for primers designed in our laboratory or reference to literature for previously published primer sequences
2.6. Real-time PCR (qPCR)
Real-time PCR was performed in a 20 μl reaction mixture consisting of 10 μl SYBR Green PCR Master Mix (Applied Biosystems), 2 μl cDNA and 0.3 μmol/l of each primer for a specific target on a Opticon II cycler (Biorad). The specific primers for qPCR are listed in Table 1. The initial step in the reaction was 95°C for 10 min, followed by 50 cycles of 95°C for 15 s and 60°C for 1 min. To obtain a calibration curve, serial dilutions of rat cDNA were used. The individual targets for each sample were quantified by determining the cycle threshold (Ct) and by using a calibration curve. The relative amount of the target was normalized with the housekeeping gene GAPDH.
2.7. Assays
Protein concentrations were measured using the Bradford reagent (Sigma, St. Louis, MO) with bovine serum albumin as a standard (Bradford, 1976). Serum glucose, triglycerides and cholesterol were measured on Synchron LX20 analyzer (Beckman Coulter Inc, Fullerton, CA). Serum, insulin, leptin and adiponectin levels were determined by commercial RIA kits (Linco Research, St. Charles, MO). Serum TNFα was assayed with the Super Sensitive Rat TNFα Elisa kit (Biosource International, Camarillo, CA). Non-esterified fatty acids in serum were measured with a commercial acyl-CoA oxidase-based colorimetric kit (NEFA-C, Wako Chemicals U.S.A., Inc., Richmond, VA). To measure the triglycerides in adipose tissue, lipids were extracted by the method of Folch et al., (1957). The lipid extracts were dried under nitrogen and dissolved in isopropanol. The triglyceride content was determined enzymatically using a commercial kit (Roche, Basel, Switzerland).
The cell density, or number of cells per gram of adipose tissue, was calculated as a ratio of the triglyceride content per gram of tissue to the triglyceride content per cell. The triglyceride content per cell was estimated as the mean volume of adipocytes, calculated from their average cell diameter of 100 cells per rat, measured under a microscope in individual animals, multiplied by density of triglycerides, represented by the density of glyceryl trioleate, 0.91 g/ml.
2.8. Statistical analysis
The data were expressed as the mean ± S.E.M.. Statistical comparisons were performed using unpaired Student’s t-test. Two-way ANOVA followed by Bonferroni analysis was used for in vivo data (body weight, water and food intake). A value of P<0.05 was considered statistically significant. All the statistical analyses were performed using GraphPad Prism 3.03 software (GraphPad Software, Inc, San Diego, CA).
3. Results
3.1. Blood pressure, body weight, and food and water intake
Candesartan cilexetil administration produced a limited but significant decrease in blood pressure, from 115.0 ± 5.6 to 86.7 ± 6.1 mmHg for groups of six animals (P< 0.01). The body weight was significantly lower in the group treated with Candesartan cilexetil from the fourth week of treatment till the end of experiment (Fig. 1A). The difference in body weight at the end of experiment was approximately 16%. Food intake expressed as ratio of ingested food per body weight was reduced in Candesartan cilexetil group from the second to the sixth week of the treatment (Fig. 1B). No significant differences between both groups were noticed afterwards with the exception of the 15-th week when food intake was slightly but significantly elevated in Candesartan cilexetil-treated rats.
FIG. 1. Effect of long-term angiotensin II AT1 receptor blockade on body weight, food and water intake.
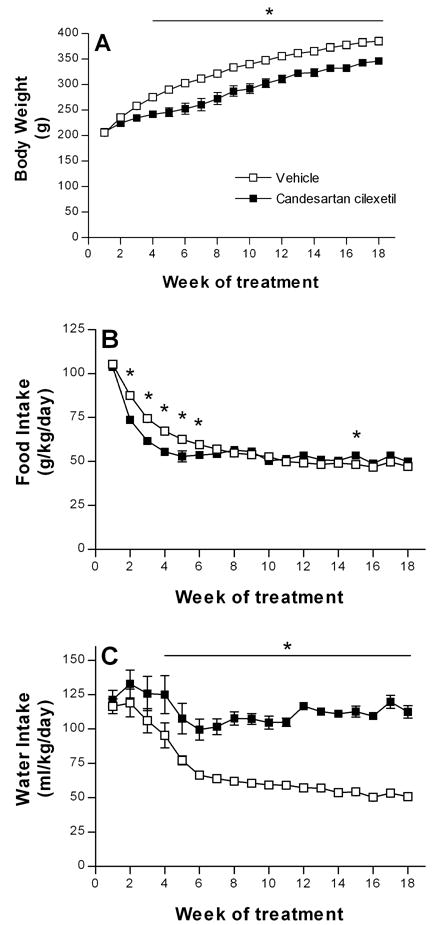
Two groups of 10 male Wistar Kyoto rats were treated with either angiotensin II AT1 receptor blocker, Candesartan cilexetil or vehicle for 18 weeks. During the treatment the animals were housed two per cage. Body weight, food and water intake were evaluated once a week. A. Body weight expressed in g as mean ± S.E.M., n = 10 for each group. B. Food intake expressed in g per kg of body weight per day. Food intake was measured individually for each cage housing two rats and normalized for body weight. Results are expressed as mean ± S.E.M., n = 5 for each group. C. Water intake expressed in ml per kg of body weight per day. Water intake was measured individually for each cage housing two rats and normalized for body weight. Results are expressed as mean ± S.E.M., n = 5 for each group. * P<0.05
Candesartan cilexetil prevented the normal age-related decrease in water intake expressed as a ratio of the amount of water per body weight (Fig.1C). The difference between both groups were statistically significant from the fourth week of treatment till the end of experiment.
3.2. Fat tissue and adipocytes
The mass of epididymal and retroperitoneal adipose tissue was significantly reduced by almost 50% in Candesartan cilexetil treated animals (Fig. 2A). Candesartan cilexetil administration caused a significant decrease in adipocyte diameter in cells from epididymal adipose tissue (Fig. 2B). The triglyceride content in epididymal adipose tissue was the same in both groups of animals (vehicle: 0.88±0.03 μg/μg tissue vs. Candesartan cilexetil-treated: 0.85±0.02 μg/μg tissue, NS). The cell density of epididymal adipose tissue was much higher in treated animals (vehicle: 5.8 ± 0.5 million/g vs. Candesartan cilexetil-treated: 12.3 ± 0.6 million/g, P<0.0001). The total number of cells in whole tissue was the same in both groups (vehicle treated: 21.6±1.8 million vs. Candesartan cilexetil-treated: 23.0±1.1 million, NS). The gene expression of preadipocyte differentiation markers Pref-1 and aP2 was not changed by Candesartan cilexetil treatment (Pref-1: 0.26±0.03 vs. 0.28±0.05 and aP2: 0.26±0.02 vs. 0.26±0.01 for vehicle vs. Candesartan cilexetil-treated rats, respectively).
FIG. 2. Effect of long-term angiotensin II AT1 receptor blockade on adiposity index and diameter of adipocytes.

Two groups of 10 male Wistar Kyoto rats were treated with either Candesartan cilexetil or vehicle for 18 weeks. At the end of treatment the rats were sacrificed by decapitation and epididymal and retroperitoneal fat was used for adipocyte isolation. A. Adiposity index for epididymal and retroperitoneal adipose tissue expressed as a percent of adipose tissue weight relative to body weight. B. Diameter of adipocytes isolated from epididymal fat pads. The adipocytes were isolated from epididymal adipose tissue by collagenase digestion and evaluated by light microscopy. The diameter of at least 100 cells from each rat was used to calculate average value. C. Representative pictures of adipocytes isolated from control and Candesartan cilexetil-treated animals. Scale bar = 100 μm. Results are expressed as mean ± S.E.M., n=10 for each group. *** P < 0.001
3.3. Serum glucose and lipids
Serum glucose levels were the same in both groups of animals (Table 2). Serum cholesterol, triglycerides and free fatty acids were elevated in the Candesartan cilexetil-treated animals (Table 2).
TABLE 2.
Glucose, Insulin and Lipids in serum
| Parameter | Vehicle | Candesartan cilexetil |
|---|---|---|
| Glucose (mmol/l) | 6.42 ± 0.12 | 6.42 ± 0.10 |
| Insulin (ng/ml) | 0.80 ± 0.07 | 0.83 ± 0.10 |
| Cholesterol (mmol/l) | 0.96 ± 0.04 | 1.10 ± 0.04 a |
| Triglycerides (mmol/l) | 0.56 ± 0.02 | 0.79 ± 0.06 b |
| Free fatty acids (mmol/l) | 0.56 ± 0.16 | 0.78 ± 0.18 a |
Results are expressed as mean ± S.E.M., n=10 for each group
P<0.05 for Vehicle vs. Candesartan cilexetil
P< 0.01 for Vehicle vs. Candesartan cilexetil
3.4. Hormones in blood and adipose tissue
Serum insulin was not changed by Candesartan cilexetil treatment (Table 2). There were opposite changes in blood and adipose tissue leptin and adiponectin. After Candesartan cilexetil treatment, serum levels of leptin decreased, and the gene expression of leptin in epididymal adipose tissue was reduced (Fig. 3A and 3C). Conversely, there was a twofold increase in adiponectin levels in the serum of Candesartan cilexetil treated rats, and an elevation of adiponectin gene expression in epididymal adipose tissue (Fig. 3B and 3D). TNFα gene expression in epididymal adipose tissue decreased significantly after Candesartan cilexetil treatment (Fig. 6A). The serum levels of TNFα were below the limit of detection (2.3 pg/ml).
FIG. 3. Effect of long term angiotensin II AT1 receptor blockade on leptin and adiponectin plasma levels and gene expression in white adipose tissue.

Two groups of 10 male Wistar Kyoto rats were treated with either Candesartan cilexetil or vehicle for 18 weeks. Animals were sacrificed by decapitation and their serum was used for determination of leptin (A) and adiponectin (C) levels by RIA. Results are expressed as mean ± S.E.M. in ng/ml and μg/ml for leptin and adiponectin, respectively. Epididymal fat pads were used for RNA isolation and determination of leptin (B) and adiponectin (D) gene expression by RT-PCR. Results from RT-PCR are normalized to the expression of GAPDH mRNA and expressed as mean ± S.E.M., n=10 for each experimental group. * P < 0.05, *** P < 0.001
FIG. 6. Effect of long-term angiotensin II AT1 receptor blockade on expression of TNFα, PPARγ, FAS and HSL genes in white adipose tissue.

Two groups of 10 male Wistar Kyoto rats were treated with either Candesartan cilexetil or vehicle for 18 weeks. At the end of treatment the rats were sacrificed by decapitation and epididymal fat was used for RT-PCR analysis. Expression of TNFα (A), PPARγ (B), FAS (C), and HSL (D) mRNA was normalized to the expression of GAPDH mRNA. Results are expressed as mean ± S.E.M., n = 10 for each experimental group. * P < 0.05
3.5. Renin-angiotensin system components in adipose tissue
Angiotensinogen and ACE mRNA levels in epididymal adipose tissue were not significantly different between the two groups of rats (Angiotensinogen: 2.11±0.04 vs. 2.03±0.04 and ACE: 1.20±0.02 vs. 1.22±0.04 for vehicle vs. Candesartan cilexetil-treated rats, respectively). Renin expression was not detectable by conventional RT-PCR. The real-time PCR revealed the presence of renin mRNA in epididymal adipose tissue but the expression did not differ between both experimental groups (Fig.5A). The expression of renin receptor mRNA was significantly reduced in Candesartan cilexetil-treated rats (Fig. 5B). There were no changes in angiotensin II AT1 receptor mRNA or protein in epididymal adipose tissue after Candesartan cilexetil treatment (Fig. 4A and 4C). The expression of angiotensin II AT2 receptor mRNA and protein significantly increased after Candesartan cilexetil treatment in epididymal adipose tissue (Fig. 4B and 4D).
FIG. 5. Effect of long-term angiotensin II AT1 receptor blockade on expression of Renin and (Pro)Renin receptor genes in white adipose tissue.

Two groups of 10 male Wistar Kyoto rats were treated with either Candesartan cilexetil or vehicle for 18 weeks. At the end of treatment the rats were sacrificed by decapitation and epididymal fat was used for real time PCR analysis. Expression of Renin (A) and (Pro)Renin receptor (B) was normalized to the expression of GAPDH mRNA. Results are expressed as mean ± S.E.M., n = 10 for each experimental group. *P < 0.05
FIG. 4. Effect of long-term angiotensin II AT1 receptor blockade on gene and protein expression of angiotensin II AT1 and AT2 receptors in white adipose tissue.

Two groups of 10 male Wistar Kyoto rats were treated with either Candesartan cilexetil or vehicle for 18 weeks. At the end of treatment the rats were sacrificed by decapitation and epididymal fat was used for RT-PCR and Western Blot analysis. Expression of angiotensin II AT1 (A) and AT2 receptor mRNA (B) was normalized to the level of GAPDH mRNA. Protein levels of angiotensin II AT1 (C) and AT2 (D) receptors were normalized to the level of β-actin. Results are expressed as mean ± S.E.M., n = 10 for each experimental group. * P < 0.05
3.6. GLUT4, PPARγ, FAS, and HSL in epididymal adipose tissue
PPARγ gene expression in epididymal adipose tissue was significantly higher after Candesartan cilexetil treatment when compared with vehicle treated rats (Fig. 6B). The treatment with Candesartan cilexetil increased the level of FAS but did not change expression of HSL (Fig. 6C and 6D, respectively). The expression of GLUT4 was not changed after Candesartan cilexetil treatment (1.93±0.05 vs. 1.90±0.07 for vehicle vs. Candesartan cilexetil-treated rats, respectively).
4. Discussion
To study the influence of the renin-angiotensin system on adipose tissue we selected a sustained treatment of rats with the angiotensin II AT1 receptor antagonist Candesartan cilexetil (Pollock and Morsing, 1999). Candesartan cilexetil has about 10% oral bioavailability and the dose administered (10 mg/kg/day) ensures maximal blockade of angiotensin II AT1 receptors (Pollock and Morsing, 1999). At this dose, Candesartan cilexetil normalized blood pressure in spontaneously hypertensive rats (SHR), protected their end organs from hypertension-induced damage (Inada et al., 1997; Pollock and Morsing, 1999) and significantly extended their life span (Baiardi et al., 2004). In the normotensive rats used here, Candesartan cilexetil produced a limited but significant blood pressure decrease and abolished the age-dependent reduction in water intake, without any overt signs of toxicity. This is in agreement with the reported increase in water intake in SHR and normotensive Wistar rats after angiotensin II AT1 receptor blockade, an effect possibly related to inhibition of antidiuretic hormone release (Armando et al., 2001; Pollock and Morsing, 1999).
Long term administration of Candesartan cilexetil significantly reduced body weight gain mainly due to a profound decrease in adipose tissue mass. This resulted from a substantial increase in the proportion of small adipocytes while the number of cells did not change. The effects on adipose tissue mass were accompanied by increased expression of PPARγ and marked changes in adipokine and cytokine production. Gene expression and plasma levels of leptin were decreased, in agreement with a previously reported correlation between leptin and adipose tissue mass (Friedman and Halaas, 1998). Adiponectin mRNA in adipose tissue and adiponectin plasma levels significantly increased after Candesartan cilexetil treatment. This adipokine, exclusively synthesized by white adipocytes, is induced during adipocyte differentiation and is a potent enhancer of insulin sensitivity (Fasshauer et al., 2004). Both leptin and adiponectin gene expressions are under the control of PPARγ. PPARγ represses the expression of leptin ob gene (Hollenberg et al., 1997) and induces the expression of adiponectin gene (Gustafson et al., 2003; Iwaki et al., 2003). Observed changes in the production of both proteins are therefore likely a direct consequence of PPARγ activation. In addition, angiotensin II AT1 receptor blockade inhibited expression of TNFα, a pro-inflammatory cytokine (Hotamisligil et al., 1993) impairing the action of insulin (Peraldi and Spiegelman, 1998). This agrees with a previous report of TNFα stimulation by an angiotensin II AT1 receptor-dependent pathway in human endothelial cells (Arenas et al., 2004). Since TNFα suppresses PPARγ expression (Xing et al., 1997), it is possible that inhibition of TNFα production contributed to the increase in PPARγ mRNA. Inhibition of TNFα production could even further potentiate effect of PPARγ on adiponectin production, because TNFα suppresses the promoter activity of the adiponectin gene (Maeda et al., 2001). We conclude that in normotensive, non-obese rats, angiotensin II AT1 receptor stimulation exerts a profound influence on adipose tissue remodeling and production of adipokines, cytokines and PPARγ, and that these effects appear to be interconnected.
There is a general consensus that angiotensin II has a trophic role in adipose tissue (Boustany et al., 2004; Hainault et al., 2002; Saint-Marc et al., 2001). However, the effects of angiotensin II on adipocyte metabolism and differentiation are not conclusive. Some reports indicate that angiotensin II inhibits adipocyte differentiation (Janke et al., 2002; Massiera et al., 2001), while other show that angiotensin II promotes it (Darimont et al., 1994a,b; Saint-Marc et al., 2001).
A major finding in our study is that Candesartan cilexetil enhanced gene expression of PPARγ, a key regulator of adipocyte differentiation (Lazar, 2005). There are reports that angiotensin II AT1 receptor blockers irbesartan and telmisartan, are partial agonists of PPARγ able to induce its activity and adipocyte differentiation independently of angiotensin II AT1 receptor blockade (Schupp et al., 2004). Since Candesartan is less lipophilic than other angiotensin II AT1 receptor blockers (Morsing et al., 1999) it probably does not activate PPARγ directly. Activation of PPARγ by Candesartan cilexetil could be due to angiotensin II AT1 receptor blockade and/or effects unrelated to angiotensin II.
Enhanced gene expression of PPARγ and fatty acid synthase, a key lipogenic enzyme, and a dramatic increase in the percentage of small adipocytes suggest that Candesartan cilexetil promoted adipocyte differentiation in our study. However, the number of adipocytes was not affected and the ratio of preadipocyte (Pref-1) and adipocyte (aP2) markers did not change. PPARγ agonist rosiglitazone has been previously shown to stimulate both adipocyte differentiation and apoptosis, thereby preventing adipocyte hypertrophy in mice (Yamauchi et al., 2001). The balance between differentiation and apoptosis of large adipocytes may therefore be a reasonable explanation for the unchanged number of cells and the unchanged ratio of preadipocytes to adipocytes. However, the treatment with rosiglitazone despite induction of apoptosis resulted in increased number of cells in adipose tissue and concomitant increase in adipose tissue mass (Yamauchi et al., 2001). In our study, we observed the opposite effect on the mass of adipose tissue indicating that balance may be shifted toward apoptosis. Another possible explanation is that the effect of Candesartan cilexetil is time-dependent as indicated by our observation of early decrease in food intake that was normalized after 6 weeks of treatment. Thus, the steady state achieved at the end of study may not reflect changes occurring at the early stages of treatment. Multiple measurements of differentiation markers during the treatment period would be necessary to clarify the effects of Candesartan cilexetil on adipocyte differentiation.
Adipocyte differentiation is accompanied by activation of lipogenetic pathways (Gregoire et al., 1998) resulting in accumulation of triglycerides in adipose tissue and redistribution of lipids from ectopic distribution to adipose tissue. This leads to increased peripheral insulin-sensitivity. However, our findings of moderately increased serum levels of free fatty acids and triglycerides after Candesartan cilexetil treatment are not consistent with this expected effect and indicate involvement of other mechanisms. Enhanced lipolysis would offer another possible explanation for adipocyte size reduction and increased levels of serum lipids after Candesartan cilexetil treatment. However, unchanged content of triglycerides and unchanged expression of hormone-sensitive lipase in adipose tissue are not in favor to this possibility. This is further supported by observation that angiotensin II AT1 receptor blocker losartan reduces angiotensin II-induced lipolysis in subcutaneous and visceral fat in rats (Cabassi et al., 2005).
These complex effects of Candesartan cilexetil may be in part the result of alterations in the expression of other local renin angiotensin system components. Candesartan cilexetil treatment profoundly affects the circulating, hormonal renin angiotensin system. Angiotensin II AT1 receptor blockers increase circulating angiotensin II levels by eliminating the negative feedback effect of angiotensin II AT1 receptor signaling on renin production (Campbell et al., 1995). Higher circulating angiotensin II can increase angiotensin AT2 receptor activity in various sites, including adipose tissue (Crandall et al., 1994). We show here that Candesartan cilexetil increased angiotensin II AT2 receptor mRNA and protein in adipose tissue, supporting the hypothesis that the treatment may result in over-stimulation of angiotensin II AT2 receptors. Angiotensin II AT2 receptor activation stimulates adipocyte differentiation (Darimont et al., 1994a, Saint-Marc et al., 2001) while increasing hepatic triglyceride production and release and fatty acid mobilization in WKY rats (Ran et al., 2005). We show here that Candesartan cilexetil-treated rats have increased serum lipids. It is possible that enhanced angiotensin II AT2 receptor stimulation may be a contributing factor. We also report decreased mRNA expression of the (pro)renin receptor in epididymal fat of Candesartan cilexetil-treated rats. This receptor activates circulating renin and prorenin, increasing local formation of angiotensin II, and it also stimulates signal transduction mechanisms independently of angiotensin II (Nguyen et al., 2004). In human mesangial cells the (pro)renin receptor activates extracellular signal-regulated kinases (ERK) 1/2 (Nguyen et al., 2002). ERK 1/2 inhibit adipocyte differentiation through phosphorylation of PPARγ and reduction of its transcriptional activity (Camp and Tafuri., 1997; Hu et al., 1996). Moreover, the (pro)renin receptor activation increases the production of transforming growth factor-β1 (Huang et al., 2006), a potent inhibitor of adipocyte differentiation (Torti et al., 1989). Thus, despite expected increase in plasma renin levels after the treatment, decreased expression of renin receptor in adipose tissue may reduce local renin activity and its effect on adipocyte differentiation.
Our results are of interest since they raise several questions regarding the long term effect of angiotensin II AT1 receptor blockade. We have found increased expression of PPARγ associated with dramatically increased levels of adiponectin and reduced expression of TNFα. These changes toward increased insulin-sensitivity were, however, accompanied by increased circulating levels of free fatty acids and triglycerides known as a risk factor for the development of insulin resistance. Since we did not perform glucose tolerance tests in this study, we cannot clarify whether the moderate increase in serum lipids affects the insulin-sensitivity in WKY rats. However, from the unchanged levels of blood glucose and insulin, we would not expect changes in insulin sensitivity, in spite of increases in circulating lipids.
Previous studies have clearly shown that the treatment with angiotensin II AT1 receptor antagonists is beneficial in obese, insulin-resistant or hypertensive animals and humans (Jandeleit-Dahm et al., 2005). In animal studies, angiotensin II AT1 receptor blockers enhanced insulin-sensitivity and improved the serum lipid profile in obese (Ran et al., 2004) or high fructose-fed (Okada et al., 2004) rats, both models of insulin-resistance, but not in their healthy controls. Thus, the insulin-sensitizing effect of angiotensin II AT1 receptor blockade appears to be restricted to the insulin-resistant state. We suggest that complex interactions between PPARγ and angiotensin II AT2-mediated lipid distribution may explain the fact that in non-obese and non-insulin resistant rats after long-term angiotensin II AT1 receptor blockade triglyceride production and free fatty acid mobilization reaches a steady state different from that in obese or insulin-resistant subjects.
Questions remain on the clinical significance of our findings. Although long lasting angiotensin II AT1 receptor blockade may negatively alter lipid parameters in non-obese and/or non-insulin resistant subjects, the significant increase in adiponectin formation and release argues against the development of insulin resistance. In addition, the increase in anti-inflammatory adiponectin and decrease in proinflammatory TNFα supports earlier reports of strong anti-inflammatory effects of angiotensin II AT1 receptor blockade, which are independent of blood-pressure regulation (Ando et al., 2004; Bregonzio et al., 2003; Zhou et al., 2005).
Acknowledgments
This study was supported by Division of Intramural Research Programs, National Institute of Mental Health and by the project 2/5090/25 of the Grant Agency of Ministry of Education and Slovak Academy of Sciences (VEGA). Candesartan cilexetil was kindly provided by AstraZeneca, Mölndal, Sweden.
References
- Ando H, Jezova M, Zhou J, Saavedra JM. Angiotensin II AT1 receptor blockade decreases brain artery inflammation in a stress-prone rat strain. Ann NY Acad Sci. 2004;1018:345–350. doi: 10.1196/annals.1296.043. [DOI] [PubMed] [Google Scholar]
- Arenas IA, Xu Y, Lopez-Jaramillo P, Davidge ST. Angiotensin II-induced MMP-2 release from endothelial cells is mediated by TNF-alpha. Am J Physiol Cell Physiol. 2004;286:C779–C784. doi: 10.1152/ajpcell.00398.2003. [DOI] [PubMed] [Google Scholar]
- Armando I, Carranza A, Nishimura Y, Hoe KL, Barontini M, Terrón JA, Falcón-Neri A, Ito T, Juorio AV, Saavedra JM. Peripheral administration of an angiotensin II AT1 receptor antagonist decreases the hypothalamic-pituitary-adrenal response to stress. Endocrinology. 2001;142:3880–3889. doi: 10.1210/endo.142.9.8366. [DOI] [PubMed] [Google Scholar]
- Baiardi G, Bregonzio G, Jezova M, Armando I, Saavedra JM. Angiotensin II AT1 receptor blockade prolongs lifespan of spontaneously hypertensive rats and reduces stress-induced release of catecholamines, glucocorticoids, and vasopressin. Ann N Y Acad Sci. 2004;1018:131–136. doi: 10.1196/annals.1296.015. [DOI] [PubMed] [Google Scholar]
- Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physio. 2004;287:R943–R949. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bregonzio C, Armando I, Ando H, Jezova M, Baiardi G, Saavedra JM. Anti-inflammatory effects of angiotensin II AT1 receptor antagonism prevents stress-induced gastric injury. Am J Physiol Gastrointest Liver Physiol. 2003;285:G414–G423. doi: 10.1152/ajpgi.00058.2003. [DOI] [PubMed] [Google Scholar]
- Bunag RD, Butterfield J. Tail-cuff blood pressure measurement without external preheating in awake rats. Hypertension. 1982;4:898–903. doi: 10.1161/01.hyp.4.6.898. [DOI] [PubMed] [Google Scholar]
- Cabassi A, Coghi P, Govoni P, Barouhiel E, Speroni E, Cavazzini S, Cantoni AM, Scandroglio R, Fiaccadori E. Sympathetic modulation by carvedilol and losartan reduces angiotensin II-mediated lipolysis in subcutaneous and visceral fat. J Clin Endocrinol Metab. 2005;90:2888–2897. doi: 10.1210/jc.2004-1995. [DOI] [PubMed] [Google Scholar]
- Camp HS, Tafuri SR. Regulation of peroxisome proliferator-activated receptor γ activity by mitogen-activated protein kinase. J Biol Chem. 1997;272:10811–10816. doi: 10.1074/jbc.272.16.10811. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Kladis A, Valentijn AJ. Effects of losartan on angiotensin and bradykinin peptides and angiotensin-converting enzyme. J Cardiovasc Pharmacol. 1995;26:233–240. doi: 10.1097/00005344-199508000-00009. [DOI] [PubMed] [Google Scholar]
- Cassis LA, English VL, Bharadwaj K, Boustany CM. Differential effects of local versus systemic angiotensin II in the regulation of leptin release from adipocytes. Endocrinology. 2004;145:169–174. doi: 10.1210/en.2003-0767. [DOI] [PubMed] [Google Scholar]
- Crandall DL, Herzlinger HE, Saunders BD, Armellino DC, Kral JG. Distribution of angiotensin II receptors in rat and human adipocytes. J Lipid Res. 1994;35:1378–1385. [PubMed] [Google Scholar]
- Darimont C, Vassaux G, Ailhaud G, Negrel R. Differentiation of preadipose cells: paracrine role of prostacyclin upon stimulation of adipose cells by angiotensin II. Endocrinology. 1994a;135:2030–2036. doi: 10.1210/endo.135.5.7956925. [DOI] [PubMed] [Google Scholar]
- Darimont C, Vassaux G, Gaillard D, Ailhaud G, Negrel R. In situ microdialysis of prostaglandins in adipose tissue: stimulation of prostacyclin release by angiotensin II. Int J Obes Relat Metab Disord. 1994b;18:783–788. [PubMed] [Google Scholar]
- Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, Ailhaud G, Teboul M, Massiera F, Sharma AM. The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int J Biochem Cell Biol. 2003;35:807–825. doi: 10.1016/s1357-2725(02)00311-4. [DOI] [PubMed] [Google Scholar]
- Fasshauer M, Paschke R, Stumvoll M. Adiponectin, obesity, and cardiovascular disease. Biochimie. 2004;86:779–784. doi: 10.1016/j.biochi.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Friedman JF, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Goossens GH, Blaak EE, van Baak MA. Possible involvement of the adipose renin-angiotensin system in the pathophysiology of obesity and obesity-related disorders. Obes Rev. 2003;4:43–55. doi: 10.1046/j.1467-789x.2003.00091.x. [DOI] [PubMed] [Google Scholar]
- Gorzelniak K, Janke J, Engeli S, Sharma AM. Validation of endogenous controls for gene expression studies in human adipocytes and preadipocytes. Horm Metab Res. 2001;33:625–627. doi: 10.1055/s-2001-17911. [DOI] [PubMed] [Google Scholar]
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- Guerre-Millo M. Adipose tissue and adipokines: for better or worse. Diabetes Metab. 2004;30:13–19. doi: 10.1016/s1262-3636(07)70084-8. [DOI] [PubMed] [Google Scholar]
- Gustafson B, Jack MM, Cushman SW, Smith U. Adiponectin gene activation by thiazolidinediones requires PPARγ2, but not C/EBPα-evidence for differential regulation of the aP2 and adiponectin genes. Biochem Biophys Res Commun. 2003;308:933–939. doi: 10.1016/s0006-291x(03)01518-3. [DOI] [PubMed] [Google Scholar]
- Hainault I, Nebout G, Turban S, Ardouin B, Ferre P, Quignard-Boulange A. Adipose tissue-specific increase in angiotensinogen expression and secretion in obese (fa/fa) Zucker rat. Am J Physiol Endocrinol Metab. 2002;282:E59–E66. doi: 10.1152/ajpendo.2002.282.1.E59. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Akimoto K, Gross SS, Hattori S, Kasai K. Angiotensin-II-induced oxidative stress elicits hypoadiponectinemia in rats. Diabetologia. 2005;48:1066–1074. doi: 10.1007/s00125-005-1766-7. [DOI] [PubMed] [Google Scholar]
- Hollenberg AN, Susulic VS, Madura JP, Zhang B, Moller DE, Tontonoz P, Sarraf P, Spiegelman BM, Lowell BB. Functional antagonism between CCAAT/Enhancer binding protein-alpha and peroxisome proliferator-activated receptor-gamma on the leptin promoter. J Biol Chem. 1997;272:5283–5290. doi: 10.1074/jbc.272.8.5283. [DOI] [PubMed] [Google Scholar]
- Hosaka Y, Tawata M, Kurihara A, Ohtaka M, Endo T, Onaya T. The regulation of two distinct glucose transporter (GLUTl and GLUT4) gene expressions in cultured rat thyroid cells by thyrotropin. Endocrinology. 1992;131:159–165. doi: 10.1210/endo.131.1.1319316. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- Huang Y, Wongamorntham S, Kasting J, McQuillan D, Owens RT, Yu L, Noble NA, Border W. Renin increases mesangial cell transforming growth factor-β1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int. 2006;69:105–113. doi: 10.1038/sj.ki.5000011. [DOI] [PubMed] [Google Scholar]
- Inada Y, Wada T, Ojima M, Sanada T, Shibouta Y, Kanagawa R, Ishimura Y, Fujisawa Y, Nishikawa K. Protective effects of candesartan cilexetil (TCV-116) against stroke, kidney dysfunction and cardiac hypertrophy in stroke-prone spontaneously hypertensive rats. Clin Exp Hypertens. 1997;19:1079–1099. doi: 10.3109/10641969709083206. [DOI] [PubMed] [Google Scholar]
- Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, Shimomura I. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655–1663. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- Jandeleit-Dahm KA, Tikellis C, Reid CM, Johnston CI, Cooper ME. Why blockade of renin-angiotensin system reduces the incidence of new-onset diabetes. J Hypertens. 2005;23:463–473. doi: 10.1097/01.hjh.0000160198.05416.72. [DOI] [PubMed] [Google Scholar]
- Janke J, Engeli S, Gorzelniak K, Luft FC, Sharma AM. Mature adipocytes inhibit in vitro differentiation of human preadipocytes via angiotensin type 1 receptors. Diabetes. 2002;51:1699–1707. doi: 10.2337/diabetes.51.6.1699. [DOI] [PubMed] [Google Scholar]
- Kim S, Whelan J, Claycombe K, Reath DB, Moustaid-Moussa N. Angiotensin II increases leptin secretion by 3T3-L1 and human adipocytes via a prostaglandin-independent mechanism. J Nutr. 2002;132:1135–1140. doi: 10.1093/jn/132.6.1135. [DOI] [PubMed] [Google Scholar]
- Lazar MA. PPARγ, 10 years later. Biochimie. 2005;87:9–13. doi: 10.1016/j.biochi.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Llorens-Cortes C, Greenberg B, Huang H, Corvol P. Tissular expression and regulation of type 1 angiotensin II receptor subtypes by quantitative reverse transcriptase-polymerase chain reaction analysis. Hypertension. 1994;24:538–548. doi: 10.1161/01.hyp.24.5.538. [DOI] [PubMed] [Google Scholar]
- Maeda N, Takahashi M, Funahashi T, Kihara S, Hishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y. PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–2099. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- Massiera F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, Quignard-Boulange A, Negrel R, Ailhaud G, Seydoux J, Meneton P, Teboul M. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001;15:2727–2729. doi: 10.1096/fj.01-0457fje. [DOI] [PubMed] [Google Scholar]
- Morsing P, Adler G, Brandt-Eliasson U, Karp L, Ohlson K, Renberg L, Sjoquist PO, Abrahamsson T. Mechanistic differences of various AT1-receptor blockers in isolated vessels of different origin. Hypertension. 1999;33:1406–1413. doi: 10.1161/01.hyp.33.6.1406. [DOI] [PubMed] [Google Scholar]
- Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen G, Burckle CA, Sraer JD. Renin/prorenin receptor biochemistry and functional significance. Curr Hypertens Rep. 2004;6:129–132. doi: 10.1007/s11906-004-0088-3. [DOI] [PubMed] [Google Scholar]
- Okada K, Hirano T, Ran J, Adachi M. Olmesartan medoxomil, an angiotensin II receptor blocker ameliorates insulin resistance and decreases triglyceride production in fructose-fed rats. Hypertens Res. 2004;27:293–299. doi: 10.1291/hypres.27.293. [DOI] [PubMed] [Google Scholar]
- Peach MJ. Renin-Angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 1977;57:313–370. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- Peraldi P, Spiegelman B. TNF-α and insulin resistance: Summary and future prospects. Mol Cell Biochem. 1998;182:169–175. [PubMed] [Google Scholar]
- Pinterova L, Zelezna B, Fickova M, Macho L, Krizanova O, Jezova D, Zorad S. Elevated AT1 receptor protein but lower angiotensin II-binding in adipose tissue of rats with monosodium glutamate-induced obesity. Horm Metab Res. 2001;33:708–712. doi: 10.1055/s-2001-19132. [DOI] [PubMed] [Google Scholar]
- Pollock DM, Morsing P. Combined treatment with ibuprofen and the AT1 receptor antagonist Candesartan in young spontaneously hypertensive rats. J Am Soc Nephrol. 1999;10:S116–S119. [PubMed] [Google Scholar]
- Ran J, Hirano T, Adachi M. Angiotensin II type 1 receptor blocker ameliorates overproduction and accumulation of triglyceride in the liver of Zucker fatty rats. Am J Physiol Endocrinol Metab. 2004;287:227–232. doi: 10.1152/ajpendo.00090.2004. [DOI] [PubMed] [Google Scholar]
- Ran J, Hirano T, Adachi M. Angiotensin II infusion increases hepatic triglyceride production via its type 2 receptor in rats. J Hypertens. 2005;23:1525–1530. doi: 10.1097/01.hjh.0000174077.88121.19. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press; Totowa, NJ: 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- Saint-Marc P, Kozak LP, Ailhaud G, Darimont C, Negrel R. Angiotensin II as a trophic factor of white adipose tissue: Stimulation of adipose cell formation. Endocrinology. 2001;142:487–492. doi: 10.1210/endo.142.1.7883. [DOI] [PubMed] [Google Scholar]
- Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation. 2004;109:2054–2057. doi: 10.1161/01.CIR.0000127955.36250.65. [DOI] [PubMed] [Google Scholar]
- Shyu KG, Chen JJ, Shih NL, Chang H, Wang DL, Lien WP, Liew CC. Angiotensinogen gene expression is induced by cyclic mechanical stretch in cultured rat cardiomyocytes. Biochem Biophys Res Commun. 1995;211:241–248. doi: 10.1006/bbrc.1995.1802. [DOI] [PubMed] [Google Scholar]
- Skurk T, van Harmelen V, Hauner H. Angiotensin II stimulates the release of interleukin-6 and interleukin-8 from cultured human adipocytes by activation of NF-kB. Arterioscler Thromb Vasc Biol. 2004;24:1199–1203. doi: 10.1161/01.ATV.0000131266.38312.2e. [DOI] [PubMed] [Google Scholar]
- Torti FM, Torti SV, Larrick JW, Ringold GM. Modulation of adipocyte differentiation by tumor necrosis factor and transforming growth factor beta. J Cell Biol. 1989;108:1105–1113. doi: 10.1083/jcb.108.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda T, Ueno Y, Aoki H, Koda T, Horio F, Takahashi N, Kawada T, Osawa T. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem Biophys Res Commun. 2004;316:149–157. doi: 10.1016/j.bbrc.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabeteologia. 2000;43:1498–1506. doi: 10.1007/s001250051560. [DOI] [PubMed] [Google Scholar]
- Xing H, Northrop JP, Grove JR, Kilpatrick KE, Su JL, Ringold GM. TNF alpha-mediated inhibition and reversal of adipocyte differentiation is accompanied by suppressed expression of PPAR gamma without effects on Prof-1 expression. Endocrinology. 1997;138:2776–2783. doi: 10.1210/endo.138.7.5242. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Murakami K, Motojima K, Komeda K, Ide T, Kubota N, Terauchi Y, Tobe K, Miki H, Tsuchida A, Akanuma Y, Nagai R, Kimura S, Kadowaki T. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor gamma (PPARgamma) deficiency and PPARgamma agonist improve insulin resistance. J Biol Chem. 2001;44:41245–41254. doi: 10.1074/jbc.M103241200. [DOI] [PubMed] [Google Scholar]
- Zhou J, Ando H, Macova M, Dou J, Saavedra JM. Angiotensin II AT1 receptor blockade abolishes brain microvascular inflammation and heat shock protein responses in hypertensive rats. J Cereb Blood Flow Metab. 2005;25:878–886. doi: 10.1038/sj.jcbfm.9600082. [DOI] [PubMed] [Google Scholar]
- Zorad S, Macho L, Jezova D, Fickova M. Partial characterization of insulin resistance in adipose tissue of monosodium glutamate-induced obese rats. Ann NY Acad Sci. 1997;827:541–545. doi: 10.1111/j.1749-6632.1997.tb51867.x. [DOI] [PubMed] [Google Scholar]


