Abstract
The mammalian soluble epoxide hydrolase (sEH) plays a role in the regulation of blood pressure and vascular homeostasis through its hydrolysis of the endothelial-derived messenger molecules, the epoxyeicosatrienoic acids. This study reports the cloning and expression of a sEH homolog from chicken liver. The resulting 63-kDa protein has an isoelectric point of 6.1. The recombinant enzyme displayed epoxide hydrolase activity when assayed with [3H]-trans-1,3-diphenylpropene oxide (t-DPPO), as well as trans-9,10-epoxystearate and the cis-8,9-, 11,12-, and 14,15- epoxyeicosatrienoic acids. The chicken enzyme displayed a lower kcat:Km for t-DPPO than the mammalian enzymes. The enzyme was sensitive to urea-based inhibitors developed for mammalian sEH. Such compounds could be used to study the role of chicken sEH in conditions in which endothelial-derived vasodilation is believed to be impaired, such as pulmonary hypertension syndrome.
Keywords: soluble epoxide hydrolase, epoxyeicosatrienoic acid, pulmonary hypertension syndrome
INTRODUCTION
Pulmonary hypertension syndrome (PHS) in chicken includes a number of conditions such as right-sided congestive heart failure, hypoxemia, pulmonary hypertension (PH), and acites (Wideman, 2000). It is estimated that PHS afflicts 4% of the broilers worldwide. Interestingly, broilers are more susceptible to PHS than to Leghorns (Julian, 1993). One contributing factor is thought to be impaired endothelium-dependent vasodilation in broilers relative to Leghorns (Martinez-Lemus et al., 1999; Martinez-Lemus et al., 2003). Endothelium-derived factors such as NO (nitric oxide), endothelin-1, and some eicosanoids have been shown to have vasoactive properties in chicken (Wideman et al., 1999; Villamor et al., 2002; Martinez-Lemus et al., 2003). It was found that NO attenuates PH induced by hypoxia or endotoxin in experiments employing NO synthase inhibitors and supplementation of diet with l-arginine, a precursor of NO (Wideman et al., 1995; Odom et al., 2004; Wideman and Chapman, 2004). Endothelin-1 has been shown to constrict chicken pulmonary arteries (Martinez-Lemus et al., 2003). Examination of the eicosanoids has focused on the actions of thromboxane and prostacyclin. Thromboxane has a vasoconstrictive effect in chicken pulmonary and cardiac microvessels (Wideman et al., 1999, 2001). However, cyclooxygenase inhibitors have failed to produce an effect on hypoxia-induced hypertension or isolated pulmonary coronary artery rings (Martinez-Lemus et al., 1999; Villamor et al., 2002; Odom et al., 2004). Taken together, these results suggest that blood pressure regulation in chicken may have similarities to that of mammals.
In mammals, the epoxyeicosatrienoic acids (EET) are paracrine- and autocrine-signaling molecules involved in the regulation of vascular homeostasis, blood pressure, and inflammation (Roman, 2002). They are produced from arachidonic acid by cytochrome P450 enzymes in the endothelium of lung, cardiac, and renal microvessels (Rosolowsky and Campbell, 1993; Zou et al., 1996; Gebremedhin et al., 1998). They have been shown to have vasodilatory actions in renal and cardiac microvessels through activation of large conductance Ca2+-activated K+ channels (Zou et al., 1996; Zhang et al., 2001; Boardman et al., 2002). The EET also have vasodilatory effects in bronchial smooth muscle (Zeldin et al., 1995). In general, the EET display vasodilatory effects and appear to function as endogenous antiinflammatory and hypotensive agents in most vascular beds.
The mammalian soluble epoxide hydrolase (sEH) catalyzes the hydrolysis of aliphatic epoxides such as the EET to their corresponding diols, the dihydroxyeicosatrienoic acids (DHET; Zeldin et al., 1993). This converts the EET into compounds that resist incorporation into lipid bilayers and can be excreted by the organism (Weintraub et al., 1999). Soluble epoxide hydrolase has been implicated in the metabolism of EET in human vasculature (Fang et al., 2004). It has also been shown to have a hypotensive effect in porcine coronary endothelial cells using an sEH inhibitor (Fang et al., 2001). In vivo experiments employing rat and mouse models have confirmed the role of sEH in the regulation of blood pressure. Treatment with sEH inhibitors have shown that the enzyme mediates blood pressure in rat models of hypertension (Yu et al., 2000; Imig et al., 2002). Recently, sEH inhibitors have been shown to reduce lung inflammation induced by tobacco smoke in a spontaneous hypertensive rat model (Smith et al., 2005).
Chickens produce the EET, as well as the EET hydrolysis products, the DHET (Nakai et al., 1992). This raises the question of whether there is a sEH homologue in chicken with epoxide hydrolase activity similar to the mammalian enzymes. Discovery of a sEH homolog would have implications for the study and management of PHS in chicken, especially if inhibitors of this enzyme are identified. Therefore, we report the cloning and characterization of a chicken sEH homolog.
MATERIALS AND METHODS
Total RNA and cDNA Library Preparation
Liver (0.5 g) from a 6- to 8-wk male Cobb broiler chicken was homogenized in 7.0 mL of TRIzol reagent (Invitrogen, Carlsbad, CA) with a Polytron grinder rotating at 9,000 rpm for 1 min, and then left at room temperature for 5 min. The sample was centrifuged at 12,000 × g for 15 min at 4°C, and then 1.4 mL of chloroform were added. The sample was left at room temperature for 5 min and then centrifuged at 12,000 × g for 15 min at 4°C. The upper phase was transferred to a new tube, and 3.5 mL of isopropanol were added. After a 10-min incubation at room temperature, the sample was spun at 12,000 × g for 10 min at 4°C. The supernatant was discarded, and 7 mL of 75% ethanol were added. The sample was vortexed for 30 s and centrifuged at 7,500 × g for 5 min at 4°C. This 75% ethanol wash was repeated one more time, and then the pellet was allowed to air dry for 10 min. The pellet was resuspended in diethyl pyrocarbonate (DEPC)-treated deionized, distilled H2O, and mRNA enrichment was performed using the Oligo-tex mRNA kit (Invitrogen). A first strand cDNA library was constructed using the Superscript First-Strand Synthesis System for reverse transcription-PCR (Invitrogen).
5′ and 3′ RACE Experiments and PCR
A 5′ RACE experiment was performed on the total RNA sample with the 5′ RACE System for Rapid Amplification of cDNA Ends kit (Invitrogen) using the nested primers 5R1: 5′-CTGAAGCCAGACCTCTGGAA-3′, 5R2: 5′-CCGTGCAGGATGAGGCTCTCA GGAATGT-3′, and 5R3: 5′-CCCTCGCTCCTGGACACCAAGCA-3′. A 3′ RACE experiment was performed on the total RNA sample with the 3′ RACE System for Rapid Amplification of cDNA Ends kit (Invitrogen) using the nested primers 3R1: 5′-AAGCCCTTATCCGTTCCACCCGCC-3′, 3R2: 5′-TGCTTGGTGTCCAGGAGCGAGGG-3′, and 3R3: 5′-ACATTCCTGAGAGCCTCATCCTGCAC-3′. Polymerase chain reaction was performed on the chicken liver cDNA library using the primers CHXF: 5′-GCGGCC GCATGGCGCGGAGGTTTGCGTTGTTC-3′ and CHXR: 5′-GCGGCCGCTCACAGCCGGGATACCC TCAGCATG-3′ and Pfu polymerase (Stratagene, La Jolla, CA) according to standard technique (Sambrook and Russell, 2001). The clone was inserted into the vector pCR-Blunt using the Zero Blunt PCR cloning kit (Invitrogen).
Baculovirus Expression
Baculovirus construction was performed using the Bac-to-Bac Baculovirus Expression System (Invitrogen). The Sf21 insect cells (Invitrogen) were used to amplify the virus. Baculovirus titer was determined using the BD BakPAK Baculovirus Rapid Titer kit (BD Biosciences Clontech, Palo Alto, CA). A 100-mL culture of Trichoplusia ni cells were infected at 0.1 multiplicity of infection (MOI) and incubated for 1 h at 28°C; then, 400 mL of ESF921 media (Expression Systems, Woodland, CA) supplemented with 1× penicillin-streptomycin solution (Sigma-Aldrich, St. Louis, MO) were added to the infected cells, and the culture was incubated for 72 h at 28°C.
Protein Purification
Infected T. ni cells (250 mL) were pelleted and resuspended in phosphate buffer with 10 mM imidazole. The cells were homogenized with an Ultra-Turax T25 homogenizer (IKA Works, Wilmington, NC) at 17,500 rpm for three 30-s intervals with 15 s of rest on ice between each grinding. The homogenate was centrifuged at 100,000 × g for 1 h at 4°C. Ni-NTA HisBind Resin (0.3 mL; EMD Biosciences, Inc., Madison, WI) was rinsed with 25 mL of 10 mM phosphate buffer (pH 7.4). The supernatant was then gently mixed with the resin at 4°C for 1 h. The supernatant and resin mixture was poured into a 25-mL Biorad disposable column (Biorad, Hercules, CA) and allowed to drain by gravity flow. The column was then washed with 45 mL of phosphate buffer containing 60 mM imidazole at a rate of 1 mL/min. The bound protein was eluted with 5 mL of phosphate buffer containing 250 mM imidazole at a rate of 1 mL/min. The eluant was concentrated to 1.5 mL on a 30-kDa cut Centricon centrifugation filtration device (Millipore, Billerica, MA). The concentrated sample was applied to a 5-mL desalting column (Amersham, Piscataway, NJ) and eluted in 2 mL of 25 mM Tris-HCL (pH 7.5). Aliquots (100 μL) were frozen in liquid nitrogen and stored at −80°C for future use.
Extract Preparation
Fresh chicken liver was obtained from a 3-wk-old male Cobb × Cobb broiler fed a corn/soy broiler starter diet that met NRC requirements. Liver was cut in small pieces and suspended in 40 mL of 20 mM sodium phosphate buffer at 4°C (pH 7.4) containing 5 mM EDTA and 1 mM phenylmethylsulfonyl fluoride and dl-dithiothreitol. The suspension was homogenized with a Polytron grinder rotating at 9,000 rpm for 1 min. The homogenate was centrifuged at 10,000 × g for 20 min at 4°C. The supernatant was then centrifuged at 100,000 × g for 60 min at 4°C. The supernatant (the cytosol) was frozen at −80°C until used as enzyme extract.
Protein Analysis
Protein concentration measurements were made using the BCA assay (Pierce, Rockford, IL) with BSA fraction V protein (Sigma-Aldrich) to derive a standard curve. Polyacrylamide gel electrophoresis was performed using Novex precast polyacrylamide gels (Invitrogen) for both SDS-PAGE analysis and isoelectric focusing. The SDS-PAGE gels were stained with Coomassie Brilliant Blue. Bands from isoelectric focusing gels (3 to 10 pH) were excised and tested for epoxide hydrolase activity using the radioactive epoxide hydrolase assay described subsequently. Protein purity was estimated from a SDS-PAGE gel stained with Coomassie Brilliant Blue with the public domain ImageJ software v1.33 (http://rsb.info.nih.gov/ij/).
Radiotracer Based Epoxide Hydrolase Activity Assay
Epoxide hydrolase activity was measured using racemic [3H]-trans-1,3-diphenylpropene oxide (t-DPPO) as substrate (Borhan et al., 1995). The t-DPPO was previously synthesized and purified (Borhan et al., 1995). One microliter of a 5 mM solution of [3H]t-DPPO in dimethylformamide was added to 100 μL of enzyme preparation in sodium phosphate buffer (0.1 M; pH 7.4) containing 0.1 mg/mL of BSA ([S]final = 50 μM). The enzyme was incubated at 30°C for 10 min, and the reaction was quenched by the addition of 60 μL of methanol and 200 μL of isooctane, which extracts the remaining epoxide from the aqueous phase. Extractions with 1-hexanol were performed in parallel to assess the possible presence of glutathione transferase activity, which could also transform the substrate (Borhan et al., 1995). The activity was followed by measuring the quantity of radioactive diol formed in the aqueous phase using a scintillation counter (Wallac Model 1409, Gaithersburg, MD). Assays were performed in triplicate.
IC50 Determination
The IC50 values reported herein were determined using racemic [3H]t-DPPO as a substrate (Borhan et al., 1995). The inhibitors were synthesized as described (Morisseau et al., 2002). Extracts of broiler hepatic cytosol or partially purified enzyme was diluted 50-fold in pH 7.4, 0.1 M sodium phosphate buffer containing 0.1 mg/mL of BSA and then incubated with the inhibitors for 5 min at 30°C prior to substrate introduction. Prepared samples were incubated at 30°C for 10 min and stopped as indicated previously. Conditions used gave rates that were linear, both with time and enzyme concentration. Assays were performed in triplicate. By definition, IC50 is the concentration of inhibitor that reduces enzyme activity by 50%. IC50 was determined by regression with a minimum of 2 points in the linear region of the curve on either side of the IC50 (for a total of 5 points). The curve was generated from at least 3 separate studies conducted in triplicate to obtain the standard deviation given in the results section.
Determination of Kinetic Parameters
A solution of [3H]t-DPPO (1 μL in dimethylformamide) was added to 100 μL of enzyme preparation in sodium phosphate buffer (0.1 M; pH 7.4) containing 0.1 mg/mL of BSA ([S]final = 50 μM). The Km determination was performed by fitting the data to the Michaelis-Menten equation using the nonlinear regression algorithm in SigmaPlot (SPSS, Inc., Chicago, IL) with an R2 value of ≥0.97. The standard deviation was generated by performing the experiment 3 separate times in triplicate.
Synthesis and Purification of EET Regioisomers
The EET isomeric mixture (8,9-, 11,12-, and 14,15-EET) was synthesized from arachidonic acid methyl ester by a previously described method (Newman et al., 2002; Smith et al., 2005). The mixture was separated to 14,15- EET fraction (tR = 24.7 min) and 8,9- and 11,12-EET mixture fraction (tR = 29.5 min) with reverse-phase preparative HPLC (C18, 22 × 250 mm) at a flow rate of 18 mL/min [75% of solvent A in solvent B; solvent A: acetonitrile-water-methanol, 51:40:9 (vol/vol/vol) with 0.01% formic acid and solvent B: acetonitrile-methanol, 85:15 (vol/vol) with 0.01% formic acid]. The mixture of 8,9-and 11,12-EET was separated to 8,9- EET fraction and 11,12-EET fraction with normal-phase preparative HPLC (silica, 22 × 250 mm) at a flow rate of 18 mL/min using 1% iso-propanol in n-hexane. Each fraction was finally purified with normal-phase HPLC with the same conditions described as previous to yield pure each isomer (8,9-EET: tR = 17.3 min, 11,12-EET: tR = 13.5 min, 14,15-EET: tR = 11.2 min).
Non-Radioactive Epoxide Hydrolase Assays
The trans-9,10-epoxystearate was purchased (Sigma-Aldrich). A 5 mM solution of each substrate was made in ethanol for the EET and methanol for the epoxystearate. The substrate solution (1 μL) was added to 100 μL of enzyme preparation in sodium phosphate buffer (0.1 M; pH 7.4) containing 0.1 mg/mL of BSA ([S]final = 50 μM). The enzyme was incubated with the substrate at 30°C for 10 min, and the reaction was quenched by addition of 400 μL of methanol. The products were analyzed by HPLC-MS/MS as previously described (Newman et al., 2002) with the following exceptions. A 2.0-mm × 20-mm, 3-μm Luna C18 Mercury MS column (Phenomenex, Torrance, CA) was used with a 350-μL/min isocratic flow of 68:28:11 (vol/vol/vol) acetonitrile/water/methanol with 0.1% glacial acetic acid for 2.5 min. Assays were performed in triplicate.
Phosphatase Assay
The phosphatase substrate, threo-9,10-phosphonooxy-hydroxy-octadecanoic acid, was synthesized as previously described (Newman et al., 2003). The assay was performed and analyzed by HPLC-MS/MS as described by Newman et al. (2003).
RESULTS AND DISCUSSION
This study reports the cloning, expression, and characterization of a homolog of sEH in chicken. Sequence fragments homologous to reported mammalian cDNA sequences were discovered in 2 expressed sequence tag databases. Sequences corresponding to the N-terminal region of mammalian sEH were found in the University of Delaware ChikEST database at http://www.chickest.udel.edu/ (Clone IDs: pgf2n.pk002.g24, pgf2n.pk001.d21, and pgl1n.pk005.l13), and sequences corresponding to the C-terminal region of mammalian sEH were found in the Biotechnology and Biological Sciences Research Council ChikEST database (template ID: 341537.2; http://www.chick.umist.ac.uk/; Boardman et al., 2002). Primers for 5′ and 3′ rapid amplification of cDNA ends (RACE) experiments were designed based on these expressed sequence tag sequences. The RACE experiments indicated that these fragments came from a single cDNA sequence. The primers CHXF and CHXR were designed based on the predicted 5′ and 3′ end of this cDNA sequence, and a 1686 base cDNA was cloned from chicken liver (Figure 1).
Figure 1.
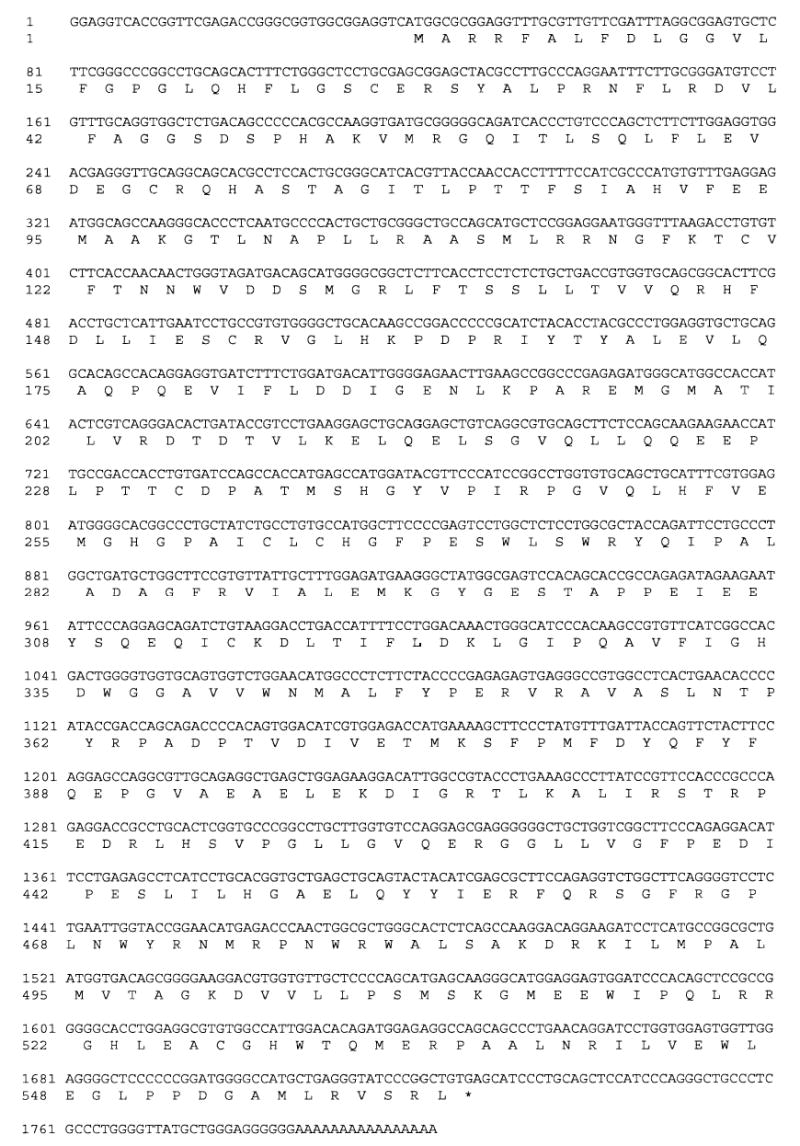
Nucleotide sequence and translated protein sequence of the chicken soluble epoxide hydrolase cDNA. This DNA sequence has accession number DQ120010 in the Genbank database.
This sequence was used to probe the first draft chicken genome assembly determined by whole genome shotgun at the Genome Sequencing Center at Washington University, St. Louis. The majority of the cDNA sequence is located on Chromosome 3 of the chicken genome. The first 100 base pairs of the cDNA sequence are located in unplaced sequences of the chicken genome. These 100 bases are contiguous and so may comprise the first exon of the gene. The last 92 base pairs of the cDNA sequence were not located in this draft of the chicken genome. There is a gap in the genome downstream from the last predicted exon of the sEH homolog gene, and it is possible that the missing base pairs fall within this gap. This will be discussed further after a closer examination of the sequence identities between the translated sequence from chicken and reported mammalian sEH sequences.
The nucleotide sequence of this clone displays a high degree of homology to mammalian and frog sEH sequences when aligned by the University of Virginia software LALIGN (http://fasta.bioch.virginia.edu/fasta/align.htm; data not shown). It displays a 62.2% identity to human sEH, a 60.5% identity to mouse sEH, and a 63.7% identity to frog sEH. The translated sequence is 51% identical to the human sequence, 50.8% identical to mouse sEH, and 62.3% identical to frog sEH. Alignment of the amino acid sequences reveals a number of important structural similarities between the chicken and the human enzyme (Figure 2). The mammalian sEH epoxide hydrolase catalytic triad is composed of a catalytic nucleophile, a histidine, and an orienting acid. In the human enzyme, this triad is represented by Asp334, His523, and Asp495 (Morisseau and Hammock, 2005). The chicken enzyme preserves the identity and spacing of these residues (marked with an arrow in Figure 2). Two tyrosines (Tyr382 and Tyr465) polarize the epoxide in the human enzyme (Morisseau and Hammock, 2005). These residues and their approximate spacing are also conserved in the chicken enzyme as Tyr383 and Tyr471, respectively (marked by the circle in Figure 2).
Figure 2.

Alignment of the chicken soluble epoxide hydrolase (sEH) with human, mouse, and frog sEH. The mammalian epoxide hydrolase “catalytic triad” residues are marked by arrows. Residues that polarize the epoxide moiety of the epoxide hydrolase substrate are marked by circles. The catalytic nucleophile of the sEH phosphatase activity is marked by a triangle.
In addition to this epoxide hydrolase catalytic site, there is a second catalytic site on the N-terminal region of the mammalian enzyme that has been shown to display phosphatase activity (Cronin et al., 2003; Newman et al., 2003). The crystal structure of human sEH has implicated Asp11 and Arg99 as having roles in this phosphatase activity through their involvement in the coordination of a magnesium atom in the active site (Gomez et al., 2004). These residues are not conserved in the chicken enzyme (marked by the triangles in Figure 2).
As mentioned previously, the last 92 base pairs of the cDNA transcript were not found in the first draft of the chicken genome. This region of the transcript encodes for residues important for epoxide hydrolase activity in mammalian enzymes. Specifically, an enzyme lacking these residues would not possess the histidine that aligns with His523 in the mouse sequence. Mutation of this residue in the mouse sEH abolishes epoxide hydrolase activity (Pinot et al., 1995). The recombinant protein possesses epoxide hydrolase activity, providing evidence that the transcript represents the correct sequence of the chicken sEH homolog and was not the result errors introduced during cloning.
Both the specific and general homology between the mammalian and chicken enzymes suggest that the gene cloned is a chicken sEH homolog. A 6 histidine tag was encoded on the 3′ end of the construct for purification purposes. Recombinant enzyme was then produced to see whether the transcript encoded for a protein with epoxide hydrolase activity.
The tagged construct was expressed in a baculovirus expression system and purified on a nickel chelation column to a maximum purity of 75% (Figure 3). Radioactively labeled t-DPPO was chosen as the initial substrate to test for epoxide hydrolase activity (Borhan et al., 1995). The resulting purified recombinant enzyme was found to have a lower specific activity than either mouse or human sEH when assayed for epoxide hydrolase activity using t-DPPO. The specific activity of the chicken enzyme was approximately 20 times lower than values previously reported for mouse and 5 times lower than values reported for human (Table 1). It was possible that the histidine tag added to the recombinant enzyme interfered with the epoxide hydrolase activity. It was decided to test the effect of the tag on epoxide hydrolase activity by examining the pattern of inhibition of epoxide hydrolase activity in chicken liver crude extract compared with the purified recombinant enzyme.
Figure 3.
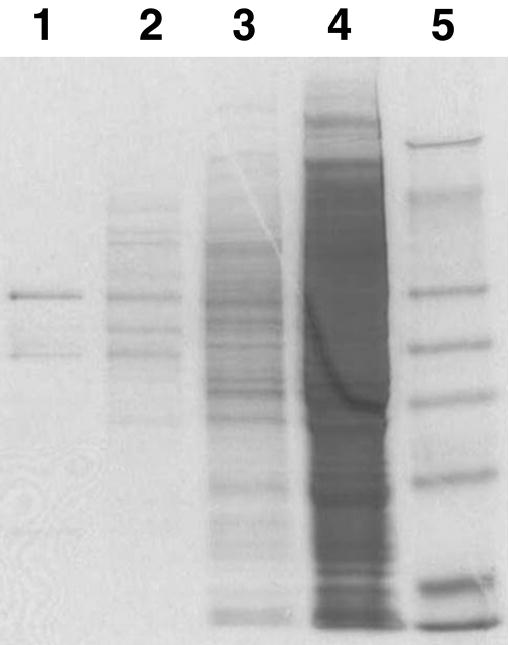
SDS-PAGE analysis of recombinant chicken soluble epoxide hydrolase (sEH) purification. Samples were run on a Novex precast 12% Tris-Glycine gel (Invitrogen, Carlsbad, CA) and stained with Coomassie Brilliant Blue. Lane 1: 1 μg of 250 mM imidazole eluant. Lane 2: 7 μg of 50 mM imidazole wash. Lane 3: 30 μg of unbound fraction. Lane 4: 30 μg of 100,000-g supernatant. Lane 5: 5 μL of SeeBlue Plus 2 (Invitrogen, Carlsbad, CA) molecular weight marker.
Table 1.
Kinetic parameters using trans-1,3-diphenylpropene oxide (t-DPPO) as a substrate
| Structure of t-DPPO | Kinetic parameter | Recombinant chicken sEH1 | Recombinant mouse sEH | Recombinant human sEH |
|---|---|---|---|---|
|
|
Specific activity (nmol/min per mg) | 823.1 ± 27.3 | 17,000 ± 300.0 | 4,500 ± 200.0 |
| Km (μM) | 25.3 ± 0.9 | 4.3 ± 0.6 | 6.2 ± 0.6 | |
| kcat (/s) | 0.9 ± 0.03 | 18.0 ± 0.3 | 4.3 ± 0.3 | |
| kcat/Km(μM/s) | 0.04 | 4.2 | 0.7 |
Recombinant chicken soluble epoxide hydrolase (sEH) was partially purified as described. Assay conditions are described in the Materials and Methods section. Results are presented as the means ± standard deviations of 3 separate experiments. Values for the human and mouse enzyme are from Morisseau et al. (2000).
Six inhibitors were chosen that have been shown to have high, moderate, and low IC50 when tested against human enzyme (Figure 4) (Morisseau et al., 2002). The t-DPPO activity was used as a measure of epoxide hydrolase activity for both the crude extract and purified recombinant enzyme. It was found that the recombinant enzyme possessed the same relative response to inhibition as the chicken liver crude extract, giving evidence that the tag did not interfere with epoxide hydrolase activity. In some cases the IC50 values obtained with the cytosol were different than the values obtained with the purified recombinant enzyme, for example, with N,N′-dicyclohexylurea (DCU). This difference could be due to a number of factors. The purified protein solution lacks enzymes that might bind or degrade the inhibitor. This purified prep also lacks protiens or small molecules that might interact with sEH and modulate the catalytic activity of the enzyme. For these reasons, some difference in IC50 values between the cytosol and purified recombinant enzyme can be expected.
Figure 4.
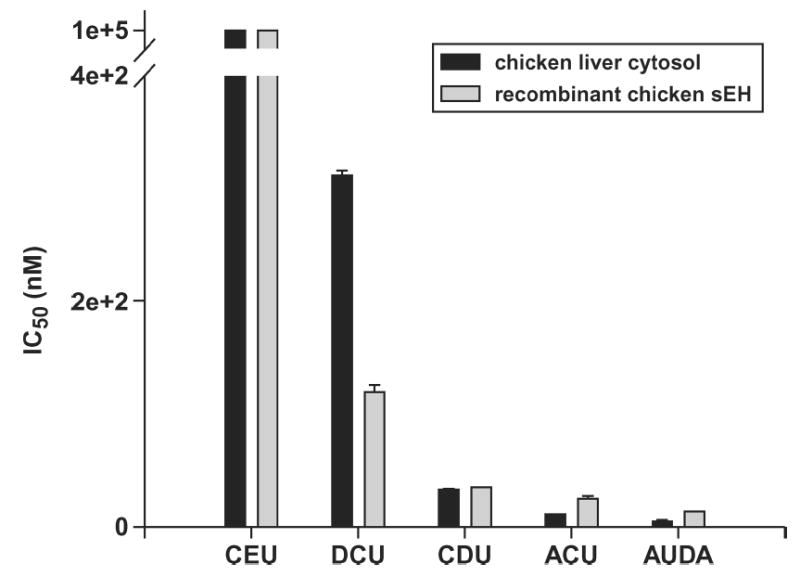
IC50 values for the urea-based inhibitors N-cyclohexyl-N′-ethylurea (CEU), N,N′-dicyclohexylurea (DCU), N-cyclohexyl-N′-dodecylurea (CDU), N-adamantyl-N′-cyclohexylurea (ACU), and 12-(3-adamantane-1-yl-ureido)-dodecanoic acid (AUDA). Recombinant chicken soluble epoxide hydrolase (sEH) was partially purified as described. Assay conditions are described in the Materials and Methods section. Error bars represent the standard deviations.
Of the inhibitors tested with the recombinant enzyme, 12-(3-adamantane-1-yl-ureido)-dodecanoic acid (AUDA) was the most potent. It possessed an IC50 of 13.7 nM when assayed with the recombinant enzyme. This indicates that this urea-based inhibitor may be a good choice for in vivo inhibition of the chicken enzyme.
Because the histidine tag did not interfere with epoxide hydrolase activity, the reduced activity of the chicken enzyme is probably due to structural differences between the mammalian and chicken sEH. The spacing between catalytic triad residues is highly conserved among mammalian enzymes (Beetham et al., 1995). The distance between the catalytic aspartate and the orienting acid is 160 to 161 residues in rat, mouse, and human sEH. This distance in the chicken sEH is 165 residues. It is possible that this difference in spacing is responsible for the attenuated epoxide hydrolase activity in the chicken enzyme.
To determine whether the identified sEH homolog was responsible for the majority of the epoxide hydrolase activity detected in chicken crude extract, purified recombinant enzyme and chicken liver crude extract were run side by side on an isoelectric focusing gel. Each lane was cut into 0.5- and 0.2-cm bands and assayed for t-DPPO activity. All of the recovered activities in both the purified recombinant and liver crude extract lanes were located in single 0.2-cm bands corresponding to the isoelectric point range of 6.0 to 6.2 (data not shown).
The purified recombinant enzyme has an experimentally determined molecular weight of 63 kDa and an isoelectric point of 6.1 (data not shown). When assayed with t-DPPO, the enzyme displays maximal epoxide hydrolase activity around pH 7.4 (data not shown). The half-life of epoxide hydrolase activity is >6 d when the enzyme is kept at 4°C. The half-life at 25°C is between 9 and 24 h, and the half-life at 37°C is <3 h (data not shown).
The kcat and Km of the enzyme for t-DPPO was then determined. The chicken enzyme has a higher Km and lower kcat for t-DPPO than either the recombinant mouse or human sEH (Table 1). Examining the kcat:Km, it was found that t-DPPO was not a good substrate for the chicken enzyme compared with the mouse or human enzyme, having a value of 100- and 20-fold lower when compared with the mouse or human enzymes, respectively (Table 1). The t-DPPO is not an endogenous substrate for the mammalian sEH. It does not possess the long alkyl chain present in proposed endogenous fatty acid substrates of the mammalian enzymes, such as the EET (Table 1). For this reason, the chicken enzyme was tested for epoxide hydrolase activity using a number of the EET, as well as the fatty acid epoxide trans-9,10-epoxystearate.
The avian enzyme did not hydrolyze any EET or the epoxystearate substrate as readily as it did t-DPPO (Tables 1 and 2). The enzyme showed the least activity toward cis-9,10-epoxystearate. The enzyme hydrolyzed the EET at higher rates; 14,15-EET was the best substrate (Table 2), albeit the activity was <1/30th the activity measured with t-DPPO. The enzyme hydrolyzed 11,12-and 8,9-EET at nearly equal rates (Table 2). Both the mouse and chicken enzymes hydrolyze 14,15- EET at over twice the rate of 11,12- and 8,9-EET (Table 2).
Table 2.
Specific activity of substrates
| Specific activity (nmol/min per mg)
|
|||
|---|---|---|---|
| Compound name | Structure | Recombinant chicken sEH1 | Recombinant mouse* and mouse |
| cis-9,10-epoxystearic acid |
|
3.1 ± 0.2 | 1,139 ± 34* |
| 14,15-EET |
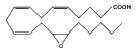
|
24.5 ± 2.1 | 1,260.0 |
| 11,12-EET |
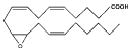
|
12.0 ± 1.2 | 640.0 |
| 8,9-EET |
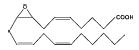
|
11.6 ± 0.1 | 370.0 |
Recombinant chicken soluble epoxide hydrolase (sEH) was partially purified as described. Assay conditions are described in the Materials and Methods section. Results are presented as the means ± standard deviations of 3 separate experiments. Values for the mouse enzyme assayed with cis-9,10-epoxystearic are from Morisseau et al. (2000). Values for the mouse enzyme assayed with the EET (epoxyeicosatrienoic acid) are from Chacos et al. (1983).
Although residues thought to be important to the mammalian sEH phosphatase activity were not conserved in the chicken sEH, this activity has a potential role in the regulation of blood pressure (Arand et al., 2003). For this reason, the phosphatase activity of the chicken enzyme was assayed using the substrate threo-9,10-phosphonooxy-hydroxy-octadecanoic acid. The chicken enzyme did not hydrolyze this substrate under the conditions developed for the mammalian enzyme phosphatase activity assay.
The EET are important endothelial-derived vasoactive signaling molecules in mammals. The mammalian sEH has been shown to convert the EET to their corresponding diols, the DHET. Through this epoxide hydrolase activity, the mammalian sEH plays a role in blood pressure regulation. In this study, a chicken sEH homolog was identified, and the epoxide hydrolase activity of the recombinant enzyme was assayed using the EET and other substrates of the mammalian enzyme. It was found that the chicken enzyme had similar activities to the mammalian enzymes. It was also found that the recombinant enzyme was inhibited by a number of urea-based inhibitors. The AUDA was the most potent of these inhibitors and could be used to inhibit the enzyme in vivo. This would be a valuable tool to probe the role of sEH in endothelial-derived vasodilation in chicken. In particular, a sEH inhibitor could be used in chicken models of PH, where it is believed that endothelial-derived vasodilation has been impaired.
Acknowledgments
The authors thank Nick Anthony and Kirk Klasing. Funding was provided by NIEHS Grant R37 ES02710; NIEHS Superfund Basic Research Program Grant P42 ES04699; NIEHS Advanced Training in Environmental Toxicology Grant T32 ES007059; Texas Agricultural Experiment Station Project #8738; Center for Environmental and Rural Health P30ES09106; Center for Nutrition, Health, and Food Genomics (TAMU); and the Jastro-Shields Graduate Research Scholarship.
References
- Arand M, Cronin A, Oesch F, Mowbray SL, Jones TA. The telltale structures of epoxide hydrolases. Drug Metab Rev. 2003;35:365–383. doi: 10.1081/dmr-120026498. [DOI] [PubMed] [Google Scholar]
- Beetham JK, Grant D, Arand M, Garbarino J, Kiyosue T, Pinot F, Belknap WR, Shinozaki K, Hammok BD. Gene evolution of epoxide hydrolases and recommended nomenclature. DNA Cell Biol. 1995;14:61–71. doi: 10.1089/dna.1995.14.61. [DOI] [PubMed] [Google Scholar]
- Boardman PE, Sanz-Ezquerro J, Overton IM, Burt DW, Bosch E, Fong WT, Tickle C, Brown WR, Wilson SA, Hubbard SJ. A comprehensive collection of chicken cDNAs. Curr Biol. 2002;12:1965–1969. doi: 10.1016/s0960-9822(02)01296-4. [DOI] [PubMed] [Google Scholar]
- Borhan B, Mebrahtu T, Nazarian S, Kurth MJ, Hammock BD. Improved radiolabeled substrates for soluble epoxide hydrolase. Anal Biochem. 1995;231:188–200. doi: 10.1006/abio.1995.1520. [DOI] [PubMed] [Google Scholar]
- Chacos N, Capdevila J, Falck JR, Manna S, Martin-Wixtrom C, Gill SS, Hammock BD, Estabrook RW. The reaction of arachidonic acid epoxides (epoxyeico-satrienoic acids) with a cytosolic epoxide hydrolase. Arch Biochem Biophys. 1983;223:639–648. doi: 10.1016/0003-9861(83)90628-8. [DOI] [PubMed] [Google Scholar]
- Cronin A, Mowbray S, Durk H, Homburg S, Fleming I, Fisslthaler B, Oesch F, Arand M. The n-terminal domain of mammalian soluble epoxide hydrolase is a phosphatase. Proc Natl Acad Sci USA. 2003;100:1552–1557. doi: 10.1073/pnas.0437829100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Kaduce TL, Weintraub NL, Harmon S, Teesch LM, Morisseau C, Thompson DA, Hammock BD, Spector AA. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells. Implications for the vascular effects of soluble epoxide hydrolase inhibition. J Biol Chem. 2001;276:14867–14874. doi: 10.1074/jbc.M011761200. [DOI] [PubMed] [Google Scholar]
- Fang X, Weintraub NL, McCaw RB, Hu S, Harmon SD, Rice JB, Hammock BD, Spector AA. Effect of soluble epoxide hydrolase inhibition on epoxyeicosatrienoic acid metabolism in human blood vessels. Am J Physiol Heart Circ Physiol. 2004;287:H2412–H2420. doi: 10.1152/ajpheart.00527.2004. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D, Harder DR, Pratt PF, Campbell WB. Bioassay of an endothelium-derived hyperpolarizing factor from bovine coronary arteries: Role of a cytochrome P450 metabolite. J Vasc Res. 1998;35:274–284. doi: 10.1159/000025594. [DOI] [PubMed] [Google Scholar]
- Gomez GA, Morisseau C, Hammock BD, Christianson DW. Structure of human epoxide hydrolase reveals mechanistic inferences on bifunctional catalysis in epoxide and phosphate ester hydrolysis. Biochemistry. 2004;43:4716–4723. doi: 10.1021/bi036189j. [DOI] [PubMed] [Google Scholar]
- Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- Julian RJ. Ascites in poultry. Avian Pathol. 1993;22:419–454. doi: 10.1080/03079459308418934. [DOI] [PubMed] [Google Scholar]
- Martinez-Lemus LA, Hester RK, Becker EJ, Jeffrey JS, Odom TW. Pulmonary artery endothelium-dependent vasodilation is impaired in a chicken model of pulmonary hypertension. Am J Physiol. 1999;277:R190–R197. doi: 10.1152/ajpregu.1999.277.1.R190. [DOI] [PubMed] [Google Scholar]
- Martinez-Lemus LA, Hester RK, Becker EJ, Ramirez GA, Odom TW. Pulmonary artery vasoactivity in broiler and leghorn chickens: An age profile. Poult Sci. 2003;82:1957–1964. doi: 10.1093/ps/82.12.1957. [DOI] [PubMed] [Google Scholar]
- Morisseau C, Beetham JK, Pinot F, Debernard S, Newman JW, Hammock BD. Cress and potato soluble epoxide hydrolases: Purification, biochemical characterization, and comparison to mammalian enzymes. Arch Biochem Biophys. 2000;378:321–332. doi: 10.1006/abbi.2000.1810. [DOI] [PubMed] [Google Scholar]
- Morisseau C, Goodrow MH, Newman JW, Wheelock CE, Dowdy DL, Hammock BD. Structural refinement of inhibitors of urea-based soluble epoxide hydrolases. Biochem Pharmacol. 2002;63:1599–1608. doi: 10.1016/s0006-2952(02)00952-8. [DOI] [PubMed] [Google Scholar]
- Morisseau C, Hammock BD. Epoxide hydrolases: Mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol. 2005;45:311–333. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- Nakai K, Ward AM, Gannon M, Rifkind AB. Beta-naphthoflavone induction of a cytochrome P450 arachidonic acid epoxygenase in chick embryo liver distinct from the aryl hydrocarbon hydroxylase and from phenobarbital-induced arachidonate epoxygenase. J Biol Chem. 1992;267:19503–19512. [PubMed] [Google Scholar]
- Newman JW, Morisseau C, Harris TR, Hammock BD. The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proc Natl Acad Sci USA. 2003;100:1558–1563. doi: 10.1073/pnas.0437724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JW, Watanabe T, Hammock BD. The simultaneous quantification of cytochrome P450 dependent linoleate and arachidonate metabolites in urine by HPLC-MS/MS. J Lipid Res. 2002;43:1563–1578. doi: 10.1194/jlr.d200018-jlr200. [DOI] [PubMed] [Google Scholar]
- Odom TW, Martinez-Lemus LA, Hester RK, Becker EJ, Jeffrey JS, Meininger GA, Ramirez GA. In vitro hypoxia differentially affects constriction and relaxation responses of isolated pulmonary arteries from broiler and leghorn chickens. Poult Sci. 2004;83:835–841. doi: 10.1093/ps/83.5.835. [DOI] [PubMed] [Google Scholar]
- Pinot F, Grant DF, Beetham JK, Parker AG, Borhan B, Landt S, Jones AD, Hammock BD. Molecular and biochemical evidence for the involvement of the Asp-333-His-523 pair in the catalytic mechanism of soluble epoxide hydrolase. J Biol Chem. 1995;270:7968–7974. doi: 10.1074/jbc.270.14.7968. [DOI] [PubMed] [Google Scholar]
- Roman RJ. P450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Rosolowsky M, Campbell WB. Role of PGI2 and epoxyeicosatrienoic acids in relaxation of bovine coronary arteries to arachidonic acid. Am J Physiol. 1993;264:H327–H335. doi: 10.1152/ajpheart.1993.264.2.H327. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., and D. W. Russell. 2001. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Smith KR, Pinkerton KE, Watanabe T, Pedersen TL, Ma SJ, Hammock BD. Attenuation of tobacco smoke-induced lung inflammation by treatment with a soluble epoxide hydrolase inhibitor. Proc Natl Acad Sci USA. 2005;102:2186–2191. doi: 10.1073/pnas.0409591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamor E, Ruijtenbeek K, Pulgar V, De Mey JG, Blanco CE. Vascular reactivity in intrapulmonary arteries of chicken embryos during transition to ex ovo life. Am J Physiol Regul Integr Comp Physiol. 2002;282:R917–R927. doi: 10.1152/ajpregu.00369.2001. [DOI] [PubMed] [Google Scholar]
- Weintraub NL, Fang X, Kaduce TL, VanRollins M, Chatterjee P, Spector AA. Epoxide hydrolases regulate epoxyeicosatrienoic acid incorporation into coronary endothelial phospholipids. Am J Physiol. 1999;277:H2098–H2108. doi: 10.1152/ajpheart.1999.277.5.H2098. [DOI] [PubMed] [Google Scholar]
- Wideman RF. Cardio-pulmonary hemodynamics and ascites in broiler chickens. Avian Poult Rev. 2000;11:21–43. [Google Scholar]
- Wideman RF, Chapman ME. N(omega)-nitro-l-arginine methyl ester (L-NAME) amplifies the pulmonary hypertensive response to endotoxin in broilers. Poult Sci. 2004;83:485–494. doi: 10.1093/ps/83.3.485. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Jr, Erf GF, Chapman ME. Intravenous endotoxin triggers pulmonary vasoconstriction and pulmonary hypertension in broiler chickens. Poult Sci. 2001;80:647–655. doi: 10.1093/ps/80.5.647. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Jr, Kirby YK, Ismail M, Bottje WG, Moore RW, Vardeman RC. Supplemental L-arginine attenuates pulmonary hypertension syndrome (ascites) in broilers. Poult Sci. 1995;74:323–330. doi: 10.3382/ps.0740323. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Jr, Maynard P, Bottje WG. Thromboxane mimics the pulmonary but not systemic vascular responses to bolus HCl injections in broiler chickens. Poult Sci. 1999;78:714–721. doi: 10.1093/ps/78.5.714. [DOI] [PubMed] [Google Scholar]
- Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87:992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- Zeldin DC, Kobayashi J, Falck JR, Winder BS, Hammock BD, Snapper JR, Capdevila JH. Regio-and enantiofacial selectivity of epoxyeicosatrienoic acid hydration by cytosolic epoxide hydrolase. J Biol Chem. 1993;268:6402–6407. [PubMed] [Google Scholar]
- Zeldin DC, Plitman JD, Kobayashi J, Miller RF, Snapper JR, Falck JR, Szarek JL, Philpot RM, Capdevila JH. The rabbit pulmonary cytochrome P450 arachidonic acid metabolic pathway: characterization and significance. J Clin Invest. 1995;95:2150–2160. doi: 10.1172/JCI117904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Oltman CL, Lu T, Lee HC, Dellsperger KC, VanRollins M. EET homologs potently dilate coronary microvessels and activate BK(Ca) channels. Am J Physiol Heart Circ Physiol. 2001;280:H2430–H2440. doi: 10.1152/ajpheart.2001.280.6.H2430. [DOI] [PubMed] [Google Scholar]
- Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K(+)-channel activity. Am J Physiol. 1996;270:F822–F832. doi: 10.1152/ajprenal.1996.270.5.F822. [DOI] [PubMed] [Google Scholar]


