Abstract
Malaria parasite infection in anopheline mosquitoes is limited by inflammatory levels of nitric oxide metabolites. To assess the mechanisms of parasite stasis or toxicity, we investigated the biochemistry of these metabolites within the blood-filled mosquito midgut. Our data indicate that nitrates, but not nitrites, are elevated in the Plasmodium-infected midgut. Although levels of S-nitrosothiols do not change with infection, blood proteins are S-nitrosylated after ingestion by the mosquito. In addition, photolyzable nitric oxide, which can be attributed to metal nitrosyls, is elevated following infection and, based on the abundance of hemoglobin, likely includes heme iron nitrosyl. The persistance of oxyhemoglobin throughout blood digestion and changes in hemoglobin conformation in response to infection suggest that hemoglobin catalyzes the synthesis of nitric oxide metabolites in a reducing environment. Provision of urate, a potent reductant and scavenger of oxidants and nitrating agents, as a dietary supplement to mosquitoes increased parasite infection levels relative to allantoin-fed controls, suggesting that nitrosative and/or oxidative stresses negatively impact developing parasites. Collectively, our results reveal a unique role for nitric oxide in an oxyhemoglobin-rich environment. In contrast to facilitating oxygen delivery by hemoglobin in the mammalian vasculature, nitric oxide synthesis in the blood-filled mosquito midgut drives the formation of toxic metabolites that limit parasite development.
Keywords: malaria, Plasmodium, mosquito, immunity, Anopheles, nitric oxide, hemoglobin
ABBREVIATIONS: AP, alkaline phosphatase; AsNOS, Anopheles stephensi nitric oxide synthase; DTT, dithiothreitol; GSNO, S-nitrosoglutathione; ICR, Institute of Cancer Research; I, malaria parasite-infected; kD, kilodalton; metHb, deoxygenated hemoglobin; NAME, NG-nitro-L-arginine methyl ester; NB, non-bloodfed; NOx, nitrogen oxides; NTYR, nitrotyrosine; oxyHb, oxygenated hemoglobin; pBM, post-bloodmeal or post-bloodfeeding; PN, peroxynitrite; RNNOs, N-nitroso compounds; ROS, reactive oxygen species; PAGE, polyacrylamide gel electrophoresis; SEM, standard error of the mean; SNAP, S-nitroso-N-acetylpenicillamine; SNO, S-nitrosothiol; U, uninfected
INTRODUCTION
The mosquito Anopheles stephensi, a primary vector of human malaria in India and the Middle East, possesses a single copy of an ·NO synthase gene (AsNOS) that is inducible by malaria parasite (Plasmodium spp.) infection [1, 2]. Parasite development in the mosquito begins with the ingestion of blood containing sexual-stage gametocytes. Mobile zygotes or ookinetes penetrate the midgut epithelium 24–36h later and transform into vegetative oocysts under the basal lamina in the hemolymph-filled, open circulatory system of the mosquito. Oocysts grow and develop for 10–12d, then release thousands of haploid sporozoites which invade the salivary glands and are released into the saliva during subsequent blood feeding.
During malaria parasite infection, induction of AsNOS expression in the midgut is biphasic, with >2-fold inductions at 6h and 36–48h after feeding [2], times associated with parasite development in the blood mass prior to invasion and after ookinete invasion of the midgut epithelium, respectively. Induction of NOS expression has been correlated to ookinete invasion as revealed by diaphorase staining [1] and immunofluorescence [3]. Synthesis of ·NO limits parasite development in the mosquito midgut as shown by dietary provision of a NOS inhibitor (L-NAME),in the infectious blood meal [1]. The effect of midgut ·NO synthesis on parasite viability occurs prior to and during midgut invasion. In parallel studies, provision of L-NAME, but not D-NAME, to A. stephensi significantly decreased the percentage of apoptotic Plasmodium berghei ookinetes in the midgut lumen [4]. At later times during midgut invasion, ~5% of ookinetes travel 5–6 cell diameters in the epithelium, perhaps in an effort to escape the toxic diffusible ·NO and ·NO metabolites [3]. This extensive movement may explain the observation that ~95% of ookinetes appear to escape the epithelium [3] yet only a few remain to survive as oocysts [reviewed in 5].
The direct and indirect effects of ·NO and ·NO metabolites on malaria parasite viability were previously only known from studies of mammal-dwelling asexual stage parasites. Minimal toxicity of free ·NO suggested that killing of Plasmodium occurs via the formation of nitrogen oxides (NOx) [6]. Specifically, Rockett et al. [6] found that low molecular weight S-nitrosothiols (SNOs) displayed a 1,000-fold greater toxicity to Plasmodium falciparum than nitrate, which was 3-fold more toxic than nitrite. In addition at lower concentrations some NOx were cytostatic to P. falciparum [7, 8]. In contrast to our knowledge of asexual parasites, the effects of NOx on mosquito-stage parasites are unknown.
In the present study, we have characterized the levels and types of NOx produced in the mosquito midgut. In this context it is necessary to consider the nature of blood digestion within the mosquito midgut. During feeding, A. stephensi ingests 2–10μL of blood that is concentrated to a volume of 1–2μL by diuresis. The pH of the mosquito midgut is slightly less than 7 prior to the blood meal, but increases up to ~8 at 6–24h post-bloodmeal (pBM) [9]. Net consumption of protein from blood is in the range of 550μg, ~90% of which is Hb [10]. Over a period of 48h, digestion occurs from the periphery of the blood bolus to the center. Intact erythrocytes are visible in the blood bolus center up to 24h pBM. Free heme from Hb digestion is converted to hematin that adheres to the peritrophic membrane (PM), a polysaccharide matrix which surrounds the blood mass. At the completion of digestion, the remaining blood bolus and PM are excreted.
In previous work, we demonstrated that parasite-induced synthesis of ·NO generates an inflammatory state in the A. stephensi midgut [2]. Here we show that enhanced levels of nitric oxide metabolites are consistent with a toxic rather than a static effect on developing parasites. In addition, photolyzable ·NO, which can be attributed to metal nitrosyls, likely includes Hb-associated heme iron nitrosyl based on the molar abundance of Hb. The persistance of oxyHb throughout the course of blood digestion and changes in Hb conformation in response to parasite infection suggest that Hb may act to catalyze the synthesis of nitric oxide metabolites in a reducing environment.
EXPERIMENTAL PROCEDURES
Insect rearing and parasite infection
Anopheles stephensi Liston (Indian wild type) mosquitoes were maintained on 10% sucrose at 27°C and 75% relative humidity under a 12h light/dark cycle. Mosquitoes 4–7d old were bloodfed on anesthetized naïve (uninfected) or P. berghei-infected (5–14% parasitemia; strain NK65) Institute of Cancer Research (ICR) mice. Following the blood meal, mosquitoes were maintained at 21°C for parasite development. For feeding studies, 1mM urate and 1mM allantoin were dissolved in phosphate-buffered saline (PBS; 1.06mM KH2PO4, 0.15M NaCl, 5.6mM Na2HPO4) and the pH adjusted to 6.7. The NOS inhibitor L-NAME (1mg/ml) or the inactive stereoisomer D-NAME (1mg/ml) were provided as dietary supplements to the mosquitoes as described [1]. Fresh NAME solutions were prepared daily in sterile distilled, deionized water (ddH2O); ddH2O was used for controls. Sterile cotton balls soaked with dietary treatment solutions (changed twice daily) and sugar cubes (changed daily) were administered from three days before blood feeding until termination of the experiment. For urate and NAME feeds, P. berghei-infected mice were rotated among mosquito cartons (15 min per carton) to avoid mouse-to-mouse variability in parasite infection. Mosquito infections were monitored by counting oocysts on midguts stained with 0.1% mercurochrome at 10d post-infection.
Chemiluminescent detection of ·NO
Free and photolyzable ·NO were measured by photolysis-chemiluminescence detection [11]. Midguts (10 per sample) were dissected into 0.1M sodium phosphate buffer (0.09M Na2HPO4, 0.006M NaH2PO4; pH 8) containing 100μM DTPA or into 0.15M NaCl (pH 7.4) containing 100μM DTPA and injected into the manual injection valve of an Isco 2350 HPLC pump connected to a photolysis chamber (Nitrolite, Thermedics, Inc., Woburn, MA). Degassed (with N2 or He) ddH2O was used as the mobile phase (1ml/min flow rate). ·NO was released by photolysis when the sample passed through a quartz coil surrounding a mercury arc lamp emitting broad wave band UV light (Hanovia-Colight, Inc., Newark, NJ). The output passed through two cold traps (0°C and −75°C) to condense the liquid and less volatile gasses (such as nitrite and nitrate). In the chemiluminescence spectrometer (Model 510, Thermal Energy Analyzer, Thermedics, Inc.), decay of activated state ·NO2* was detected by a photomultiplier and the electrical output analyzed by an integrator (5890 series II Plus, Gas Chromatograph, Hewlett Packard). Values obtained from the integrator were compared to a standard curve made from samples of known concentrations of S-nitrosoglutathione (GSNO). GSNO was prepared immediately prior to use by reacting equimolar concentrations (0.5M) of glutathione in water with 100μM DTPA and nitrite in 1M HCl with 100μM DTPA. Concentrations of midgut bound-·NO were adjusted to heme concentration in each sample. All buffers were analyzed for NOx; detectable levels were subtracted from the sample data.
For nitrite and SNO analyses, chemical reductions were used to liberate ·NO prior to chemiluminescence detection. Mosquito midguts (50 per sample) were dissected into PBS with 1% Nonidet P-40, 4mM K3Fe(CN)6, 10mM NEM, and 0.1mM DTPA, pH 7.4 [12]. Prior to analysis, midgut lysate sample proteins were concentrated by microfiltration (10kD or 100kD Microcon; Millipore Corp., Bedford, MA) at ~2,000 x g for 20 min; this treatment was essential to minimize sample foaming during analysis. In addition, 100μL diluted antifoam agent (166μL in 5 ml water; Sievers) was added to the reaction vessel to further reduce foaming.
For nitrite analyses, sample aliquots were injected into an anaerobic (He-purged) reaction vessel containing room temperature acidified KI (50mg KI in 5ml glacial acetic acid) for conversion of sample nitrites to ·NO [13]. For SNO analyses, samples were pretreated with HgCl2 [1 part 0.2% HgCl2 in water (or water alone for controls): 2 parts mosquito lysate: 3 parts PBS] for 30 min at room temperature. Acidified KI was used to chemically reduce nitrites from SNOs to ·NO. Liberated ·NO from KI-treated and from HgCl2/KI-treated samples was measured with a Sievers ·NO analyzer (NOA; Sievers Instruments, Boulder, CO). Sample values were compared to standard curves of sodium nitrite (NaNO2) and normalized against heme concentrations of matched sample aliquots to account for differences in blood meal volumes among mosquitoes. Induction of nitrite and SNO levels in midguts of P. berghei-infected A. stephensi relative to those in uninfected A. stephensi at each time point were analyzed with Student’s t test.
Immunohistochemical staining for nitrotyrosine (NTYR)
Detection of NTYR was adapted from protocols of Crow and Ischiropoulos et al. [14]. Non-bloodfed, P. berghei-infected and uninfected A. stephensi were collected and preserved in Lillie’s neutral buffered formalin (29mM NaH2PO4·H2O, 46mM Na2HPO4, 4% formalin) at 1d, 3d and 6d pBM. Ten-micron sections of paraffin-embedded mosquitoes were prepared and mounted on poly-lysine coated slides by American HistoLabs, Inc. After sequential de-paraffinization with xylenes and rehydration through graded alcohols, the sections were rinsed with buffer S (0.5M NaCl, 2.7mM KCl, 4.3mM Na2HPO4·7H2O, 1.4mM KH2PO4; pH 7.2) and incubated for 5 min in buffer S with 0.3% Triton X-100. The salt content of buffer S reduces non-specific staining; washes between treatments used buffer S with 1% Tween-20 (BST). Endogenous peroxidases were blocked using a 5% H2O2 in 95% methanol, while endogenous avidin and biotin were blocked by incubating sections buffer S supplemented with streptavidin (30 min at 37°C) followed by buffer S supplemented with d-biotin (30 min at 37°C). Blocked sections were incubated with 5% normal goat serum (Vector) in BST for 30 min at 37°C to minimize nonspecific cross-reactivity. Polyclonal anti-nitrotyrosine (αNTYR; Upstate Biotechnology) was used as the primary antibody (1:700 for 90 min at 37°C) and a biotinylated anti-rabbit IgG (Vector Labs) was used as the secondary antibody (1:200 for 40 min at 37°C). Antibodies were diluted in BST. The ABC (Avidin Biotin Complex) kit and VIP (Very Intense Purple) substrate (Vector Labs) were used for detection. Sections incubated for 10 min at room temperature with peroxynitrite (PN) diluted 1:10 in buffer S were used as positive controls. Negative controls consisted of sections detected with αNTYR pre-absorbed overnight with NTYR or sections treated for 5 min at room temperature with 0.5M sodium hydrosulfite in N2-purged 0.01N NaOH to convert NTYR to undetectable aminotyrosine.
Hemoglobin analysis
At various times pBM, mosquitoes were collected and midguts dissected into the appropriate buffer (see below; 9μL per midgut). Midguts were homogenized by sonication, centrifuged at 2,000 x g for 2 min and the supernatant collected and immediately stored at −80°C. Midgut lysate heme concentrations were calculated based on absorbance values at 300–800nm; heme concentrations were calculated from the absorbance peaks and the respective extinction coefficients for either oxyHb (ɛsoret = 125 mM−1cm−1, ɛα band = 14.6 mM−1cm−1, ɛβ band = 13.8 mM−1cm−1) or methemoglobin (metHb; ɛsoret= 124 mM−1cm−1).
Anopheles stephensi midguts (10 per sample) were dissected into PBS at 1h, 12.5h, and 24h pBM from infected and uninfected bloodfed mosquitoes and from non-bloodfed mosquitoes. Mouse tail blood samples were collected from both infected and uninfected mice immediately prior to bloodfeeding and diluted 1:10 in PBS. Heme concentrations in midgut lysates and mouse blood samples were measured and samples containing 3nmol heme in 5μl PBS were mixed with 5μl loading buffer (62.5mM Tris-HCl, pH 6.8, 40% glygerol, with or without 2% β-mercaptoethanol). Proteins were electrophoretically separated on 7.5% polyacrylamide gels under native conditions. Hemoglobins were visible without staining as heme-containing red-brown bands. The electrophoretically separated proteins were transferred to Immobilon™-P (Millipore) using wet transfer (EC140 Mini Blot Module; E-C Apparatus Corporation) and Towbin transfer buffer (25mM Tris-HCl, 192mM glycine, 20% methanol, 0.1% SDS, pH 8.3) as described by the manufacturer. Blots were treated with 1mM levamisole (pH 7.5) for 20 min to block endogenous alkaline phosphatases (AP), then blocked for 1h in TBS (0.14M NaCl, 2.7mM KCl, 25mM Tris base, pH 7.4) containing 5% non-fat dry milk powder and 0.02% Tween-20. For detection, western blots were incubated with rabbit anti-mouse Hb antisera (Research Plus, Inc.) at a 1:100,000 dilution overnight at 4°C followed by AP-conjugated goat anti-rabbit IgG secondary antibody (Southern Biotechnology Associates) at 1:10,000 for 2h at room temperature. The colorimetric 5-bromo-4-chloro-3-indolyl-phosphate (BCIP)/nitro blue tetrazolium (NBT) substrates (Vector) were used for detection.
SNO-biotin-switch western blotting
SNO-biotin switch western blots were prepared using a protocol adapted from Jaffrey and Snyder [15]. Briefly, midguts from uninfected and P. berghei-infected mosquitoes collected at 1h, 6h, 12h and 24h pBM were dissected into buffer A (69mg NEM, 4.3mg DTPA, 49mg potassium acetate in 10 ml H2O, pH 6.5). Immediately prior to bloodfeeding, samples of mouse tail blood were obtained and diluted 1:10 into buffer A. Heme concentrations of midgut lysates and mouse blood samples were measured and samples containing 1.5nmol heme in 3μl buffer were mixed with equal volumes of 50μM NEM containing 2.5% SDS and incubated for 30 min at 37°C. An equal volume of 1mM CuCl was added to each sample; samples were then incubated for 30 min at 37°C. Subsequently, 0.5 volume of 4mM biotin-HPDP (N-[6-(biotinamido)hexyl]-3′-(2′-pyridyidithiol) propionamide, US Alchemy, Inc.) in DMSO was added to each sample; samples were then incubated for 30 min at room temperature. For controls, a duplicate sample set was processed identically except that DMSO without biotin-HPDP was added in the last step. All reactions were performed in the dark. Sample proteins were mixed without heating with non-reducing Laemmli’s sample buffer, electrophoretically separated on 10–20% Tris-HCl SDS-PAGE Ready gels (BioRad), then transferred to Immobilon™-P (Millipore) using semidry transfer (BioRad). Membranes were blocked with 10% milk before incubation with a 1:30,000 dilution of peroxidase-conjugated anti-biotin monoclonal antisera (Clone BN-34; Sigma). Peroxidase activity was detected using SuperSignal® West Pico Chemiluminescent substrate (Pierce).
Statistics
Statistical significance was evaluated using Student’s t test (Microsoft Excel).
RESULTS
Nitrites and SNOs in the mosquito midgut are not increased by parasite infection
We previously reported levels of total ·NO metabolites in midgut lysates from P. berghei-infected and uninfected A. stephensi [2]. These data were derived from samples analyzed utilizing vanadium chloride reduction and thus reflect levels of total nitrogen oxides. At 12.5h pBM, total NOx levels were 5.9 ± 1.0μM/mM heme in P. berghei-infected A. stephensi and 4.0 ± 0.6μM/mM heme in uninfected A. stephensi [2]. At 24 h pBM, these levels were 13.2 ± 3.9μM/mM heme in infected and 8.7 ± 1.9μM/mM heme in uninfected A. stephensi [2].
During blood digestion, heme concentrations decreased predictably over time due to blood digestion but did not differ between midgut lysates from parasite-infected and uninfected mosquitoes within any timepoint (Fig. 1A). At 12.5h, the average midgut lysate heme concentration was ~17mM and at 24h, the average heme concentration was ~15mM (Fig. 1A). Therefore, one can conclude that the increases in total ·NO metabolites resulted from increased ·NO production [2] rather than infection-induced changes in digestion. However, the chemical nature of the ·NO metabolite(s) within the midgut remained unclear. Therefore we examined the proportion of these metabolites existing as nitrites and SNOs (Fig. 1B). The proportions of nitrites and SNOs to total ·NO metabolites in uninfected and infected midgut lysates were not significantly different at 12.5h and 24h after feeding and, in fact, followed a trend of lower proportions in infected lysates relative to uninfected lysates at 12.5h (Fig. 1B). These observations indicated that enhanced levels of total ·NO metabolites in infected midguts relative to uninfected midguts were due to significantly enhanced levels of nitrates and higher NOx.
Figure 1.
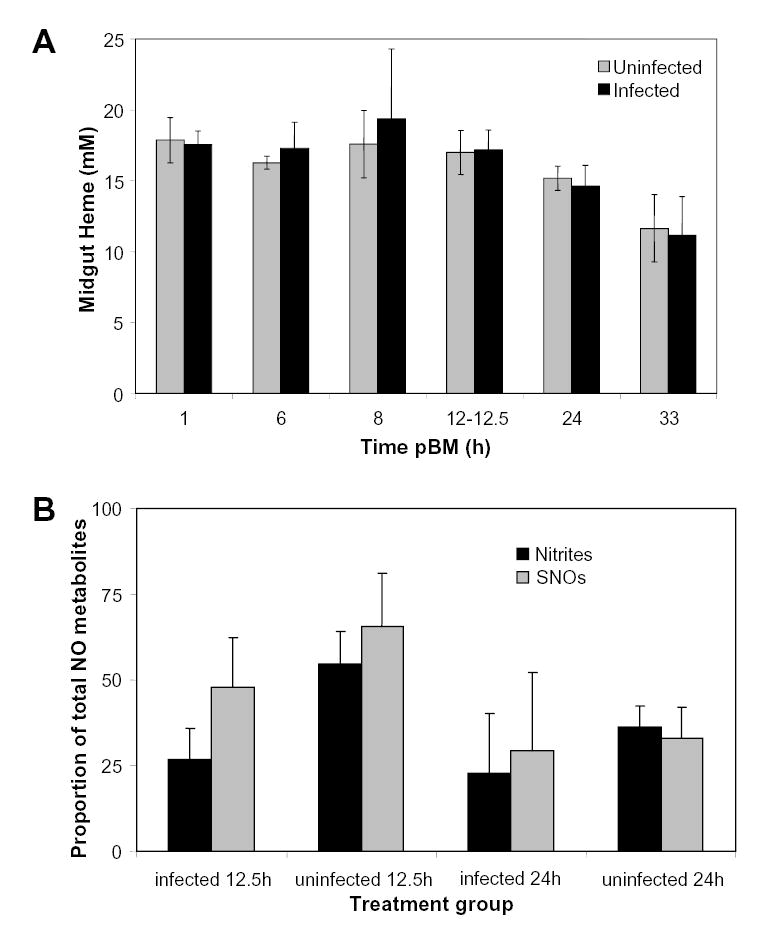
Heme concentration and relative proportions of nitrites and nitrosothiols (SNOs) in uninfected and infected mosquito midguts. A, Uninfected and infected midgut heme concentrations were measured at various times pBM. Values are represented as mean ± SEM. Although heme concentration appeared to decrease beyond 12h pBM, heme concentrations in infected compared to uninfected midguts were not significantly different at all time points tested (Student’s t test; p>0.5; n = 4–12). B, Proportions of nitrites and SNOs were calculated as ratios to total ·NO metabolites; unity is defined as 100. For each timepoint, mean nitrite concentration and mean SNO concentration were divided by mean total ·NO metabolites for both uninfected and infected midguts (n = 8). Untransformed data are shown as mean ± SEM. Arcsine transformed proportional data were used for t test analyses within each timepoint.
In addition to a lack of differences in proportions at 12.5h and 24h following infection, absolute levels of nitrites did not increase in response to parasite infection. Specifically, at 12.5h pBM, midgut lysate nitrite levels were 1.6 ± 0.3μM/mM heme in P. berghei-infected mosquitoes and 2.2 ± 0.4 μM/mM heme in uninfected mosquitoes. At 24h pBM, midgut lysate nitrite levels were 3.0 ± 0.5μM/mM heme in P. berghei-infected mosquitoes and 3.1 ± 0.8μM/mM heme in uninfected mosquitoes.
As observed for nitrites, absolute levels of SNOs did not increase significantly in response to parasite infection. At 12.5h pBM, midgut lysate SNO levels were 2.9 ± 1.1μM/mM heme in P. berghei-infected mosquitoes and 2.6 ± 0.9μM/mM heme in uninfected mosquitoes. At 24h pBM, midgut lysate SNO levels were 3.9 ± 1.7μM/mM heme in P. berghei-infected mosquitoes and 2.9 ± 1.0μM/mM heme in uninfected mosquitoes.
Tyrosine nitration is enhanced by parasite infection
The prior results suggested that chemistry of higher oxides of nitrogen might prevail within the mosquito midgut. As a marker of such chemistry [16], we selected nitrotyrosine (NTYR). Much greater NTYR levels were observed in tissues of P. berghei-infected versus uninfected A. stephensi at 24h pBM (Fig. 2), suggesting that parasite-induced higher oxides of nitrogen observed at this time post-infection ([2] and Fig. 1) were temporally associated with nitration. Staining was particularly evident in the fat body, ovarian tissue, midgut epithelium and blood bolus (Fig. 2). Staining was also more intense in the distal portion of the midgut, consistent with observations that parasite invasion and oocyst development are concentrated in this area [reviewed in 17]. Together, midgut NOx levels and NTYR data suggested that ·NO-mediated stress is enhanced in and around the infected midgut during the time of ookinete invasion of the midgut epithelium and oocyst development.
Figure 2.
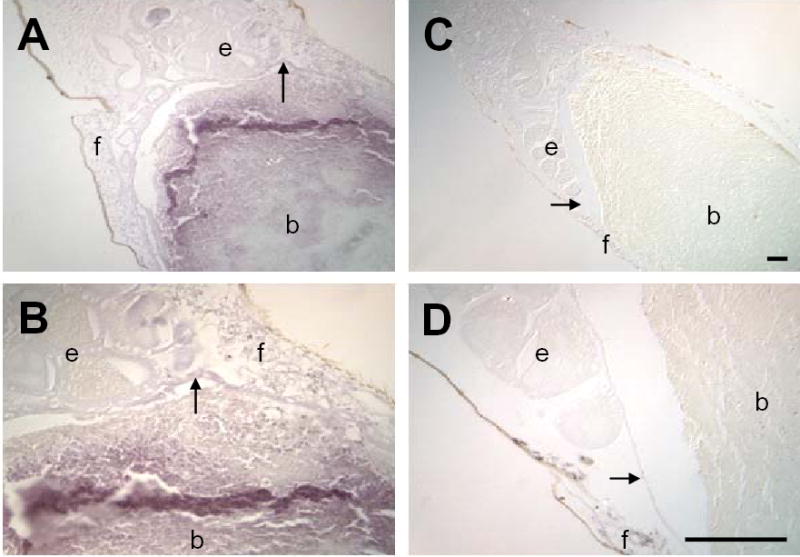
Nitrotyrosine (NTYR) levels in the midgut and surrounding tissues are increased in response to P. berghei infection. Immunohistochemical staining of 10μm sections of bloodfed A. stephensi was performed using polyclonal anti-nitrotyrosine (αNTYR). At 24h pBM, increased staining for NTYR (purple) was observed in tissues of P. berghei-infected (A, B) compared to uninfected A. stephensi (C, D). Staining was observed in the (b) blood mass, (e) ovarian tissue, (f) fat body and (→) midgut epithelium). The most pronounced staining occurred in blood meal bolus in the posterior midgut. Tissue samples were observed under 100x (A, C) and 400x (B, D) magnification; scale bar = 75μm.
Hemoglobin in the mosquito midgut is oxygenated throughout digestion
UV-visible spectra of midgut lysates from 1–49h pBM (Fig. 3) were used to calculate midgut heme concentrations (Fig. 1). From spectral analyses, we noted that Hb within the mosquito midgut, regardless of time pBM or presence or absence of infection, appeared to be maintained in a ferrous liganded or oxyHb state without detectable heme oxidation to 33h pBM (Fig. 3). The lack of oxidized heme in A. stephensi midgut lysates suggested that the midgut is a reducing environment [18]. As oxyHb reacts readily with ·NO, we chose to examine whether ·NO remained bound to Hb in the midgut by means of photolysis with chemiluminescence and western blotting.
Figure 3.
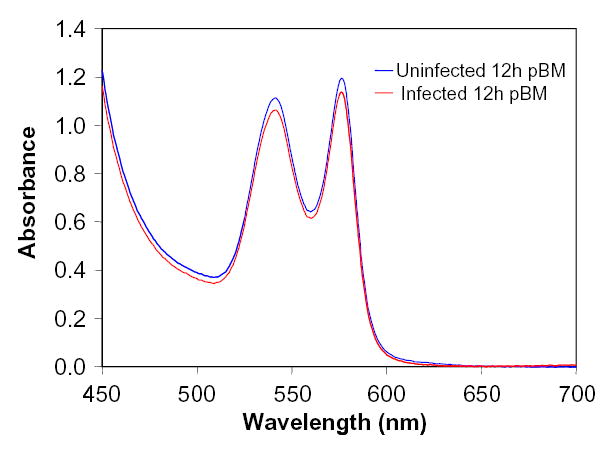
Absorbance spectra of midgut hemoglobins (Hb). Spectra of lysates from parasite-infected and uninfected midguts collected from 1–33h pBM were consistent with spectra for oxyHb and contained little to no detectable metHb. Representative spectra from 12.5h pBM are shown here.
Photolyzable adducts of ·NO in the mosquito midgut are increased by parasite infection
Photolysis coupled with chemiluminescent detection is a sensitive measure of SNOs and metal-nitrosyl adducts and to some extent N-nitroso compounds [19]. Photolyzed-·NO levels in midgut lysates were measured immediately after (0h) to 33.5h after A. stephensi feeding on uninfected or P. berghei-infected mice. In all samples, concentrations of photolyzed-·NO were 3.5 – 17-fold lower than corresponding concentrations of nitrites and SNOs in both parasite-infected and uninfected A. stephensi midgut lysates, indicating a lack of background detection of SNOs generated spuriously by reactions of abundant blood-derived thiols with nitrate. Photolyzed-·NO levels of infected and uninfected midgut lysates were not significantly different at collection times <10h (Fig. 4A). However, at 12.5–13.5 h and 24–25.5 h pBM, the concentrations of photolyzed-·NO were significantly higher in infected compared to uninfected midgut lysate samples (Fig. 4B).
Figure 4.
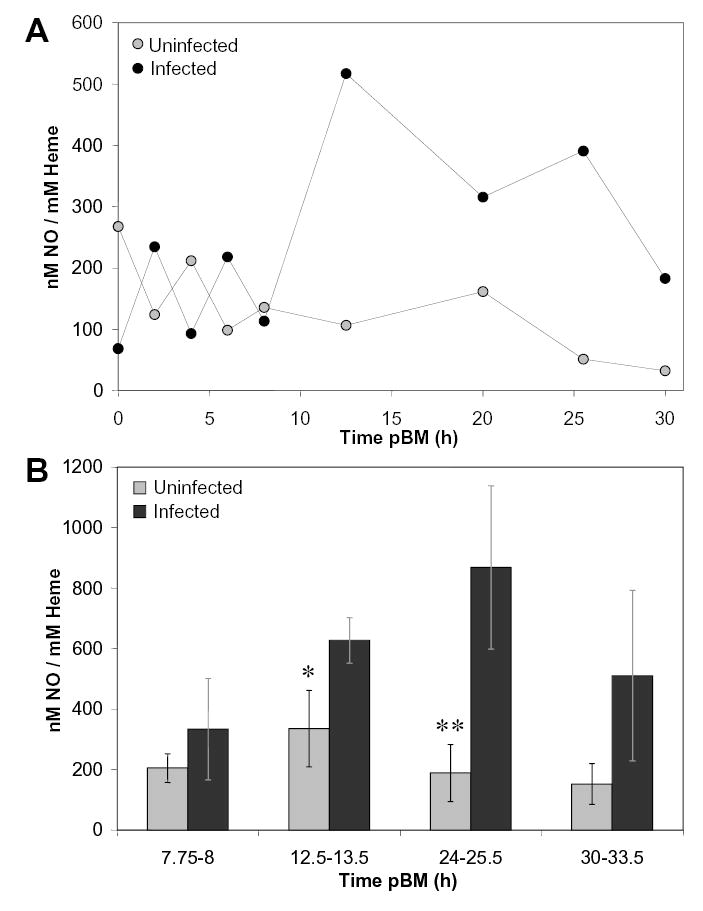
Midgut levels of photolyzed-·NO were increased in response to P. berghei-infection. Photolyzable ·NO was quantified using photolysis-chemiluminescence spectroscopy. For midgut lysates, ·NO concentration was normalized against sample heme concentration to account for variation in ingested blood meal volume. A, A representative timed series demonstrates that from immediately after (0h) to 8h pBM, photolyzed-·NO levels in uninfected and P. berghei-infected midguts were not significantly different. In contrast, elevated photolyzed-·NO levels were detected in infected midguts between 12.5h and 30h pBM. B, Parasite infection increased levels of photolyzed-·NO in midgut lysates collected from 8–33.5 h pBM (n = 3). Values are represented as mean ± SEM. At 12.5–13.5 h and 24–25.5 h pBM, infected midgut levels were significantly higher than those from uninfected blood meals (*p = 0.02 and **p = 0.03).
Anopheles stephensi midguts contain SNO-proteins and cross-linked Hb
Based on the detection of significant levels of SNOs within the blood-filled A. stephensi midgut we chose to examine the formation of SNO-proteins as a function of time post-feeding because the nature of SNO-modified peptides and proteins could influence the toxicity of ·NO in the midgut [6, 11]. To assess the profile of S-nitrosylated midgut proteins in A. stephensi, lysates were assayed using the “SNO-biotin switch” method [15]. Western blots of heme-normalized midgut lysate samples from P. berghei-infected and uninfected A. stephensi showed little to no differences in the profiles of SNO-modified proteins at 1h, 6h, 12h, and 24h following bloodfeeding in two independent trials (lanes 3 and 4, Fig. 5A). Equivalent concentrations of heme-normalized mouse blood samples, drawn from mice prior to bloodfeeding, were used as controls (lanes 1 and 2, Fig. 5A). SNO-modified mouse proteins were detected and no obvious differences were noted between infected and uninfected blood, consistent with the fact that ICR mice do not produce ·NO in response to parasite infection [20]. Although SNO-protein profiles in the mosquito midgut did not change with parasite infection, SNO-proteins in mouse blood differed from SNO-proteins in blood-fed mosquito midguts at all times examined (lane 1 versus 3 and lane 2 versus 4 for each panel, Fig. 5A). These observations suggested that exposure of blood to the mosquito midgut alters SNO-protein content and that production of ·NO by the mosquito midgut leads to S-nitrosylation of proteins within the blood meal.
Figure 5.

Parasite infection does not alter the profile of SNO-modified proteins, which may include Hb, in the A. stephensi midgut. A, SNO-biotin-switch westerns revealed S-nitrosated protein profiles in infected versus uninfected midgut lysates collected from 1h – 24h pBM. Although uninfected and infected midgut lysate profiles were not different (lane 3 versus 4 in each panel), SNO-modified proteins in mouse blood were altered following ingestion by A. stephensi (lane 1 versus 3 and lane 2 versus 4 for each panel). B, A Coomassie-stained gel (a) and western blot (b) using anti-Hb antisera for 12h pBM T2 midgut lysate samples (from panel A). Migration of tetrameric (t), dimeric (d) and monomeric (m) Hb are indicated by arrows. Lanes: 1 = uninfected mouse blood, 2 = P. berghei infected mouse blood, 3 = uninfected midgut lysate, 4 = infected midgut lysate, and 5 = molecular weight markers (kD). T1 and T2 refer to two independent trials.
As expected, digestion of midgut blood over time appeared to reduce protein complexity in the samples (lanes 3 and 4 at 24h versus at 1h, Fig. 5A). A strong cross-reacting band at ~64 kD, the molecular mass of tetrameric Hb, was evident in the SNO-biotin-switch blots at 12h (T2, lanes 3 and 4) and particularly at 24h in both mosquito lysates and mouse blood samples (lanes 1–4, Fig. 5A). It is probable that this protein is tetrameric Hb, as this is the predominant protein at that molecular weight within these samples (Fig. 5B).
To further examine the structure of Hb in midguts lysates from parasite-infected and uninfected A. stephensi, lysate proteins were separated electrophoretically under native conditions. Hemoglobin can be directly observed under these conditions without the use of staining dyes due to the red-brown color derived from heme. Tetrameric Hb was evident as the most abundant band in normal mouse blood and in most midgut lysates (Fig. 6A, B). However, in midgut lysates from parasite-infected A. stephensi collected 12.5h and 24h pBM, tetrameric Hb was faint or absent while a much slower migrating red-brown protein was abundant (arrows, Fig. 6A). This slow-migrating protein was not present in normal mouse blood, uninfected midgut lysates, or samples collected at 1h pBM (Fig. 6A). When samples were pre-treated prior to electrophoresis with β-mercaptoethanol, the slow migrating protein disappeared and a protein with migration identical to that of tetrameric Hb appeared in midgut lysates from 12.5h and 24h parasite-infected mosquitoes (Fig. 6B). As expected, pre-treatment with β-mercaptoethanol had no effect on tetrameric Hb in the remaining samples (Fig. 6B). These results suggested that disulfide bonds were responsible for retarding the migration of an aggregated Hb.
Figure 6.
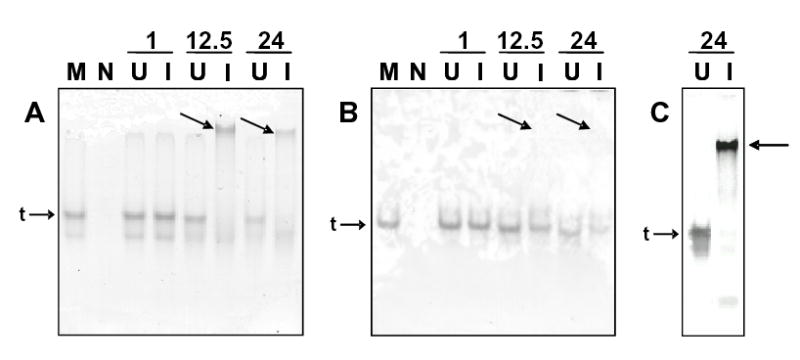
Midguts from parasite-infected mosquitoes contain a disulfide-linked, slow migrating Hb at 12.5h and 24h pBM. Uninfected mouse blood (M) and midgut lysates from non-bloodfed A. stephensi (N), and P. berghei-infected (I) and uninfected (U) A. stephensi collected at 1h, 12.5h and 24h pBM were electrophoretically separated through 7.5% polyacrylamide. The heme-containing Hbs were visible as red-brown bands on unstained gels. A, Under native conditions, a slow migrating Hb (arrows) relative to the expected tetrameric Hb (t) was visible in infected midgut lysates only at 12.5h and 24h pBM. B, Sample pre-treatment with β-mercaptoethanol eliminated the slow migrating Hb (arrows) and was coincident with the reappearance of tetrameric Hb (t), indicating that altered protein migration was due to disulfide bonds. C, Western blot analysis using mouse anti-Hb antisera confirmed that the slow migrating protein was Hb (arrow).
To confirm that the slow migrating protein was Hb, anti-Hb western blots were performed on midgut lysates collected at 24h pBM and separated and transferred under native conditions. As expected, the red-brown proteins in midgut lysates from both uninfected and infected A. stephensi were clearly identifiable as Hb (Fig. 6C). The association of this slow migrating Hb with only infected midguts at later times pBM suggested that nitrosative or oxidative stress may be associated with its formation. Interestingly, a minority of infected midgut lysates lacked the slow migrating Hb (not shown), suggesting that variation in parasite infection intensity and, hence, nitrosative stress can be translated into variation in disulfide-bonded Hb formation.
Dietary supplementation with urate, but not allantoin, increased parasite infection levels in A. stephensi
The observation of increased NTYR staining in the mosquito midgut demonstrated that parasite infection leads to an increase in both nitrosative and oxidative stress. Uric acid/urate is a product of purine metabolism with limited membrane permeability and is a potent reductant and nonspecific scavenger of nitroxyl [21], PN [22] and ·OH [23]. In other systems, PN and nitroxyl can induce NTYR formation, although nitroxyl does so only weakly [24]. In mice, urate administration to animals with symptoms of multiple sclerosis significantly decreased NTYR formation in brain tissue and greatly reduced disease severity relative to control animals [22].
We examined the effects of administration of urate, or its non-redox active metabolite allantoin, by dietary supplementation to adult female mosquitoes. In preliminary assays, mosquito resting and flight behavior, mortality, and bloodfeeding success among the urate-, allantoin-, and PBS-treated control groups were not markedly different. As such, we concluded that urate and allantoin were not overtly toxic to treated mosquitoes. The mean numbers of P. berghei oocysts from mosquitoes fed PBS and those fed 1mM allantoin were not significantly different from each other (Fig. 7A). However, mean numbers of oocysts in both PBS and allantoin control groups were significantly lower than the mean number of oocysts in A. stephensi fed 1mM urate (Fig. 7A). Human plasma contains urate at concentrations up to 500μM [25], while mouse plasma contains ~20μM urate [22]. If A. stephensi could utilize urate ingested with blood as a reductant and antioxidant, significant differences in infection could result from feeds that utilize human versus mouse blood. To test this possibility, a follow-up assay included dietary supplementation of urate at 500μM and 1mM. As observed previously, the mean number of oocysts in mosquitoes fed 1mM urate was significantly higher than the mean number of oocysts in mosquitoes fed PBS (Fig. 7B). However, the number of oocysts from mosquitoes fed 500μM urate was not significantly different from the PBS-fed controls (Fig. 7B).
Figure 7.

Dietary supplementation with urate, but not allantoin, increased parasite infection levels in A. stephensi. Values are represented as mean ± SEM. A, Dietary urate (1 mM) in PBS, but not allantoin (1 mM) in PBS, or PBS alone, increased P. berghei infection levels in A. stephensi as measured by 10d oocyst count (p = 0.006 for PBS versus urate, p = 0.296 for PBS versus allantoin and p = 0.008 for urate versus allantoin; n = 29). B, Dietary urate increased parasite infection levels when provided at 1 mM but not at 0.5 mM relative to control PBS (0 mM) treatment (p = 0.04 for PBS versus 1 mM urate, p = 0.33 for PBS versus 0.5 mM urate, and p = 0.03 for 0.5 mM versus 1 mM urate; n = 20).
DISCUSSION
Previously we have shown that ·NO synthesis limits Plasmodium development in A. stephensi. In the present study we have investigated the nature of the ·NO metabolites involved in this form of insect immunity. During parasite infection, the lower oxides of nitrogen, namely nitrites and SNOs, were not increased relative to levels in uninfected insects, suggesting that these metabolites are either not involved in the anti-parasite response or form a more labile pool that was not detected in our assays. Further, given that autooxidation of ·NO leads to a 50:50 ratio of nitrites and nitrates, the skewed production of nitrates indicates that there is an elevated production of higher oxides of nitrogen. Parasite infection in A. stephensi also resulted in increased levels of NTYR and photolyzable-·NO adducts. Formation of these ·NO metabolites in response to parasite infection appears to involve Hb, which is structurally modified in infected insects, yet maintained in a liganded form. The importance of oxidative and/or nitrosative stress to the control of parasite development is emphasized by the ability of dietary urate relative to control dietary allantoin to increase parasite loads.
Free ·NO and ·NO-adducts, including SNOs, N-nitroso compounds (RNNOs), and metal-nitrosyls can contribute to signal output of photolysis with chemiluminescent detection [26]. Under conditions found in the A. stephensi midgut, it is likely that free ·NO reacts rapidly to generate a variety of ·NO metabolites. Although RNNOs may be generated endogenously [27], it is unlikely that they are involved in host defense as they do not possess antiplasmodial activity [28]. Because the steady state concentration of SNOs was not elevated following infection, metal nitrosyls would appear to account for the increase in photolyzable-·NO. Due to the high concentration of Hb within the midgut, it is reasonable to propose that the predominant metal nitrosyl is iron nitrosylHb. Alternatively, iron released from Hb during digestion prior to sequestration in hematin could react with ·NO to form dinitrosyl iron complexes (DNICs) [29]. DNICs, although perhaps formed at lower levels than iron nitrosylHb, could mediate toxic effects through redox destabilization [30].
Possible mechanisms for iron nitrosylHb formation in the mosquito midgut
Classically, ·NO can react with oxyHb to form metHb and nitrate or with deoxyHb to form iron nitrosylHb [31]. The levels of nitrosylHb in mammalian circulation have been reported to be ~5μM in mixed venous blood and ~2.5μM in arterial blood [32]. When mosquitoes feed, blood is drawn from the peripheral venous circulation, thus the blood meal likely contains nitrosylHb. However, the observation that midgut blood contains and maintains oxyHb suggests that ingestion of the blood meal is analogous to blood entering the lungs. The introduction of oxygen to partially nitrosylated Hb leads to a loss of the NO moiety through oxidation or conversion to SNO. However, under alkaline oxygenated conditions such as those found in the midguts of Anopheles spp. [9], iron nitrosylHb is reasonably stable and undergoes reversible oxygen binding with moderate affinity [33].
The addition of superoxide dismutase (SOD) to Hb increases the yield of total ·NO bound to Hb [34]. SOD activity is increased by bloodfeeding in A. gambiae by 0.95–1.94-fold at 12h pBM and 2.1–2.5-fold at 24h pBM relative to unfed controls [35], suggesting that mosquito SOD could facilitate ·NO binding to Hb. Finally, SOD can decrease the conversion of oxyHb to metHb [36], which would be consistent with the lack of observed metHb in bloodfed midguts.
All reactions of ·NO with Hb in the vasculature are decreased by the RBC membrane [37], as ·NO reacts at least 1,000 times faster with free Hb than with Hb contained by the RBC membrane [38]. The midgut conditions resemble that of a cell-free Hb system since the RBCs nearest to the midgut epithelium, the source of ·NO, are actively digested and lysed. The higher concentrations of Hb in the midgut together with high local concentrations of ·NO produced by the midgut epithelium, could support nitrosylHb formation as described by Gow et al. [34]. Further, the reaction of ·NO with oxyHb to form nitrosylHb may be favored in the parasite-infected midgut where in addition to high local ·NO fluxes, Hb is freed from RBCs and sequestered by disulfide bond-dependent aggregation during digestion.
NTYR formation in the A. stephensi midgut
In addition to NOS, a putative dual function oxidase, Ag-Duox, is highly expressed in the A. gambiae midgut in response to ookinete invasion [39]. Ag-Duox encodes an N-terminal peroxidase domain and a C-terminal NADPH oxidase domain [39]. As such, the local induction of Ag-Duox could lead to high local levels of O2·− as has been shown in An. albimanus upon injection of ookinetes into midgut explants [40]. Based on these observations, we predicted that higher oxides of nitrogen are formed in Anopheles mosquitoes and that these metabolites could induce NTYR formation. In this regard it is important to note that the combination of ·NO and O2·− has been show to kill 75% of P. berghei ookinetes in vitro [40]. Our data showed that NTYR levels were significantly higher in parasite-infected A. stephensi relative to bloodfed, uninfected mosquitoes at 24h pBM. In parasite-infected A. stephensi, elevated NOx were detected in the blood meal at 12.5h and 24h pBM [2], a time period consistent with enhanced NTYR staining at 24h and that spans the final stages of ookinete maturation in the midgut and the beginning of ookinete invasion of the midgut epithelium.
The presence of NTYR suggests PN-like reactivity and it is likely that PN or its redox congeners directly affect parasite development. Diffusion of ·NO into the blood mass and the subsequent formation of toxic NOx would occur in the same physical environment in which critical stages of parasite development occur. While NOx in general would be predicted to damage several classes of parasite biomolecules, we can speculate that some parasite proteins may be key targets for inhibition. For example, P. falciparum glutathione reductase (PfGR) can be inactivated by PN through nitration of Tyr 106 and Tyr 114 [41]. These findings have contributed to strategies for developing irreversible PfGR inhibitors for novel antimalarial drugs [41], suggesting that similar investigations of mosquito-stage parasites could lead to the development of novel transmission-blocking strategies.
In light of the induction of SOD expression in the An. gambiae midgut following bloodfeeding [35], it is necessary to consider reactions independent of PN that may contribute to NTYR formation. For example, nitroxyl, heme proteins, transition metals and/or peroxidases can catalyze NTYR formation. Intense NTYR staining in the blood bolus may, therefore, reflect heme iron catalysis of tyrosine nitration in an environment of Hb digestion and high ·NO flux [42]. Kumar et al. [39] hypothesized that ·NO synthesis following parasite infection in An. gambiae results in accumulation of nitrite, which is a substrate for myeloperoxidase (MPO)-mediated tyrosine nitration [43]. However, all of these reactive pathways rely on the formation of higher NOx and thus NTYR can be considered a marker of nitrative stress.
Urate enhances parasite development by reducing nitrative and oxidative stress
Provision of urate as a dietary supplement increased parasite loads in A. stephensi relative to allantoin-fed control insects. Uric acid inhibits nitration reactions and has been used as a scavenger of PN [22]. In addition, urate can alter the chemistry of nitroxyl [21] which could indirectly influence PN formation. Urate can also scavenge ·OH [23], suggesting that urate supplementation could have increased parasite infection by reducing levels of cytotoxic ·OH. In contrast to the direct effects of urate on NOx and ROS, urate does not interfere with peroxidase-mediated nitration. As such, the effects of urate supplementation suggest that PN rather than an alternative metabolite is responsible for the bulk of the tissue nitration and limiting parasite development.
Toxicity of NOx predicted to occur in the A. stephensi midgut
In the redox-active mosquito midgut, ·NO synthesis in the context of blood digestion results in the formation of NOx that likely mediate the ·NO-dependent reduction in Plasmodium development. Specifically, we propose that iron nitrosylHb and perhaps DNICs formed during blood digestion preserve the bioactivity of ·NO in the blood-filled midgut, while toxic nitrates and higher oxides of nitrogen comprise the majority of Plasmodium-induced NOx. Further, the patterns of SNO-modified proteins change over time in the mosquito midgut, indicating that mosquito physiology drives dynamic S-nitrosylation of ingested blood proteins and perhaps endogenous mosquito proteins. These changes occur during the first 24h of parasite development, a critical time in which parasites undergo rapid developmental changes within the blood mass. Importantly, ·NO synthesis occurs in the context of ROS produced in the midgut after feeding, a situation which would potentiate nitrative stress. In addition to mosquito-generated ROS, the ingested blood itself may be a source of oxygen radicals. Within 20 min of feeding, erythrocyte hemolysis frees up to 10% of the total ingested hemoglobin in the midgut lumen [44]. The globins are dissociated from heme and digested, whereas the heme is polymerized to insoluble hematin under oxygenated, alkaline conditions [45]. Polymerization, however, is not instantaneous, nor does it completely abolish radical chemistry [46]. Thus, significant quantities of heme and hematin can react with iron and oxygen in the bloodmeal to form ROS that could react with ·NO to induce nitration in the blood mass and in the surrounding tissues. Intriguingly, elevated levels of nitrosylHb and NTYR are detected during the inflammatory response of two mouse strains to the protozoan Leishmania amazonensis [47], revealing a parallel to the chemistry and toxic effects of ·NO synthesis in Plasmodium-infected A. stephensi.
Nitroxyl and PN are strongly reactive and can oxidize thiols and amines, nitrosylate metals, induce DNA strand breaks and base oxidation, oxidize lipids and proteins, deaminate DNA, oxidize sugars, and significantly alter protein activity through nitration [48], suggesting that overproduction of toxic NOx in A. stephensi mediates NOS-dependent parasite apoptosis [4]. At the same time, however, induction of mosquito 2-Cys peroxiredoxin, which protects against ROS- and NOx-mediated cell death [49], would tip the balance in favor of the mosquito. As such, a complex immunological system within A. stephensi produces reactive species to limit infection and cytoprotective enzymes to preserve host cell function. Enhancement of anti-parasite ·NO synthesis in the context of increased expression of host peroxiredoxin, therefore, may form the basis for a novel transmission blocking strategy for malaria control.
Acknowledgments
This work was supported by grants to SL from the National Institutes of Health (AI50663, AI60664). We thank Dr. Jonathan S. Stamler for his support of this work and for his critical review of the manuscript.
References
- 1.Luckhart S, Vodovotz Y, Cui L, Rosenberg R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc Natl Acad Sci USA. 1998;95:5700–5705. doi: 10.1073/pnas.95.10.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luckhart S, Crampton AL, Zamora R, Lieber MJ, Dos Santos PC, Peterson TML, Emmith N, Lim J, Wink DA, Vodovotz Y. Mammalian transforming growth factor-β1, activated after ingestion by Anopheles stephensi, modulates mosquito immunity. Infect Immun. 2003;71:3000–3009. doi: 10.1128/IAI.71.6.3000-3009.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han YS, Thompson J, Kafatos FC, Barillas-Mury C. Molecular interactions between Anopheles stephensi midgut cells and Plasmodium berghei: the time bomb theory of ookinete invasion of mosquitoes. EMBO J. 2000;19:6030–6040. doi: 10.1093/emboj/19.22.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Olayan EM, Williams GT, Hurd H. Apoptosis in the malaria protozoan, Plasmodium berghei: a possible mechanism for limiting intensity of infection in the mosquito. Int J Parasitol. 2002;32:1133–1143. doi: 10.1016/s0020-7519(02)00087-5. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh A, Edwards MJ, Jacobs-Lorena M. The journey of the malaria parasite in the mosquito: hopes for the new century. Parasitol Today. 2000;16:196–201. doi: 10.1016/s0169-4758(99)01626-9. [DOI] [PubMed] [Google Scholar]
- 6.Rockett KA, Awburn MM, Cowden WB, Clark IA. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun. 1991;59:3280–3283. doi: 10.1128/iai.59.9.3280-3283.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balmer P, Phillips HM, Maestre AE, McMonagle FA, Phillips RS. The effect of nitric oxide on the growth of Plasmodium falciparum, P. chabaudi and P. berghei in vitro. Parasite Immunol. 2000;22:97–106. doi: 10.1046/j.1365-3024.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- 8.Taylor-Robinson AW. Antimalarial activity of nitric oxide: cytostasis and cytotoxicity towards Plasmodium falciparum. Biochem Soc Trans. 1997;25:262S. doi: 10.1042/bst025262s. [DOI] [PubMed] [Google Scholar]
- 9.Billker O, Miller AJ, Sinden RE. Determination of mosquito bloodmeal pH in situ by ion-selective microelectrode measurement: implications for the regulation of malarial gametogenesis. Parasitology. 2000;120:547–551. doi: 10.1017/s0031182099005946. [DOI] [PubMed] [Google Scholar]
- 10.Briegel H, Rezzonico L. Concentration of host blood protein during feeding by anopheline mosquitoes (Diptera: Culicidae) J Med Entomol. 1985;22:612–618. doi: 10.1093/jmedent/22.6.612. [DOI] [PubMed] [Google Scholar]
- 11.Stamler JS, Jaraki O, Osborne J, Simon DI, Keaney J, Vita J, Singel D, Valeri CR, Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci USA. 1992;89:7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gladwin MT, Wang X, Reiter CD, Yang BK, Vivas EX, Bonaventura C, Schechter AN. S-Nitrosohemoglobin is unstable in the reductive erythrocyte environment and lacks O2/NO-linked allosteric function. J Biol Chem. 2002;277:27818–27828. doi: 10.1074/jbc.M203236200. [DOI] [PubMed] [Google Scholar]
- 13.Ewing JF, Janero DR. Specific S-nitrosothiol (thionitrite) quantification as solution nitrite after vanadium(III) reduction and ozone-chemiluminescent detection. Free Radic Biol Med. 1998;25:621–628. doi: 10.1016/s0891-5849(98)00083-5. [DOI] [PubMed] [Google Scholar]
- 14.Crow JP, Ischiropoulos H. Detection and quantitation of nitrotyrosine residues in proteins: in vivo marker of peroxynitrite. Methods Enzymol. 1996;269:185–94. doi: 10.1016/s0076-6879(96)69020-x. [DOI] [PubMed] [Google Scholar]
- 15.Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Science STKE. 2001;PL1 doi: 10.1126/stke.2001.86.pl1. [DOI] [PubMed] [Google Scholar]
- 16.Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys. 1998;356:1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- 17.Cociancich SO, Park SS, Fidock DA, Shahabuddin M. Vesicular ATPase-overexpressing cells determine the distribution of malaria parasite oocysts on the midguts of mosquitoes. J Biol Chem. 1999;274:12650–12655. doi: 10.1074/jbc.274.18.12650. [DOI] [PubMed] [Google Scholar]
- 18.Takeoka S, Sakai H, Kose T, Mano Y, Seino Y, Nishide H, Tsuchida E. Methemoglobin formation in hemoglobin vesicles and reduction by encapsulated thiols. Bioconjug Chem. 1997;8:539–544. doi: 10.1021/bc970091y. [DOI] [PubMed] [Google Scholar]
- 19.Alpert C, Ramdev N, George D, Loscalzo J. Detection of S-nitrosothiols and other nitric oxide derivatives by photolysis-chemiluminescence spectrometry. Anal Biochem. 1997;245:1–7. doi: 10.1006/abio.1996.9947. [DOI] [PubMed] [Google Scholar]
- 20.Murata K, Takano F, Fushiya S, Oshima Y. Potentiation by febrifugine of host defense in mice against Plasmodium berghei NK65. Biochem Pharmacol. 1999;58:1593–1601. doi: 10.1016/s0006-2952(99)00244-0. [DOI] [PubMed] [Google Scholar]
- 21.Espey MG, Miranda KM, Thomas DD, Wink DA. Ingress and reactive chemistry of nitroxyl-derived species within human cells. Free Radic Biol Med. 2002;33:827–834. doi: 10.1016/s0891-5849(02)00978-4. [DOI] [PubMed] [Google Scholar]
- 22.Hooper DC, Scott GS, Zborek A, Mikheeva T, Kean RB, Koprowski H, Spitsin SV. Uric acid, a peroxynitrite scavenger, inhibits CNS inflammation, blood-CNS barrier permeability changes, and tissue damage in a mouse model of multiple sclerosis. FASEB J. 2000;14:691–698. doi: 10.1096/fasebj.14.5.691. [DOI] [PubMed] [Google Scholar]
- 23.Becker BF. Towards the physiological function of uric acid. Free Radic Biol Med. 1993;14:615–631. doi: 10.1016/0891-5849(93)90143-i. [DOI] [PubMed] [Google Scholar]
- 24.Ohshima H, Celan I, Chazotte L, Pignatelli B, Mower HF. Analysis of 3-nitrotyrosine in biological fluids and protein hydrolyzates by high-performance liquid chromatography using a postseparation, on-line reduction column and electrochemical detection: results with various nitrating agents. Nitric Oxide. 1999;3:132–141. doi: 10.1006/niox.1999.0216. [DOI] [PubMed] [Google Scholar]
- 25.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alpert C, Ramdev N, George D, Loscalzo J. Detection of S-nitrosothiols and other nitric oxide derivatives by photolysis-chemiluminescence spectrometry. Anal Biochem. 1997;245:1–7. doi: 10.1006/abio.1996.9947. [DOI] [PubMed] [Google Scholar]
- 27.Ohshima H, Bartsch H. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res. 1994;305:253–264. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 28.Nagasawa HT, Fraser PS, Yuzon DL. A new method for nitrosation of proline and related sec-α-amino acids to N-nitrosamino acids with possible oncogenic activity. J Med Chem. 1973;16:583–585. doi: 10.1021/jm00263a048. [DOI] [PubMed] [Google Scholar]
- 29.Vanin AF, Malenkova IV, Serezhenkov VA. Iron catalyzes both decomposition and synthesis of S-nitrosothiols: optical and electron paramagnetic resonance studies. Nitric Oxide. 1997;1:191–203. doi: 10.1006/niox.1997.0122. [DOI] [PubMed] [Google Scholar]
- 30.Keese MA, Bose M, Mulsch A, Schirmer RH, Becker K. Dinitrosyl-dithiol-iron complexes, nitric oxide (NO) carriers in vivo, as potent inhibitors of human glutathione reductase and glutathione-S-transferase. Biochem Pharmacol. 1997;54:1307–1313. doi: 10.1016/s0006-2952(97)00348-1. [DOI] [PubMed] [Google Scholar]
- 31.Thomas DD, Miranda KM, Colton CA, Citrin D, Espey MG, Wink DA. Heme proteins and nitric oxide (NO): the neglected, eloquent chemistry in NO redox signaling and regulation. Antioxid Redox Signal. 2003;5:307–317. doi: 10.1089/152308603322110887. [DOI] [PubMed] [Google Scholar]
- 32.McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, Gow AJ, Pawloski JR, Watke P, Singel DJ, Piantadosi CA, Stamler JS. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8:711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 33.Yonetani T, Tsuneshige A, Zhou Y, Chen X. Electron paramagnetic resonance and oxygen binding studies of alpha-Nitrosyl hemoglobin. A novel oxygen carrier having no-assisted allosteric functions. J Biol Chem. 1998;273:20323–20333. doi: 10.1074/jbc.273.32.20323. [DOI] [PubMed] [Google Scholar]
- 34.Gow AJ, Luchsinger BP, Pawloski JR, Singel DJ, Stamler JS. The oxyhemoglobin reaction of nitric oxide. Proc Natl Acad Sci USA. 1999;96:9027–9032. doi: 10.1073/pnas.96.16.9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Christophides GK, Cantera R, Charles B, Han YS, Meister S, Dimopoulos G, Kafatos FC, Barillas-Mury C. The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proc Natl Acad Sci USA. 2003;100:14139–14144. doi: 10.1073/pnas.2036262100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang T, Olsen KW. Enzymatic protection from autoxidation for crosslinked hemoglobins. Artif Cells Blood Substit Immobil Biotechnol. 1994;22:709–717. doi: 10.3109/10731199409117902. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Miller MJ, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR., Jr Diffusion-limited reaction of free nitric oxide with erythrocytes. J Biol Chem. 1998;273:18709–18713. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 38.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, 3rd, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 39.Kumar S, Gupta L, Han YS, Barillas-Mury C. Inducible peroxidases mediate nitration of Anopheles midgut cells undergoing apoptosis in response to Plasmodium invasion. J Biol Chem. 2004;279:53475–53482. doi: 10.1074/jbc.M409905200. [DOI] [PubMed] [Google Scholar]
- 40.Lanz-Mendoza H, Hernandez-Martinez S, Ku-Lopez M, Rodriguez Mdel C, Herrera-Ortiz A, Rodriguez MH. Superoxide anion in Anopheles albimanus hemolymph and midgut is toxic to Plasmodium berghei ookinetes. J Parasitol. 2002;88:702–706. doi: 10.1645/0022-3395(2002)088[0702:SAIAAH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 41.Savvides SN, Scheiwein M, Bohme CC, Arteel GE, Karplus PA, Becker K, Schirmer RH. Crystal structure of the antioxidant enzyme glutathione reductase inactivated by peroxynitrite. J Biol Chem. 2002;277:2779–2784. doi: 10.1074/jbc.M108190200. [DOI] [PubMed] [Google Scholar]
- 42.Bian K, Gao Z, Weisbrodt N, Murad F. The nature of heme/iron-induced protein tyrosine nitration. Proc Natl Acad Sci USA. 2003;100:5712–7. doi: 10.1073/pnas.0931291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monzani E, Roncone R, Galliano M, Koppenol WH, Casella L. Mechanistic insight into the peroxidase catalyzed nitration of tyrosine derivatives by nitrite and hydrogen peroxide. Eur J Biochem. 2004;271:895–906. doi: 10.1111/j.1432-1033.2004.03992.x. [DOI] [PubMed] [Google Scholar]
- 44.Chege GM, Beier JC. Blood acquisition and processing by three Anopheles (Diptera: Culicidae) species with different innate susceptibilities to Plasmodium falciparum. J Med Entomol. 1998;35:319–323. doi: 10.1093/jmedent/35.3.319. [DOI] [PubMed] [Google Scholar]
- 45.Berner R, Rudin W, Hecker H. Peritrophic membranes and protease activity in the midgut of the malaria mosquito, Anopheles stephensi (Liston) (Insecta: Diptera) under normal and experimental conditions. J Ultrastruct Res. 1983;83:195–204. doi: 10.1016/s0022-5320(83)90077-1. [DOI] [PubMed] [Google Scholar]
- 46.Dix TA, Fontana R, Panthani A, Marnett LJ. Hematin-catalyzed epoxidation of 7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene by polyunsaturated fatty acid hydroperoxides. J Biol Chem. 1985;260:5358–5365. [PubMed] [Google Scholar]
- 47.Giorgio S, Linares E, Ischiropoulos H, Von Zuben FJ, Yamada A, Augusto O. In vivo formation of electron paramagnetic resonance-detectable nitric oxide and of nitrotyrosine is not impaired during murine leishmaniasis. Infect Immun. 1998;66:807–814. doi: 10.1128/iai.66.2.807-814.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fukuto JM, Switzer CH, Miranda KM, Wink DA. Nitroxyl (HNO): chemistry, biochemistry, and pharmacology. Annu Rev Pharmacol Toxicol. 2005;45:335–55. doi: 10.1146/annurev.pharmtox.45.120403.095959. [DOI] [PubMed] [Google Scholar]
- 49.Peterson TML, Luckhart S. A mosquito 2-Cys peroxiredoxin protects against nitrosative and oxidative stresses associated with malaria parasite infection. Free Rad Biol Med. 2006;40:1067–82. doi: 10.1016/j.freeradbiomed.2005.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]


