Abstract
Objective: To examine the change in Framingham risk score (FRS) arising from short-term treatment with ziprasidone or olanzapine.
Method: Hospitalized adults with a primary DSM-IV diagnosis of schizophrenia or schizo-affective disorder were randomly assigned to 6 weeks of double-blind treatment with ziprasidone or olanzapine from November 21, 1998 to September 28, 2000. Data on fasting lipid levels were collected at screening and endpoint, and blood pressure was measured at screening and baseline and weekly until week 6 of treatment (or last visit). FRS for patients aged ≥30 years was calculated using an algorithm derived from the Framingham Heart Study. Baseline-to-endpoint least-squares mean changes in age-adjusted FRS by gender were compared using analysis of covariance (baseline adjusted).
Results: Men who received olanzapine demonstrated a mean increase in their total cholesterol levels (+18.5 mg/dL; N = 53) and low-density lipoprotein cholesterol levels (+13.0 mg/dL; N = 45), whereas men who received ziprasidone demonstrated a mean decrease in their total cholesterol levels (−8.5 mg/dL; N = 44) and low-density lipoprotein cholesterol levels (−7.2 mg/dL; N = 40) (p = .0006 and p = .004, respectively). Additionally, men who received olanzapine showed an increase in baseline FRS (+7.69%; N = 53), whereas men who received ziprasidone showed a decrease in baseline FRS (−11.06%; N = 42) (p = .09). In women, treatment differences in FSR numerically favored ziprasidone but were not statistically significant. Neither treatment had a significant effect on blood pressure.
Conclusion: In short-term treatment, olanza-pine was associated with a significant worsening of lipid profile compared with ziprasidone, with a consequent increase in FRS versus ziprasidone. These findings, coupled with the significant weight gain in patients treated with olanzapine versus ziprasidone, warrant investigation in longer-term trials.
Schizophrenia is a chronic, disabling mental illness that affects approximately 1 in every 100 persons and is associated with increased medical comorbidity and mortality.1,2 In addition to an increased incidence of suicide, the rates of death from natural causes are higher for persons with schizophrenia than in the general population.2–5 In a study of 370 patients with schizophrenia followed for 13 years, there was a 3-fold increase in all-cause mortality, two thirds of which was attributable to natural causes.3 Much of this excess mortality is a result of cardiovascular disease (CVD), including coronary heart disease (CHD) and stroke.1,3,5,6 In patients with schizophrenia, rates of CVD are higher, with CVD mortality roughly double that in the general population.2 In a recent 5-year retrospective study of CVD risk in 3022 Canadian patients with schizophrenia, risk-adjusted odds increased significantly for ventricular arrhythmia, heart failure, stroke, diabetes, and all-cause mortality.7 The investigators noted that it was unclear whether this increased risk was attributable to an underlying biological factor associated with schizophrenia, lifestyle habits, poor health care in this population, or drug therapy.
It is well established that weight gain, diabetes, dyslipidemias, and hypertension are risk factors for CVD.8 Patients with schizophrenia demonstrate higher rates of obesity, diabetes, dyslipidemia, and hypertension than do individuals in the general population,2,9,10 possibly as a result of lifestyle factors. The schizophrenia population also has demonstrated increased rates of smoking, poor diet, lack of exercise, and alcohol and substance abuse, which are thought to be related to the development of metabolic disorders.1,10 One study found rates of type 2 diabetes as high as 18% to 30% among family members of patients with schizophrenia11 versus 4.8% in the U.S. population,12 which suggests a strong link between schizophrenia and type 2 diabetes. Evidence of a link between schizophrenia and type 2 diabetes is further supported by a recent report of impaired fasting glucose tolerance, insulin resistance, and high levels of plasma glucose, cortisol, and insulin in first-episode, drug-naive patients with schizophrenia.13
Patients with schizophrenia are likely to require long-term antipsychotic medication, and, given that they have an increased baseline risk of diabetes and dyslipidemia, it is important that their medication does not further increase the risk for these conditions.14 Atypical antipsychotic agents are currently the treatment of choice for schizophrenia because they are effective in treating positive and negative symptoms15,16 and have improved tolerability profiles over older, conventional antipsychotic agents (e.g., a lower incidence of extrapyramidal side effects and tardive dyskinesia).15 Although some atypical antipsychotic agents have been associated with weight gain and dyslipidemia,17–19 this is not the case for all agents.18 The American Diabetes Association/American Psychiatric Association guidelines published in 2004 in Diabetes Care and The Journal of Clinical Psychiatry divided the atypical antipsychotic agents into 3 groups based on their propensity to cause weight gain and dyslipidemia.20 Olanzapine and clozapine are associated with the highest level of risk, quetiapine and risperidone with an intermediate level of risk, and ziprasidone and aripiprazole with the lowest level of risk.
In some cases, the weight gain associated with antipsychotic medication can be sufficiently severe to result in an increased risk of new-onset diabetes and dyslipidemias in treated patients.18,19,21 Olanzapine has demonstrated the greatest effect on weight, with mean gain exceeding 10 kg (22 lb) during the first year of exposure at commonly prescribed doses.2 Olanzapine is also associated with disturbances in glucose metabolism, including diabetes and ketoacidosis,22 and with increases in fasting total cholesterol, low-density lipoprotein cholesterol (LDL-C), and triglyceride levels.19,23
Simpson et al.24 found that while olanzapine and ziprasidone were equally effective in patients with an acute exacerbation of schizophrenia, significant baseline-to-endpoint increases in fasting total cholesterol, LDL-C, triglyceride levels, weight, and body mass index (BMI) were seen in the olanzapine group and not the ziprasidone group. The unfavorable metabolic changes seen with olanzapine were sustained for 6 months and have been replicated in a separate 6-month, randomized, double-blind trial.24,25 Consistent with these studies, antipsychotic-induced weight and metabolic changes were reversed in a study of patients switched to ziprasidone from conventional antipsychotic agents, olanzapine, or risperidone.26 Switching patients to ziprasidone was associated with significant reductions in BMI during a 6-week period that were sustained at a 1-year follow-up.26 Similar substantial decreases in total cholesterol and triglyceride levels were seen in patients switched to ziprasidone from conventional antipsychotic agents, olanzapine, and risperidone.
The current study evaluated the change in Framingham risk score (FRS)27 after short-term treatment with ziprasidone or olanzapine in hospitalized adults with schizophrenia. This measure of CHD risk has been used extensively in clinical and epidemiologic studies, and its utility is well established.28–30 Use of the FRS in schizophrenia populations is relatively new, although one prior study used it to determine CHD risk of 689 patients with schizophrenia from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study as compared with age-, race-, and gender-matched controls.31 They found, as expected, that the FRS was significantly elevated in male and female patients with schizophrenia, compared with controls, and suggested that increased CHD is an important contributor to excess mortality in persons with schizophrenia. Randomized controlled trials, such as the present study, are necessary to help us understand the differences in risk posed by different antipsychotic agents.
METHOD
Study Background
Data for this post hoc analysis were obtained from a 6-week multicenter, randomized, double-blind trial24 that compared the safety and efficacy of ziprasidone and olanzapine in 269 hospitalized adults (aged 18 years and over) with a primary diagnosis of schizophrenia or schizoaffective disorder as defined in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). The inclusion and exclusion criteria for the original clinical trial limited the sample to subjects with normal laboratory findings at baseline, no significant abnormalities on electrocardiography, and no evidence of insulin-dependent (type 1) diabetes mellitus. All subjects provided written informed consent to participate in the study.
Subjects
A subset of 206 subjects, 129 men and 77 women over age 30 who participated in the clinical trial, were included in this analysis, which was conducted from November 21, 1998 to September 28, 2000. Subjects younger than 30 years were excluded because the Framingham risk algorithms do not include individuals younger than 30 years; therefore, it was not possible to establish valid estimates of CHD risk in younger populations using these risk equations.27,32
Treatment
Subjects were randomly assigned to ziprasidone (N = 136; mean dosage = 129.9 mg/day) or olanzapine (N = 133; mean dosage = 11.3 mg/day). Treatment was initiated with a fixed titration schedule to 160 mg/day for ziprasidone and 10 mg/day for olanzapine during week 1. At weeks 2 through 6, treatment was continued with flexible doses of ziprasidone, 80 to 160 mg/day, or olanzapine, 5 to 15 mg/day. Treatment compliance was measured by pill counts on returned medication. Vital signs (blood pressure, pulse) and body weight were evaluated at screening and baseline and weekly until week 6 of treatment (or last visit). Laboratory tests were completed at screening and week 6 (or last visit) and included the fasting fractionated serum lipids (total cholesterol, LDL-C, high-density lipoprotein cholesterol [HDL-C], and triglyceride levels).
FRS Analysis
Framingham risk score was calculated using algorithms developed by Wilson et al.27 for subjects aged 30 years and older. Change in FRS from baseline to endpoint was calculated separately for men and women in each treatment group. Variables included in the risk equations were age, total cholesterol or LDL-C levels, HDL-C levels, diabetes, smoking history, and blood pressure. Serum lipid levels and blood pressure were reevaluated at week 6, and any change in FRS was calculated based on changes in these parameters.
An analysis of covariance, with terms for treatment group, gender, treatment-by-gender interaction, and baseline value, was used to compare between-treatment differences in baseline-to-endpoint changes in total cholesterol, LDL-C, HDL-C, and triglyceride levels and FRS.
RESULTS
Subjects at Baseline
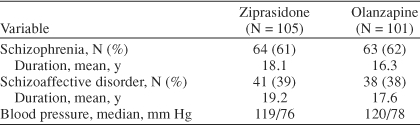
Subjects included in this analysis were randomly assigned to either ziprasidone (N = 105) or olanzapine (N = 101). The 2 treatment groups were comparable at baseline, with no clinically significant differences in age or weight. Both treatment groups had similar illness characteristics (Table 1), with roughly two thirds of each group having a primary diagnosis of schizophrenia and one third, schizoaffective disorder. The mean duration of illness was 18.5 years in the ziprasidone group and 16.8 years in the olanzapine group (p = NS). Baseline mean systolic and diastolic blood pressure findings were also similar between groups.
Table 1.
Illness History and Baseline Blood Pressure
Changes in Lipids and Blood Pressure
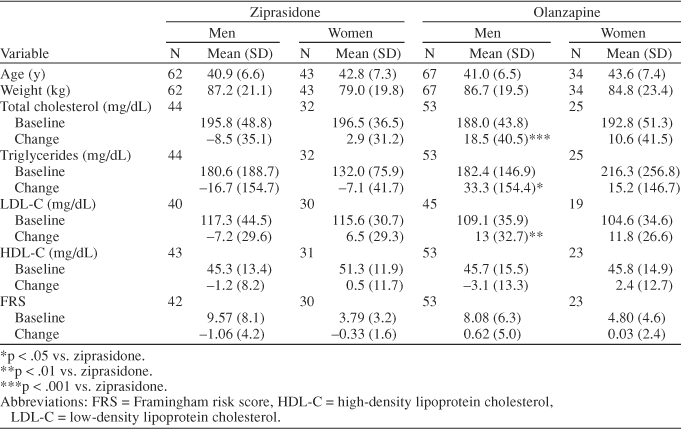
As shown in Table 2, men receiving olanzapine demonstrated increases in total cholesterol, LDL-C, and triglyceride levels from baseline to endpoint, whereas men receiving ziprasidone demonstrated decreases in total cholesterol, LDL-C, and triglyceride levels. The between-group differences in these changes were significant (p < .001 for total cholesterol, p < .01 for LDL-C, and p < .05 for triglyceride levels). Women receiving ziprasidone demonstrated a slight increase in total cholesterol and LDL-C levels, whereas women receiving olanzapine demonstrated a larger increase in these levels. Furthermore, women receiving ziprasidone demonstrated a slight decrease in triglyceride levels, whereas women taking olanzapine demonstrated an increase in triglyceride levels. There were no statistically significant between-group changes in HDL-C levels in men or women. There were no significant changes in blood pressure with either treatment.
Table 2.
Age, Weight, Lipids, and FRS and Change in Lipids and FRS in a 6-Week Study
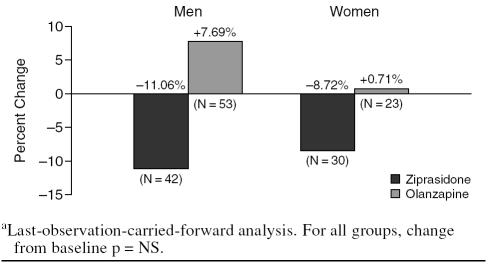
Change in FRS
Change in FRS showed a similar pattern of results, with men in the olanzapine group demonstrating greater increases in risk scores than men in the ziprasidone group, although this difference was not statistically significant at conventional levels (p = .09) (Table 2). Men treated with olanzapine (N = 53) showed a 7.69% increase in FRS over a 6-week period, whereas men treated with ziprasidone (N = 42) showed an 11.06% decrease in FRS over the same period (Figure 1). In women, numerical differences in FRS favored ziprasidone, but these changes were not statistically significant (p = .44) (Table 2). Women treated with olanzapine (N = 23) showed a 0.71% increase in FRS relative to baseline, whereas women treated with ziprasidone (N = 30) showed an 8.72% decrease in FRS relative to baseline (Figure 1).
Figure 1.
Percent Change in Framingham Risk Score (total cholesterol method)a
DISCUSSION AND CONCLUSIONS
Our findings indicated that men and women treated with olanzapine displayed an increase in FRS compared with patients treated with ziprasidone, although this difference was not statistically significant for women and reached only the trend level of significance for men (p = .09). The increase in FRS in men treated with olanzapine was attributable to significant increases from baseline in total cholesterol (p = .0006), LDL-C (p = .004), and triglyceride (p < .05) levels. Among women, there were numerically greater increases in total cholesterol, LDL-C, and triglyceride levels in patients receiving olanzapine than in patients receiving ziprasidone. These differences were not statistically significant, perhaps because the number of women in the study was smaller than men.
Interpretation of these results is limited by the short duration of the study and the small number of patients. The extent to which the lipid increases in olanzapine patients would persist with ongoing treatment cannot be determined from this trial, although medication-related changes in lipid levels have been observed over sustained periods in other trials.26,33 Furthermore, data from a double-blind, 6-month extension of this trial indicated that differences between ziprasidone and olanzapine in terms of lipid profile were sustained.34 Taken together with substantial evidence from large-scale clinical trials35–38 and meta-analyses38 showing the importance of lipid levels to subsequent CHD risk, these results suggest that the sustained use of olanzapine may elevate CHD risk, as measured by the Framingham risk equations. Further study of FRS change using larger populations and with extended follow-up assessments is required to confirm these conclusions.
Another limitation stemming from the short trial duration is that increases in CHD were assessed indirectly through a well-established prediction model. The FRS has been validated in numerous large-scale clinical and epidemiologic studies of CHD and has been compared with other proposed measures of risk (e.g., metabolic syndrome and natural logarithm of BMI).30,32 Although the present trial involved a small number of patients, baseline FRS values for men were in accord with those of the larger CATIE schizophrenia trial31 (9.4 in the CATIE trial and 8.1 to 9.6 in the present study).
The interrelationship of weight gain, dyslipidemia, type 2 diabetes, and cardiovascular disease has been well established and underscores the importance of minimizing the long-term metabolic consequences of antipsychotic treatment.39 The prevalence of diabetes has been estimated to be 2 to 6 times higher among individuals with schizophrenia than in the general U.S. adult population (6.5%).20 In response to increasing concern regarding the adverse metabolic effects of antipsychotic treatment, 4 medical associations, including the American Diabetes Association and the American Psychiatric Association, issued a consensus statement on antipsychotic drugs and obesity and diabetes.20 The panel recommended that patients be monitored for increases in weight and for the development or worsening of dyslipidemia at initiation and during the course of treatment. Patients who gain a clinically significant amount of weight or who experience worsening of dyslipidemia or glycemia should be switched to an antipsychotic agent that is not associated with weight gain or other metabolic adverse effects.20 Close monitoring and management of lipid levels is particularly important in patients with multiple risk factors for CHD.40
In summary, differential effects of ziprasidone and olanzapine treatment on FRSs were observed in a short-term, randomized, schizophrenia trial. These findings are consistent with known differences in the propensity of each agent for weight gain, dyslipidemia, and glucose dysregulation.20 Longer-term follow-up is needed to examine the adverse cardiovascular comorbidity and mortality implications of these findings for patients receiving atypical antipsychotic medications.
Drug names: aripiprazole (Abilify), clozapine (FazaClo, Clozaril, and others), olanzapine (Zyprexa), quetiapine (Seroquel), risperidone (Risperdal), ziprasidone (Geodon).
Footnotes
Supported by funding from Pfizer Inc., New York, N.Y.
The authors are employees of Pfizer.
REFERENCES
- Davidson M. Risk of cardiovascular disease and sudden death in schizophrenia. J Clin Psychiatry. 2002 63suppl 9. 5–11. [PubMed] [Google Scholar]
- Casey DE, Haupt DW, and Newcomer JW. et al. Antipsychotic-induced weight gain and metabolic abnormalities: implications for increased mortality in patients with schizophrenia. J Clin Psychiatry. 2004 65suppl 7. 4–18. [PubMed] [Google Scholar]
- Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212–217. doi: 10.1192/bjp.177.3.212. [DOI] [PubMed] [Google Scholar]
- Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11–53. doi: 10.1192/bjp.173.1.11. [DOI] [PubMed] [Google Scholar]
- Osby U, Correia N, and Brandt L. et al. Mortality and causes of death in schizophrenia in Stockholm county, Sweden. Schizophr Res. 2000 45:21–28. [DOI] [PubMed] [Google Scholar]
- Kendrick T. Cardiovascular and respiratory risk factors and symptoms among general practice patients with long-term illness. Br J Psychiatry. 1996;169:733–739. doi: 10.1192/bjp.169.6.733. [DOI] [PubMed] [Google Scholar]
- Curkendall SM, Mo J, and Glasser DB. et al. Cardiovascular disease in patients with schizophrenia in Saskatchewan, Canada. J Clin Psychiatry. 2004 65:715–720. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, and Black HR. et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003 42:1206–1252. [DOI] [PubMed] [Google Scholar]
- Dixon L, Postrado L, and Delahanty J. et al. The association of medical comorbidity in schizophrenia with poor physical and mental health. J Nerv Ment Dis. 1999 187:496–502. [DOI] [PubMed] [Google Scholar]
- Lambert TJ, Velakoulis D, and Pantelis C. Medical comorbidity in schizophrenia. Med J Aust. 2003 178(suppl). S67–S70. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Schnur DB, Reddy R. Family history of type 2 diabetes in schizophrenic patients. Lancet. 1989;1:495. doi: 10.1016/s0140-6736(89)91392-5. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Diabetes Surveillance System. Prevalence of Diabetes. Crude and Age-Adjusted Prevalence of Diagnosed Diabetes per 100 Population, United States, 1980–2004. Available at: http://www.cdc.gov/diabetes/statistics/prev/national/tprevage.htm. Accessed Jan 17, 2006. [Google Scholar]
- Ryan MC, Collins P, Thakore JH. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am J Psychiatry. 2003;160:284–289. doi: 10.1176/appi.ajp.160.2.284. [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Hennekens AR, and Hollar D. et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005 150:1115–1121. [DOI] [PubMed] [Google Scholar]
- Kane J. Progress defined: short-term efficacy, long-term effectiveness. Int Clin Psychopharmacol. 2001 16suppl 1. S1–S8. [DOI] [PubMed] [Google Scholar]
- Dossenbach M, Erol A, and el Mahfoud Kessaci M. et al. Effectiveness of antipsychotic treatments for schizophrenia: interim 6-month analysis from a prospective observational study (IC-SOHO) comparing olanza-pine, quetiapine, risperidone, and haloperidol. J Clin Psychiatry. 2004 65:312–321. [PubMed] [Google Scholar]
- Allison DB, Mentore JL, and Heo M. et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999 156:1686–1696. [DOI] [PubMed] [Google Scholar]
- Meyer JM. Effects of atypical antipsychotics on weight and serum lipid levels. J Clin Psychiatry. 2001 62suppl 27. 27–34. [PubMed] [Google Scholar]
- Meyer JM, Koro CE. The effects of antipsychotic therapy on serum lipids: a comprehensive review. Schizophr Res. 2004;70:1–17. doi: 10.1016/j.schres.2004.01.014. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- Ollendorf DA, Joyce AT, Rucker M. Rate of new-onset diabetes among patients treated with atypical or conventional antipsychotic medications for schizophrenia. MedGenMed. 2004;6:5. [PMC free article] [PubMed] [Google Scholar]
- Koller EA, Doraiswamy PM. Olanzapine-associated diabetes mellitus. Pharmacotherapy. 2002;22:841–852. doi: 10.1592/phco.22.11.841.33629. [DOI] [PubMed] [Google Scholar]
- Weiden PJ, Simpson GM, and Potkin SG. et al. Effectiveness of switching to ziprasidone for stable but symptomatic outpatients with schizophrenia. J Clin Psychiatry. 2003 64:580–588. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Glick ID, and Weiden PJ. et al. Randomized, controlled, double-blind multicenter comparison of the efficacy and tolerability of ziprasidone and olanzapine in acutely ill inpatients with schizophrenia or schizoaffective disorder. Am J Psychiatry. 2004 161:1837–1847. [DOI] [PubMed] [Google Scholar]
- Breier A, Berg PH, and Thakore JH. et al. Olanzapine versus ziprasidone: results of a 28-week double-blind study in patients with schizophrenia. Am J Psychiatry. 2005 162:1879–1887. [DOI] [PubMed] [Google Scholar]
- Weiden PJ, Loebel A, and Yang R. et al. Course of weight and metabolic benefits 1 year after switching to ziprasidone. Poster presented at 157th annual meeting of the American Psychiatric Association; May 1–6, 2004; New York, NY. [Google Scholar]
- Wilson PW, D'Agostino RB, and Levy D. et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998 97:1837–1847. [DOI] [PubMed] [Google Scholar]
- Kieltyka L, Urbina EM, and Tang R. et al. Framingham risk score is related to carotid artery intima-media thickness in both white and black young adults: the Bogalusa Heart Study. Atherosclerosis. 2003 170:125–130. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Wilson PW, and Larson MG. et al. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am J Cardiol. 2004 94:20–24. [DOI] [PubMed] [Google Scholar]
- Wannamethee SG, Shaper AG, and Lennon L. et al. Metabolic syndrome vs Framingham risk score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005 165:2644–2650. [DOI] [PubMed] [Google Scholar]
- Goff DC, Sullivan LM, and McEvoy JP. et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005 80:45–53. [DOI] [PubMed] [Google Scholar]
- Mahoney LT, Burns TL, and Stanford W. et al. Usefulness of the Framingham risk score and body mass index to predict early coronary artery calcium in young adults (Muscatine Study). Am J Cardiol. 2001 88:509–515. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, and McEvoy JP. et al. Effectiveness of antipsy-chotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005 353:1209–1223. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Weiden PJ, and Pigott T. et al. Six-month, blinded, multicenter continuation study of ziprasidone versus olanzapine in schizophrenia. Am J Psychiatry. 2005 162:1535–1538. [DOI] [PubMed] [Google Scholar]
- Turnbull F. Blood Pressure Lowering Treatment Trialists Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003 362:1527–1535. [DOI] [PubMed] [Google Scholar]
- Sever PS, Dahlof B, and Poulter NR. et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003 361:1149–1158. [DOI] [PubMed] [Google Scholar]
- Julius S, Kjeldsen SE, and Weber M. et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004 363:2022–2031. [DOI] [PubMed] [Google Scholar]
- Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Merz CN. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel 3 Guidelines. J Am Coll Cardiol. 2004;44:720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel 3) Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]