Abstract
Binge ethanol (EtOH) consumption suppresses inflammatory responses and resistance to infection, but paradoxically it is associated with increased levels of acute phase proteins (which are indicators of inflammation) and an increased risk of inflammation mediated pathologies such as cardiovascular disease and cirrhosis of the liver. The latter effect may be mediated by increased translocation of bacteria leading to activation of toll-like receptor 4 (TLR4). In this study, the dose-response and time course of the effects of EtOH alone or EtOH in conjunction with a TLR 4 agonist (LPS) were evaluated in mice. Ethanol alone at a dosage of 6 g/kg induced an acute phase response (as indicated by ELISA for serum amyloid A and serum amyloid P) that was maximal 24 hr after dosing. Lower dosages of EtOH did not have this effect but did suppressed the acute phase response to LPS and the production of IL-6 up to 3 hr after dosing. EtOH at 6 g/kg did not induce an acute phase response in C3H/HeJ (TLR4 mutant) mice, indicating that this response is mediated through TLR4. These results provide a resolution for the apparently paradoxical pro- and anti-inflammatory actions of EtOH with regard to acute phase responses.
Keywords: ethanol, cardiovascular disease, inflammation, acute phase response, animal model
Introduction
Results from a number of studies indicate that consumption of moderate amounts of ethanol decrease the risk of heart disease (Stewart 2002; Imhof & Koenig 2003; de Lange et al. 2004). The beneficial effects of moderate ethanol consumption may result from ethanol's effect on inflammation. Ethanol alters concentrations of acute phase proteins in human subjects, and these are among the most stable and predictive inflammatory markers with regard to cardiovascular disease (Imhof et al. 2001). Non-drinkers and heavy drinkers have elevated levels of C-reactive protein (CRP, a major acute phase protein in humans) as compared to moderate drinkers (Imhof et al. 2001; Stewart 2002; Volpato et al. 2004). The pattern of drinking may be as important as the amount, and binge drinking is associated with an increased risk of cardiovascular disease (Britton & McKee 2000). Although it may seem that this is merely a matter of suppression of inflammation by ethanol at low dosages and enhancement at high dosages, the situation is more complex. For example, many experimental studies in animals (Kolls et al. 1995; Vinson et al. 1998) and a few in human subjects (Gluckman & MacGregor 1978) indicate that binge EtOH consumption is anti-inflammatory for at least a few hours after high dosages. However, excessive consumption of EtOH by humans is associated with increased risk of conditions that are basically inflammatory in nature such as cirrhosis of the liver and cardiovascular disease. Resolving this apparent paradox was the major goal of this study.
Acute phase proteins can be induced by the binding of certain foreign ligands to toll-like receptors (TLRs) (Michelsen et al. 2004). TLRs are transmembrane receptors that normally bind to microbial components such as lipopolysaccharide (LPS) and subsequently activate a signaling pathways that lead to production of cytokines, some of which can induce an acute phase response (Michelsen et al. 2004). In particular, TLR4, which recognizes LPS, is closely associated with cardiovascular disease (Arroyo-Espliguero et al. 2004; Michelsen et al. 2004). Therefore, it was of interest to determine the role, if any, for TLR4 (and hence for LPS) in any increases in acute phase proteins that may be associated with ethanol consumption.
One cytokine (IL-6) and two acute phase proteins (serum amyloid A and serum amyloid P, SAA and SAP) were selected for evaluation in this study. Activation of acute phase responses by a variety of stimuli (including LPS) is mediated largely by stimulation of the production of IL-6 (though other pro-inflammatory cytokines are upregulated to compensate in its absence), which then stimulates hepatocytes to produce acute phase proteins and release them into the circulation (Fattori et al. 1994). SAA is a representative acute phase protein that is conserved between human and mouse. Mouse SAP is a structural analog of and has considerable sequence homology with human CRP, and these two proteins are quantitatively the major acute phase proteins in mice and humans, respectively (Bottazzi et al. 2006).
Experimental studies of the relationships between ethanol consumption and acute phase responses in humans are severely limited by ethical considerations, and epidemiological studies have inherent limitations with regard to clearly establishing cause-effect relationships and detailed mechanisms. Therefore, it is surprising that the effects of ethanol and the role of ethanol-mediated increases in circulating LPS in the induction or suppression of acute phase responses have been examined in very few studies in an animal model (as indicated by lack of references identified in PubMed using the search terms: rats or mice, acute phase or serum amyloid A or P, and ethanol). Results reported here with a mouse model were consistent with results of human epidemiological studies, indicating that high dosages of ethanol induce an acute phase response that peaks at 24 hr, after the initial suppression of inflammation. However, low dosages do not induce an acute phase response but do inhibit acute phase responses induced by other stimuli.
Methods
Mice and treatments
Female B6C3F1 mice were obtained from Charles River Labs through the National Cancer Institute's Animal Program. Female mice for evaluation of the role of TLR4 were C3H/OuJ (wild type TLR4) and C3H/HeJ (mutant, hyporesponsive TLR4) were obtained from Jackson Labs (Hopkins et al. 1996). All mice were used at 8-12 weeks of age, and they were housed under specific pathogen free conditions in an AAALAC accredited facility. All animal procedures were done in accord with the NIH Guide and with LSU Health Sciences Center policies.
Mice were given ethanol as a 32% solution in tissue culture grade water (Sigma Chemical Co.) by oral gavage. This mouse model for binge drinking has been used for several years in this laboratory (Han et al. 1993; Han & Pruett 1995; Carson & Pruett 1996; Weiss et al. 1996; Wu & Pruett 1997; Collier & Pruett 2000). The peak blood ethanol concentration induced by various dosages of ethanol in this model have been determined: 6 g/kg, 0.5%; 5 g/kg, 0.4%; 4 g/kg, 0.2%; 3 g/kg, 0.15%; 2 g/kg, 0.1% (Carson & Pruett 1996). However, it should be noted that ethanol is cleared 2-3 times faster in mice than in humans (Carson & Pruett 1996). Therefore, the total exposure (expressed as area under the concentration vs. time curve) for mice will be less than for humans at an equivalent peak blood level. Consequently, a higher peak level must be attained in mice than in humans to provide equivalent total exposure. Even the upper range of blood levels in this mouse model (0.4-0.5%) is not rare in human beings (Urso et al. 1981). In some experiments, some groups of mice were given LPS (from Escherichia coli 0128:B5 from Sigma Chemical Co.) at 25 μg/mouse, intravenously. At the end of each experiment, mice were anesthetized and bled to obtain serum, which was then analyzed for IL-6 or acute phase proteins.
ELISA for IL-6, Serum Amyloid A protein (SAA), and Serum Amyloid P protein (SAP)
The serum concentration of IL-6 and SAA was determined using ELISA kits from BD Pharmingen and Biosource International, respectively, exactly according to manufacurer's instructions.
An ELISA was used that takes advantage of the affinity of SAP for trinitrophenol (TNP) (Serban & Rordorf-Adam 1986). The wells of flat bottom 96 well microtiter plates were coated with a solution of keyhole limpet hemocyanin-TNP (Biosearch Technologies, Catalog No. T-5060-5) at 10 μg/ml in 0.1 M sodium carbonate buffer, pH 9.6. Samples were diluted in a buffer containing calcium (necessary for binding of SAP to TNP) (1.42 g of Tris hydroxymethyl aminomethane hydrochloride (Fisher Scientific Cat. No. BP153-1), 0.109 g of tris hydroxymethyl aminomethane (Fisher Scientific Cat. No. BP154-1), 8.49 g NaCl, and 0.74 g of calcium chloride per liter). The standard was purified mouse SAP (Calbiochem, Cat. No. 565193), and standards and samples were diluted in the sample buffer and diluted appropriately before being added to the coated plate. Wells were washed 4 times between each reagent using sample buffer with 0.2% bovine serum albumin (Sigma Chemical Co.). This buffer was also used for antibodies. After one hour of incubation with standards and samples, plates were washed and sheep anti-mouse SAP antibody (Alpha Diagnostic International, Cat. No. SAP14-S) diluted 1/10000 was added to all wells (except blanks). After a 1 hr incubation and 4 washes, a peroxidase conjugated anti-sheep Ig at 1/5000 was added to the wells. After 1 hr of incubation and 4 washes, Tetramethylbenzidine (TMB) substrate (Sigma Prod. No. T8665) was added to the wells. After color developed, 50 μl of stop solution (2 N H2SO4) was added to each well. This method was based on a previously published procedure (Serban & Rordorf-Adam 1986).
Statistical evaluation of results
In experiments in which more than two groups needed to be compared, analysis of variance followed by Newman-Keul's post hoc test was used to compare each group to every other group. This was done using Prism 4.0 software (GraphPad, San Diego, CA), and the test for the normality of the data implemented by Prism did not indicate significant deviations from normality in the data shown here. Experiments in which 2 groups were compared were analyzed by a two-tailed, unpaired Student's t test (also using Prism 4.0).
Results
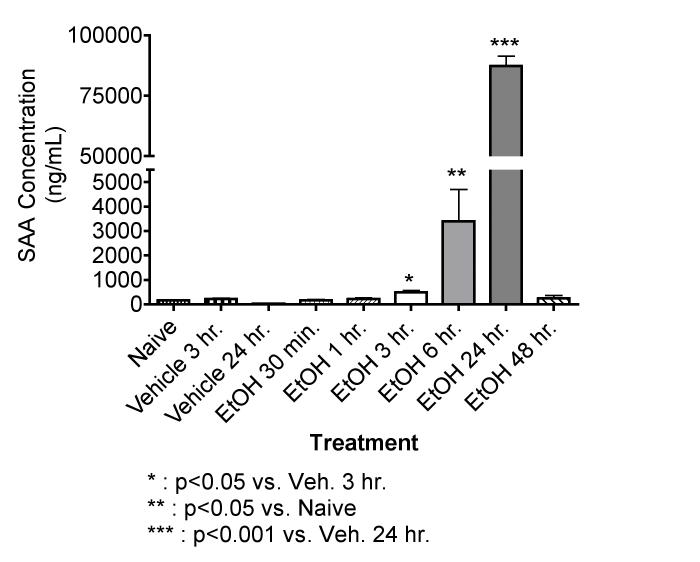
EtOH at 6 g/kg induces the production of SAA in a time-dependent manner
As shown in Figure 1, EtOH by gavage at 6 g/kg caused a significant increase in SAA measured beginning as early as 3 hr after dosing, peak levels are noted at 24 hr, and values had returned to normal by 48 hr. On the basis of this result, 24 hr was selected as the time for analysis of SAA and SAP in subsequent experiments. Basal levels of SAA shown here are comparable to those reported by others (1-5 μg/ml) (Lindhorst et al. 1997).
Figure 1.
Effects of EtOH at 6 g/kg on the Acute Phase Protein SAA in mice. EtOH treated mice were given 32 % EtOH solution by oral gavage at 6 g/kg. Vehicle treated mice received a volume of water by oral gavage corresponding to a 6 g/kg dose of 32 % EtOH solution. The naive group received no treatment. At the time points indicated, the mice were bled, and the SAA concentrations were determined by ELISA. The results shown are means ± SEM for groups of 5 mice each. These results were obtained in two separate experiments (30 min, 1 hr and 2 hr; 6 hr, 24 hr, and 48 hr).
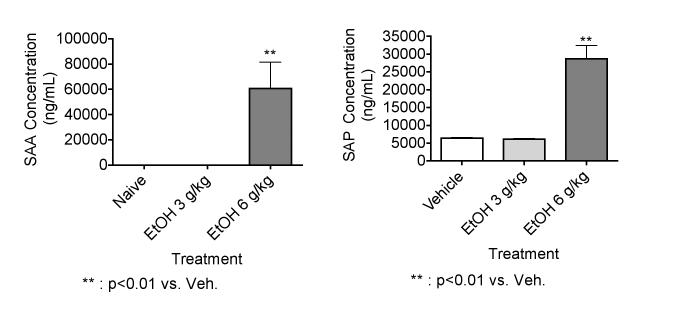
EtOH at 6 g/kg but not at 3 g/kg induces the production of SAA and SAP
Results shown in Figure 2 indicate that EtOH at 6 g/kg significantly increased SAA and SAP levels measured 24 hr after EtOH administration. However, EtOH at 3 g/kg did not have this effect, suggesting that the induction of an acute phase response by EtOH is dose-dependent. In a previous study, we reported that a 32% solution of EtOH at 7 g/kg caused obvious hemorrhaging and necrosis over broad areas of the stomach and duodenum in mice. EtOH at 6 g/kg (the concentration used in this study as well) caused only minor and sporadic hemorrhage, and lower dosages caused no detectable histological changes (Carson & Pruett 1996). However, this may be sufficient to allow LPS or other microbial components into the circulation and induce an acute phase response.
Figure 2.
Effects of EtOH at 3 g/kg and 6 g/kg on SAA and SAP. EtOH treated mice were given 32 % EtOH solution by oral gavage at 3 or 6 g/kg. Vehicle treated mice received a volume of water by oral gavage corresponding to a 6 g/kg dose of 32 % EtOH solution. The naive group received no treatment. Mice were bled 24 hr after EtOH administration, and SAA and SAP concentrations were determined by ELISA. The results shown are means ± SEM for groups of 5 mice each.
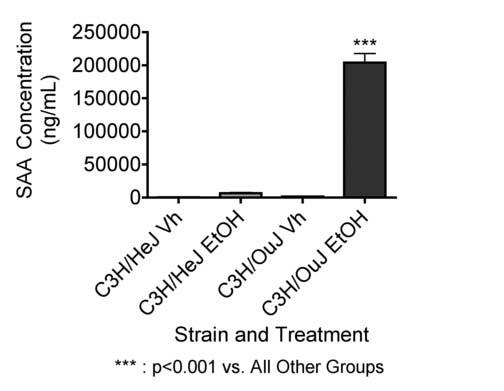
The SAA response to EtOH at 6 g/kg is mediated through toll-like receptor 4 (TLR4)
Results shown in Figure 3 indicate that C3H/OuJ mice, which have a functional wild-type TLR4, responded to EtOH at 6 g/kg with a substantial increase in the concentration of SAA measured 24 hr after EtOH treatment. In contrast, C3H/HeJ mice, which have a mutant hyporesponsive TLR4, did not respond with a significant increase in SAA under the same conditions. These results indicate that TLR4 is important in the acute phase response induced by EtOH administration. The major ligand for TLR4 is bacterial lipopolysaccharide (LPS) (Hirschfeld et al. 2000), so these results suggest a role for LPS, which has previously been implicated in liver diseases associated with EtOH consumption (Bode & Bode 2005). However, there is evidence that other ligands can also act through TLR4, so the role of LPS cannot be considered definitively established. The concentration of SAA was greater in C3H/OuJ mice than in previous experiments with C3H/HeN × C57Bl/6 mice (Figures 1 and 2). Although the cause of this difference is not certain, strain differences in the acute phase response to LPS have been reported (Mortensen et al. 1983).
Figure 3.
Role of TLR4 in the acute phase response to EtOH. EtOH treated mice were given 32 % EtOH solution by oral gavage at 5 g/kg. Vehicle treated mice received a volume of water by oral gavage corresponding to a 5 g/kg dose of 32 % EtOH solution. At 24 hr, the mice were bled, and the SAA concentrations were determined by ELISA. The results shown are means ± SEM and the group size for the vehicle groups was 3 and the group size for the EtOH groups was 7.
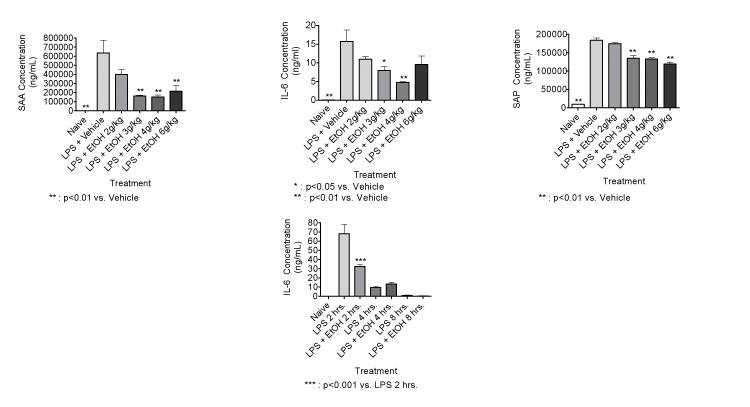
EtOH dose-responsively inhibits LPS-induced IL-6 production and production of acute phase proteins at 3 hr after administration
Results shown in Figure 4 indicate that EtOH at 3-6 g/kg significantly suppresses the IL-6 response to LPS (25 μg/mouse) as well as the acute phase response (indicated by SAA and SAP) 3 hr after LPS administration. The trend was generally dose-responsive, but there was less inhibition at 6 g/kg than at the next lower dosage. This may reflect simultaneous inhibition of response and induction of response as noted in Figure 1 at 3 hr in mice treated with EtOH only at 6 g/kg. Inhibition of LPS-induced IL-6 production by EtOH was observed at 2 hr (Figure 4, lower panel) and 3 hr (Figure 4, upper panel), but not at later times (Figure 4, lower panel). The concentration of IL-6 was almost undetectable by 8 hr. Thus, the inhibition of production of the cytokine that is primarily involved in induction of the acute phase response is short-lived, whereas the effects of EtOH alone on the acute phase response peaks much later (Figure 1). We did not evaluate the effects of EtOH on LPS-induced SAA production at later time points, because we wanted to evaluate IL-6 as well as SAA levels, and LPS-induced IL-6 production decreases to background levels by 8 hr after dosing, with a peak at 2 hr. Because SAA and SAP are induced primarily by IL-6, we wanted to focus on these early time points. The results are consistent with the idea that EtOH inhibits SAA and SAP production in part by inhibiting IL-6 production.
Figure 4.
EtOH dose-responsively inhibits SAA production in response to LPS (25 μg/mouse, iv) at 3 hr (in the upper panels), and the duration of the suppression of IL-6 is relatively brief (lower panel). Mice were treated with EtOH (using a 32 % EtOH solution by oral gavage) to achieve the indicated dosages. Vehicle treated mice received a volume of water by oral gavage corresponding to a 6 g/kg dose of 32 % EtOH solution. The naive group received no treatment. The mice were bled, and the SAA and IL-6 concentrations were determined by ELISA. The results shown are means ± SEM for groups of 5 mice each.
Discussion
As noted in the Introduction, there have been very few previous reports of the effects of ethanol on acute phase responses in mice or rats. However, one study does indicate that the rate of synthesis of acute phase proteins, but not the concentration of these proteins in blood is suppressed by ethanol in a rat model using turpentine to induce the acute phase response (Nadkarni & Pestonjamasp 1985). These results are difficult to compare with the results in the present study because none of the same acute phase proteins were examined in the two studies. To our knowledge the present report is the first report of the effects of EtOH on an LPS-induced acute phase response.
Previous studies in rodent models have demonstrated that acute EtOH administration generally suppresses inflammatory responses associated with resistance to infection (D'Souza et al. 1995; Greenberg et al. 1995; Mason et al. 1997). However, it has also been reported that EtOH consumption is associated with increases in inflammatory processes associated with liver disease (Bode & Bode 2005) and cardiovascular disease (Britton & McKee 2000). The results presented in the present study indicate that both of these effects can occur in response to the same treatment (EtOH at 6 g/kg). For example, EtOH at 6 g/kg activates an acute phase response 6-24 hr after administration in a manner that is TLR4-dependent. Although other ligands may be involved, it is likely that the major ligand involved in this activation is LPS. This would be consistent with previous reports indicating increased LPS in the circulation following EtOH consumption (Bode & Bode 2005). A comparison of results in Figures 1 and 4 indicates that EtOH alone and LPS alone at 3 hr after administration yield 486 and ∼65,000 ng/ml of SAA in the serum, respectively. Both of these responses are highly significant compared to untreated controls, but this difference suggests that the amount of LPS exposure due to increased intestinal permeability after acute ethanol, is much less than the amount administered in Figure 4. This is not surprising, because 25 μg is a substantial dose for of LPS for mice. Thus, our working hypothesis is that ethanol causes minor tissue damage in the upper gastrointestinal tract (Carson & Pruett 1996) which allows LPS from resident bacteria access to the circulation, leading to production of cytokines such as IL-6 that stimulate production of acute phase proteins in the liver.
However, we also noted that the same dosage of EtOH can inhibit the acute phase response induced by exogenous LPS when evaluated 3 hr after treatment. Also, the cytokine most often implicated in the induction of acute phase responses (IL-6) is only inhibited by EtOH for 3 hr, with no inhibition evident at 4 hr (Figure 4). This would seem to at least partially resolve the paradox that has been discussed for many years that excessive consumption of EtOH both suppresses inflammation in some situations and contributes to inflammation in others. In particular, it is interesting that the peak appearance of acute phase proteins occurs at 24 hr, well after EtOH would have been cleared (Carson & Pruett 1996). Thus, suppression of inflammation may predominate at lower dosages of EtOH, which do not induce an acute phase response, and increased inflammation or acute phase responses may predominate following higher dosages, but only after the EtOH is cleared. The duration of suppression (3 hr) is much less than the duration of the acute phase response (beginning at 6 hr and ending by 48 hr), so it would be expected that the net effect of high dosages of EtOH over a long period of exposure is to increase rather than decrease acute phase responses and inflammation.
Most studies involving EtOH and LPS in rodents have involved relatively long term exposures with EtOH in the drinking water or in a liquid diet preparation. The maximum EtOH concentration in these studies is about 20%. These studies are clearly relevant with regard to alcohol-dependent persons who do not primarily drink distilled beverages (with an EtOH content greater than 20%). However, there are many more binge drinkers than alcohol-dependent chronic drinkers (Wechsler et al. 2000), and many of these individuals drink distilled beverages (which have a high percentage of EtOH). Thus, a model is needed to reflect both the gastrointestinal damage associated with drinking EtOH at high concentrations and the suppressed inflammatory response that has been characterized in humans. The model described here seems to fill this need.
Acknowledgements
This work was supported by grant R01 AA009505 from the National Institute on Alcohol Abuse and Alcoholism.
References
- Arroyo-Espliguero R, Avanzas P, Jeffery S, Kaski JC. CD14 and toll-like receptor 4: a link between infection and acute coronary events? Heart. 2004;90(9):983–8. doi: 10.1136/hrt.2002.001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res. 2005;29(11 Suppl):166S–71S. doi: 10.1097/01.alc.0000189280.19073.28. [DOI] [PubMed] [Google Scholar]
- Bottazzi B, Garlanda C, Salvatori G, Jeannin P, Manfredi A, Mantovani A. Pentraxins as a key component of innate immunity. Curr. Opin. Immunol. 2006;18:10–15. doi: 10.1016/j.coi.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Britton A, McKee M. The relation between alcohol and cardiovascular disease in Eastern Europe: explaining the paradox. J Epidemiol Community Health. 2000;54(5):328–32. doi: 10.1136/jech.54.5.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson EJ, Pruett SB. Development and characterization of a binge drinking model in mice for evaluation of the immunological effects of ethanol. Alcoholism: Clin. Exp. Res. 1996;20:132–138. doi: 10.1111/j.1530-0277.1996.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Collier SD, Pruett SB. Mechanisms of suppression of poly I:C-induced activation of NK cells by ethanol. Alcohol. 2000;21(1):87–95. doi: 10.1016/s0741-8329(00)00087-2. [DOI] [PubMed] [Google Scholar]
- D'Souza NB, Mandujano JF, Nelson S, W.R. S, Shellito JE. Alcohol ingestion impairs host defenses predisposing otherwise healthy mice to Pneumocystis carinii infection. Alcohl. Clin. Exp. Res. 1995;19:1219–1225. doi: 10.1111/j.1530-0277.1995.tb01604.x. [DOI] [PubMed] [Google Scholar]
- de Lange DW, Hijmering ML, Lorsheyd A, Scholman WL, Kraaijenhagen RJ, Akkerman JW, van de Wiel A. Rapid intake of alcohol (binge drinking) inhibits platelet adhesion to fibrinogen under flow. Alcohol Clin Exp Res. 2004;28(10):1562–8. doi: 10.1097/01.alc.0000141808.62230.75. [DOI] [PubMed] [Google Scholar]
- Fattori E, Cappelletti M, Costa P, Sellitto C, Cantoni L, Carelli M, Faggioni R, Fantuzzi G, Ghezzi P, Poli V. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994;180(4):1243–50. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman SJ, MacGregor RR. Effect of acute alcohol intoxication on granulocyte mobilization and kinetics. Blood. 1978;52:551–559. [PubMed] [Google Scholar]
- Greenberg S, Xie J, Kolls J, Nelson S, Didier P, Mason C. Ethanol suppresses Mycobacteria tuberculosis-induced mRNA for nitric oxide synthase in alveolar macrophages, in vivo. Alcohol. Clin. Exp. Res. 1995;19:394–401. doi: 10.1111/j.1530-0277.1995.tb01521.x. [DOI] [PubMed] [Google Scholar]
- Han Y-C, Pruett SB. Mechanisms of ethanol-induced suppression of a primary antibody response in a mouse model for binge drinking. J. Pharmacol. Exp. Ther. 1995;275:950–957. [PubMed] [Google Scholar]
- Han YC, Lin T-L, Pruett SB. Thymic atrophy caused by ethanol in a mouse model for binge drinking: involvement of endogenous glucocorticoids. Toxicol. Appl. Pharmacol. 1993;123:16–25. doi: 10.1006/taap.1993.1216. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165(2):618–22. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- Hopkins W, Gendron-Fitzpatrick A, McCarthy DO, Haine JE, Uehling DT. Lipopolysaccharide-responder and nonresponder C3H mouse strains are equally susceptible to an induced Escherichia coli urinary tract infection. Infect Immun. 1996;64(4):1369–72. doi: 10.1128/iai.64.4.1369-1372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001;357(9258):763–7. doi: 10.1016/S0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]
- Imhof A, Koenig W. Alcohol inflammation and coronary heart disease. Addict Biol. 2003;8(3):271–7. doi: 10.1080/13556210310001602176. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Xie J, Lei D, Greenburg S, Summer WR, Nelson S. Differential effects of in vivo ethanol on LPS-induced TNF and nitric oxide production in the lung. Am. J. Physiol. 1995;268:L991–L998. doi: 10.1152/ajplung.1995.268.6.L991. [DOI] [PubMed] [Google Scholar]
- Lindhorst E, Young D, Bagshaw W, Hyland M, Kisilevsky R. Acute inflammation, acute phase serum amyloid A and cholesterol metabolism in the mouse. Biochim Biophys Acta. 1997;1339(1):143–54. doi: 10.1016/s0167-4838(96)00227-0. [DOI] [PubMed] [Google Scholar]
- Mason CM, Dobard E, Summer WR, Nelson S. Intraportal lipopolysaccharide suppresses pulmonary antibacterial defense mechanisms. J. Infect. Dis. 1997;176:1293–1302. doi: 10.1086/514125. [DOI] [PubMed] [Google Scholar]
- Michelsen KS, Doherty TM, Shah PK, Arditi M. TLR signaling: an emerging bridge from innate immunity to atherogenesis. J Immunol. 2004;173(10):5901–7. doi: 10.4049/jimmunol.173.10.5901. [DOI] [PubMed] [Google Scholar]
- Mortensen RF, Beisel K, Zeleznik NJ, Le PT. Acute-phase reactants of mice. II. Strain dependence of serum amyloid P-component (SAP) levels and response to inflammation. J Immunol. 1983;130(2):885–9. [PubMed] [Google Scholar]
- Nadkarni GD, Pestonjamasp KN. Effect of ethanol on turpentine-induced acute phase response in rats. Biochem Pharmacol. 1985;34(4):525–7. doi: 10.1016/0006-2952(85)90184-4. [DOI] [PubMed] [Google Scholar]
- Serban D, Rordorf-Adam C. Quantitation of serum amyloid P component by an enzyme-linked immunoassay. J Immunol Methods. 1986;90:159–164. doi: 10.1016/0022-1759(86)90071-2. [DOI] [PubMed] [Google Scholar]
- Stewart SH. Alcohol and inflammation: a possible mechanism for protection against ischemic heart disease. Nutr Metab Cardiovasc Dis. 2002;12(3):148–51. [PubMed] [Google Scholar]
- Urso T, Gavaler JS, Van Thiel DH. Blood ethanol levels in sober alcohol users seen in an emergency room. Life Sci. 1981;28(9):1053–6. doi: 10.1016/0024-3205(81)90752-9. [DOI] [PubMed] [Google Scholar]
- Vinson RB, Carroll JL, Pruett SB. Mechanism of supressed neutrophil mobilization in a mouse model for binge drinking: role of glucocorticoids. American Physiological Society. 1998;275:R1049–R1057. doi: 10.1152/ajpregu.1998.275.4.R1049. [DOI] [PubMed] [Google Scholar]
- Volpato S, Pahor M, Ferrucci L, Simonsick EM, Guralnik JM, Kritchevsky SB, Fellin R, Harris TB. Relationship of alcohol intake with inflammatory markers and plasminogen activator inhibitor-1 in well-functioning older adults: the Health, Aging, and Body Composition study. Circulation. 2004;109(5):607–12. doi: 10.1161/01.CIR.0000109503.13955.00. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Lee JE, Kuo M, Lee H. College binge drinking in the 1990s: a continuing problem. Results of the Harvard School of Public Health 1999 College Alcohol Study. J Am Coll Health. 2000;48(5):199–210. doi: 10.1080/07448480009599305. [DOI] [PubMed] [Google Scholar]
- Weiss PA, Collier SD, Pruett SB. Role of glucocorticoids in ethanol-induced decreases in expression of MHC class II molecules on B cells and selective decreases in spleen cell number. Toxicol. Appl. Pharmacol. 1996;139:153–162. doi: 10.1006/taap.1996.0154. [DOI] [PubMed] [Google Scholar]
- Wu W-J, Pruett SB. Involvement of catecholamines and glucocorticoids in ethanol-induced suppression of splenic natural killer cell activity in a mouse model for binge drinking. Alcohol. Clin. Exp. Res. 1997;21:1030–1036. [PubMed] [Google Scholar]