Abstract
Currently, there is a lot of interest in cannabis use as a risk factor for the development of schizophrenia. Cognitive dysfunction associated with long-term or heavy cannabis use is similar in many respects to the cognitive endophenotypes that have been proposed as vulnerability markers of schizophrenia. In this overview, we examine the similarities between these in the context of the neurobiology underlying cognitive dysfunction, particularly implicating the endogenous cannabinoid system, which plays a significant role in attention, learning and memory, and in general, inhibitory regulatory mechanisms in the brain. Closer examination of the cognitive deficits associated with specific parameters of cannabis use and interactions with neurodevelopmental stages and neural substrates will better inform our understanding of the nature of the association between cannabis use and psychosis. The theoretical and clinical significance of further research in this field is in enhancing our understanding of underlying pathophysiology and improving the provision of treatments for substance use and mental illness.
Medical subject headings: cannabis, cognitive dysfunction, endophenotypes, schizophrenia, endocannabinoids
Abstract
La consommation de cannabis comme facteur de risque d'apparition de la schizophrénie suscite actuellement beaucoup d'intérêt. Le dysfonctionnement de la cognition associé à la consommation de longue durée ou importante de cannabis ressemble à de nombreux égards aux endophénotypes cognitifs que l'on a proposés comme marqueurs de la vulnérabilité à la schizophrénie. Dans cet aperçu, nous analysons les similitudes entre ces facteurs dans le contexte de la neurobiologie qui soustend le dysfonctionnement de la cognition, en mettant en cause particulièrement le système cannabinoïde endogène qui joue un rôle important dans l'attention, l'apprentissage et la mémoire et, en général, dans les mécanismes régulateurs de l'inhibition dans le cerveau. Une étude plus attentive des déficits de la cognition associés à des paramètres particuliers de la consommation de cannabis et aux interactions avec les stades neurodéveloppementaux et les substrats nerveux nous aidera à mieux comprendre la nature du lien entre la consommation de cannabis et la psychose. L'importance théorique et clinique de recherches plus poussées dans ce domaine vise à nous aider à mieux comprendre la pathophysiologie sous-jacente et à améliorer la prestation des traitements contre les toxicomanies et les maladies mentales.
Introduction
There have been sporadic hypotheses regarding an association between cannabis use and schizophrenia for over 3 decades.1–4 Interest in this putative link has seen a recent resurgence as a result of further evidence from large-scale epidemiological studies,5–8 developments in understanding the neurobiological effects of cannabis9–11 and the neural substrates and predictors of schizophrenia.12–14 A meta-analysis of prospective studies determined a pooled estimated odds ratio of 2.1 (95% confidence interval [CI] 1.7–2.5) for prior cannabis use leading to the subsequent development of psychosis, an association that could not be explained by confounds or reverse causality, suggesting that cannabis is a component cause in the development and prognosis of schizophrenia.15 The purpose of this overview is not to provide another critique of the evidence for cannabis use as a risk factor in the development of schizophrenia — this has been admirably covered by several recent reviews16–18 and discussed at length in the epidemiological studies cited above. Rather, this paper focuses on a specific aspect pertinent to this association, that of cognitive dysfunction.
Cannabis intoxication impairs cognitive processes. There is an increasing body of evidence demonstrating that cannabis users show persistent deficits in specific cognitive functions beyond the period of acute intoxication. The extent of persistence of these deficits is still a matter of contention. Further, recent neurobiological studies have uncovered mechanisms involving the endogenous cannabinoid (eCB) system that inform the neural substrates underlying persistent deficits in cognition after repeated exposure to cannabis. In this paper, we integrate this evidence within the framework of endophenotypes of schizophrenia and propose that the similarity between the cognitive dysfunctions associated with cannabis use and schizophrenia is more than purely coincidental.
The endogenous cannabinoid system
The discovery of the eCB system over a decade ago spurred substantial animal research on the effects of exogenous and endogenous cannabinoids on receptor and overall brain function. Cannabinoid receptors (CB1) are the most abundant metabotropic receptors in the brain and are involved in many important physiological and behavioural events.9,11 They occur in high density at presynaptic terminals in regions involved in cognition, particularly learning and memory, in the hippocampus, prefrontal cortex (PFC), anterior cingulate, basal ganglia and cerebellum. The eCB system, via its endogenous ligands anandamide and 2-arachidonoyl-glycerol (2-AG), mediates the flow of information in the brain through retrograde signalling, modulating inhibitory and excitatory neurotransmitter release crucial for synaptic plasticity, depolarization-induced suppression of inhibition or excitation, long-term potentiation (and hence learning), memory and other higher cognitive functions.10,11,19,20 eCBs are synthesized on demand through cleavage of membrane precursors and are involved in various short-range signalling processes.19 Research has demonstrated alterations in the functioning of the brain in CB1-rich regions and in cognitively relevant neuromodulator systems (e.g., dopaminergic, cholinergic, serotonergic, gamma-aminobutyric acid [GABA]-ergic, glutamatergic) as a result of exposure to cannabinoids.19–21 Alterations in the functionality of the eCB system, such as receptor downregulation, desensitization and downstream effector changes accompanying the development of tolerance, dependence and resultant regional neuroadaptations, occur after the chronic administration of cannabinoids.22,23 There is also good evidence for alterations in the eCB system in schizophrenia, with an increased density of CB1 receptors in the dorsolateral prefrontal cortex (DLPFC)24 and anterior cingulate25 of postmortem brains of patients with schizophrenia and elevated levels of anandamide in the cerebrospinal fluid (CSF) in acute schizophrenia.26 Further, patients with schizophrenia show an enhanced sensitivity to the cognitive effects of delta- 9-tetrahydrocannabinol (THC).27
Endophenotypes of schizophrenia
Impaired cognition is a fundamental feature of schizophrenia. The impact on patients' daily lives is considerable, restricting functional capacity and contributing to social disability. Cognitive impairments are more strongly predictive of functional outcome than any other symptomatic measure, including overt psychotic symptoms,28 and residual impairments remain even with atypical antipsychotic medication.29,30
In a comprehensive meta-analyis of multiple aspects of schizophrenia, Heinrichs31 identified cognitive and psychophysiological aspects of brain function as the most powerful and robust case-control differences, rather than neuroanatomical or neurochemical alterations. Thirteen measures were found to produce effect sizes (ESs) large enough to describe abnormalities that occur in 50% of the schizophrenia population. Eight of these were cognitive psychometric findings, and another 3 used psychophysiological measures of cognition. The 2 measures with the largest ESs were the P50 evoked potential deficit (1.55; CI 1.21–1.89) and impaired general verbal memory (1.41; CI 1.20–1.62). Other cognitive measures pertained to tasks requiring learning, reasoning, selective attention, visual or auditory perception and expressive language. Only 2 neuroanatomical measures yielded ESs large and stable enough for inclusion, both pertaining to the hippocampus postmortem: reduced volume (0.92; CI 0.63–1.21) and reduced cell count (0.86; CI 0.38–1.34). Remarkably, no neurotransmitter receptor density differences (dopaminergic, glutamatergic or serotonergic) or neurodevelopmental findings yielded large or stable enough ESs for inclusion, despite the prominence of these in current hypotheses of schizophrenia. Only 1 neuroimaging measure, of reduced frontal brain metabolism (hypofrontality) during mental activity, met the minimum requirements for validity and stability. Accordingly, recent attention has focused on the characterization of putative cognitive endophenotypes of schizophrenia.
Endophenotypes are internal markers, that is, biochemical, physiological, neuroanatomical, neuropsychological, perceptual or cognitive measures of functional capacity. In complex disorders such as schizophrenia, endophenotypes are conceptualized as quantitative traits intermediate between the predisposing genes (genotype) and overt signs and expressed symptoms (phenotype), reflecting more proximal effects of gene action and being closer to the underlying neuropathology of the disorder.32,33 Research on endophenotypes can, in principle, assist in identifying aberrant genes conferring vulnerabilities to schizophrenia, because the number of genes producing variations in endophenotypes may be smaller, as endophenotypes are more elementary than the complex spectrum of symptoms and signs of psychiatric disorders.
For a marker to be defined as a genetically mediated endophenotype, certain criteria need to be met.32 The endophenotype must be associated with illness in the population, be heritable, be primarily state-independent, cosegregate with illness within families and be found in nonaffected family members at a higher rate than in the general population (see Snitz and others34). Several putative cognitive endophenotypes have been identified for schizophrenia. None of these have been shown to meet all criteria for a true endophenotype. Several measures show promise, and further research may validate their candidacy. Here we propose a taxonomy of endophenotypes based on conceptual distinctions about the domain of cognition that is most affected. The primary purpose of this taxonomy is to provide a means of organizing substantial literature on the nature of the cognitive deficits in schizophrenia into a smaller subset by capturing the most salient of the presumed mechanisms. However, while the proposed endophenotype clusters are conceptually distinguishable, they may not reflect the same cognitive mechanisms within clusters. Further, there may be a good deal of overlap between clusters. Future research will need to empirically evaluate this and other proposed taxonomies of cognitive deficits in schizophrenia.
Pre-attentive or automatic endophenotype
Three primary psychophysiological measures comprise evidence toward a pre-attentive endophenotype characterized by abnormalities in automatic processing of auditory stimuli: P50 suppression, prepulse inhibition (PPI) and the mismatch negativity (MMN) of the event-related potential (ERP). Each of these measures can be elicited in the absence of active attention and are therefore characterized as pre-attentive or automatic. Each has been shown to meet the most stringent of the criteria for an endophenotype — that is, they have been observed in nonaffected first-degree family members.
The P50, a positive component of the auditory evoked potential peaking around 50 ms poststimulus, provides a measure of sensory motor gating, because P50 amplitude is reduced to a test click when preceded by an identical conditioning click. The degree of suppression, indexed by the test:conditioning P50 ratio, reflects the brain's ability to automatically inhibit irrelevant repetitive sensory input. P50 test:conditioning ratios are larger in patients with schizophrenia35,36 and their first-degree relatives,36,37 consistent with impaired sensory gating mechanisms. A recent meta-analysis of 25 schizophrenia studies of P50 suppression determined an effect size of 1.56.38 P50 suppression shows linkage to the chromosome 15q14 locus of the α-7-nicotinic receptor gene.39 The neural substrates of the P50 effect have been identified as temporo-parietal (peri-Sylvian area near the auditory cortex), prefrontal cortical in an early pre-attentive phase and hippocampal in later attentive steps of the sensory gating process.40
Prepulse inhibition (PPI) of the startle reflex refers to the diminished response to a startling sound when it is preceded by a weaker sound and is also regarded as an index of sensory motor gating. PPI reduction in schizophrenia patients is the basis of most rodent models of schizophrenia.41 The neural substrates of PPI include the hippocampus, amygdala, thalamus and basal ganglia. PPI is modulated by an increase of mesolimbic dopamine and can be used as a marker of central serotonergic functioning in rodents and in humans.42
MMN is an ERP elicited by any discriminable change by a deviant stimulus within a regular background of repetitive auditory stimuli while attention is directed elsewhere. It is automatic or pre-attentive in that it is not reliant on active attention but on an intact auditory sensory memory. The neural generator of MMN is well established as the superior temporal gyrus, with a probable additional frontal generator. MMN amplitude is reduced in patients,43,44 and a meta-analysis of MMN studies in schizophrenia established an effect size of 0.99 (95% CI 0.79–1.29).45
Inhibition endophenotype
This endophenotype is characterized primarily by effortful response inhibition processes measured by well-validated behavioural inhibition tasks, such as the Go/No-Go Task and Stop-Signal Task, and others that require interference control of a prepotent response, such as the Stroop Task and the antisaccade task. Impairments on these tasks are not unique to schizophrenia, but the nature of the deficit in schizophrenia may be unique and involves the anterior cingulate cortex and inhibitory control networks in the PFC.46–50
Attention/working memory/dysexecutive endophenotype
Evidence for this endophenotype comes from deficits in tasks of sustained attention, working memory and other executive functions. Sustained attention is the capacity to maintain attention over a relatively prolonged period to detect infrequent targets, ensuring that goals of behaviour are maintained over time. This is most often measured with variants of continuous performance tasks (CPTs); versions differ in complexity and demands on other processes, such as working memory and sensory processing. People with schizophrenia are impaired on simple and complex versions, whereas nonaffected relatives are impaired only on more demanding versions of the task.51,52 Effect sizes of over 1.0 have been reported.53 The event-related P300 component elicited in auditory oddball attention tasks (essentially variants of CPT tasks), is also a strong candidate for endophenotypic status, with meta-analyses of patient54 and relative38,55 studies showing moderate effect sizes for reduced amplitudes and delayed latencies. We have recently confirmed reduced P300 amplitude in patient and family member groups but could find no evidence of latency changes in either group.36
Working memory is a multicomponent system involving active maintenance and manipulation of stored information critical for planning and guiding behaviour. It is, therefore, a core component of executive functions of cognition and is subserved by a network of prefrontal, parietal and subcortical regions of the brain. Several tasks are widely used to assess working memory, including the visual span subtest of the Wechsler Memory Scale; the spatial span and spatial working memory tasks of the Cambridge Neuropsychological Test Automated Battery (CANTAB); other spatial working memory tasks, such as oculomotor delayed response tasks; digits backward and letter-number sequencing subtests of the Wechsler Adult Intelligence Scale III; and n-back tasks. People with schizophrenia are consistently impaired on spatial working memory tasks, less reliably on verbal working memory tasks.56,57 Visuo-spatial working memory and attentional deficits in schizophrenia may also be regulated by α-7-nicotinic receptor stimulation, because cigarette smoking enhances performance on such tasks in patients, possibly explaining the high rate of nicotine use among patients.58 Functional magnetic resonance imaging (fMRI) studies of working memory have shown altered activation of the DLPFC in people with schizophrenia and their unaffected siblings with similar performance levels to control subjects.32 This suggests there is a functional inefficiency of the DLPFC. It has therefore been suggested that DLPFC activation during working memory may be a more sensitive endophenotype than performance on working memory tasks.32
People with schizophrenia are also impaired on executive tasks associated with frontal lobe function, such as the Wisconsin Card Sorting Test (WCST), verbal fluency tasks and the Tower of London.53,59
Verbal memory endophenotype
Evidence underlying this endophenotype comes from verbal declarative memory tasks where people with schizophrenia typically show learning deficits in acquisition or encoding.31,60 Increased rates of forgetting are present but mild. Verbal declarative memory is among the most impaired cognitive domains in schizophrenia.31,53 Impaired attention, symptom fluctuations and medication status do not account for the deficit.60 Verbal learning and recall of word lists and stories produce large effect sizes of around 1.4, distinguishing more than 70% of patients from control subjects.53 Verbal learning deficits are present in first-episode psychosis,61 remain stable over the course of illness62 and are evident in nonaffected relatives.63 They are underpinned by the medial temporal lobe and hippocampus and connections with PFC as part of a dysfunctional network in schizophrenia.
Eye movement control endophenotype
People with schizophrenia show abnormal eye movement control. Smooth pursuit eye movement deficits when tracking an object moving at a fixed speed have been reported.64 Typically, patients show low gain, that is, a slower speed of eye movement relative to the speed of the object, and exhibit increased catch up saccades.32 Other oculomotor disturbances in schizophrenia also contribute to this endophenotype, including antisaccade performance.
Evidence linking cannabinoid function to schizophrenia endophenotypes
In the sections below, we present evidence from studies of cannabis users and studies in which cannabinoids were administered to animals. We address the specific measures outlined above for each endophenotype of schizophrenia, with additional evidence from the cannabis literature pertinent to the conceptualization of each endophenotype. A recent review focused on the overlap between the acute effects of cannabis (and ketamine) on verbal and episodic memory and similar deficits in schizophrenia,65 emphasizing that these drug models can offer insights into the core pathophysiology of the disorder. Here we examine a more comprehensive range of cognitive functions within the schema of endophenotypes of schizophrenia and report on acute and chronic cannabinoid effects in human and preclinical research, considering neurobiological underpinnings with a focus on the eCB system. Table 1 summarizes the findings, with the evidence detailed below.
Table 1
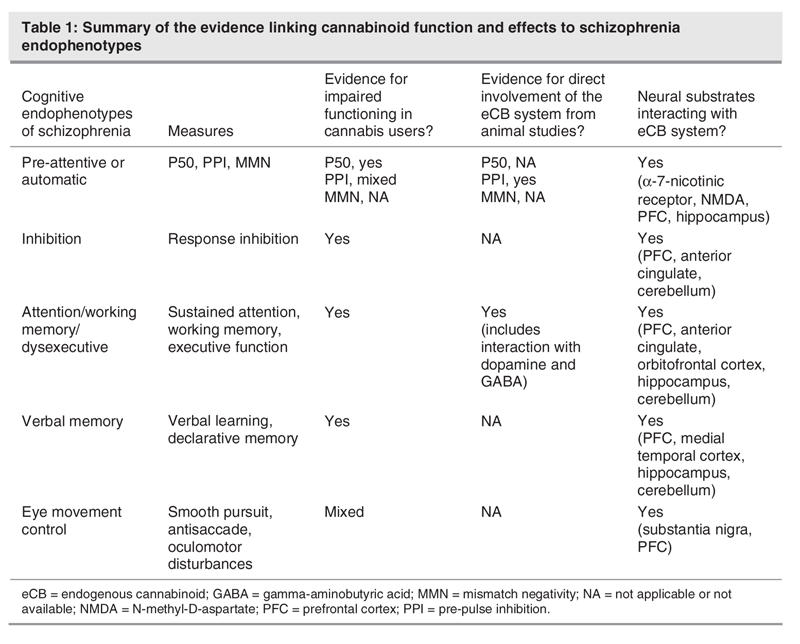
Preattentive/automatic endophenotype
A series of replication studies have shown P50 suppression to be reduced in chronic cannabis users who were rigorously screened and medically and psychiatrically normal and who did not use any substances other than cannabis.66–68 Those with the greatest extent of exposure to cannabis showed the greatest degree of reduction in P50 suppression. Anandamide modulates the α-7-nicotinic receptor,69 which is linked to P50 suppression, suggesting longer-term effects of smoked cannabis on this system.
Evidence for PPI reduction in human cannabis users has been mixed. PPI was reduced in longer-term cannabis users in one study and correlated with duration but not recency of cannabis use,70 whereas 2 studies found some evidence of altered startle reflex71 or no evidence of alteration in PPI in abstinent cannabis users.42 Effects on PPI from administration of cannabinoid agonists to animals have been mixed as well but do indicate some modulation of sensorimotor gating by the eCB system with apparent differences after short-versus longer-term administration. For example, cannabinoid agonists such as CP55,940 or WIN55,212-2 (WIN) have been shown to increase PPI,72 decrease PPI73,74 and reduce the startle response itself in the absence of a prepulse,74,75 confounding the ability to determine an effect on PPI. Several studies have shown that antagonism of the CB1 receptor by SR141716A alone has no effect on PPI, although this cannabinoid antagonist has been shown to reverse the disruption of PPI induced by dopamine or N-methyl-D-aspartate (NMDA) agonists or antagonists in some studies76,77 but not others.74 One study showed that acute and chronic administration of the anandamide reuptake and degradation inhibitor AM404 disrupted PPI in mice and that this effect was blocked by SR141716A.78 The authors interpreted these findings as indicative of a psychosis-like state after enhancement of anandamide bioavailability. Most recently, Bortolato and colleagues79 demonstrated no acute or chronic effects of WIN on PPI at any dose in Sprague-Dawley rats, although previous studies had demonstrated a disruption of PPI by this compound acutely in Wistar rats,73 suggesting that genetic differences may be critical for the development of cannabis-induced cognitive dysfunction.
Critical periods of neurodevelopment may also underlie cannabis-induced effects on PPI and, indeed, other cognitive functions. A long-lasting PPI deficit was found in adult rats after long-term (25 d) administration of WIN during puberty but not when WIN was administered during adulthood, suggesting that cannabinoids interfere with the development of the eCB system during puberty.80 The pubertal-treated rats also showed deficits in object recognition memory and performance on a progressive ratio operant behaviour task, whereas those treated in adulthood did not. The authors proposed cannabinoid administration during puberty as a model for the etiology of schizophrenia.
Interestingly, Malone and colleagues81 used an animal model to show how cannabis might precipitate psychosis in vulnerable individuals with compromised dopaminergic function. They found that THC alone did not affect PPI in mice, but when apomorphine was administered before THC, there was a significantly greater disruption than that caused by this dopamine agonist alone. Thus when sufficient dopaminergic stimulation is present, THC will exacerbate altered sensorimotor gating and could result in a compromised system being more vulnerable to the development of psychosis. While PPI continues to be widely used in animal models of psychosis, Braff and others41 caution that much work is required to clarify the degree of correspondence between pharmacological manipulation of PPI in animals and humans given the evidence of species differences.
There are no published studies of MMN in human cannabis users or in animal models of cannabinoid administration. MMN reduction is thought to be an index of deficient NMDA receptor functioning.82 NMDA antagonists, such as ketamine, reduce MMN in monkey models,83 and similarities between cognitive effects of ketamine and cannabis have been highlighted elsewhere.65 Research has shown that anandamide modulates NMDA receptor activity directly through proexcitatory potentiating effects as well as indirectly inhibiting activity through cannabinoid receptor–mediated inhibition of voltage-sensitive calcium channels.84 A dysfunction in the eCB system, for example, owing to cannabis use or to pathological processes in schizophrenia, might therefore be expected to impact upon MMN and other preattentive processes. Since cannabinoids modulate NMDA receptor activity, an investigation of MMN and other preattentive processes in cannabis users may prove interesting.
Inhibition endophenotype
Substance abuse disorders are generally thought to be characterized by behavioural disinhibition and low impulse control resulting from reduced neural inhibition.85 Inhibitory control is discussed below in the context of aberrant incentive salience. Few studies have investigated the effects of cannabis on the specific measures contributing to the inhibition endophenotype of schizophrenia, although many studies have determined effects of cannabinoids on various inhibitory processes and neural systems. These are mentioned throughout this paper. Antisaccade performance is discussed under the eye-movement endophenotype, below. Several recent neuroimaging studies of long-term, heavy cannabis users (or young adults prenatally exposed to cannabis) have found evidence of altered inhibitory processing (in the Stroop, No/No-Go and decision-making tasks involving response selection and inhibition) and are reviewed separately below.
The Stroop Task has frequently been assessed in human studies of cannabis users; impairments are found inconsistently.86–90 Where impaired performance on the Stroop was not clinically significant or did not differ from nonusers, performance decrements were nevertheless found to be related to cannabis use parameters, such as duration of cannabis use90 or dosage (joints/wk) interacting with lower IQ.89 Imaging studies show altered frontal cortical activation (DLPFC and anterior cingulate) during the interference condition of the Stroop Task, despite the reasonable task performance in cannabis users91 and 1-month abstinent cannabis users.92
One human study found that acute administration of THC increased impulsive responding on a Stop Signal Task but did not affect Go/No-Go Task performance,93 whereas another found evidence of a greater incidence of premature responding during intoxication, which was discussed in terms of failures of inhibitory control over inappropriate responses.94
Attention/working memory/dysexecutive endophenotype
Cannabis has been shown to affect sustained attention, as measured by the CPT after acute administration, as well as in some studies of long-term cannabis users (for review, see Solowij87). Pope and colleagues88 found CPT performance to be insensitive to long-term cannabis use, whereas Jacobsen and others95 found that adolescent cannabis users made significantly fewer correct hits than did nonusers. There was also a trend toward more false alarms with greater exposure to cannabis. A recent study of relative regional cerebral glucose metabolism in abstinent methamphetamine users performing a CPT task found that those who also regularly used cannabis showed lower glucose metabolism in orbitofrontal, temporal, hippocampal and parahippocampal regions during task performance, in the absence of overt performance deficits.96 Carefully controlled longitudinal studies of children prenatally exposed to cannabis have found impaired performance on CPT tasks between ages 6 and 12 years, with greater errors of commission and impulsivity errors.97–101 These deficits continue through adolescence (13–16 yr), with factor analysis of a range of attentional mechanisms demonstrating a specific impairment of stability of attention over time.102,103 Deficits in learning and memory are also apparent.101,104
Several studies have assessed sustained attention by means other than the CPT or have made inferences regarding sustained attentional processes from combined test data. For example, a recent study reported a disruption of sustained and transient attention after smoked cannabis in human volunteers105 that resulted in impaired memory task performance. Pope and Yurgelun-Todd86 interpreted the pattern of results from a large neuropsychological test battery administered to college students who used cannabis as reflecting a primary effect on the attentional/executive system, in particular, abilities to shift or sustain attention.
Other kinds of attentional processes, such as selective and divided attention, have also been investigated in cannabis users and were found to be impaired. Ehrenreich and colleagues106 found that cannabis users differed from control subjects on phasic alertness and divided attention and that early-onset cannabis use (before age 16 yr) was the strongest predictor of attentional dysfunction in adulthood on a visual scanning test. They attributed this to vulnerable periods during brain development that are subject to persistent alterations by exogenous cannabinoids. Fletcher and colleagues107 reported a 17-year follow-up of long-term cannabis users, in which older (i.e., 45 years of age) long-term users were found to perform more poorly than were older nonusers on complex tasks of selective and divided attention associated with working memory; no differences were found between younger (i.e., 28 years of age) users and nonusers. Conversely, Skosnik and others108 found that even light cannabis use (once/wk) in college students can result in increased disinhibition on a negative priming task, a measure of automatic inhibition of irrelevant information in an attention task. Negative priming performance is also impaired in patients with schizophrenia.109
We have identified specific deficits in selective attention processes in cannabis users, whereby the ability to focus attention and filter irrelevant information was progressively impaired with the number of years that cannabis was used.87,110,111 This was indexed by frontal brain ERP measures, whereas speed of information processing indexed by the latency of the P300 component was increasingly slower with increasing frequency of cannabis use, suggesting differential impairments associated with shorter-versus longer-lasting effects of cannabis. People with schizophrenia also inappropriately allocate attention to task-irrelevant stimuli.112 A recent study found evidence of impaired attentional processes in a similar ERP task in cannabis users,113 and a reduced P300 amplitude was more pronounced in early-onset cannabis users. P300 amplitude is thought to reflect the allocation of attentional resources as well as inhibitory brain processes. An association between a polymorphism of the cannabinoid receptor gene and the P300 component has been reported, with the gene contributing to 20% of the variance in frontal P300 amplitude.114 Several brain imaging studies of cannabis users have employed attentional tasks and are described below.
Impaired attentional processing has also been demonstrated in animal studies after the administration of cannabinoids.115–117 Some elegant studies pertinent to attentional deficits were conducted by Verrico and colleagues.118,119 They found that acute administration of THC potently increased dopamine metabolism and release in PFC but that repeat administration led to a persistent anatomically selective reduction of dopamine metabolism in PFC. This was found to underlie impairments on a visuospatial attention task that persisted for at least 14 days after the last drug administration (longer time periods were not tested). Interestingly, these deficits were transiently reversed by acute amphetamine, suggesting monoaminergic dysfunction related to the attentional deficits.
Cannabis alters the perception of time,87,90,93 and temporal processing is significantly disrupted in schizophrenia.120–123 Neural substrates implicated in these processes include the cerebellum, basal ganglia, PFC and parietal cortex. Animal studies have confirmed the involvement of the cannabinoid system in temporal processing, with specific mediation by cannabinoid receptors.114,124,125 Computational modelling suggested that the reduction in sensitivity to time induced by cannabinoids could be attributed to dysfunction in attentional mechanisms.125 Alternatively, it has been suggested that distortions in time judgement may be caused by deficits in strategic processing in cortical systems involved in encoding or rehearsal,126 once again suggesting the involvement of executive processes.
Working memory is also disrupted by cannabis. D'Souza and colleagues127 conducted a rigorous investigation of the effects of intravenous THC administered to healthy volunteers who had experience with cannabis use but who were not heavy users. THC impaired working memory, distractibility and verbal fluency and induced transient positive and negative schizophrenia-like symptoms. In other studies, performance, electroencephalography (EEG) and ERP measures were impaired on a spatial n-back task after smoked cannabis,105 and short-term THC administration impaired delay-dependent discrimination within working memory in a delayed-matching-to-sample (DMTS) task.128 Conversely, acute THC was found to spare perceptual priming and working memory but produced a riskier speed-accuracy trade off and impaired episodic memory, with no residual effects 24 or 48 hours later in infrequent cannabis users.129 Similarly, simple measures of working memory were relatively unimpaired by a low dose of THC administered to heavy, presumably tolerant cannabis users,94 but evidence of greater impulsivity in responding during intoxication may have reflected failures of inhibitory control.
Neuropsychological studies of long-term users in the unintoxicated state86,88–90,130 have generally reported various memory, attention and executive functions to be impaired (e.g., verbal fluency, WCST, Ravens Progressive Matrices, Stroop Task), but few have specifically assessed working memory. We have preliminary evidence of impaired working memory processes on several CANTAB measures.131 Several neuroimaging studies of cannabis users have used n-back and other working memory and executive function tasks, as reported below.
Despite a relative paucity of human studies, there is a substantial body of evidence from animal studies that establishes an unequivocal role of the eCB system in working memory and associated functions. A large number of studies conducted in the 1990s reported generally dose-dependent impairments from cannabinoid administration on radial arm and Morris water maze tests and DMTS tasks in rats and mice and showed that these were cannabinoid receptor-mediated, because they were reversed by SR141716A. These studies have been reviewed elsewhere132; we cite the more recent studies here. In several studies, Hampson and Deadwyler133,134 established dose-dependent cannabinoid reduction in hippocampal cell ensemble firing and impairment of DMTS performance that resembles hippocampal removal. Studies continue to confirm that the deficits are delay-dependent.135
Chronic exposure to cannabinoids has been found to result in lasting impairment of working memory in an object recognition task and social interaction (increased anxiety) in adolescent but not adult rats 21 drug-free days after 21 days of drug administration.136 Several studies have demonstrated impairments after short-and longer-term cannabinoid administration to rats and mice in the hippocampal-dependent Morris water maze task,137–139 and Varvel and colleagues140 have shown that these cannabinoid-induced impairments are dependent on interactions with GABA(A) receptors. THC administration pretest specifically impairs the acquisition of spatial learning and working memory performance on this task, while consolidation and retrieval of previously learned material is delay-dependent.139 Interestingly, impaired reversal learning and increased perseveratory behaviour in this task (as induced by stress) were accompanied by the downregulation of CB1 receptors and reduced 2AG levels in the hippocampus and were reversed by the administration of an exogenous cannabinoid (HU-210).141 Task acquisition was unimpaired by stress. Varvel and Lichtman138 also showed that CB1 knockout mice did not differ from wild type mice on acquisition but showed significant deficits in reversal learning, most likely because of perseveration. The evidence from this study strongly suggested that the eCB system may have a role in facilitating extinction or forgetting processes, as confirmed by subsequent studies, described below.
Learning on a virtual Morris water maze task in humans has been shown to be impaired by ketamine, and this impairment was related to the induction of schizophrenia-like symptoms.142 However, the authors distinguished the learning and memory mechanisms involved in this task from simple working memory processes that were unimpaired. This virtual task has been demonstrated to be impaired in humans with hippocampal damage143 and in people with schizophrenia.144 NMDA antagonism impairs learning by disrupting long-term potentiation (LTP) in the hippocampus, a hallmark of exogenous cannabinoid activity133; eCB-mediated modulation of NMDA receptor activity was discussed above. Studies have also determined the importance of cannabinoid receptor-mediated inhibition of hippocampal extracellular acetylcholine and D2 receptor activation in cannabinoid effects on working memory in rats.145,146 Fadda and others147 showed that potentiation and antagonism of THC-induced spatial working memory deficits in rats are dependent on the ratio between cannabidiol and THC.
Although interactions between cannabinoid receptors and their endogenous ligands have been shown to play an essential role in the extinction of aversive memories,148 involvement of the cannabinoid receptor has now been shown to be inessential for the extinction of positively reinforced memories.149 This may have implications for eCB involvement in reward mechanisms, and the CB1 knockout mice showed alterations in motivation.142 Further work by Alvares and colleagues150 supported a selective action, suggesting that the eCB system requires some degree of aversiveness to be recruited; effects were demonstrated in an aversive inhibitory avoidance task but not in an open-field habituation task. However, in this study, CB1 antagonism by AM251 was shown to disrupt memory consolidation, whereas antagonists such as SR141716A have generally shown facilitation of working memory (see Lichtman151) and agonists result in impaired memory function. The various compounds tested in animal studies have complex actions that are not yet fully understood, and may be acting as partial or inverse agonists or acting on a putative CB3 receptor.11 Differences are also observed between systemic administration and direct intrahippocampal injection. Alvares and colleagues150 proposed that increased levels of eCBs in the hippocampus that occur immediately after training contribute to facilitate memory consolidation, perhaps by decreasing the activity of GABAergic inhibitory networks. It appears that the eCB system is involved in modulating memory processes in a fine-tuned regulation; dysfunction either by excess or deficit may have adverse consequences in a task-dependent manner. For example, although pharmacological administration of cannabinoid agonists inhibits hippocampal LTP and impairs memory, eCBs can facilitate LTP at the single-cell level,152 possibly by eCB-mediated depolarization induced suppression of inhibition.10,19 Chronic exposure to THC blocks synaptic plasticity,22 but even a single exposure transiently modifies functional properties of cannabinoid receptors and abolishes the retrograde signalling that underlies eCB-mediated synaptic plasticity in the hippocampus and nucleus accumbens.153
The fine tuning role of the eCB system in regulating cortical information processing is increasingly apparent. Melis and others154 report a novel eCB-mediated self-regulatory role of dopamine neurons by which they release 2-AG selectively to suppress PFC-stimulation-evoked activity. They infer that a dysfunction in the eCB system may be involved in altered stress responses and contributes to inappropriate incentive salience to irrelevant stimuli. More specifically, they suggest that a functional eCB system might be a candidate for the modulation of the cortical afferents that provide a filter for nonsalient information and that are disrupted by long-term cannabis use manifested as a difficulty in filtering irrelevant information evident in our ERP studies of selective attention in cannabis users.87,110,111,155,156
Verbal memory endophenotype
Cannabinoids exert a profound influence on synaptic plasticity underlying learning and memory. Verbal learning and memory have been, perhaps, the most consistently impaired cognitive functions in studies of acute cannabis administration, as well as in long-term cannabis users. Recent research is highlighted here, while Solowij87 provides an extensive review of the literature on short-and longer-term effects on verbal memory. Performance on word list learning tasks, such as the Rey Auditory Verbal Learning Test (RAVLT), California Verbal Learning Test, Buschke's Selective Reminding task and variants has been demonstrated to be impaired in multiple neuropsychological studies of heavy or long-term cannabis users in the unintoxicated state,86,88–90,107,157,158 and impaired learning and retrieval of information were the only cognitive domains to demonstrate a significant effect size in a meta-analysis of a small number of select studies of cannabis users.159 Fletcher and others107 found that only older (≥ 45 yr) cannabis users differed from control subjects in list learning, whereas (≤ 28 yr) users were unaffected. Deficits in verbal learning and memory tasks in long-term heavy cannabis users have variously been attributed to duration of cannabis use,90 frequency of cannabis use88 or cumulative dosage effects.89 The most pronounced effects are found on recall after interference or delay, but impaired learning is apparent also in flatter learning curves, fewer words learned (recalled) on each trial and poorer total recognition performance. Intrusion errors are frequent. Block and colleagues'160 brain imaging study of verbal memory is described below. We are currently investigating verbal learning and memory processes in cannabis users and people with schizophrenia with and without comorbid cannabis use in a series of ongoing fMRI studies.161,162
Immediate and delayed recall of words has also been shown to be impaired by acute intravenous administration of THC to human volunteers.127 Recognition performance was spared in D'Souza et al's study,127 whereas Ilan and colleagues105 found that acute intoxication resulted in greater intrusion errors during recognition. Those subjects who were most affected by cannabis showed a reduced ERP difference between previously studied words and new distracter words, suggesting a disruption of neural mechanisms underlying memory for recent study episodes.105 Curran and colleagues129 found that a high dose of THC (15 mg) resulted in no learning occurring over a 3-trial selective reminding task.
Eye movement control endophenotype
Evidence for involvement of the eCB system in high-level control of eye movements and associated cognitive functions comes from Ploner and others'163 extensive investigation of oculomotor effects after acute oral administration of THC to human volunteers. They found that THC affected specific aspects of saccade control, namely, spatial attentional shifts, fine tuning of volitional saccades, spatial working memory and inhibition of inappropriate saccades. They suggested that the pattern of effects implied modulation of neuronal activity in substantia nigra pars reticulata and/or DLPFC, reflecting distribution of cannabinoid receptors and their involvement in inhibitory control of inappropriate saccades, rather than the eye fields and final motor pathway for saccades. Smooth pursuit eye movements, however, are not impaired by short-term THC administration to humans.164,165
Visual search has been investigated in several studies of cannabis users and of acute cannabinoid administration. Huestegge and colleagues166 found that longer-term cannabis users with an early age of onset of use showed less effective search behaviour, including longer response times and more fixations, conservative search patterns and frequent reinspections of previously fixated areas. They interpreted these findings, however, as reflecting an impairment in visual short-term memory and less effective visual processing at a more strategic, top–down controlled level, rather than specific eye-movement control deficits. Visual search processes have also been investigated in association with effects on driving ability.167,168
Recent neuroimaging studies of cannabis users
There has been a growing interest in the application of structural and functional brain imaging methods to gain insight into the neurobiology of cannabis effects on cognition, and neuroimaging techniques are providing a sensitive means of investigating the genetics of schizophrenia and its behavioural manifestation.169 Further, regional brain activation may more sensitively inform endophenotypes than performance measures on cognitive tasks.32 Positron emission tomography (PET) and regional cerebral blood flow (rCBF) studies of cannabis users have been used to assess neural activation during attention and memory tasks. O'Leary and colleagues170,171 examined acute and chronic effects of cannabis on rCBF using PET during dichotic listening (auditory attention) tasks. They found that cannabis intoxication resulted in increased blood flow in paralimbic regions and in the anterior cingulate and cerebellum, which they suggested was associated with the intoxicating and mood enhancing effects of the drug.170,171 Decreased blood flow was observed in temporal lobe regions sensitive to auditory attention, visual cortex and an attentional network consisting of frontal and parietal lobe regions and thalamus. Despite an intact performance on the relatively simple dichotic task, they interpreted the decreased flow as being related to perceptual and cognitive changes that occur with intoxication. Mathew and others172 found that acute intravenous administration of THC increased activity primarily in the right hemisphere in the orbitofrontal cortex, insula, cingulate gyrus and subcortical structures in a dose-dependent manner related to the degree of subjective intoxication.
Memory-related rCBF in frequent users was examined after at least 26 hours of supervised abstinence.160 Subjects learned a list of words (from the RAVLT) over multiple trials to a criterion of 2 perfect recalls, with Buschke's selective reminding technique, 1 day before the PET session. Cannabis users required significantly more trials than did control subjects to achieve the learning criterion, and they showed decreased memory-related blood flow in PFC, increased flow in memory-relevant regions of the cerebellum and altered lateralization in the hippocampus, with the greatest differences apparent in episodic encoding during new list learning. Users relied more on short-term memory, recalling more words than control subjects from the end of the word list and fewer from the middle. This pattern of altered distribution of memory processes contributes to poor list learning over trials. We have preliminary data from an fMRI study of verbal learning and memory in long-term cannabis users that suggests altered activation of frontal, medial temporal, parietal and cerebellar regions during encoding and retrieval of words learned from the RAVLT.161,162
Other paradigms employed in recent fMRI research with cannabis users have included visual attention, working memory, response inhibition and decision-making tasks. Chang and colleagues173 found similar task performance between current and former cannabis users and control subjects on a visual-attention task, but both user groups showed altered activation of frontal, parietal, occipital and cerebellar regions, some of which normalized with duration of abstinence. Earlier age of first use and greater cumulative dose of cannabis exposure were related to lower frontal and cerebellar activation and suggested neuroadaptive processes and greater use of reserve networks. Kanayama and colleagues174 assessed spatial working memory in heavy cannabis users with fMRI. Users made nonsignificantly more errors on the task and showed increased activation of brain regions typically used in spatial working memory tasks, such as the PFC and anterior cingulate, with the additional recruitment of areas not typically used in such tasks, such as the basal ganglia regions. The authors interpreted their findings in terms of cannabis users experiencing subtle neurophysiological deficits for which they compensate by working harder and calling on additional brain regions to meet the demands of the task. Increased activation of the anterior cingulate in particular was thought to reflect an increased effort to overcome cannabis-induced attentional impairments and to coordinate activity from the wide range of regions recruited to perform the task.
Two studies have assessed cannabis users on a decision-making task requiring intact executive functions, the Iowa Gambling Task. In contrast to Kanayama and colleagues' findings, Porrino and others175 found underactivation specifically of the anterior cingulate in heavy cannabis users in an fMRI study. The users showed poorer performance on the task, reflecting an inability to learn from previous experience. This was correlated with age of first cannabis use. The authors interpreted these findings in terms of cannabis use being associated with impaired decision making and learning, reflected by a failure to activate the anterior cingulate. Bolla and colleagues176 used PET to assess heavy and moderate cannabis users after 25 days of supervised abstinence. They found dose-related impairments in performance and regional brain activation. All users showed greater activation in the left cerebellum and reduced activation in the right lateral orbitofrontal cortex and right DLPFC than control subjects. The heavy group showed less activation in the left medial orbitofrontal cortex but greater activation of a large left hemisphere region, including the cerebellum, para-hippocampus/lingual gyrus and posterior cingulate, than the moderate users. A threshold effect was suggested, such that dysfunction may only become apparent after a certain amount of drug exposure is reached. Right cerebellar activation decreased as the number of years of cannabis use increased. In accordance with Porrino and colleagues' study175 learning was observed in moderate users and control subjects but was absent in heavy users. The pattern of behaviour on the task led Bolla and colleagues176 to interpret the faulty decision making in terms of heavy cannabis users focusing on immediate reinforcing aspects of a situation while ignoring the negative consequences. Differences in activation observed between these 2 studies may be a result of the different imaging technologies or the fact that Bolla and colleagues' cohort was abstinent for 25 days before testing.
Activation elicited by a modified Stroop Task has been examined in 2 studies. Eldreth and others92 used PET to assess 25-day abstinent cannabis users and found hypoactivity in the left anterior cingulate cortex and left PFC and hyperactivity in the bilateral hippocampus, compared with control subjects, despite intact task performance. The authors discussed possible recruitment of an alternative neural network as a compensatory strategy to overcome persistent cannabis-induced functional deficits. In an fMRI study, Gruber and Yurgelun-Todd91 found that cannabis users showed significantly lower anterior cingulate activity, higher midcingulate activity and altered activation of the DLPFC than did control subjects. The users made more errors of commission in the interference condition of the Stroop Task, but their task performance was good overall, suggesting again that they used different cortical processes to achieve similar task performance to control subjects. The altered frontal neural functioning during the performance of this task requiring inhibition and performance monitoring has implications for decision making ability.91
An fMRI study of adolescent cannabis users performing an n-back working memory task with additional selective attention load focused analyses on the hippocampus.95 Cannabis users were less accurate and failed to deactivate the right hippocampus across task conditions, compared with control subjects. The authors interpreted this as a dysfunction of inhibitory hippocampal interneurons that “may be mediated by cannabis-induced inhibition of neurotransmitter release disrupting hippocampal synaptic plasticity or by cannabis-induced apoptosis of hippocampal neurons.”95 Smith and colleagues177 used fMRI to investigate the effects of prenatal exposure to cannabis in adolescents aged 18 to 22 years on a Go/No-Go response inhibition task. They found increased activity of bilateral PFC and right premotor cortex and attenuation of left cerebellar activity with increasing prenatal exposure to cannabis. Exposed offspring also made more errors of commission. It was suggested that prenatal exposure to cannabis may induce long-lasting alterations (into young adulthood) in neural substrates underlying response inhibition.
Thus, the neuroimaging studies have shown altered blood flow or activation in the PFC, orbitofrontal cortex, anterior cingulate, basal ganglia, cerebellum and hippocampus. Alterations in the PFC, orbitofrontal and anterior cingulate (among other regions) support altered inhibitory processing in cannabis users and are interesting in light of the increased density of cannabinoid receptors that have been determined in these regions in schizophrenia patients postmortem.24,25 Activation of these regions is also altered in neuroimaging studies of schizophrenia using similar paradigms (see Niznikiewicz and colleagues178 for a review). Differential findings of overactivation or underactivation of brain regions are evident in these studies of human cannabis exposure and may reflect differences in tasks, performances and populations tested, and differing effects associated with various parameters of cannabis use, just as in the schizophrenia literature, hypoactivity and hyperactivity of brain regions may be task-, performance-or medication-dependent.178 For example, the DLPFC shows either increased (e.g.,179) or decreased activation (e.g.,180) in working memory tasks in people with schizophrenia, compared with control subjects. Reduced activation of specific brain regions in patients versus control subjects is observed more frequently than increased activation of the same regions when performance decrements are also present, but the latter more often accompanies similar performance between groups. This implies a functional inefficiency of that region, which is compensated for by recruiting additional regions to perform the task or the application of different strategies. Differential regional activation may also be explained by faulty functional connectivity (e.g., between temporal and frontal regions) contributing to functional abnormalities in schizophrenia.178 The complexity of effects from the vast neuroimaging literature in schizophrenia remains to be integrated, clarified and fully understood; neuroimaging studies of cannabis users are in their infancy and hold much promise for elucidating cognitive dysfunction associated with cannabis use and for exploring interactions with the neuropathology of schizophrenia.
Recovery of cognitive function / persistence of cognitive deficits
Investigations of recovery of cognitive function with abstinence from cannabis have produced conflicting evidence, with some studies suggesting full recovery after 28 days of abstinence,88 others showing partial early recovery after a mean 2 years abstinence87,155,156 and still others finding no recovery after 25 to 28 days of abstinence.89,92,176 The reasons for these differences are unclear but may be partly owing to varying tasks assessed and differing characteristic populations. Few studies have assessed very long-term users (histories of 20–30 years or more of use) as in our ERP and neuropsychological studies. In a reanalysis of their 2001 study, Pope and others157 found that deficits were more likely to persist beyond 28 days in participants who had commenced cannabis use before age 17. Recent studies of 1-month abstinent heavy cannabis users have reported elevated blood flow velocities181 and tissue composition changes, as measured by voxel-based morphometry.182 The latter study found grey and white matter density changes in the same regions (e.g., parahippocampal gyrus) that showed altered activation in a decision-making task176,182 with some density changes correlating with duration of cannabis use.
Changes associated with the number of years of cannabis use could reflect long-term neuroadaptations requiring significant time to revert to normal functioning. Changes associated with frequency or dosage of cannabis use may reflect adaptations associated with accumulation of drug residues that should resolve once these cannabinoids have been fully eliminated from the body/brain. In most cases, elimination occurs within 4 to 6 weeks of cessation of use. Where deficits have been shown to persist beyond this period of abstinence, neuroadaptations may differ in nature to those associated with cannabis use duration and may or may not be shorter lasting. Further research is required to elucidate the mechanisms involved in persistence or recovery of cognitive deficits in human cannabis users. Human patterns of use can extend to 20–30 or more years of heavy use. This has not been well-modelled by animal research on the long-term effects of cannabinoid administration. While the neurophysiology of the eCB system that might explain persistent cognitive deficits is exceedingly complex, G-protein coupled receptor dependent signalling involving metabotropic glutamate receptors (which initiate eCB release) can produce permanent changes in hippocampal synaptic transmission if cannabinoid stimulation is sufficiently prolonged.11
Genetic linkage to cognitive dysfunction and schizophrenia
Despite the multiplicity of evidence for multiple genes involved in schizophrenia, Coolen and colleagues183 have recently demonstrated in an animal model how a subtle imbalance in the expression of a single gene protein that is involved in a wide variety of developmentally important signalling pathways may be sufficient to form the molecular basis of a complex phenotype such as schizophrenia. The diversity of functional roles of the eCB system imply that a subtle imbalance in this system could manifest as a complex phenotype. The CB1 receptor gene is located on chromosome 6q14-q15.184 Suggestive evidence links global cognitive impairment in schizophrenia to susceptibility genes on chromosome 6,185 albeit in the 6p24 region, with CPT and RAVLT performance showing linkages to this region. Genetic variants of the CB1 receptor have been shown to differ between substance using and nonusing patients with schizophrenia.186 A triple repeat polymorphism of the CB1 receptor gene has been reported to be significantly associated with the hebephrenic subtype of schizophrenia.187 In that study, a 9- repeat allele of an AAT-repeat polymorphism of the cannabinoid receptor gene was associated with a 2.3-fold higher susceptibility to schizophrenia. Other studies have, however, failed to support an association between CB1 receptor polymorphisms and schizophrenia, psychotic symptoms or psychosis proneness.188–190
There is growing interest in epigenetic influences within the genotype-endophenotype-phenotype pathways in schizophrenia, whereby multiple genetic and environmental factors become integrated over time through dynamic processes.33 Levenson and Sweatt191 have described epigenetic mechanisms in memory formation and define epigenetics as “a set of self-perpetuating, post-translational modifications of DNA and nuclear proteins that produce lasting alterations in chromatin structure as a direct consequence, and lasting alterations in patterns of gene expression as an indirect consequence.”191 Alterations of DNA protein (chromatin) structure, which, in turn, regulates gene expression through histone acetylation, may mediate long-lasting behavioural changes in the context of learning and memory that require a highly coordinated pattern of gene expression. Exposure to learning paradigms that result in the formation of long-term memories leads to changes in histone acetylation. Levenson and Sweatt191 describe how exposure to various environmental conditions leads to changes in the epigenetic profile of the genome in relevant brain regions and propose that drugs that target the epigenome may be viable therapies for treating neurocognitive disorders. Mechanisms inherent in these processes include synaptic plasticity, LTP, depolarization-induced suppression of inhibition and NMDA receptor activation, all of which the eCB system is known to be directly involved in. This suggests a rich area for research on cannabinoid-mediated epigenetic mechanisms in schizophrenia from both a therapeutic perspective (synthetic cannabinoid agonist/antagonist medications) but also from a deleterious perspective in terms of the potentially negative effects of exogenous cannabinoids on the epigenome. Susceptible genes may influence vulnerability to environmental pathogens.192,193
Short-and long-term administration of antipsychotic drugs have been shown to alter the expression of genes involved in synaptic plasticity and intracellular calcium regulation.194,195 Calcineurin is a calcium-and calmodulin-dependent protein phosphatase that plays a significant role in brain development and synaptic plasticity and affects working memory. Calcineurin-knockout mice display behaviours that have been likened to schizophrenia, and alterations affecting calcineurin signalling have been proposed as a contributing factor in the pathogenesis of schizophrenia.195,196 Decreased expression of calcineurin subunits in the hippocampus has been reported in schizophrenia.197 Cannabinoid effects on calcium channels are modulated by cyclic AMP-dependent protein kinase and calmodulin.198 Adaptations in the signalling pathways involved in the development of tolerance to cannabinoids have been shown to involve the activity of protein kinases,199 and increased adenylyl cyclase activity is stimulated by calcium/calmodulin during cannabinoid withdrawal.200 The involvement of the eCB system in the extinction of aversive memories appears to involve the activity of kinases and phosphatases such as calcineurin.201 Intriguingly, the extract of hemp seed has been shown to activate calcineurin and improve memory function in mice.202
The following sections examine evidence of 2 common gene variants that have been associated with specific cognitive processes, cannabinoid effects and schizophrenia. These are the catechol O-methyltransferase (COMT) polymorphism associated with PFC-based executive functions and neurophysiology and a brain-derived neurotrophic factor (BDNF) polymorphism associated with medial-temporal-cortex based declarative memory processes.203 BDNF is a cyclic-AMP response-element binding protein (CREB)-regulated gene. Interference with the function of CREB impairs long-term memory formation. Significant numbers of genes and proteins are altered after short-and long-term exposure to cannabinoids, including CREB, BDNF, calmodulin and GABA receptor subunit proteins.204 Large doses of THC applied directly to cultured hippocampal neuronal slices have been shown to cause significant toxicity, shrinkage of cell bodies and DNA strand breaks characteristic of neuronal apoptosis.205 Currently, there is also significant interest in the DISC1 gene in relation to schizophrenia, memory and hippocampal function,169,206,207 and DISC1 regulates cyclic–AMP signalling.208 However, allelic variation of DISC1 has not yet been investigated in relation to cannabis use or the eCB system.
COMT and tonic/phasic dopamine
The COMT gene, located on chromosome 22q11, is essential for the metabolic degradation of dopamine in PFC and has been implicated in schizophrenia.209,210 Evidence for an association between COMT genotype and schizophrenia has, however, been mixed211–213 (with some studies failing to find support for a link214,215). COMT involvement in dopamine metabolism and specific cognitive functions affected in schizophrenia have prompted ongoing interest in a potential association. Dopamine dysregulation and a functional Val158Met polymorphism in the COMT gene have each been associated with deficits in attention, working memory and other executive functions and in PFC pathophysiology.216,217 Goldberg and colleagues218 found n-back working memory tasks to be associated with the COMT polymorphism in the same manner across schizophrenia, healthy siblings and control subjects. They suggested “an additive genetic model in which the effect of allele load is similar in its effects on prefrontally based working memory irrespective of the genetic or environmental background in which it is expressed.”218 Variations in the COMT gene have also been linked to episodic and semantic memory with better recall (but not recognition) performance by Met homozygotes.219 Bearden and colleagues220 investigated the COMT genotype as a predictor of executive functioning in Velocardiofacial (22q11.2 deletion) syndrome, one of the highest known risk factors for schizophrenia. They found that Met-hemizygous patients performed significantly better on a composite measure comprising set-shifting, verbal fluency, attention and working memory than Val-hemizygous patients. Nolan and others221 found that the Met allele may promote cognitive stability in schizophrenia by increasing tonic dopamine but may limit cognitive flexibility: Met homozygotes showed better rule acquisition but poorer ability to switch to reversal learning. Gallinat and colleagues222 found smaller frontal P300 amplitudes in Met homozygous individuals, particularly those with schizophrenia. Conversely, another study demonstrated an association between COMT and executive functioning in healthy siblings but not their counterparts with schizophrenia.223
Meyer-Lindenberg and colleagues224 have recently shown that the COMT Val/Met polymorphism predicted reduced dopamine synthesis in the midbrain and affected the interaction with PFC, implicating a dopamine tuning mechanism in PFC and suggesting “a systems-level mechanism for cognitive and neuropsychiatric associations with COMT.”224 The activity of dopamine neurons in the midbrain is under both excitatory and inhibitory control of the PFC, and a marked increase in prefrontal dopamine is seen in COMT-knockout mice.224 An eCB-mediated self-regulatory role of dopamine neurons in the ventral tegmental area to suppress PFC-stimulation-evoked activity was described above.154 Melis and colleagues154 highlight how finely the eCB system might regulate dopamine modulation of cortical information processing, explaining a relation between unbalanced eCB signalling and altered dopamine-dependent processes associated with stress, substance abuse and psychiatric disorders such as schizophrenia. Bilder and colleagues192 discuss the COMT polymorphism findings in the context of tonic/phasic dopamine activity and resultant effects on cognitive stability versus flexibility in working memory, sustained attention and mismatch tasks. These tonic and phasic actions of dopamine may explain why people with schizophrenia (and perhaps cannabis users) fluctuate between impaired and unimpaired performance over time, suggesting influences that are perhaps transitory in nature.31
From a longitudinal birth cohort study, Caspi and colleagues193 reported a significant interaction between the COMT genotype and early onset cannabis use in the risk of developing psychosis. They showed that Val carriers were most likely to exhibit psychotic symptoms and develop a schizophreniform disorder if they used cannabis in adolescence. Those with the Val/Val genotype and early onset cannabis use (before age 15 or monthly use by age 18) had the highest risk of developing adult schizophreniform disorder (OR 10.9, 95% CI 2.2–54.1), followed by Val/Met individuals with early onset cannabis use (OR 2.9, CI 0.78–8.2), but not Met/Met individuals (OR 1.1, CI 0.21–5.4). Adult onset cannabis use (> 18 yr) did not interact with genotype in predicting psychosis outcomes. This exceptionally well-controlled study ruled out alternative explanations for the demonstration of a susceptibility gene by environment interaction in which adolescent, but not adult-onset, cannabis use interacts with the COMT gene polymorphism to predict the emergence of adult psychosis. The genetic polymorphism alone, and adolescent cannabis use alone, did not predict the development of psychosis. This may explain the inconsistent findings with regard to the COMT gene polymorphism association with schizophrenia and underscores the conditional exposure to an environmental pathogen,193 in this instance, adolescent cannabis use. Caspi and colleagues discuss possible neurobiological interactions between cannabinoids and dopamine underpinning this association. Most recently, the COMT genotype has been shown to moderate the effects of cannabis on inducing positive psychotic symptoms, with Val homozygotes being most susceptible.225 Psychosis liable (patients or relatives) Val carriers were more sensitive to acute THC-induced psychotic experiences and impairment of memory and attention.226,227
BDNF
The BDNF gene, located on chromosome 11p13, plays a critical role in activity-dependent neuroplasticity underlying learning and memory (e.g., LTP) in the hippocampus, where its expression and protein levels are highest in the brain, followed by the PFC. BDNF has also been implicated in the neurobiology of schizophrenia.228 Hariri and colleagues229 used fMRI to examine the relation between BDNF Val66Met polymorphism and hippocampal activity during episodic memory processing. Val homozygotes showed greater memory-related hippocampal activity during both encoding and retrieval and better recognition memory performance than Met carriers. The interaction between genotype and left hippocampal activity during encoding accounted for 25% of the variance in recognition memory performance, indicating a key role for BDNF modulation of hippocampal engagement in the acquisition of information. Val/val homozygotes have also been shown to have larger hippocampal volumes than val/met heterozygotes, and the val/met polymorphism of the BDNF gene accounted for a greater proportion of the variance in hippocampal volumes in first-episode schizophrenia patients than in control subjects.228 Neurotrophins such as BDNF also play a critical role in neurodevelopment, neuronal survival and plasticity of dopaminergic, cholinergic and serotonergic neurons and, as such, have been implicated in the pathophysiology of schizophrenia.230,231 However, the evidence is mixed. Some studies report no association of the BDNF gene polymorphism with schizophrenia,232 whereas others have shown reduced BDNF levels and receptor mRNA in DLPFC of schizophrenia patients, which may compromise the function and plasticity of the PFC.233 Hashimoto and colleagues234 found reduced PFC BDNF levels in schizophrenia and reported that signalling mediated by BDNF contributes to altered inhibitory GABA-related gene expression that may underlie cognitive deficits.
Bayatti and others235 report inhibition of BDNF expression by short-term in vitro application of WIN. CB1 receptors are negatively coupled with the cAMP signalling cascade and that their activation inhibits CREB phosphorylation which might explain reduced BDNF expression. However, Butovsky and colleagues236 report that long-term administration of THC to rats resulted in increased expression of both mRNA and protein levels of BDNF in specific brain regions associated with reward, notably a tenfold increase in nucleus accumbens. Smaller increases were found in the ventral tegmental area, medial PFC and paraventricular nucleus. There was no change in the hippocampus. The authors suggest that THC-induced upregulation of BDNF expression has an important role in the neuroadaptive processes resulting from exposure to cannabinoids.
Jockers-Scherübl and colleagues237,238 report raised levels of the neurotrophins nerve growth factor (NGF) and BDNF in serum of unmedicated schizophrenia patients with past chronic cannabis use, compared with nonusing patients, and the patient using cannabis had an earlier onset of the disorder. They interpret the raised NGF and BDNF as neuroprotective mechanisms to counter putative neurotoxic damage to vulnerable brains by cannabis and other drug use (polydrug users also showed raised NGF and BDNF serum concentrations, and BDNF levels are high after traumatic brain injury). Otherwise healthy control subjects who were using cannabis in this study did not differ from nonusing control subjects or patients. Other studies have shown reduced serum neurotrophin levels in schizophrenia patients.239
Aberrant incentive salience and inhibitory control
Schizophrenia may be conceptualized as a “state of aberrant salience” induced by dysregulated neurochemistry, particularly of the dopaminergic system.240 Phasic dopamine transmission has been linked to the updating, resetting or gating of relevant novel information and, specifically, to incentive-reward signals and uncertainty in these.192 This role extends to conditioned learning to update links between stimulus and response when an unexpected reward does not occur.192 Similar mechanisms involving attribution of aberrant incentive salience and reward processes have long been posited to underlie substance use and addiction.241–243 Tsapakis and colleagues244 discuss how the development of dopamine sensitization underlies both a craving for drugs and the positive symptoms of schizophrenia. Attribution of aberrant incentive salience to stimuli entails the kinds of dysfunction in cognitive processes in schizophrenia that have been discussed above also in relation to cannabis effects. Hypersensitization of drug incentives and of the dopaminergic reward system is accompanied by cognitive impairments associated with PFC function, difficulties in decision making, impulse control and judgement of consequences associated with further drug seeking and is linked to the efficiency of learning and memory.245,246 Accordingly, there has been a shift in the conceptualization of addiction mechanisms, from a subcortical pleasure and reward system focus to an acknowledged dysfunction in cortically mediated response selection and inhibition processes.247 Deficient inhibitory control may be a central feature of addiction, with dysfunction of anterior cingulate and orbitofrontal cortices affecting the regulation of the reward system.247 Jentsch and Taylor248 comprehensively reviewed the evidence that suggests that altered dopaminergic activity, impaired frontal cortical inhibitory response control and cognitive dysfunction resulting from long-term drug use, together with impulsivity and altered incentive motivational processes owing to limbic/amygdala dysfunction, underlie continued drug-seeking behaviour. Further, the neuroadaptations associated with sensitization are independent of those associated with physiological dependence and persist long after drug-use cessation.249
Impaired functioning in various cognitive tasks by people with schizophrenia could collectively be considered within a framework of failure to inhibit dominant responses in the face of pervasive generalized noise. The complex role of the eCB system in inhibitory processes and identified mechanisms by which eCBs may mediate key processes involved in incentive salience implicate this system in schizophrenia and serve to highlight the potential for deregulation of the eCB system as a consequence of long-term or heavy cannabis use. eCBs are involved in the PFC-mediated development of inappropriate incentive salience and resultant effects on attention.154 Cannabinoids potently increase dopamine metabolism and release in PFC, but repeat administration leads to a persistent anatomically selective reduction of dopamine metabolism in PFC that underlies attentional deficits.118,119 Cannabinoids have a profound influence on learning and memory via effects on eCB-mediated hippocampal metaplasticity.153 A dysfunction in hippocampal eCB signalling, and resultant effects on related circuitry (e.g., PFC), may underlie impairments of learning and memory in long-term cannabis users.90 There is a good degree of overlap between a dysfunction of these cognitive mechanisms in schizophrenia and in long-term cannabis use.
Nonspecificity of cognitive dysfunction and neural substrates
The deficits in cognitive functions highlighted in this paper, as well as their purported underlying neural substrates, are not unique to schizophrenia or to cannabis. Many other clinical disorders and substance using populations show similar kinds of neuroadaptations and cognitive deficits. Multiple substances have been shown to affect PPI, saccadic and smooth pursuit eye movements and attention, learning and memory and are associated with COMT polymorphism and altered expression of BDNF. Cannabinoid receptors have also been implicated in Alzheimer's disease250 and Parkinson's disease,251 largely in terms of neuroprotection; in addition, the eCB system has been demonstrated to promote neural progenitor cell proliferation in the hippocampus.252 Long-term but not short-term administration of the potent but nonselective cannabinoid agonist HU-210 increased adult rat hippocampal neurogenesis and produced anxiolytic and antidepressant-like effects.253 Other endogenous compounds, such as neurosteroids, have been shown to display neuroprotective properties in rodents but exacerbate psychotic symptoms in humans.254 The complexity of sometimes opposite effects of short-and long-term endogenous and exogenous cannabinoids of different types and possible inverted U dose–response actions on cognition, neurobiology and related systems provide a wealth of apparent discrepancy for future research to disentangle and interpret.
The concept of endophenotypes in schizophrenia has itself been contested.255 However, as Heinrichs31 acknowledged,
Several endophenotypes could underpin any single clinical syndrome or diagnostic classification. Moreover, there is no reason to assume that illnesses defined by consensus or convention represent unique or mutually exclusive combinations of endophenotypes. Psychiatric disorders often overlap in terms of symptoms and they may overlap in their biological underpinnings as well. Although the endophenotype concept is rooted in genetic theory it is possible to broaden the idea to include environmental and complex interacting etiologies.
A broader focus of research on endophenotypes that underlie multiple psychiatric disorders and psychopathology in general will facilitate identification of biological substrates and conferred risks and provide clinical relevance to factors that increase risk by 2- to 3-fold, such as cannabis.256,257 The particular association between cannabis and schizophrenia identified in epidemiological studies and the degree of overlap of their cognitive effects and associated brain neurochemistry support the focus of the current paper in highlighting their similarities. In some studies, an apparent relation between cannabis use and depression, for example, did not hold after controlling for symptoms of psychosis, but not the reverse (e.g.,258). Combining cognitive tasks and neuroimaging techniques, exploring gene-by-environment interactions and neurochemical and neurobiological underpinnings in the context of specific endophenotypes has the potential to further elucidate our understanding of the complexity of the schizophrenic disorder and its relation with cannabis use and the eCB system.
Cannabis use by schizophrenia patients
An ongoing puzzle is the high rate of cannabis use by people with schizophrenia, with 30% to 70% of the population with schizophrenia using cannabis.259–262 The primary reasons given by patients for their use are similar to those reported by the general population, including enhancement of affect, coping with unpleasant affect and social reasons, with limited support for relief of symptoms and side effects.263 The expectation that cannabis use will improve affect maintains use and potentially leads to the development of dependence and worsening of symptoms and course of the illness.264,265 There is little direct evidence for a self-medication hypothesis,8,258,266 although Hambrecht and Häfner's267,268 hypotheses regarding self-medication have found some support from Ferdinand and colleagues'269 14-year follow-up of a youth cohort from the general population. They found that not only did cannabis use predict future psychotic symptoms (CI 1.79–4.43) but that the onset of psychotic symptoms predicted future cannabis use in people who had never used cannabis before (CI 1.13–2.57). This suggests self-medication (and also provides support for reverse causality theories, although cannabis use has most often been found to precede the development of schizophrenia rather than the reverse). Neurobiologically, it is possible that the initial enhancement of prefrontal dopamine by cannabis might ameliorate certain negative symptoms. The persistent reduction of PFC dopamine with long-term cannabis use would, however, most likely further impair cognition and other negative symptoms; the increased mesolimbic dopamine transmission associated with cannabis ingestion explains a general worsening of positive symptoms. There is a convincing body of evidence that cannabis use triggers more psychotic episodes in patients and worsens the course of the disorder (eg.,264). Given the complexity of cannabinoid effects and the multiplicity of cannabinoid compounds in cannabis plant matter and products (e.g., the antioxidant cannabidiol that possesses some anxiolytic and antipsychotic properties), it is possible that aspects of smoked cannabis do serve to temporarily relieve certain symptoms or serve potential neuroprotective functions. However, more research is required to understand these complex actions in the context of this complex disorder.
Limited evidence that patients who use cannabis may be more functional before the onset of illness and that higher levels of functionality are required to maintain substance use (e.g.,270–272), may explain the preliminary results of 3 studies that found either no or minor additional adverse cognitive effects on cognitive functions in patients with psychoses and comorbid cannabis use compared with those without cannabis use.273–275 Another small study of schizophrenia patients with dual diagnosis of abuse or dependence on any drug found better performance on frontal tasks such as the WCST and verbal fluency tasks.276 The authors conjectured that better planning and organizational abilities are required to initiate, procure and maintain drug use. Stirling and colleagues,277 however, showed in a 10- to 12-year follow-up of first-episode psychosis admissions that ongoing cannabis use preserved neurocognitive function in several domains, with users outperforming nonusers and groups being indistinguishable with respect to premorbid adjustment or social functioning. These studies counter the intuitive hypothesis that cognitive impairments known to be associated with cannabis use in a healthy population could well exacerbate deficits in the already cognitively compromised schizophrenia patients who also use cannabis, but there has been a surprising dearth of specific research in this area. There is preliminary evidence that a history of cannabis use significantly predicted poorer semantic clustering scores in a verbal learning task in adolescents with early onset schizophrenia.278 We are currently exploring these interactions in ongoing neuropsychological and neuroimaging studies of patients with and without comorbid cannabis use. It has also been hypothesized that there are socialization benefits to maintaining a peer network of cannabis users and that this is a factor in recreational cannabis use by patients before they develop aberrant inhibitory and incentive-sensitization mechanisms that accompany drug dependence and associated problems. D'Souza and colleagues27 discuss the problems inherent in retrospective studies based on self-report. They also speculate that patients may derive some immediate benefits of using cannabis at the expense of negative consequences.
D'Souza and colleagues27 have definitively demonstrated exacerbation of cognitive and psychotic symptoms in a well-controlled study of acute intravenous THC administration to schizophrenia patients. They found transient adverse effects on verbal learning and recall; perceptual alteration; vigilance; positive, negative, general and extrapyramidal symptoms; and plasma prolactin and cortisol that were often dose-dependent. Patients were more vulnerable to THC effects on learning and memory than were healthy control subjects, and no beneficial effects were observed. The authors discuss multiple neurobiological mechanisms that might explain the enhanced sensitivity to the cognitive effects of THC. Among these are intriguing links between the growing focus on deficits in synchronous neural activity in schizophrenia (e.g.,279–281) and the involvement of eCBs in hippocampal oscillations, synchronous activity, integration and binding in the gamma range (e.g.,9,282) and high-frequency ripple and theta range.283 Disruption of this synchronous activity could result from either activation of CB1 receptors by exogenous cannabinoids or a dysfunctional eCB system. Thus, exacerbation of cognitive deficits by cannabis use in patients may be explained in neurobiological terms, whereas the onus is on researchers to replicate and explain potential preservation of neurocognition in cannabis using patient cohorts. In this vein, a recent study demonstrated that hyperdopaminergia in an animal model of schizophrenia is accompanied by decreased eCB signalling and that administration of indirect agonists (anandamide reuptake inhibitors) alleviated the accompanying hyperlocomotion.284 Clearly, additional research is required to fully understand the fine tuning role of the eCB system and complex interactions with exogenous cannabinoids in this disorder.
Cannabinoid consequences for neurodevelopment and adolescence
The nature of adverse consequences of cannabis use may differ across the life span,285 with educational and psychosocial effects apparent in adolescents and young adults and long-term cognitive or physiological effects becoming manifest only after many years of exposure to the drug. However, an increasing number of studies are detecting cognitive impairments in adolescent users (e.g.,95) as well as a greater incidence of psychotic symptoms (e.g.,286).
Evidence is growing for greater adverse cognitive consequences of cannabis when use begins during early adolescence (e.g., before age 16 or 17) as opposed to young adulthood. Early onset cannabis use was shown to impair attentional processes measured by reaction time during visual scanning,106 visual search and short-term memory166,287 and result in the most reduced P300 amplitudes in an attention task.113 Early onset cannabis users were found to have smaller whole brain volume, lower percentage of cortical grey matter, higher percentage of white matter and increased resting CBF, compared with late onset users.288 Cannabis users who commenced use before age 17 were least likely to show recovery of cognitive functions after 28 days abstinence.130
Rey and colleagues286 review the evidence that early regular cannabis use has substantial negative effects on psychosocial functioning and psychopathology in the context of developing juvenile psychiatric disorders. Early-onset cannabis use confers the greatest risk of developing psychosis, either in its own right (e.g.,258) or as a gene-by-environment interaction with the COMT Val/Met polymorphism most associated with cognitive deficits.193 Fergusson and Horwood289,290 have shown adverse outcomes associated with adolescent cannabis use, including the development of psychotic symptoms, and recently showed that the direction of causality is from cannabis use to symptoms and not the reverse291 (although Ferdinand and others269 do provide evidence for the latter). There is evidence that adolescent-onset cannabis use is associated with more rapid development of dependence and a greater incidence of dependence and associated problems than when onset occurs during adulthood.292,293 Thus individuals who begin to use cannabis when the brain is still developing may be most vulnerable to its deleterious effects.193
This is of concern when these adverse neurodevelopmental effects are posited to occur at the same time that neurodevelopmental changes and cognitive decline may be occurring in the development of schizophrenia or its prodrome. Davalos and colleagues294 studied children genetically at risk for schizophrenia and found deficits in verbal skills, working memory and inhibition that they suggest may progress into more generalized or global deficits with the development of the disorder. The neuropsychological performance of adolescents referred for prodromal symptoms was shown to be intermediate between population norms and the performance of established schizophrenia patients.295 This suggests that the cognitive decline that accompanies first psychotic episodes may not only be prevented or lessened by prodromal interventions but may also be exacerbated by concomitant cannabis use. Adolescence is increasingly being viewed as a unique period of brain development, during which the functional organization of the brain responsible for higher-order cognitive processes, mature level control and decision making and social information processing is not yet fully mature, with ongoing myelination and synaptic pruning toward achieving greater synchronous and collaborative activity.296–298 Dysregulation of these developing networks, by substance use, for example, may contribute to the onset of mood, anxiety, depressive and psychotic disorders. There is a growing recognition that substances affect the brain in different ways during adolescence versus adulthood (e.g.,299). Insufficient research has investigated the unique effects of cannabis during this neurodevelopmentally vulnerable period.
Animal studies have demonstrated greater adverse consequences when cannabinoids are administered to adolescent rats (see for example80,136,300,301). Early life experiences have been shown to increase the likelihood of developing schizophrenia later in life. It has been conjectured that an early insult (e.g., a lesion or maternal deprivation), when compounded by cannabinoid exposure during puberty, may confer the greatest risk of psychopathological consequences in adulthood. Evidence for this, however, is scant, and there are mixed potentially harmful versus beneficial effects of exposure to cannabinoids during development (e.g.,302–304).
Prenatal (or neonatal) exposure to cannabinoids has, however, unequivocally been shown to be harmful in multiple animal (e.g.,305–308) and human studies, as reviewed above (e.g.,101,104). Mereu and colleagues305 found that in utero exposure to WIN disrupted retention in a passive-avoidance task in 40- and 80-day-old rats, and this was accompanied by decreased hippocampal LTP and glutamate release, suggesting long-lasting, if not permanent, impairment of memory processes and their neural substrates by exposure to cannabinoids during a critical developmental period. The authors surmised that these mechanisms may explain the observations of cognitive impairments in humans exposed to cannabis in utero. The eCB system is intimately involved in neurodevelopment. Cannabinoid receptors are present in the brain from early stages of gestation and play a vital role in the developing organism.306,307
Despite the convincing evidence for periods of neurodevelopmental vulnerability, we have not found any evidence of age of onset effects in our neuropsychological or ERP studies of adult long-term heavy cannabis users, finding the number of years of cannabis use to be the most robust predictor of impairment (e.g.,87,111,132). It is clear that early onset cannabis users have a longer duration of exposure to cannabis than their age-matched counterparts with later onset of use, and these interactions can be difficult to disentangle. However, some studies report that age of onset effects held after controlling for duration of exposure to cannabis (e.g.,288). Studies of adult cannabis users, most of whom commenced use during adolescence, continue to show fairly consistent cognitive deficits, but these are variously related either to duration, frequency or dose/quantity of cannabis use. Further research on the parameters of cannabis use that result in dysfunction, monitoring of age of commencement of cannabis use and their interactions is of utmost importance. This is of particular concern given the growing evidence for an association between cannabis use and the development of psychosis in young people.
Conclusion
In this paper, we highlight the similarities between cognitive dysfunction associated with cannabis use and the cognitive endophenotypes of schizophrenia, drawing on recent developments in the understanding of the neurobiology of cannabinoid effects. We propose that the use of cannabis leads to cognitive deficits of a similar nature to those seen in schizophrenia but of a lower magnitude. We further propose that the neurobiology underpinning the development of cognitive deficits in cannabis users may overlap with the neurobiological underpinnings of schizophrenia. We have reviewed a multitude of evidence that taken together could inform our understanding of the potential for cannabis use to trigger the onset of psychosis in vulnerable individuals and explain the exacerbation of symptoms in schizophrenia patients.
Our central tenet in this paper is that the eCB system is involved, either directly or through its interactions with other neuromodulators (and, critically, dopamine), in both the development of similar cognitive deficits associated with cannabis use and schizophrenia, respectively, and in the pathophysiology and symptoms of psychiatric disorders in general. We concur with evidence that suggests that cannabis is but one of many causal factors that may precipitate psychosis, and its propensity to do so needs to be better understood within the context of predisposing genetic and environmental vulnerabilities. It has been proposed that the psychosis phenotype may exist as a continuous distribution throughout the general population.309 There are critical periods of neurodevelopment in which cannabis use may exert greater adverse effect (e.g., during adolescence) to combine with other vulnerabilities and precipitate the onset of psychosis. As suggested by Weiser and colleagues,256 cannabis use itself may not necessarily lie along a causal pathway to psychosis, but an association with schizophrenia may be due to dysfunction of the eCB system. This may lead to an increased propensity to use cannabis and an increased risk for the development of psychosis in cannabis users. Van Os and colleagues310 point out how genetic factors that influence the sensitivity to the psychosis-increasing effects of cannabis may also influence the probability of initiation of cannabis use. Leweke and colleagues311 report preliminary evidence that anandamide levels in CSF differ between patients who use and do not use cannabis, and we have preliminary evidence for altered functionality of the eCB system in patients who were former cannabis users versus those who have never used cannabis,312 with further research in progress. It is possible that contradictory evidence in the field may be explained by genetic differences in subpopulations or by cannabinoids having differential effects in people with dysfunctional versus intact eCB systems. In line with the trend toward classification of clusters of cognitive measures as endophenotypes of psychiatric disorders and investigations of their underlying neurobiology, we propose that a closer consideration of the cognitive deficits associated with specific parameters of cannabis use, and interactions with neural substrates and age may better inform the nature of the association between cannabis use, the eCB system, and schizophrenia or psychosis or psychiatric disorders of multiple kinds.
Not all of the cognitive endophenotypes of schizophrenia classified in this paper have been found to be impaired by cannabis, but not all have been studied directly or investigated in large-scale, well-controlled studies. Even if the cognitive measures in our taxonomy fail to meet all criteria for endophenotype status,32 the consistency of their association with schizophrenia and the increasing evidence of similar deficits associated with cannabis use warrant an exploration of the potential involvement of the eCB system. As the eCB system is largely inhibitory in its modulation of neurotransmitter release (decreasing the release of either excitatory or inhibitory transmitters10), and since drug dependence entails a loss of inhibitory control,247,248 acute and chronic effects of cannabinoids on inhibitory processes might be expected to underlie many of the cognitive deficits outlined above. The putative endophenotypes of schizophrenia are also not mutually exclusive. A particular cognitive mechanism may contribute to more than one endophenotype and multivariate endophenotypes, for example, as demonstrated based on a combination of electrophysiological measures,36 may provide improved diagnostic classification than any single endophenotype. Further elucidation of cognitive deficits associated with short65 and long-term drug use has important theoretical and clinical significance in terms of understanding fundamental pathophysiology and improving treatment and rehabilitation programs for substance use and mental illness.313
In addition to combining multidisciplinary research approaches (e.g., cognitive, neuroimaging, neurochemical and genetic), future directions for this field include empirical evaluation of the putative endophenotypes of schizophrenia; more refined investigations in animal models and human studies of short-and long-term effects of different endogenous and exogenous cannabinoids on specific and multiple measures contributing to endophenotypes, particularly where these have not been investigated (for example, MMN and inhibitory processes); determination of cognitive effects of varying parameters of cannabis use by humans, and their recovery, as may be impacted also by genotype and neurodevelopmental stage, with complimentary preclinical studies; assessment of the effects of exogenous cannabinoids on the functionality of the eCB system and of cannabinoid effects in general in hyperdopaminergic states and other animal models of psychosis; and greater exploration of the fine tuning role of the eCB system and implications of excess versus deficits in the system and on other neuromodulators.
The prevalence of cannabis use among people with mental illnesses, the potential for cannabis to trigger psychotic symptoms and episodes, and the neurobiological interactions between the eCB system and the neuropathology associated with psychotic disorders suggest a need to focus greater attention on investigating the nature of and mechanisms underlying cognitive impairments associated with cannabis use.
Acknowledgments
The authors are grateful to Sharon Monterrubio for assistance with manuscript preparation and to anonymous reviewers for their helpful suggestions.
Footnotes
Contributors: Dr. Solowij acquired the data and wrote the article. Both Drs. Solowij and Michie designed the review, analyzed the data, revised the article and gave final permission for it to be published.
Competing interests: None declared.
Correspondence to: Dr. Nadia Solowij, School of Psychology, University of Wollongong, Wollongong, NSW 2522 Australia; fax 61 2 4221 4163; nadia@uow.edu.au
References
- 1.Bernhardson G, Gunne L-M. Forty-six cases of psychosis in cannabis abusers. Int J Addict 1972;7:9-16. [DOI] [PubMed]
- 2.Thacore VR, Shukla SRP. Cannabis psychosis and paranoid schizophrenia. Arch Gen Psychiatry 1976;33:383-6. [DOI] [PubMed]
- 3.Rottanburg D, Robins AH, Ben-Arie O, et al. Cannabis-associated psychosis with hypomanic features. Lancet 1982;2:1364-6. [DOI] [PubMed]
- 4.Andreasson S, Allebeck P, Engstrom A, et al. Cannabis and schizophrenia. Lancet 1987;2:1483-6. [DOI] [PubMed]
- 5.Arseneault L, Cannon M, Poulton R, et al. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ 2002;325:1212-3. [DOI] [PMC free article] [PubMed]
- 6.van Os J, Bak M, Hanssen M, et al. Cannabis use and psychosis: A longitudinal population-based study. Am J Epidemiol 2002;156:319-27. [DOI] [PubMed]
- 7.Zammit S, Allebeck P, Andreasson S, et al. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ 2002;325:1199-201. [DOI] [PMC free article] [PubMed]
- 8.Henquet C, Krabbendam L, Spauwen J, et al. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. BMJ 2005;330:11-4. [DOI] [PMC free article] [PubMed]
- 9.Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science 2002;296:678-82. [DOI] [PubMed]
- 10.Howlett AC, Breivogel CS, Childers SR, et al. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology 2004;47:345-58. [DOI] [PubMed]
- 11.Alger BE. Endocannabinoid identification in the brain: Studies of breakdown lead to breakthrough, and there may be NO hope. Sci STKE 2005;309:pe51. [DOI] [PubMed]
- 12.Harrison PJ. The neuropathology of schizophrenia: a critical review of the data and their interpretation. Brain Cogn 1999;122(Pt 4):593-624. [DOI] [PubMed]
- 13.Pantelis C, Yücel M, Wood SJ, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull 2005;31:672-96. [DOI] [PubMed]
- 14.Mäki P, Veijola J, Jones PB, et al. Predictors of schizophrenia - a review. Br Med Bull 2005;73-74:1-15. [DOI] [PubMed]
- 15.Henquet C, Murray R, Linszen D, et al. The environment and schizophrenia: The role of cannabis use. Schizophr Bull 2005;31:608-12. [DOI] [PubMed]
- 16.Hall W, Degenhardt L, Teesson M. Cannabis use and psychotic disorders: an update. Drug Alcohol Rev 2004;23:433-43. [DOI] [PubMed]
- 17.Smit F, Bolier L, Cuijpers P. Cannabis use and the risk of later schizophrenia: a review. Addiction 2004;99:425-30. [DOI] [PubMed]
- 18.Hall W. Is cannabis use psychotogenic? Lancet 2006;367:193-5. [DOI] [PubMed]
- 19.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 2003;4:873-84. [DOI] [PubMed]
- 20.Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci 2006;29:37-76. [DOI] [PubMed]
- 21.Iversen L. Cannabis and the brain. Brain 2003;126:1252-70. [DOI] [PubMed]
- 22.Hoffman AF, Oz M, Caulder T, et al. Functional tolerance and blockade of long term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J Neurosci 2003;23:4815-20. [DOI] [PMC free article] [PubMed]
- 23.Sim-Selley L. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol 2003;15:91-119. [DOI] [PubMed]
- 24.Dean B, Sundram S, Bradbury R, et al. Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience 2001;103:9-15. [DOI] [PubMed]
- 25.Zavitsanou K, Garrick T, Huang XF. Selective antagonist [3H]SR141716A binding to cannabinoid CB1 receptors is increased in the anterior cingulate cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2004;28:355-60. [DOI] [PubMed]
- 26.Giuffrida A, Leweke FM, Gerth CW, et al. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology 2004;29:2108-14. [DOI] [PubMed]
- 27.D'Souza DC, Abi-Saab WM, Madonick S, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: Implications for cognition, psychosis, and addiction. Biol Psychiatry 2005;57:594-608. [DOI] [PubMed]
- 28.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996;153:321-30. [DOI] [PubMed]
- 29.Hughes C, Kumari V, Soni W, et al. Longitudinal study of symptoms and cognitive function in chronic schizophrenia. Schizophr Res 2003;59:137-46. [DOI] [PubMed]
- 30.Rund BR, Melle I, Friis S, et al. Neurocognitive dysfunction in first-episode psychosis: correlates with symptoms, premorbid adjustment, and duration of untreated psychosis. Am J Psychiatry 2004;161:466-72. [DOI] [PubMed]
- 31.Heinrichs RW. Meta-analysis, and the science of schizophrenia: variant evidence or evidence of variants? Neurosci Biobehav Rev 2004;28:379-94. [DOI] [PubMed]
- 32.Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry 2003;160:636-45. [DOI] [PubMed]
- 33.Gottesman II, Hanson DR. Human development: Biological and genetic processes. Annu Rev Psychol 2005;56:263-86. [DOI] [PubMed]
- 34.Snitz BE, MacDonald AW, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: A meta-analytic review of putative endophenotypes. Schizophr Bull 2006;32:179-94. [DOI] [PMC free article] [PubMed]
- 35.Adler LE, Pachtman E, Franks RD, et al. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry 1982;17:639-54. [PubMed]
- 36.Price GW, Michie PT, Johnston J, et al. A multivariate electrophysiological endophenotype, from a unitary cohort, shows greater research utility than any single feature in the Western Australian Family Study of Schizophrenia. Biol Psychiatry 2006;60:1-10. [DOI] [PubMed]
- 37.Siegel C, Waldo M, Mizner G, et al. Deficits in sensory gating in schizophrenic-patients and their relatives - Evidence obtained with auditory evoked-responses. Arch Gen Psychiatry 1984;41:607-12. [DOI] [PubMed]
- 38.Bramon E, Rabe-Hesketh S, Sham P, et al. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res 2004;70:315-29. [DOI] [PubMed]
- 39.Freedman R, Coon H, Myles-Worsley M, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A 1997;94:587-92. [DOI] [PMC free article] [PubMed]
- 40.Grunwald T, Boutros NN, Pezer N, et al. Neuronal substrates of sensory gating within the human brain. Biol Psychiatry 2003;53:511-9. [DOI] [PubMed]
- 41.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234-58. [DOI] [PubMed]
- 42.Quednow BB, Kuhn KU, Hoenig K, et al. Prepulse inhibition and habituation of acoustic startle response in male MDMA (‚ecstasy') users, cannabis users, and healthy controls. Neuropsychopharmacology 2004;29:982-90. [DOI] [PubMed]
- 43.Shelley AM, Ward PB, Catts SV, et al. Mismatch negativity - an index of a preattentive processing deficit in schizophrenia. Biol Psychiatry 1991;30:1059-62. [DOI] [PubMed]
- 44.Michie PT. What has MMN revealed about the auditory system in schizophrenia? Int J Psychophysiol 2001;42:177-94. [DOI] [PubMed]
- 45.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res 2005;76:1-23. [DOI] [PubMed]
- 46.Kiehl KA, Smith AM, Hare RD, et al. An event-related potential investigation of response inhibition in schizophrenia and psychopathy. Biol Psychiatry 2000;48:210-21. [DOI] [PubMed]
- 47.Weisbrod M, Kiefer M, Marzinzik F, et al. Executive control is disturbed in schizophrenia: evidence from event-related potentials in a Go/NoGo task. Biol Psychiatry 2000;47:51-60. [DOI] [PubMed]
- 48.Badcock JC, Michie PT, Johnson L, et al. Acts of control in schizophrenia: dissociating the components of inhibition. Psychol Med 2002;32:287-97. [DOI] [PubMed]
- 49.Braff DL, Light GA. Preattentional and attentional cognitive deficits as targets for treating schizophrenia. Psychopharmacology (Berl) 2004;174:75-85. [DOI] [PubMed]
- 50.Kerns JG, Cohen JD, MacDonald AW, et al. Decreased conflict-and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry 2005;162:1833-9. [DOI] [PubMed]
- 51.Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull 1994;20:31-46. [DOI] [PubMed]
- 52.Michie PT, Kent A, Stienstra R, et al. Phenotypic markers as risk factors in schizophrenia: neurocognitive functions. Aust N Z J Psychiatry 2000;34:S74-85. [DOI] [PubMed]
- 53.Heinrichs RW. The primacy of cognition in schizophrenia. Am Psychol 2005;60:229-42. [DOI] [PubMed]
- 54.Jeon Y-W, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology 2003;40:684-701. [DOI] [PubMed]
- 55.Bramon E, McDonald C, Croft RJ, et al. Is the P300 wave an endophenotype for schizophrenia? A meta-analysis and a family study. Neuroimage 2005;27:960-8. [DOI] [PubMed]
- 56.Cannon TD, Huttunen MO, Lonnqvist J, et al. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. Am J Hum Genet 2000;67:369-82. [DOI] [PMC free article] [PubMed]
- 57.Conklin HM, Curtis CE, Calkins ME, et al. Working memory functioning in schizophrenia patients and their first-degree relatives: cognitive functioning shedding light on etiology. Neuropsychologia 2005;43:930-42. [DOI] [PubMed]
- 58.Sacco KA, Termine A, Seyal A, et al. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia - involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry 2005;62:649-59. [DOI] [PubMed]
- 59.Pantelis C, Barnes TRE, Nelson HE, et al. Frontal-striatal cognitive deficits in patients with chronic schizophrenia. Brain 1997;120:1823-43. [DOI] [PubMed]
- 60.Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: From clinical assessment to genetics and brain mechanisms. Neuropsychol Rev 2003;13:43-77. [DOI] [PubMed]
- 61.Hill SK, Beers SR, Kmiec JA, et al. Impairment of verbal memory and learning in antipsychotic-naive patients with first-episode schizophrenia. Schizophr Res 2004;68:127-36. [DOI] [PubMed]
- 62.Hoff A, Svetina C, Shields G, et al. Ten year longitudinal study of neuropsychological functioning subsequent to a first episode of schizophrenia. Schizophr Res 2005;78:27-34. [DOI] [PubMed]
- 63.Sitskoorn M, Aleman A, Ebisch S, et al. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophr Res 2004;71:285-95. [DOI] [PubMed]
- 64.Levy DL, Holzman PS, Matthysse S, et al. Eye tracking dysfunction and schizophrenia - a critical perspective. Schizophr Bull 1993;19:461-536. [DOI] [PubMed]
- 65.Fletcher PC, Honey GD. Schizophrenia, ketamine and cannabis: evidence of overlapping memory deficits. Trends Cogn Sci 2006;10:167-74. [DOI] [PubMed]
- 66.Patrick G, Struve FA. Reduction of auditory P50 gating response in marihuana users: Further supporting data. Clin Electroencephalogr 2000;31:88-93. [DOI] [PubMed]
- 67.Patrick G, Struve F. Reduced P50 sensory gating in heavy marihuana users. Clin Electroencephalogr 2000;31(2):88-93. [DOI] [PubMed]
- 68.Patrick G, Straumanis JJ, Struve FA, et al. Reduced P50 auditory gating response in psychiatrically normal chronic marihuana users: a pilot study. Biol Psychiatry 1999;45:1307-12. [DOI] [PubMed]
- 69.van der Stelt M, Di Marzo V. Anandamide as an intracellular messenger regulating ion channel activity. Prostaglandins Other Lipid Mediat 2005;77:111-22. [DOI] [PubMed]
- 70.Kedzior KK, Martin-Iverson MT. Chronic cannabis use is associated with attention-modulated reduction in prepulse inhibition of the startle reflex in healthy humans. J Psychopharmacol 2006;20:471-84. [DOI] [PubMed]
- 71.Morsen CP, Wolfing K, Ziegler S, et al. Visual evoked potentials and startle reflex: a comparison between alcohol, cannabis and heroin addicts. Psychophysiology 2004;41(Suppl 1):S69.
- 72.Stanley-Cary CC, Harris C, Martin-Iverson MT. Differing effects of the cannabinoid agonist, CP 55,940, in an alcohol or Tween 80 solvent, on prepulse inhibition of the acoustic startle reflex in the rat. Behav Pharmacol 2002;13:15-28. [DOI] [PubMed]
- 73.Schneider M, Koch M. The cannabinoid agonist WIN 55,212-2 reduces sensorimotor gating and recognition memory in rats. Behav Pharmacol 2002;13:29-37. [DOI] [PubMed]
- 74.Martin RS, Secchi RL, Sung E, et al. Effects of cannabinoid receptor ligands on psychosis-relevant behavior models in the rat. Psychopharmacology (Berl) 2003;165:128-35. [DOI] [PubMed]
- 75.Mansbach RS, Rovetti CC, Winston EN, et al. Effects of the cannabinoid CB1 receptor antagonist SR141716A on the behavior of pigeons and rats. Psychopharmacology (Berl) 1996;124:315-22. [DOI] [PubMed]
- 76.Musty RE, Deyo RA, Baer JL, et al. Effects of SR141716 on animal models of schizophrenia. 10th Annual Symposium on the Cannabinoids; 2000; Burlington, VT.
- 77.Malone DT, Long LE, Taylor DA. The effect of SR 141716 and apomorphine on sensorimotor gating in Swiss mice. Pharmacol Biochem Behav 2004;77:839-45. [DOI] [PubMed]
- 78.Fernandez-Espejo E, Galan-Rodriguez B. Sensorimotor gating in mice is disrupted after AM404, an anandamide reuptake and degradation inhibitor. Psychopharmacology (Berl) 2004;175:220-4. [DOI] [PubMed]
- 79.Bortolato M, Aru GN, Frau R, et al. The CB receptor agonist WIN 55,212-2 fails to elicit disruption of prepulse inhibition of the startle in Sprague-Dawley rats. Psychopharmacology (Berl) 2005;177:264-71. [DOI] [PubMed]
- 80.Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology 2003;28:1760-9. [DOI] [PubMed]
- 81.Malone D, Long L, Gampert L, et al. The effect of cannabinoids on sensorimotor gating in Swiss mice [poster]. 12th Annual Symposium on the Cannabinoids; 2002 Jul 10–14; Pacific Grove (CA).
- 82.Umbricht D, Schmid L, Koller R, et al. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers - Implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry 2000;57:1139-47. [DOI] [PubMed]
- 83.Javitt DC, Steinschneider M, Schroeder CE, et al. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: Implications for schizophrenia. Proc Natl Acad Sci U S A 1996;93:11962-7. [DOI] [PMC free article] [PubMed]
- 84.Hampson AJ, Bornheim LM, Scanziani M, et al. Dual effects of anandamide on NMDA receptor-mediated responses and neurotransmission. J Neurochem 1998;70:671-6. [DOI] [PubMed]
- 85.Gamma A, Brandeis D, Brandeis R, et al. The P3 in ‚ecstasy' polydrug users during response inhibition and execution. J Psychopharmacol 2005;19:504-12. [DOI] [PubMed]
- 86.Pope HG. YurgelunTodd D. The residual cognitive effects of heavy marijuana use in college students. JAMA 1996;275:521-7. [PubMed]
- 87.Solowij N. Cannabis and cognitive functioning. Cambridge: Cambridge University Press; 1998.
- 88.Pope HG, Gruber AJ, Hudson JI, et al. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry 2001;58:909-15. [DOI] [PubMed]
- 89.Bolla KI, Brown K, Eldreth D, et al. Dose-related neurocognitive effects of marijuana use. Neurology 2002;59:1337-43. [DOI] [PubMed]
- 90.Solowij N, Stephens RS, Roffman RA, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA 2002;287:1123-31. [DOI] [PubMed]
- 91.Gruber S, Yurgelun-Todd D. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res Cogn Brain Res 2005;23:107-18. [DOI] [PubMed]
- 92.Eldreth DA, Matochik JA, Cadet JL, et al. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage 2004;23:914-20. [DOI] [PubMed]
- 93.McDonald J, Schleifer L, Richards JB, et al. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology 2003;28:1356-65. [DOI] [PubMed]
- 94.Hart CL, van Gorp W, Haney M, et al. Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology 2001;25:757-65. [DOI] [PubMed]
- 95.Jacobsen LK, Mencl WE, Westerveld M, et al. Impact of cannabis use on brain function in adolescents. Ann N Y Acad Sci 2004;1021:384-90. [DOI] [PubMed]
- 96.Voytek B, Berman SM, Hassid BD, et al. Differences in regional brain metabolism associated with marijuana abuse in methamphetamine abusers. Synapse 2005;57:113-5. [DOI] [PubMed]
- 97.Fried PA, Watkinson B, Gray R. A follow-up-study of attentional behavior in 6-year-old children exposed prenatally to marijuana, cigarettes, and alcohol. Neurotoxicol Teratol 1992;14:299-311. [DOI] [PubMed]
- 98.Fried PA. The Ottawa Prenatal Prospective-Study (OPPS) - Methodological issues and findings - Its easy to throw the baby out with the bath water. Life Sci 1995;56:2159-68. [DOI] [PubMed]
- 99.Leech SL, Richardson GA, Goldschmidt L, et al. Prenatal substance exposure: effects on attention and impulsivity of 6-year-olds. Neurotoxicol Teratol 1999;21:109-18. [DOI] [PubMed]
- 100.Fried PA, Smith AR. A literature review of the consequences of prenatal marihuana exposure - An emerging theme of a deficiency in aspects of executive function. Neurotoxicol Teratol 2001;23:1-11. [DOI] [PubMed]
- 101.Richardson GA, Ryan C, Willford J, et al. Prenatal alcohol and marijuana exposure: Effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol 2002;24:309-20. [DOI] [PubMed]
- 102.Fried PA, Watkinson B. Differential effects on facets of attention in adolescents prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol 2001;23:421-30. [DOI] [PubMed]
- 103.Fried PA. Adolescents prenatally exposed to marijuana: examination of facets of complex behaviors and comparisons with the influence of in utero cigarettes. J Clin Pharmacol 2002;42:97S-102S. [DOI] [PubMed]
- 104.Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol 2003;25:427-36. [DOI] [PubMed]
- 105.Ilan AB, Smith ME, Gevins A. Effects of marijuana on neurophysiological signals of working and episodic memory. Psychopharmacology (Berl) 2004;176:214-22. [DOI] [PMC free article] [PubMed]
- 106.Ehrenreich H, Rinn T, Kunert HJ, et al. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl) 1999;142:295-301. [DOI] [PubMed]
- 107.Fletcher JM, Page JB, Francis DJ, et al. Cognitive correlates of long-term cannabis use in Costa Rican men. Arch Gen Psychiatry 1996;53:1051-7. [DOI] [PubMed]
- 108.Skosnik PD, Spatz-Glenn L, Park S. Cannabis use is associated with schizotypy and attentional disinhibition. Schizophr Res 2001;48:83-92. [DOI] [PubMed]
- 109.MacQueen GM, Galway T, Goldberg JO, et al. Impaired distractor inhibition in patients with schizophrenia on a negative priming task. Psychol Med 2003;33:121-9. [DOI] [PubMed]
- 110.Solowij N, Michie PT, Fox AM. Effects of long-term cannabis use on selective attention: An event-related potential study. Pharmacol Biochem Behav 1991;40:683-8. [DOI] [PubMed]
- 111.Solowij N, Michie PT, Fox AM. Differential impairments of selective attention due to frequency and duration of cannabis use. Biol Psychiatry 1995;37:731-9. [DOI] [PubMed]
- 112.Gilmore CS, Clementz BA, Buckley PF. Stimulus sequence affects schizophrenia - normal differences in event processing during an auditory oddball task. Brain Res Cogn Brain Res 2005;24:215-27. [DOI] [PubMed]
- 113.Kempel P, Lampe K, Parnefjord R, et al. Auditory-evoked potentials and selective attention: different ways of information processing in cannabis users and controls. Neuropsychobiology 2003;48:95-101. [DOI] [PubMed]
- 114.Johnson JP, Muhleman D, MacMurray J, et al. Association between the cannabinoid receptor gene (CNR1) and the P300 event-related potential. Mol Psychiatry 1997;2:169-71. [DOI] [PubMed]
- 115.Presburger G, Robinson JK. Spatial signal detection in rats is differentially disrupted by Delta-9-tetrahydrocannabinol, scopolamine, and MK-801. Behav Brain Res 1999;99:27-34. [DOI] [PubMed]
- 116.Mishima K, Fujii M, Aoo N, et al. The pharmacological characterization of attentional processes using a two-lever choice reaction time task in rats. Biol Pharm Bull 2002;25:1570-6. [DOI] [PubMed]
- 117.Arguello PA, Jentsch JD. Cannabinoid CB1 receptor-mediated impairment of visuospatial attention in the rat. Psychopharmacology (Berl) 2004;177:141-50. [DOI] [PubMed]
- 118.Verrico CD, Jentsch JD, Roth RH. Persistent and anatomically selective reduction in prefrontal cortical dopamine metabolism after repeated, intermittent cannabinoid administration to rats. Synapse 2003;49:61-6. [DOI] [PubMed]
- 119.Verrico CD, Jentsch JD, Roth RH, et al. Repeated, intermittent Delta(9)-tetrahydrocannabinol administration to rats impairs acquisition and performance of a test of visuospatial divided attention. Neuropsychopharmacology 2004;29:522-9. [DOI] [PubMed]
- 120.Rammsayer T. Temporal discrimination in schizophrenic and affective-disorders - Evidence for a dopamine-dependent internal clock. Int J Neurosci 1990;53:111-20. [DOI] [PubMed]
- 121.Davalos DB, Kisley MA, Ross RG. Effects of interval duration on temporal processing in schizophrenia. Brain Cogn 2003;52:295-301. [DOI] [PubMed]
- 122.Elvevag B, McCormack T, Gilbert A, et al. Duration judgements in patients with schizophrenia. Psychol Med 2003;33:1249-61. [DOI] [PubMed]
- 123.Brown SM, Kieffaber PD, Carroll CA, et al. Eyeblink conditioning deficits indicate timing and cerebellar abnormalities in schizophrenia. Brain Cogn 2005;58:94-108. [DOI] [PubMed]
- 124.Han CJ, Robinson JK. Cannabinoid modulation of time estimation in the rat. Behav Neurosci 2001;115:243-6. [DOI] [PubMed]
- 125.Crystal JD, Maxwell KW, Hohmann AG. Cannabinoid modulation of sensitivity to time. Behav Brain Res 2003;144:57-66. [DOI] [PubMed]
- 126.Harrington DL, Boyd LA, Mayer AR, et al. Neural representation of interval encoding and decision making. Brain Res Cogn Brain Res 2004;21:193-205. [DOI] [PubMed]
- 127.D'Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: Implications for psychosis. Neuropsychopharmacology 2004;29:1558-72. [DOI] [PubMed]
- 128.Lane S, Cherek D, Lieving L, et al. Marijuana effects on human forgetting functions. J Exp Anal Behav 2005;83:67-83. [DOI] [PMC free article] [PubMed]
- 129.Curran HV, Brignell C, Fletcher S, et al. Cognitive and subjective dose-response effects of acute oral Delta(9)-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 2002;164:61-70. [DOI] [PubMed]
- 130.Pope HG, Gruber AJ, Hudson JI, et al. Early-onset cannabis use and cognitive deficits: What is the nature of the association? Drug Alcohol Depend 2003;69:303-10. [DOI] [PubMed]
- 131.Respondek C, Solowij N, Ward PB. An investigation of neurocognitive performance, verbal learning ability and fMRI indices of cognitive functioning in long term cannabis users. Aust J Psychol 2004;56:219.
- 132.Solowij N. Marijuana and cognitive function. In: Onaivi, ES, editor. Biology of marijuana: from gene to behaviour. New York: Taylor and Francis; 2002. p. 308-32.
- 133.Hampson RE, Deadwyler SA. Cannabinoids hippocampal function and memory. Life Sci 1999;65:715-23. [DOI] [PubMed]
- 134.Hampson RE, Deadwyler SA. Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. J Neurosci 2000;20:8932-42. [DOI] [PMC free article] [PubMed]
- 135.Han CJ, Pierre-Louis J, Scheff A, et al. A performance-dependent adjustment of the retention interval in a delayed non-matching-to-position paradigm differentiates effects of amnestic drugs in rats. Eur J Pharmacol 2000;403:87-93. [DOI] [PubMed]
- 136.O'Shea M, Singh ME, McGregor IS, et al. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol 2004;18:502-8. [DOI] [PubMed]
- 137.Varvel SA, Hamm RJ, Martin BR, et al. Differential effects of Delta(9)-THC on spatial reference and working memory in mice. Psychopharmacology (Berl) 2001;157:142-50. [DOI] [PubMed]
- 138.Varvel SA, Lichtman AH. Evaluation of CB1 receptor knockout mice in the Morris water maze. J Pharmacol Exp Ther 2002;301:915-24. [DOI] [PubMed]
- 139.Hill MN, Froc DJ, Fox CJ, et al. Prolonged cannabinoid treatment results in spatial working memory deficits and impaired long-term potentiation in the CA1 region of the hippocampus in vivo. Eur J Neurosci 2004;20:859-63. [DOI] [PubMed]
- 140.Varvel SA, Anum E, Niyuhire F, et al. Delta-9-THC-induced cognitive deficits in mice are reversed by the GABA(A) antagonist bicuculline. Psychopharmacology (Berl) 2005;178:317-27. [DOI] [PubMed]
- 141.Hill MN, Patel S, Carrier EJ, et al. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology 2005;30:508-15. [DOI] [PubMed]
- 142.Rowland LM, Astur RS, Jung RE, et al. Selective cognitive impairments associated with NMDA receptor blockade in humans. Neuropsychopharmacology 2005;30:633-9. [DOI] [PubMed]
- 143.Astur RS, Taylor LB, Mamelak AN, et al. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res 2002;132:77-84. [DOI] [PubMed]
- 144.Astur RS, Mathalon DH, D'Souza DC, et al. Using virtual reality to examine hippocampus functioning in schizophrenia [lecture]. International Workshop on Virtual Rehabilitation Proceedings; 2003 Sept 21–22; Piscataway (NJ).
- 145.Braida D, Sala M. Cannabinoid-induced working memory impairment is reversed by a second generation cholinesterase inhibitor in rats. Neuroreport 2000;11:2025-9. [DOI] [PubMed]
- 146.Nava F, Carta G, Battasi AM, et al. D-2 dopamine receptors enable Delta(9)-tetrahydrocannabinol induced memory impairment and reduction of hippocampal extracellular acetylcholine concentration. Br J Pharmacol 2000;130:1201-10. [DOI] [PMC free article] [PubMed]
- 147.Fadda P, Robinson L, Fratta W, et al. Differential effects of THC or CBD-rich cannabis extracts on working memory in rats. Neuropharmacology 2004;47:1170-9. [DOI] [PubMed]
- 148.Marsicano G, Wotjak C, Azad S, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature 2002;418:530-4. [DOI] [PubMed]
- 149.Holter SM, Kallnik M, Wurst W, et al. Cannabinoid CB1 receptor is dispensable for memory extinction in an appetitively-motivated learning task. Eur J Pharmacol 2005;510:69-74. [DOI] [PubMed]
- 150.de Oliveira Alvares L, de Oliveira LF, Camboim C, et al. Amnestic effect of intrahippocampal AM251, a CB1-selective blocker, in the inhibitory avoidance, but not in the open field habituation task, in rats. Neurobiol Learn Mem 2005;83:119-24. [DOI] [PubMed]
- 151.Lichtman AH. SR141716A enhances spatial memory as assessed in a radial-arm maze task in rats. Eur J Pharmacol 2000;404:175-9. [DOI] [PubMed]
- 152.Carlson G, Wang Y, Alger BE. Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat Neurosci 2002;5:723-4. [DOI] [PubMed]
- 153.Mato S, Chevaleyre V, Robbe D, et al. A single in-vivo exposure to Delta 9THC blocks endocannabinoid-mediated synaptic plasticity. Nat Neurosci 2004;7:585-6. [DOI] [PubMed]
- 154.Melis M, Perra S, Muntoni AL, et al. Prefrontal cortex stimulation induces 2-arachidonoyl-glycerol-mediated suppression of excitation in dopamine neurons. J Neurosci 2004;24:10707-15. [DOI] [PMC free article] [PubMed]
- 155.Solowij N. Do cognitive impairments recover following cessation of cannabis use? Life Sci 1995;56(23-24):2119-26. [DOI] [PubMed]
- 156.Solowij N, Grenyer BFS, Chesher G, et al. Biopsychosocial changes associated with cessation of cannabis use: a single-case study of acute and chronic cognitive effects, withdrawal and treatment. Life Sci 1995;56:2127-34. [DOI] [PubMed]
- 157.Pope HG, Gruber AJ, Hudson JI, et al. Cognitive measures in long-term cannabis users. J Clin Pharmacol 2002;42(11 Suppl)):41S-7S. [DOI] [PubMed]
- 158.Messinis L, Kyprianidou A, Malefaki S, et al. Neuropsychological deficits in long-term frequent cannabis users. Neurology 2006;66:737-9. [DOI] [PubMed]
- 159.Grant I, Gonzalez R, Carey CL, et al. Non-acute (residual) neurocognitive effects of cannabis use: A meta-analytic study. J Int Neuropsychol Soc 2003;9:679-89. [DOI] [PubMed]
- 160.Block RI, O'Leary DS, Hichwa RD, et al. Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacol Biochem Behav 2002;72:237-50. [DOI] [PubMed]
- 161.Respondek C, Solowij N, Ward P. Functional magnetic resonance imaging indices of memory function in long-term cannabis users. World J Biol Psychiatry 2004;5(Suppl 1):S131.
- 162.Solowij N, Respondek C, Ward P. Functional magnetic resonance imaging indices of memory function in long-term cannabis users [lecture]. 2004 Symposium on the Cannabinoids; 2004 June 22–24; Paestum (Italy).
- 163.Ploner CJ, Tschirch A, Ostendorf F, et al. Oculomotor effects of delta-9-tetrahydrocannabinol in humans: Implications for the functional neuroanatomy of the brain cannabinoid system. Cereb Cortex 2002;12:1016-23. [DOI] [PubMed]
- 164.Flom MC, Brown B, Adams AJ, et al. Alcohol and marijuana effects on ocular tracking. Am J Optom Physiol Opt 1976;53:764-73. [DOI] [PubMed]
- 165.Baloh RW, Sharma S, Moskowitz H, et al. Effects of alcohol and marijuana on eye movements. Aviat Space Environ Med 1979;50:18-23. [PubMed]
- 166.Huestegge L, Radach R, Kunert HJ, et al. Visual search in long-term cannabis users with early age of onset. Prog Brain Res 2002;140:377-94. [DOI] [PubMed]
- 167.Rizzo M, Lamers CTJ, Sauer CG, et al. Impaired perception of self-motion (heading) in abstinent ecstasy and marijuana users. Psychopharmacology (Berl) 2005;179:559-66. [DOI] [PubMed]
- 168.Lamers CTJ, Ramaekers JG. Visual search and urban city driving under the influence of marijuana and alcohol. Hum Psychopharmacol Clin Exp Res 2001;16:393-401. [DOI] [PubMed]
- 169.Roffman JL, Weiss AP, Goff DC, et al. Neuroimaging-genetic paradigms: A new approach to investigate the pathophysiology and treatment of cognitive deficits in schizophrenia. Harv Rev Psychiatry 2006;14:78-91. [DOI] [PubMed]
- 170.O'Leary DS, Block RI, Flaum M, et al. Acute marijuana effects on rCBF and cognition: a PET study. Neuroreport 2000;11:3835-41. [DOI] [PubMed]
- 171.O'Leary DS, Block RI, Koeppel JA, et al. Effects of smoking marijuana on brain perfusion and cognition. Neuropsychopharmacology 2002;26:802-16. [DOI] [PubMed]
- 172.Mathew RJ, Wilson WH, Turkington TG, et al. Time course of tetrahydrocannabinol-induced changes in regional cerebral blood flow measured with positron emission tomography. Psychiatry Res 2002;116:173-85. [DOI] [PubMed]
- 173.Chang L, Yakupov R, Cloak C, et al. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain 2006;129:1096-112. [DOI] [PubMed]
- 174.Kanayama G, Rogowska J, Pope HG, et al. Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2004;176:239-47. [DOI] [PubMed]
- 175.Porrino L, Whitlow CT, Lamborn C, et al. Impaired performance on a decision-making task by heavy marijuana users: an fMRI study [poster]. 2004 Symposium on the Cannabinoids; 2004 June 22–24; Paestum (Italy).
- 176.Bolla KI, Eldreth DA, Matochik JA, et al. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage 2005;26:480-92. [DOI] [PubMed]
- 177.Smith AM, Fried PA, Hogan MJ, et al. Effects of prenatal marijuana on response inhibition: an fMRI study of young adults. Neurotoxicol Teratol 2004;26:533-42. [DOI] [PubMed]
- 178.Niznikiewicz MA, Kubicki M, Shenton ME. Recent structural and functional imaging findings in schizophrenia. Curr Opin Psychiatry 2003;16:123-47.
- 179.Callicott JH, Bertolino A, Mattay VS, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 2000;10:1078-92. [DOI] [PubMed]
- 180.Barch DM, Csernansky JG, Conturo T, et al. Working and long-term memory deficits in schizophrenia: Is there a common prefrontal mechanism? J Abnorm Psychol 2002;111:478-94. [DOI] [PubMed]
- 181.Herning RI, Better WE, Tate K, et al. Cerebrovascular perfusion in marijuana users during a month of monitored abstinence. Neurology 2005;64:488-93. [DOI] [PubMed]
- 182.Matochik JA, Eldreth DA, Cadet JL, et al. Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend 2005;77:23-30. [DOI] [PubMed]
- 183.Coolen MW, Van Loo KMJ, Van Bakel NHM, et al. Gene dosage effect on [gamma]-secretase component Aph-1b in a rat model for neurodevelopmental disorders. Neuron 2005;45:497-503. [DOI] [PubMed]
- 184.Hoehe MR, Caenazzo L, Martinez MM, et al. Genetic and physical mapping of the human cannabinoid receptor gene to chromosome-6q14-q15. New Biol 1991;3:880-5. [PubMed]
- 185.Hallmayer JF, Jablensky A, Michie P, et al. Linkage analysis of candidate regions using a composite neurocognitive phenotype correlated with schizophrenia. Mol Psychiatry 2003;8:511-23. [DOI] [PubMed]
- 186.Leroy S, Griffon N, Bourdel MC, et al. Schizophrenia and the cannabinoid receptor type 1 (CB1): Association study using a single-base polymorphism in coding exon 1. Am J Med Genet 2001;105:749-52. [DOI] [PubMed]
- 187.Ujike H, Takaki M, Nakata K, et al. CNR1, central cannabinoid receptor gene, associated with susceptibility to hebephrenic schizophrenia. Mol Psychiatry 2002;7:515-8. [DOI] [PubMed]
- 188.Hoehe MR, Rinn T, Flachmeier C, et al. Comparative sequencing of the human CB1 cannabinoid receptor gene coding exon: no structural mutations in individuals exhibiting extreme responses to cannabis. Psychiatr Genet 2000;10:173-7. [DOI] [PubMed]
- 189.Tsai SJ, Wang YC, Hong CJ. Association study of a cannabinoid receptor gene (CNR1) polymorphism and schizophrenia. Psychiatr Genet 2000;10:149-51. [DOI] [PubMed]
- 190.Tsai SJ, Wang YC, Hong CJ. Association study between cannabinoid receptor gene (CNR1) and pathogenesis and psychotic symptoms of mood disorders. Am J Med Genet 2001;105:219-21. [DOI] [PubMed]
- 191.Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci 2005;6:108-18. [DOI] [PubMed]
- 192.Bilder RM, Volavka J, Lachman HM, et al. The catechol-0-methyltransferase polymorphism: Relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology 2004;29:1943-61. [DOI] [PubMed]
- 193.Caspi A, Moffitt TE, Cannon M, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry 2005;57:1117-27. [DOI] [PubMed]
- 194.Kontkanen O, Toronen P, Lakso MA, et al. Antipsychotic drug treatment induces differential gene expression in the rat cortex. J Neurochem 2002;83:1043-53. [DOI] [PubMed]
- 195.Rushlow W, Seah Y, Belliveau D, et al. Changes in calcineurin expression induced in the rat brain by the administration of antipsychotics. J Neurochem 2005;94:587-96. [DOI] [PubMed]
- 196.Miyakawa T, Leiter LM, Gerber DJ, et al. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A 2003;100:8987-92. [DOI] [PMC free article] [PubMed]
- 197.Eastwood SL, Burnet PWJ, Harrison PJ. Decreased hippocampal expression of the susceptibility gene PPP3CC and other calcineurin subunits in schizophrenia. Biol Psychiatry 2005;57:702-10. [DOI] [PubMed]
- 198.Rubovitch V, Gafni M, Sarne Y. The cannabinoid agonist DALN dependent calcium-channels positively modulates L-type voltage-in N18TG2 neuroblastoma cells. Brain Res Mol Brain Res 2002;101:93-102. [DOI] [PubMed]
- 199.Bass CE, Welch S.P., Martin B.R. Reversal of Delta(9)-tetrahydrocannabinol-induced tolerance by specific kinase inhibitors. Eur J Pharmacol 2004;496:99-108. [DOI] [PubMed]
- 200.Tzavara ET, Valjent E, Firmo C, et al. Cannabinoid withdrawal is dependent upon PKA activation in the cerebellum. Eur J Neurosci 2000;12:1038-46. [DOI] [PubMed]
- 201.Cannich A, Wotjak CT, Kamprath K, et al. CB1 cannabinoid receptors modulate kinase and phosphatase activity during extinction of conditioned fear in mice. Learn Mem 2004;11:625-32. [DOI] [PMC free article] [PubMed]
- 202.Luo J, Yin JH, Wu HZ, et al. Extract from Fructus cannabis activating calcineurin improved learning and memory in mice with chemical drug-induced dysmnesia. Acta Pharmacol Sin 2003;24:1137-42. [PubMed]
- 203.Goldberg TE, Weinberger DR. Genes and the parsing of cognitive processes. Trends Cogn Sci 2004;8:325-35. [DOI] [PubMed]
- 204.Grigorenko E, Kittler J, Clayton C, et al. Assessment of cannabinoid induced gene changes: tolerance and neuroprotection. Chem Phys Lipids 2002;121:257-66. [DOI] [PubMed]
- 205.Chan GC-K, Hinds TR, Impey S, et al. Hippocampal neurotoxicity of 9-tetrahydrocannabinol. J Neurosci 1998;18:5322-32. [DOI] [PMC free article] [PubMed]
- 206.Callicott JH, Straub RE, Pezawas L, et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci U S A 2005;102:8627-32. [DOI] [PMC free article] [PubMed]
- 207.Cannon T, Hennah W, van Erp T, et al. Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short-and long-term memory. Arch Gen Psychiatry 2005;62:1205-13. [DOI] [PubMed]
- 208.Millar J, Pickard B, Mackie S, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science 2005;310:1187-91. [DOI] [PubMed]
- 209.Bray NJ, Buckland PR, Williams NM, et al. A haplotype implicated in schizophrenia susceptibility is associated with reduced COMT expression in human brain. Am J Hum Genet 2003;73:152-61. [DOI] [PMC free article] [PubMed]
- 210.Matsumoto M, Weickert CS, Beltaifa S, et al. Catechol O-methyltransferase (COMT) mRNA expression in the dorsolateral prefrontal cortex of patients with schizophrenia. Neuropsychopharmacology 2003;28:1521-30. [DOI] [PubMed]
- 211.Glatt SJ, Faraone SV, Tsuang MT. Association between a functional catechol O-methyltransferase gene polymorphism and schizophrenia: Meta-analysis of case-control and family-based studies. Am J Psychiatry 2003;160:469-76. [DOI] [PubMed]
- 212.Lohmueller KE, Pearce CL, Pike M, et al. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet 2003;33:177-82. [DOI] [PubMed]
- 213.Owen MJ, Williams NM, O'Donovan MC. The molecular genetics of schizophrenia: findings promise new insights. Mol Psychiatry 2004;9:14-27. [DOI] [PubMed]
- 214.Williams HJ, Glaser B, Williams NM, et al. No evidence for association between COMT genotypes and schizophrenia in two large samples. Am J Med Genet B Neuropsychiatr Genet 2004;130:84.
- 215.Fan JB, Zhang CS, Gu NF, et al. Catechol-O-methyltransferase gene Val/Met functional polymorphism and risk of schizophrenia: A large-scale association study plus meta-analysis. Biol Psychiatry 2005;57:139-44. [DOI] [PubMed]
- 216.Egan MF, Goldberg TE, Kolachana BS, et al. Effect of COMT Val(108/158) Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A 2001;98:6917-22. [DOI] [PMC free article] [PubMed]
- 217.Malhotra AK, Kestler LJ, Mazzanti C, et al. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry 2002;159:652-4. [DOI] [PubMed]
- 218.Goldberg TE, Egan MF, Gscheidle T, et al. Executive subprocesses in working memory - Relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry 2003;60:889-96. [DOI] [PubMed]
- 219.De Frias CM, Annerbrink K, Westberg L, et al. COMT gene polymorphism is associated with declarative memory in adulthood and old age. Behav Genet 2004;34:533-9. [DOI] [PubMed]
- 220.Bearden CE, Jawad AF, Lynch DR, et al. Effects of a functional COMT polymorphism on prefrontal cognitive function in patients with 22q11.2 deletion syndrome. Am J Psychiatry 2004;161:1700-2. [DOI] [PubMed]
- 221.Nolan KA, Bilder RM, Lachman HM, et al. Catechol O-methyltransferase Val(158)Met polylmorphism in schizophrenia: Differential effects of Val and Met alleles on cognitive stability and flexibility. Am J Psychiatry 2004;161:359-61. [DOI] [PubMed]
- 222.Gallinat J, Bajbouj M, Sander T, et al. Association of the G1947A COMT (Val(108/158)Met) gene polymorphism with prefrontal P300 during information processing. Biol Psychiatry 2003;54:40-8. [DOI] [PubMed]
- 223.Rosa A, Peralta V, Cuesta MJ, et al. New evidence of association between COMT gene and prefrontal neurocognitive from sibling function in healthy individuals pairs discordant for psychosis. Am J Psychiatry 2004;161:1110-2. [DOI] [PubMed]
- 224.Meyer-Lindenberg A, Kohn PD, Kolachana B, et al. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci 2005;8:594-6. [DOI] [PubMed]
- 225.Henquet C, Rosa A, Delespaul P, et al. COMT Val158Met moderation of the effect of cannabis use on “switching on” psychotic symptoms in daily life. Schizophr Res 2006;81(Suppl 1):S218-9.
- 226.Rosa A, Henquet C, Krabbendam L, et al. Experimental study of COMT Val58Met moderation of cannabis-induced effects on psychosis and cognition. Schizophr Res 2006;81(Suppl 1):S222. [DOI] [PubMed]
- 227.Van Os J, Henquet C, Rosa A, et al. Cannabis and psychosis. Schizophr Res 2006;81(Suppl 1):S306.
- 228.Szeszko PR, Lipsky R, Mentschel C, et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry 2005;10:631-6. [DOI] [PubMed]
- 229.Hariri AR, Goldberg TE, Mattay VS, et al. Brain-derived neurotrophic factor val(66)met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci 2003;23:6690-4. [DOI] [PMC free article] [PubMed]
- 230.Angelucci F, Brene S, Mathe AA. BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry 2005;10:345-52. [DOI] [PubMed]
- 231.Shoval G, Weizman A. The possible role of neurotrophins in the pathogenesis and therapy of schizophrenia. Eur Neuropsychopharmacol 2005;15:319-29. [DOI] [PubMed]
- 232.Szczepankiewicz A, Skibinska M, Czerski PM, et al. No association of the brain-derived neurotrophic factor (BDNF) gene C-270T polymorphism with schizophrenia. Schizophr Res 2005;76:187-93. [DOI] [PubMed]
- 233.Weickert CS, Ligons DL, Romanczyk T, et al. Reductions in neurotrophin receptor mRNAs in the prefrontal cortex of patients with schizophrenia. Mol Psychiatry 2005;10:637-50. [DOI] [PubMed]
- 234.Hashimoto T, Bergen SE, Nguyen QL, et al. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci 2005;25:372-83. [DOI] [PMC free article] [PubMed]
- 235.Bayatti N, Hermann H, Lutz B, et al. Corticotropin-releasing hormone-mediated induction of intracellular signaling pathways and brain-derived neurotrophic factor expression is inhibited by the activation of the endocannabinoid system. Endocrinology 2005;146:1205-13. [DOI] [PubMed]
- 236.Butovsky E, Juknat A, Goncharov I, et al. In vivo up-regulation of brain-derived neurotrophic factor in specific brain areas by chronic exposure to Delta(9)-tetrahydrocannabinol. J Neurochem 2005;93:802-11. [DOI] [PubMed]
- 237.Jockers-Scherübl MC, Matthies U, Danker-Hopfe H, et al. Chronic cannabis abuse raises nerve growth factor serum concentrations in drug-naive schizophrenic patients. J Psychopharmacol 2003;17:439-45. [DOI] [PubMed]
- 238.Jockers-Scherübl MC, Danker-Hopfe H, Mahlberg R, et al. Brain-derived neurotrophic factor serum concentrations are increased in drug-naive schizophrenic patients with chronic cannabis abuse and multiple substance abuse. Neurosci Lett 2004;371:79-83. [DOI] [PubMed]
- 239.Tan YL, Zhou DF, Cao LY, et al. Decreased BDNF in serum of patients with chronic schizophrenia on long-term treatment with antipsychotics. Neurosci Lett 2005;382:27-32. [DOI] [PubMed]
- 240.Kapur S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry 2003;160:13-23. [DOI] [PubMed]
- 241.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 2000;95:S91-S117. [DOI] [PubMed]
- 242.Robinson TE, Berridge KC. Addiction. Annu Rev Psychol 2003;54:25-53. [DOI] [PubMed]
- 243.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci 2004;5:483-94. [DOI] [PubMed]
- 244.Tsapakis EM, Guillin O, Murray RM. Does dopamine sensitization underlie the association between schizophrenia and drug abuse? Curr Opin Psychiatry 2003;16:S45-52.
- 245.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci 2001;2:695-703. [DOI] [PubMed]
- 246.Cami J, Farre M. Mechanisms of disease: drug addiction. N Engl J Med 2003;349:975-86. [DOI] [PubMed]
- 247.Lubman DI, Yücel M, Pantelis C. Addiction, a condition of compulsive behaviour? Neuroimaging and neuropsychological evidence of inhibitory dysregulation. Addiction 2004;99:1491-502. [DOI] [PubMed]
- 248.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373-90. [DOI] [PubMed]
- 249.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex 2000;10:318-25. [DOI] [PubMed]
- 250.Ramirez BG, Blazquez C, del Pulgar TG, et al. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci 2005;25: 1904-13. [DOI] [PMC free article] [PubMed]
- 251.Lastres-Becker I, Molina-Holgado F, Ramos JA, et al. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson's disease. Neurobiol Dis 2005;19:96-107. [DOI] [PubMed]
- 252.Aguado T, Monory K, Palazuelos J, et al. The endocannabinoid system drives neural progenitor proliferation. FASEB J 2005;19:1704-6. [DOI] [PubMed]
- 253.Jiang W, Zhang Y, Xiao L, et al. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic-and antidepressant-like effects. J Clin Invest 2005;115:3104-16. [DOI] [PMC free article] [PubMed]
- 254.Ritsner M, Maayan R, Gibel A, et al. Low pregnelnolone plasma concentration in patients with schizophrenia associated with symptoms, anxiety, and distress. Schizophr Res 2006;81(Suppl 1):S206.
- 255.Keri S, Janka Z. Critical evaluation of cognitive dysfunctions as endophenotypes of schizophrenia. Acta Psychiatr Scand 2004;110:83-91. [DOI] [PubMed]
- 256.Weiser M, Davidson M, Noy S. Comments on risk for schizophrenia. Schizophr Res 2005;79:15-21. [DOI] [PubMed]
- 257.Weiser M, Van Os J, Davidson M. Time for a shift in focus in schizophrenia: from narrow phenotypes to broad endophenotypes. Br J Psychiatry 2005;187:203-5. [DOI] [PubMed]
- 258.Stefanis NC, Delespaul P, Henquet C, et al. Early adolescent cannabis exposure and positive and negative dimensions of psychosis. Addiction 2004;99:1333-41. [DOI] [PubMed]
- 259.Duke PJ, Pantelis C, McPhillips MA, et al. Comorbid non-alcohol substance misuse among people with schizophrenia: epidemiological study in central London. Br J Psychiatry 2001;179:509-13. [DOI] [PubMed]
- 260.Kavanagh DJ, Waghorn G, Jenner L, et al. Demographic and clinical correlates of comorbid substance use disorders in psychosis: multivariate analyses from an epidemiological sample. Schizophr Res 2004;66:115-24. [DOI] [PubMed]
- 261.Fowler IL, Carr VJ, Carter NT, et al. Patterns of current and lifetime substance use in schizophrenia. Schizophr Bull 1998;24:443-55. [DOI] [PubMed]
- 262.Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental-disorders with alcohol and other drug-abuse: results from the Epidemiologic Catchment-Area (ECA) study. JAMA 1990;264:2511-8. [PubMed]
- 263.Spencer C, Castle D, Michie PT. Motivations that maintain substance use among individuals with psychotic disorders. Schizophr Bull 2002;28:233-47. [DOI] [PubMed]
- 264.Linszen D, Peters B, de Haan L. Cannabis abuse and the course of schizophrenia. In: Castle D, Murray R, editors. Marijuana and madness: psychiatry and neurobiology. Cambridge: Cambridge University Press; 2004. p. 119-26.
- 265.Spencer C. Motives that maintain cannabis use among individuals with psychotic disorders. In: Castle D, Murray R, editors. Marijuana and madness: psychiatry and neurobiology. Cambridge: Cambridge University Press; 2004. p. 166-85.
- 266.Goswami S, Mattoo SK, Basu D, et al. Substance-abusing schizophrenics: Do they self-medicate? Am J Addict 2004;13:139-50. [DOI] [PubMed]
- 267.Hambrecht M, Häfner H. Cannabis, vulnerability, and the onset of schizophrenia: an epidemiological perspective. Aust N Z J Psychiatry 2000;34:468-75. [DOI] [PubMed]
- 268.Hambrecht M, Hafner H. Substance abuse and the onset of schizophrenia. Biol Psychiatry 1996;40:1155-63. [DOI] [PubMed]
- 269.Ferdinand RF, Sondeijker F, van der Ende J, et al. Cannabis use predicts future psychotic symptoms, and vice versa. Addiction 2005;100:612-8. [DOI] [PubMed]
- 270.Arndt S, Tyrell G, Flaum M, et al. Comorbidity of substance abuse and schizophrenia: the role of premorbid adjustment. Psychol Med 1992;22:379-88. [DOI] [PubMed]
- 271.Mueser KT, Drake RE, Wallach MA. Dual diagnosis: a review of etiological theories. Addict Behav 1998;23:717-34. [PubMed]
- 272.Sevy S, Robinson DG, Solloway S, et al. Correlates of substance misuse in patients with first-episode schizophrenia and schizoaffective disorder. Acta Psychiatr Scand 2001;104:367-74. [DOI] [PubMed]
- 273.Jonkman CM, Hijman R, Von Dijk D, et al. The effect of cannabis on verbal memory in schizophrenia: preliminary results. Schizophr Res 2002;53(Suppl 1):S224-5.
- 274.Liraud F, Verdoux H. [Effect of comorbid substance use on neuropsychological performance in subjects with psychotic or mood disorders.] Encephale 2002;28:160-8. [PubMed]
- 275.Jockers-Scherübl MC, Rentzsch J, Wolf T, et al. Cannabis induces differing cognitive changes in schizophrenia patients and in controls. Schizophr Res 2006;81(Suppl 1):S303-4.
- 276.Joyal CC, Halle P, Lapierre D, et al. Drug abuse and/or dependence and better neuropsychological performance in patients with schizophrenia. Schizophr Res 2003;63:297-9. [DOI] [PubMed]
- 277.Stirling J, Lewis S, Hopkins R, et al. Cannabis use prior to first onset psychosis predicts spared neurocognition at 10-year follow-up. Schizophr Res 2005;75:135-7. [DOI] [PubMed]
- 278.Kranzler H. Verbal learning and memory deficits in early-onset schizophrenia. Schizophr Res 2006;81(Suppl 1):S114.
- 279.Lee KH, Williams LM, Breakspear M, et al. Synchronous Gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res Brain Res Rev 2003;41:57-78. [DOI] [PubMed]
- 280.Spencer KM, Nestor PG, Niznikiewicz MA, et al. Abnormal neural synchrony in schizophrenia. J Neurosci 2003;23:7407-11. [DOI] [PMC free article] [PubMed]
- 281.Light GA. Probing cortico-cortical interactions that underlie the multiple sensory, cognitive, and everyday functional deficits in schizophrenia. Behav Brain Sci 2004;27:799.
- 282.Hajos N, Katona I, Naiem SS, et al. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci 2000;12:3239-49. [DOI] [PubMed]
- 283.Klausberger T, Marton LF, O'Neill J, et al. Complementary roles of cholecystokinin-and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci 2005;25:9782-93. [DOI] [PMC free article] [PubMed]
- 284.Tzavara ET, Li DL, Moutsimilli L, et al. Endocannabinoids activate transient receptor potential vanilloid 1 receptors to reduce hyperdopaminergia-related hyperactivity: Therapeutic implications. Biol Psychiatry 2006;59:508-15. [DOI] [PubMed]
- 285.Solowij N, Grenyer BFS. Are the adverse consequences of cannabis use age-dependent? Addiction 2002;97:1083-6. [DOI] [PubMed]
- 286.Rey JM, Martin A, Krabman P. Is the party over? Cannabis and juvenile psychiatric disorder: The past 10 years. J Am Acad Child Adolesc Psychiatry 2004;43:1194-205. [DOI] [PubMed]
- 287.Huestegge L, Radach R, Kunert H. Effects of long-term cannabis consumption with early age of onset on oculomotor control and visual information processing [lecture]. 2004 Symposium on the Cannabinoids; 2004 June 22–24; Paestum (Italy).
- 288.Wilson W, Mathew R, Turkington T, et al. Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. J Addict Dis 2000;19:1-22. [DOI] [PubMed]
- 289.Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction 2002;97:1123-35. [DOI] [PubMed]
- 290.Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med 2003;33:15-21. [DOI] [PubMed]
- 291.Fergusson DM, Horwood LJ, Ridder EM. Tests of causal linkages between cannabis use and psychotic symptoms. Addiction 2005;100:354-66. [DOI] [PubMed]
- 292.Wagner FA, Anthony JC. From first drug use to drug dependence: developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology 2002;26:479-88. [DOI] [PubMed]
- 293.Chen CY, Anthony JC. Possible age-associated bias in reporting of clinical features of drug dependence: epidemiological evidence on adolescent-onset marijuana use. Addiction 2003;98:71-82. [DOI] [PubMed]
- 294.Davalos DB, Compagnon N, Heinlein S, et al. Neuropsychological deficits in children associated with increased familial risk for schizophrenia. Schizophr Res 2004;67:123-30. [DOI] [PubMed]
- 295.Hawkins KA, Addington J, Keefe RSE, et al. Neuropsychological status of subjects at high risk for a first episode of psychosis. Schizophr Res 2004;67:115-22. [DOI] [PubMed]
- 296.Luna B, Sweeney JA. The emergence of collaborative brain function - fMRI studies of the development of response inhibition. In: editor. Adolescent Brain Development: Vulnerabilities and Opportunities. 2004. p. 296-309. [DOI] [PubMed]
- 297.Nelson E, Leibenluft E, McClure E, et al. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med 2005;35:163-74. [DOI] [PubMed]
- 298.Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci 2005;9:60-8. [DOI] [PubMed]
- 299.White AM, Swartzwelder HS. Hippocampal function during adolescence - A unique target of ethanol effects. Ann N Y Acad Sci 2004;1021:206-20. [DOI] [PubMed]
- 300.Schneider M, Koch M. Deficient social and play behavior in juvenile and adult rats after neonatal cortical lesion: effects of chronic pubertal cannabinoid treatment. Neuropsychopharmacology 2005;30:944-57. [DOI] [PubMed]
- 301.Pistis M, Perra S, Pillolla G, et al. Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biol Psychiatry 2004;56:86-94. [DOI] [PubMed]
- 302.Del Arco I, Munoz R, Rodriguez DF, et al. Maternal exposure to the synthetic cannabinoid HU-210: effects on the endocrine and immune systems of the adult male offspring. Neuroimmunomodulation 2000;7:16-26. [DOI] [PubMed]
- 303.Biscaia M, Marin S, Fernandez B, et al. Chronic treatment with CP 55,940 during the peri-adolescent period differentially affects the behavioural responses of male and female rats in adulthood. Psychopharmacology (Berl) 2003;170:301-8. [DOI] [PubMed]
- 304.Macri S, Laviola G. Single episode of maternal deprivation and adult depressive profile in mice: interaction with cannabinoid exposure during adolescence. Behav Brain Res 2004;154:231-8. [DOI] [PubMed]
- 305.Mereu G, Fa M, Ferraro L, et al. Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long-term potentiation and glutamate release. Proc Natl Acad Sci U S A 2003;100:4915-20. [DOI] [PMC free article] [PubMed]
- 306.Fride E. The endocannabinoid-CB receptor system: importance for development and in pediatric disease. Neuroendocrinol Lett 2004;25:24-30. [PubMed]
- 307.Fride E. The endocannabinoid-CB1 receptor system in pre-and postnatal life. Eur J Pharmacol 2004;500:289-97. [DOI] [PubMed]
- 308.O'Shea M, Mallet P. Impaired learning in adulthood following neonatal delta9-THC exposure. Behav Pharmacol 2005;16:455-61. [DOI] [PubMed]
- 309.Myin-Germeys I, Krabbendam L, van Os J. Continuity of psychotic symptoms in the community. Curr Opin Psychiatry 2003;16:443-9.
- 310.Van Os J, Henquet C, Stefanis N. Cannabis-related psychosis and the gene-environment interaction. Addiction 2005;100(6):874-5. [DOI] [PubMed]
- 311.Leweke FM, Schreiber D, Koethe D, et al. Endocannabinoids in patients with first-episode schizophrenia and schizophreniform psychosis: relation to cannabis use. Schizophr Res 2006;81(Suppl 1):S304.
- 312.Monterrubio S, Solowij N, Meyer BJ, et al. Fatty acid relationships in former cannabis users with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2006;30:280-5. [DOI] [PubMed]
- 313.Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol 2001;11:250-7. [DOI] [PubMed]


