Abstract
Objective
Major depressive disorder occurs in 15%–30% of patients who have had a myocardial infarction (MI), but the neurobiological mechanisms involved are not well understood. Previously, we found early intracellular signalling changes in the limbic system after acute MI in rats. The aim of the present study was to test the presence of behavioural deficits compatible with animal models of depression after acute MI in rats and to verify whether this is associated with apoptosis vulnerability markers.
Methods
Occlusion of the left-anterior descending artery was induced for 40 minutes under anesthesia in adult male Sprague–Dawley rats. Control sham rats underwent the same surgical procedure without occlusion. After surgery, subgroups of MI and sham rats were treated with desipramine, 10 mg/kg, intraperitoneally for 14 days. All rats were tested on measures of behavioural depression 14 days after surgery with a sucrose preference test, a forced swimming test, and a memory test (Morris water maze [MWM]). The rats were sacrificed, and the MI size was determined; apoptosis was estimated in the prefrontal cortex, hypothalamus, amygdala and hippocampus by measuring Bax:Bcl-2 ratio and caspase-3 activity.
Results
Untreated MI rats drank significantly less sucrose and swam significantly less than sham rats. No difference was found on the MWM. Behavioural depression was prevented by desipramine. Bax:Bcl-2 ratio was significantly increased in the prefrontal cortex and hypothalamus of MI rats, compared with sham rats; caspase-3 activity showed no difference between the 2 groups. Bax:Bcl-2 ratio in the prefrontal cortex was correlated with swim time in the forced swim test.
Conclusion
Behavioural impairment and limbic apoptotic events observed after a myocardial infarct are consistent with a model of human post-MI depression.
Medical subject headings: anhedonia, apoptosis, Bax:Bcl-2, antidepressant, reperfusion
Abstract
Objectif
Un trouble dépressif majeur survient chez 15 à 30 % des patients qui ont subi un infarctus du myocarde (IM), mais on ne comprend pas bien les mécanismes neurobiologiques en cause. Auparavant, nous avions découvert des changements précoces de la transmission des signaux intracellulaires dans le système limbique après un IM aigu chez des rats. La présente étude visait à vérifier la présence de déficits du comportement compatibles avec des modèles animaux de la dépression après un IM aigu chez les rats et à vérifier s'il y a un lien avec les marqueurs de la vulnérabilité à l'apoptose.
Méthodes
On a provoqué l'occlusion de l'artère interventriculaire antérieure pendant 40 minutes sous anesthésie chez des rats Sprague–Dawley mâles adultes. Des rats témoins ont subi la même intervention chirurgicale sans occlusion. Après l'intervention, on a traité les sous-groupes de rats qui ont subi un IM et de rats témoins en leur administrant de la désipramine; 10 mg/kg, par voie intrapéritonéale pendant 14 jours. Tous les rats ont été soumis à des mesures de dépression du comportement 14 jours après l'intervention chirurgicale, au moyen de mesures de préférence du sucrose, de tests de natation forcée et d'un test de mémoire (labyrinthe d'eau de Morris [LEM]). On a sacrifié les rats et déterminé la taille de l'IM. On a estimé l'apoptose dans le cortex préfrontal, l'hypothalamus, les amygdales et l'hippocampe en mesurant le ratio Bax:Bcl-2 et l'activité de la caspase-3.
Résultats
Les rats qui ont subi un IM non traité ont bu beaucoup moins de sucrose et ont nagé beaucoup moins que les rats témoins. Le test LEM n'a révélé aucune différence. On a prévenu la dépression du comportement par la désipramine. Le ratio Bax:Bcl-2 avait augmenté considérablement dans le cortex préfrontal et l'hypothalamus des rats qui ont subi un IM comparativement aux rats témoins. L'activité de la caspase-3 n'a présenté aucune différence entre les deux groupes. On a établi un lien entre le ratio Bax:Bcl-2 dans le cortex préfrontal et le temps de natation au cours du test de natation forcée.
Conclusion
Le déficit du comportement et les événements d'apoptose limbique observés après un infarctus du myocarde correspondent à un modèle de dépression humaine consécutive à un IM.
Introduction
Major depressive disorder (MDD) occurs in 15%–30% of patients who have had myocardial infarction (MI),1–3 suggesting a pathophysiological cross-talk between the heart and the brain. Interface models have been proposed and have lead to various hypotheses, one of which suggests that the release of proinflammatory cytokines is involved in the pathophysiology of post-MI depression.4 This is supported by the fact that the administration of proinflammatory cytokines, such as tumour necrosis factor alpha (TNF-α), induces depressed mood in healthy humans.5,6 Proinflammatory cytokines also display pro-apoptotic properties in limbic areas such as the hippocampus, further supporting their role in the pathophysiology of depression.7,8 We have recently shown in rats that pro-inflammatory cytokine release can induce apoptosis (programmed cell death) of limbic neural tissue after acute MI.9,10 Apoptosis is regulated by different classes of proteins,11,12 including caspase-3, which can induce the cleavage of other proteins and alter cell integrity. Activation of caspase-3 is considered to be a hallmark of apoptosis.12,13 Upstream caspase-3 are anti-apoptotic proteins (e.g., Bcl-2) and pro-apoptotic proteins (e.g., Bax), which regulate the release of cytochrome C from mitochondria, activating caspase to induce apoptosis. For example, it has been shown that Bcl-2 shuts off the apoptotic signal transduction pathway upstream of caspase activation.14 The Bax:Bcl-2 ratio is used as an index of vulnerability for apoptosis.12,15
Apoptosis is suggested to be involved in mood disorders,16 and antidepressant treatments are known to prevent apoptosis17,18 and even increase neurogenesis in the rat hippocampus.19 Fluoxetine (a selective 5-hydroxytryptamine [HT] uptake inhibitor) and moclobemide (a monoamine oxidase-A inhibitor) are antidepressant drugs known to upregulate Bcl-2.20,21 Lithium and valproate, mood-regulating molecules used in bipolar disorders, also increase the expression of the anti-apoptotic protein Bcl-2.22 Thus, the present study aims to verify whether biochemical markers of apoptosis are associated with behavioural signs of depression after MI in rats.
Methods
Experimental groups
We used 30 adult male Sprague–Dawley rats. They were housed individually under constant temperature (22°C) and humidity (40%–50%); food and water were available ad libitum. Light period was 12 hours long and started at 8 am. In 14 rats, the left coronary artery was occluded for 40 minutes (MI rats); the remaining rats were sham operated and were submitted to the same protocol, except the coronary artery was not occluded. Five MI rats and 7 sham rats were treated with desipramine 10 mg/mL (Sigma, Saint Louis, Mo.) at a daily morning dosage of 10 mg/kg, intraperitoneally; the other rats received 0.9% saline in equal volume. The first dose was administered immediately after suturing, and the last dose was administered the morning before being sacrificed (i.e., 14–18 days after surgery). Behavioural tests were conducted between the 14th and the 18th day after surgery (see below). Animal care and handling procedures were approved by the Local Animal Care Committee and followed the guidelines of the Canadian Council for Animal Care.
Surgical procedure
Anesthesia was induced with ketamine/xylazine (35–50 mg/kg and 5 mg/kg intramuscularly, respectively) and maintained on isoflurane (1.5%) ventilation. Electrocardiogram (ECG) and heart rate were monitored throughout. A left thoracotomy was performed at the fifth intercostal space, and the left coronary artery was occluded for 40 minutes with a silk thread. Ischemia was confirmed by alterations of the ST segment (time of complete stimulation of the ventricles) and myocardial surface cyanosis. After the thread was removed and the thorax sutured, each animal was returned to its home cage. All surgeries were performed between 8 am and noon.
Behavioural measures
We selected the tests used on the basis of their validity regarding behavioural depression syndrome. All tests were conducted individually, in the morning, starting 14 days after surgery.
Forced swim test
The forced swim test was originally described by Porsolt23,24 and is a measure of behavioural despair.23,24 Rats were placed in a clear plastic cylindrical pool (45 cm tall × 25 cm diameter) filled with 30 cm of water maintained at 22°C–25°C. Rats were tested for 2 consecutive days (15 min on the 14th day postsurgery and 5 min on the 15th day postsurgery). An experimentor scored the time spent swimming, trying to escape and being immobile on day 15 postsurgery.
Sucrose preference test
Decreased sucrose intake is a measure of anhedonia.25,26 Rats had free access to two 250-mL bottles for 5 consecutive days (i.e., 14–18 days after surgery), one containing tap water and the other containing a 1% sucrose solution. The position of the bottles was alternated each day. Volume intake was estimated by weighing bottles each morning at light onset.
Morris water maze (MWM)
The MWM is a test of motor performance and spatial memory requiring an intact hippocampus.27 Rats were placed in a pool (150 cm diameter, 50 cm deep) filled to 25 cm with water maintained at 22°C–25°C and made opaque with powdered milk. A submerged platform is placed just below the surface of the water. The rats are tested on 4 trials each day, 5 minutes apart, for 6 consecutive days (i.e., 14–19 days after surgery). The number of quadrants crossed, the number of successful trials and the time taken to reach the platform are recorded.28 None of the rats tested with the MWM received desipramine.
Tissue analyses
Brain regions were identified according to the atlas of Paxinos and Watson.29
Infarct size
The heart was canulated via the aorta and washed with saline, and the coronary artery was reoccluded at the same site to determine the area at risk by infusing 2 mL of Evans Blue (0.5%) into the aorta. The left ventricle was placed at –80°C for 5 minutes and then sliced into four or five 2-mm transverse sections. After 5 minutes of incubation in triphenyltetrazolium chloride (1.5%), infarct size was estimated as a percent of area at risk.10
Bax:Bcl-2 content
Tissue samples were lysed in a buffer containing protease and phosphatase inhibitors (leupeptin, microcystine and benzamidine). After solubilization, equal amounts of proteins in each line were loaded on a 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE), and after migration, proteins were transferred onto a nitrocellulose membrane. Primary antibody directed against Bax or Bcl-2 (Santa Cruz Biotechnology Inc., Santa Cruz, Calif.) at a concentration of 1:1000 was followed by a secondary peroxidase-coupled antibody (antirabbit immunoglobulin-horseradish peroxidase [IgG-HRP] or antimouse IgG-HRP, from Santa Cruz Biotechnology, Calif.) at a concentration of 1:5000. A Renaissance chemiluminescence kit (Perkin Elmer, Mississauga, Ont.) was used to visualize the bands, and the quantitative analysis was conducted with a Kodak ImageStation. After quantification, membranes were placed in stripping buffer (0.1 M glycine, 1% SDS, pH 2.0, 1 hour at room temperature). The same procedures were repeated with the other antibody (Bax or Bcl-2) to obtain the Bax:Bcl-2 ratio.10
Caspase-3 activity
Cytosolic proteins were extracted in lysis buffer (1% Triton X-100, 0.32 M sucrose, 10 mmol/mL Tris [pH 8.0], 5 mmol/mL ethylenediamine tetra-acetate, 2 mmol/mL DL-1,4-dithiothreitol [DLL], 1 mmol/mL phenylmethanesulfonyl fluoride [PMSF], 10 mg/mL Leupeptin, 10 mg/mL Pepstatin A, 10 g/mL Aprotinin). Enzymatic reactions were performed in a reaction buffer (50 mmol/mL Tris [pH 7.5], 5 mmol/mL MgCl2, 1 mmol/mL ethylene glycol bis-2-aminoethyl ether-N,N',N_,n'-tetra-acetic acid, 0.1% 3-cholamidopropyl dimethylammonio]-1-propanesulfonate [CHAPS], 1 mmol/mL DTT), with 25 mg of proteins and fluorogenic substrate, N-acetyl-asp-glu-val-asp-7-amido-4 methylcoumarin (Ac-DEVD-AMC) (40 μmol/mL). Reactions were incubated at 37°C for 3 hours and stopped with the addition of 0.4 M NaOH and 0.4 M glycine buffer. Fluorescence was quantified with a spectrofluorometer (Photon Technology International, Lawrenceville, NJ) at an excitation wavelength of 365 nm and an emission wavelength of 465 nm. Linearity has been tested and observed up to 100 μg of protein.10
Statistical analysis
We performed analysis of variance (ANOVA) for factorial designs (2 × 2) for the forced swim test, the sucrose test and the Bax:Bcl-2 ratio (prefrontal cortex) to compare MI and sham rats with and without desipramine. We performed ANOVA for factorial designs with repeated-measures (2 × 6; days as repeated-measures) to analyze results obtained in the MWM. We used orthogonal contrasts according to Gram–Schmidt were performed. Linear regression coefficient was calculated with GraphPad Prism version 4b (GraphPad Software, San Diego, Calif.). Caspase-3 activity and Bax:Bcl-2 ratio (amygdala, hippocampus, hypothalamus) were analyzed with the Student's t test to compare MI and sham rats. In all cases, alpha level was set at 0.05.
Results
Behavioural measures
Forced swim test
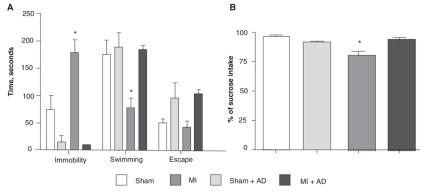
ANOVA indicated a significant interaction between MI and treatment (F1,16 = 20,33; p < 0.001) for immobility, swimming (F1,16 = 6,25; p < 0,05) and escape time (F1,16 = 15,86; p < 0.001). For immobility and swimming, further analysis indicated that the response of the untreated MI group was largely responsible for this interaction: untreated MI rats were more immobile (F1,16 = 12,42, p < 0.005) and swam less (F1,16 = 11.93, p < 0.005) than untreated sham rats. Conversely, desipramine-treated MI rats did not differ significantly from the desipramine-treated sham group with respect to immobility and swimming performance. See Figure 1a.
Fig. 1: Results (mean and standard error of the mean [SEM]) of behavioural tests in myocardial infarction (MI) and control sham rats, with and without long-term antidepressant treatment (see Methods). Forced swim test (sham n = 6, sham + desipramine treated rats [AD] n = 3, MI n = 7, MI + AD n = 4) (A). The SEM bar in the MI + AD group (immobility) is too small to be displayed. Sucrose preference test (sham n = 5, sham + AD n = 3, MI n = 5, MI + AD n = 4) (B). *Indicates a statistically significant difference between the untreated MI group and the untreated sham group.
Sucrose preference test
In the sucrose preference test, total liquid did not differ between groups. ANOVA indicated an interaction between MI and treatment in the sucrose preference test (F1,13 = 11,1; p < 0.01). Further analysis indicated that the response for the untreated MI group was largely responsible for this interaction: untreated MI rats drank significantly less sucrose (F1,13 = 21,74; p < 0.001) than did the untreated sham rats. However, desipramine-treated MI rats did not differ significantly from the desipramine-treated sham group with respect to sucrose intake. See Figure 1b.
Morris water maze
In the MWM, ANOVA indicated no difference between the MI group and the sham groups (n = 5 in each group). There was a significant (F1,40 = 12.59, p < 0.001) linear trend in the longitudinal data for days 1–6, suggesting a learning effect in both groups of rats. Higher-order polynomial terms were not statistically significant.
Tissue analyses
Infarct size
The MI size was 71.0% (standard error of the mean [SEM] 2.0%) of the area at risk.
Bax:Bcl-2 content
Bax:Bcl-2 ratios were significantly different between the MI and the sham groups in the hypothalamus (MI: 200 SEM 9.1, sham: 100 SEM 6.9; n = 4. t = 8.76; df 6; p < 0.001). In the prefrontal cortex, the ANOVA indicated a significant interaction between MI and treatment (F1,11 = 6,5; p < 0.05). Further analysis indicated that the change in Bax:Bcl-2 content for the untreated MI group was largely responsible for this interaction: compared with the untreated sham rats, untreated MI rats showed a higher Bax:Bcl-2 content (F1,11 = 19,18; p < 0.005). Treated MI rats did not differ significantly from the desipramine-treated group with respect to the Bax:Bcl-2 content. Bax:Bcl-2 ratios did not differ between MI and sham groups for the amygdala (t = 0.91; df 6; p = 0.39) and the hippocampus (t = 1,09; df 6; p = 0.32). See Figure 2.
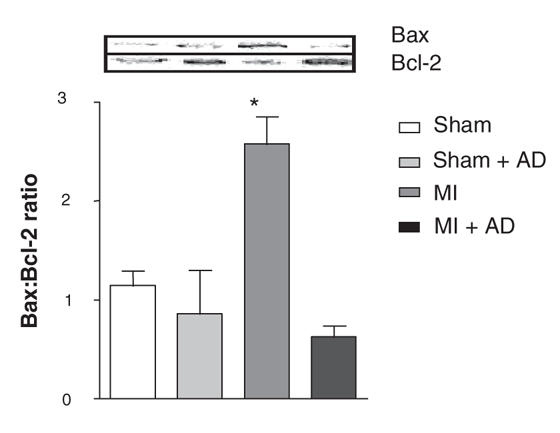
Fig. 2: Top: representative gel (Western blot analyses), acquired with a Kodak Image Station 440CF. Bottom: Bax:Bcl-2 ratio in the prefrontal cortex 3 weeks after surgery (mean and standard error of the mean). Sham n = 4, sham + desipramine treated rats [AD] n = 3, myocardial infarction (MI) n = 4, MI + AD n = 4. *Indicates a statistically significant difference between the untreated MI group and the untreated sham group.
Relation between Bax:Bcl-2 ratios and specific behavioural changes
Regarding the relation between Bax:Bcl-2 ratios and specific behavioural changes, we observed an inverse linear regression between Bax:Bcl-2 ratio and swimming time in the forced swim test: an increase in the prefrontal cortex Bax:Bcl-2 ratio was associated with a shorter period of swimming. See Figure 3.
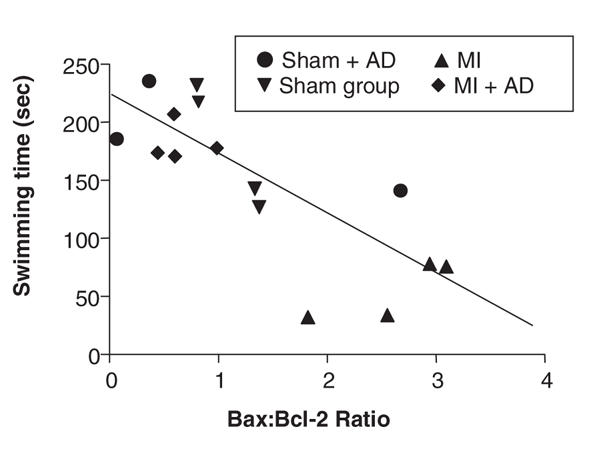
Fig. 3: Linear regression between Bax:Bcl-2 ratio in the prefrontal cortex and swimming time in the forced swim test indicates an inverse relation (r2 = 0.71; p < 0.0001). AD = desipramine treated rats; MI = myocardial infarction.
Caspase-3 activity
Caspase-3 activity showed no difference between the MI and sham rats in any of the 4 limbic structures investigated.
Discussion
This study shows that MI rats display behavioural signs compatible with depression 2 weeks after the cardiovascular event, including anhedonia (i.e., less sucrose intake25,30) and behavioural despair (i.e., decreased forced swimming23,24), and both were reversed by antidepressant treatment (desipramine 10 mg/kg, intraperitoneally, for 14 days). In the latter case, desipramine decreased the passive strategy (immobility) in favour of active strategies (escape and swim), which is consistent with previous observations in other models.31,32 However, MI rats were not impaired on the MWM, a task that requires an intact hippocampus. This is consistent with our observation of lack of hippocampal apoptosis. The lack of impairment on the MWM further suggests that MI rats did not suffer from overt motor problems or fatigue over the 6-day protocol.
The increased Bax:Bcl-2 ratio we observed in MI rats relative to sham rats is in line with the results obtained by Eilat16 in peripheral blood leukocytes of patients with major depression, compared with control subjects. Our results show that neurons of the prefrontal cortex and hypothalamus are vulnerable to apoptosis 2–3 weeks after an acute MI, while the hippocampus is spared. Surprisingly, we found no Bax:Bcl-2 ratio difference in the hippocampus and amygdala of the MI group versus the sham group. At least 2 possibilities could explain these results. First, there could be a different time course for post-MI apoptosis in these structures, since we have previously reported the presence of apoptosis in the amygdala 3 days after MI.10 Second, subdivisions of these limbic structures may need to be independently analyzed because the apoptotic signal in limbic subcompartments may be lost when averaged across an entire structure. This possibility may be less likely in the case of the hippocampus because, in the present study, MI rats displayed normal performance on the MWM.
A statistically significant inverse linear relation was observed between the Bax:Bcl-2 ratio in the prefrontal cortex and swimming time in the forced swim test. The fact that signs of behavioural despair correlate with higher Bax:Bcl-2 ratios (increased apoptosis vulnerability) suggests a link between limbic cell death and behavioural markers of depression. Because only prefrontal cortex tissue was available for this correlation analysis, more research with other apoptotic structures should be done to verify whether the present observation is neuroanatomically specific.
The absence of significant differences in caspase-3 activity between MI and sham rats at the level of prefrontal cortex and hypothalamus despite increased Bax:Bcl-2 ratios may be interpreted in several ways. First, it may indicate that this apoptotic process, that is, the stimulation of caspase-3, is not active at this point in time and that a shorter or longer post-MI time-course could be involved (see Wann10). A second possibility is that caspase-3 is activated only in a subcompartment of the structures investigated in the present experiment. Third, a dissociation between Bax:Bcl-2 activity and caspase-3 activity has been reported,12 suggesting that this is not an exceptional process. Indeed, cell death can actually occur without the activation of caspase-3, as in cases of autophagy or paraptosis. Molecules that are involved in apoptosis, such as Bcl-2, Bax and apoptosis-inducible factors, are also involved in caspase-independent cell death processes.33,34 The possibility that caspase-3–independent apoptosis is present in our model should be tested in the near future.
In conclusion, the behavioural impairment and limbic apoptotic events reported here after MI are compatible with a model of human post-MI depression, and this model follows a particular time course.
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research to GR and RG. GR is a scholar of the Fonds de recherche en santé du Québec. BPW is recipient of the J.A. DeSève PhD scholarship. We thank Pierre Fortier and Caroline Bouchard for their skilful assistance and technical expertise.
Footnotes
Contributors: Drs. Wann, Bah, Le Marec, Rousseau and Godbout designed the study. All authors acquired the data; Drs. Wann, Bah, Boucher, Le Marec, Rousseau and Godbout analyzed it. Drs. Wann, Bah, Courtemanche, Le Marec, Rousseau and Godbout wrote the article; Drs. Wann, Boucher, Le Marec, Rousseau and Godbout critically reviewed it. All authors gave final approval for the article to be published.
Competing interests: None declared.
Correspondence to: Dr. Roger Godbout, Centre de recherche, Hôpital du Sacré-Coeur, 5400 Boul. Gouin ouest, Montréal QC H4J 1C5; fax 514 338-2694; Roger.Godbout@UMontreal.ca
References
- 1.Carney R, Freedland K, Jaffe A. Insomnia and depression prior to myocardial infarction. Psychosom Med 1990;52:603-9. [DOI] [PubMed]
- 2.Frasure-Smith N, Lespérance F, Talajic M. Depression following myocardial infarction: impact on 6-months survival. JAMA 1993; 270: 1819-25. [PubMed]
- 3.Honig A, Lousberg R, Wojchiechowski F, et al. Depression following a first heart infarct: similarities with and difference from ‚ordinary' depression. Ned Tijdschr Geneeskd 1997;141:196-9. [PubMed]
- 4.Dantzer R, Aubert A, Bluthe RM, et al. Mechanisms of the effects of cytokines. In: Dantzer R, Wollman EE, Yirmiya R, editors. Cytokines, stress and depression. New York: Kluwer Academic/Plenum Publishers; 1999. p. 83-106.
- 5.Maier S, Watkins L. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev 1998;105:83-107. [DOI] [PubMed]
- 6.Musselman D, Lawson D, Gumnick J, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med 2001;344:961-6. [DOI] [PubMed]
- 7.Lucassen P, Muller M, Holsboer F, et al. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am J Pathol 2001;158:453-68. [DOI] [PMC free article] [PubMed]
- 8.Francis J, Zhang Z, Weiss M, et al. Neural regulation of the proinflammatory cytokine response to acute myocardial infarction. Am J Physiol Heart Circ Physiol 2004;287:H791-7. [DOI] [PubMed]
- 9.Boucher M, Wann B, Kaloustian S, et al. Sustained cardioprotection afforded by a2a adenosine receptor stimulation after 72 hours of myocardial reperfusion. J Cardiovasc Pharmacol 2005;45:439-46. [DOI] [PubMed]
- 10.Wann B, Boucher M, Kaloustian S, et al. Apoptosis detected in the amygdala following myocardial infarction in the rat. Biol Psychiatry 2006;59:430-3. [DOI] [PubMed]
- 11.Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997;91:479-89. [DOI] [PubMed]
- 12.Jarskog L, Selinger E, Lieberman J, et al. Apoptotic proteins in the temporal cortex in schizophrenia: high bax/bcl-2 ratio without caspase-3 activation. Am J Psychiatry 2004;161:109-15. [DOI] [PubMed]
- 13.Casciola-Rosen L, Nicholson D, Chong T, et al. Apopain/CPP32 cleaves proteins that are essential for cellular repair: a fundamental principle of apoptotic death. J Exp Med 1996;183:1957-64. [DOI] [PMC free article] [PubMed]
- 14.Chinnaiyan AM, Orth K, O'Rourke K, et al. Molecular ordering of the cell death pathway: Bcl-2 and Bcl-xL function upstream of the Ced-3 like apoptotic proteases. J Biol Chem 1996;271:4573-6. [DOI] [PubMed]
- 15.Cregan S, Dawson V, Slack R. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene 2004;23:2785-96. [DOI] [PubMed]
- 16.Eilat E, Mendlovic S, Doron A, et al. Increased apoptosis in patients with major depression: a preliminary study. J Immunol 1999; 163: 533-4. [PubMed]
- 17.Lucassen PJ, Fuchs E, Czéh B. Antidepressant treatment with tianeptine reduces apoptosis in the hippocampal dentate gyrus and temporal cortex. Biol Psychiatry 2004;55:789-96. [DOI] [PubMed]
- 18.Nahon E, Israelson A, Abu-Hamad S, et al. Fluoxetine (Prozac) interaction with the mitochondrial voltage-dependent anion channel and protection against apoptotic cell death. FEBS Lett 2005; 579: 5105-10. [DOI] [PubMed]
- 19.Malberg JE, Eisch AJ, Nestler EJ, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 2000;20:9104-10. [DOI] [PMC free article] [PubMed]
- 20.Chiou SH, Ku HH, Tsai TH, et al. Moclobemide upregulated Bcl-2 expression and induced neural stem cell differentiation into serotoninergic neuron via extracellular-regulated kinase pathway. Br J Pharmacol. 2006;148:587-98. [DOI] [PMC free article] [PubMed]
- 21.Chiou SH, Chen SJ, Peng CH, et al. Fluoxetine up-regulates expression of cellular FLICE-inhibitory protein and inhibits LPS-induced apoptosis in hippocampus-derived neural stem cell. Biochem Biophys Res Commun 2006;343:391-400. [DOI] [PubMed]
- 22.Li R, El-Mallahk RS. A novel evidence of different mechanisms of lithium and valproate neuroprotective action on human SY5Y neuroblastoma cells: caspase-3 dependency. Neurosci Lett 2000;294:147-50. [DOI] [PubMed]
- 23.Porsolt RD, Anton G, Deniel M, et al. Behavioural despair in rats: a new animal model sensitive to antidepressant treatments. Eur J Pharmacol 1978;47:379-91. [DOI] [PubMed]
- 24.Porsolt RD, Bertin A, Jalfre M. Behavioural despair in mice: a primary screening test for antidepressants. Arch Int Pharmocodyn Ther 1977;229:327-36. [PubMed]
- 25.Willner P, Towell A, Sampson D, et al. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 1987;93:358-64. [DOI] [PubMed]
- 26.Redei E, Ahmadiyeh N, Baum A, et al. Novel animal models of affective disorders. Semin Clin Neuropsychiatry 2001;6:43-67. [DOI] [PubMed]
- 27.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 1984;11:47-60. [DOI] [PubMed]
- 28.Beaulieu I, Godbout R. Spatial learning on the Morris Water Maze Test after a short-term paradoxical sleep deprivation in the rat. Brain Cogn 2000;43:27-31. [PubMed]
- 29.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1986.
- 30.Grippo AJ, Santos CM, Johnson RF, et al. Increased susceptibility to ventricular arrhythmias in a rodent model of experimental depression. Am J Physiol Heart Circ Physiol 2004;286:H619-26. [DOI] [PubMed]
- 31.Detke M, Rickels M. I IL. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 1995;121:66-72. [DOI] [PubMed]
- 32.Reneric J, Lucki I. Antidepressant behavioural effects by dual inhibition of monoamine reuptake in the rat forced swimming test. Psychopharmacology 1998;136:190-7. [DOI] [PubMed]
- 33.Bröker LE, Kruyt FAE, Giaccone G. Cell death independent of caspases: a review. Clin Cancer Res 2005;11:3155-62. [DOI] [PubMed]
- 34.Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005;122:927-39. [DOI] [PubMed]