Abstract
Mitochondrial leucyl-tRNA synthetase (LeuRS) in the yeast Saccharomyces cerevisiae provides two essential functions. In addition to aminoacylation, LeuRS functions in RNA splicing. The details of how it came to act in splicing are not known. Here we show that Mycobacterium tuberculosis and human mitochondrial LeuRSs can substitute in splicing for the S. cerevisiae mitochondrial LeuRS. Mutations of yeast mitochondrial LeuRS that had previously been shown to abolish splicing activity also eliminate splicing by the M. tuberculosis enzyme. These results suggest the role of LeuRS in splicing in yeast mitochondria results from features of the enzyme that are broadly conserved in evolution. These features are not likely to be designed for splicing per se, but instead have been adopted in yeast for that purpose.
Aminoacyl-tRNA synthetases are a family of essential enzymes (1). Prokaryotes typically contain up to 20 different tRNA synthetases, whose primary function is to establish the genetic code in the first step of protein synthesis. Eukaryotes have distinct sets of nuclear-encoded cytoplasmic and mitochondrial enzymes. Each enzyme activates and covalently links a single amino acid to its cognate tRNA that bears the anticodon triplet for that amino acid. However, many tRNA synthetases have been recruited and adapted to perform other cellular functions (2). Among these functions is facilitation of the self-splicing mechanism of group I introns.
In yeast mitochondria, leucyl-tRNA synthetase (LeuRS or NAM2p) acts as an intron-specific splicing factor to aid in the excision of two closely related group I introns (3–6). These include the bI4 and aI4α introns that respectively interrupt mRNA encoding the essential respiratory proteins, cytochrome b and subunit I of cytochrome oxidase. Two other organisms (Neurospora crassa and Podospora anserina) depend on a mitochondrial tyrosyl-tRNA synthetase (TyrRS or CYT-18p) for a critical role in RNA splicing (7, 8). CYT-18p uses its existing tRNA binding domains (9) as well as a unique N-terminal domain that is not found in other tyrosine enzymes (8, 10). This idiosyncratic N-terminal splicing element is conspicuously absent in yeast mitochondrial LeuRS. Also, the yeast LeuRS-dependent splicing reaction requires a second protein [a maturase (5, 11)], whereas the N. crassa splicing reaction solely depends on CYT-18p (9).
The question we addressed was whether the novel splicing activity of yeast mitochondrial LeuRS resulted from a common feature shared by other LeuRSs that are not known to be active in splicing of pre-mRNA. If so, LeuRSs from other organisms might substitute in splicing for the yeast mitochondrial enzyme. Alternatively, the novel splicing activity could have resulted from an idiosyncratic modification of specific motifs that were designed for another purpose (such as aminoacylation of tRNA). Were this the case it is doubtful that leucine enzymes that lacked these modifications would be adequate surrogates for the yeast enzyme.
To test these ideas, we created a null allele of the gene encoding yeast mitochondrial LeuRS. We then attempted to rescue the respiration deficiency of this null strain with transgenes for LeuRS from two widely separated organisms, Homo sapiens and M. tuberculosis. These leucine enzymes are thought to have no role in splicing in human mitochondria or M. tuberculosis, respectively. Thus, the use in yeast of genes for these two enzymes enabled us to test specifically whether determinants broadly conserved in evolution were adopted in yeast for splicing. Alternatively, the failure of either the human mitochondrial or M. tuberculosis enzymes to rescue the null allele would be consistent with alterations specific to the yeast mitochondrial enzyme being responsible for the splicing activity.
Materials and Methods
Cloning, Expression, and Purification of M. tuberculosis LeuRS.
M. tuberculosis (Erdman H37Rv) genomic DNA (a gift from P. Brennan, Colorado State University, Fort Collins) was combined with degenerate primers and Taq polymerase for amplification by PCR. A gel-purified 440-bp PCR product was cloned into plasmid pT7Blue(R) (Novagen). The M. tuberculosis DNA insert was isolated by restriction digestion with BamHI and NdeI and radiolabeled by using the Random Primer DNA Labeling Kit (Boehringer Mannheim).
R. Young (Whitehead Institute, Cambridge, MA) kindly provided the λgt11 M. tuberculosis genomic library (12). Plaques from the library were transferred to Gene Screen Hybridization Transfer nylon membranes (DuPont) and probed by using the LeuRS-encoding PCR fragment (107 cpm). Subsequent to washing and autoradiography, positive plaques were recovered followed by isolation of the phage DNA. EcoRI digestion of one (called pLEU10) of the eight isolated phage plasmids yielded four fragments, which were cloned into plasmid pBSKS+ (Stratagene) for sequencing.
PCR was used to amplify the full-length gene from pLEU10 and to introduce BglII and NotI restriction sites as well as to replace the GTG start codon with an ATG for optimal expression in Escherichia coli. The gene was cloned into plasmid pGEX-4T-2 (Amersham Pharmacia), yielding plasmid pJZL201, which was used to transform E. coli DH5α (GIBCO/BRL). Mutant enzymes were generated by using the Stratagene Quikchange Site-Directed Mutagenesis kit. Glutathione S-transferase fusion proteins were purified according to the commercial protocol (Amersham Pharmacia) and tested for aminoacylation activity (13).
Plasmid Constructs Used in Complementation Studies in the Mitochondrial LeuRS Deletion Strain.
Plasmid pG59/T1 is derived from a YEp13 library (14) and contains an 11-kbp genomic insert that encodes the mitochondrial LeuRS gene (MSL1) (15). Restriction digestions showed that the insert orientation was opposite to the published orientation (15). When pG59/T1 was digested to completion with XbaI and religated, the resulting pQB184 still contained the full MSL1 gene. Therefore, the smaller pQB184 was used interchangeably with pG59/T1 as a source of the MSL1 gene. A 2.8-kbp ScaI/SpeI fragment from pQB184 containing the full MSL1 gene was excised and ligated into the EcoRV/SpeI backbone of pBluescript SK+ (Stratagene), generating plasmid pQB185. A 2-kbp BstEII segment deleting 75% of the MSL1 gene fragment (to give mslΔ) was excised from pQB185. The BstEII ends were filled in by using Klenow fragment and ligated to “filled-in” BamHI ends of a 1.15-kbp HIS3 fragment within plasmid YDp-H (16), thereby generating plasmid pQB187. Plasmid pQB223 was constructed by ligating a 4.5-kbp SphI/XbaI fragment containing the MSL1 gene from pG59/T1 to the SphI/XbaI backbone of plasmid YEplac195 (17).
The presequence from the cytochrome oxidase IV gene (18, 19) was used to construct mitochondrial import vectors pQB111 and pQB136, which also contained a constitutive ADHI promoter (20). Plasmid pQB154 bearing the MetRS gene from M. tuberculosis was made by cloning the 1.7-kbp BamHI fragment from plasmid pSLM101 (21) into the BamHI site of pQB111, and then as a HindIII/KpnI fragment into the LEU2+ vector YEplac181 (ref. 17; F.H., X.S., P.S., and S.A.M., unpublished work). Removal of the MetRS-specific sequence (BamHI fragment) from pQB154 yielded plasmid pQB153, which was used as the vector control.
The pC3-Mtb plasmid was engineered by PCR amplification of the M. tuberculosis LeuRS gene from plasmid pLEU10 using Vent DNA polymerase (New England Biolabs) and 5′ and 3′ primers that introduced, respectively, XbaI and BglII sites. The PCR product was restriction digested and the gene was inserted into pQB153 that had been cleaved with XbaI and BamHI. Primers that contained 5′ XbaI and 3′ BamHI sites were designed to amplify human mitochondrial LeuRS by reverse transcription (RT)-PCR using the published sequence (22). Template mRNA (a gift from S. Ferrington, Harvard University, Cambridge, MA) was isolated from a human blood cell line K562 (RA6) using PolyATract Systems III (Promega). The PCR product was digested (with appropriate restriction enzymes) and inserted into the XbaI and BamHI site of plasmid pQB153 to yield plasmid pC3-Hs.
The LEU2 marker of the pC3-Mtb plasmid series encoding wild-type and mutant M. tuberculosis LeuRS genes was swapped with a TRP1 marker to carry out complementation experiments in yeast strains that contained different variants of the mitochondrial genome (11). Specifically, pC3-Mtb was cleaved with ScaI and NsiI followed by gel purification to separate the 5.3-kbp fragment encoding the LeuRS gene from the 3.2-kbp fragment containing the LEU2 marker. The TRP1 gene was excised from YEplac112 (17) by restriction digestion with ScaI and NsiI, yielding a 2.5-kb fragment and cloned into these same restriction sites (of the 5.3-kbp fragment) from pC3-Mtb, thus yielding plasmid pSBR-Mtb/Trp.
Yeast Strains and Media.
Standard methods for yeast propagation and transformation were used (23). Table 1 lists the yeast strains that were used in this work and their genotypes. Yeast strains that contained wild-type (HM410) and mutant (HM402 and HM411) mitochondrial genomes as well as plasmids containing the wild-type yeast mitochondrial LeuRS (NAM2; YCpGMC074) and G240S suppressor mutant (NAM2–1; YCpGMC075) were provided by C. Herbert (Centre National de la Recherche Scientifique, Gif-sur-Yvette, France) and used exactly as described in previous experiments (11). Strains EY699 (which is equivalent to W303 MATa ade2–1 ura3–1 his3–11,15 trp1Δ63 leu2–3,112 can1–100) and EY722 (which is equivalent to W303 MATα ade2–1 ura3–1 his3–11,15 trp1–1 leu2–3,112 can1–100) were obtained from E. Elion (Harvard Medical School, Boston, MA; ref. 24). Strains CH1271 (MATa ade6 lys1) and CH1272 (MATα ade6 lys1) were a gift from C. Holm (University of California, San Diego) and used for mating-type testing. Strains QBY51 and QBY52 were obtained, respectively, by growing strains CH1271 and CH1272 in the presence of ethidium bromide (25 μg/ml) and screening for rho0 colonies (25). Strain QBY274 (Kar1Δ15) was generated by transforming strain EY722 with 10 μg of BglII-restricted plasmid pMR1593 (26). Transformants were purified twice on 5-fluoroorotic acid plates to select for Ura- recombinants, and their genotypes were verified by Southern hybridizations.
Table 1.
Yeast strains used in this work
| Strain | Genotype | Origin |
|---|---|---|
| W303 | MATa ade2-1 ura3-1 his3-11,15 trp1Δ63 leu2-3,112 can1-100 | E. Elion (24), Harvard Medical School |
| QBY51 | MATa ade6 lys1 [ρ°] | This work |
| QBY52 | MATα ade6 lys1 [ρ°] | This work |
| QBY274 | MATα ade2-1 ura3-1 his3-11,15 trp1Δ63 leu2-3,112 can1-100 kar1Δ15 | This work |
| QBY276 | MATa ade2-1 ura3-1 his3-11,15 trp1Δ63 leu2-3,112 can1-100 msl1Δ∷HIS3 | This work |
| QBY320 | MATa ade2-1 ura3-1 his3-11,15 trp1Δ63 leu2-3,112 can1-100 msl1Δ∷HIS3 [ρ+] (pQB223) | This work |
| HM402 | MATα ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 nam2Δ∷LEU2 [Δ introns] | C. Herbert (11), CNRS-Gif-sur-Yvette |
| HM410 | MATα ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 nam2Δ∷LEU2 [wt 777-3A*] | C. Herbert (11), CNRS-Gif-sur-Yvette |
| HM411 | MATα ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 nam2Δ∷LEU2 [box7 mutant (V328)†] | C. Herbert (11), CNRS-Gif-sur-Yvette |
| S912/50 | MATa his4 NAM2-1 [ρ°] | C. Herbert (11), CNRS-Gif-sur-Yvette |
Construction of Null Strain.
Although a disruption of the yeast mitochondrial MSL1 gene (W303Δ MSL1) has been described (15), a new null strain QBY276, which has a more extensive deletion of the MSL1 ORF, and contains a HIS3 selectable marker instead of the LEU2 marker, was constructed. For this purpose, plasmid pQB187 was digested with SalI and XbaI to remove the mslΔ-containing insert and then the insert was used to transform strain EY699 (24) by the one-step gene disruption method (27). Twelve transformants were purified on complete minimal medium that lacked histidine [SC-His (23)]. Genomic DNA from these transformants was digested with PstI and analyzed by Southern hybridization using a 2.4-kbp PstI probe (from pQB185) that is internal to the MSL1 gene and spans the BstEII segment that was deleted in the pQB187 mslΔ construct. Using this probe, the unmodified strain showed a 2.4-kb hybridization signal whereas the desired mslΔ∷HIS3 allele gave a faint hybridization signal at 1.4 kb (less intense because of sharing only a 280-bp complementary DNA sequence with the probe). Strain QBY320 [QBY276 (pQB223) ρ+] was generated by transformation of the null strain QBY276 (ΔMSL1) with a maintenance plasmid pQB223 that harbors the MSL1 gene and a URA3 marker. A Ura+ transformant was purified and mated with strain QBY274 in a standard cytoduction procedure to generate a ρ+ derivative (26). Cytoductants were micromanipulated on rich glucose medium (yeast extract/peptone/dextrose) and allowed to form colonies; the colonies were later purified on complete minimal medium that lacked histidine and uracil and contained glycerol to select for rho+ derivatives of QBY276 (pQB223).
Complementation of Yeast Mitochondrial LeuRS Deletion Strain.
Plasmids pC3-Mtb, pC3-Hs, and pQB184 (each of which contains a LEU2 selectable marker) were used to transform strain QBY320 [msl1Δ∷HIS3 ρ+(pQB223)]. Six Leu+ transformants for each plasmid were isolated. Cells harboring both the maintenance plasmid pQB223 (URA3 marker) and transformed plasmid with LEU2 marker were grown on 5-fluoroorotic acid medium to select for loss of pQB223. Ura- segregants then were tested for growth on glycerol medium to determine complementation. Because QBY320 also contains an ade2 mutation, cells with intact or restored mitochondrial function develop a red pigment that also can be used as a qualitative indicator (28). Subsequent to selection on 5-fluoroorotic acid medium, growth of the msl1 deletion strain (QBY320) on glycerol medium was tested to ensure dependence on the presence of a heterologous enzyme. Single colonies complemented by pC3-Mtb or pC3-Hs were grown nonselectively in rich medium (yeast extract/peptone/dextrose) to induce low-frequency loss of the LEU2-bearing plasmids. Colonies were diluted and plated at 300 colonies/plate to verify that those that were leucine auxotrophs (having lost the LEU2-bearing plasmid) were unable to grow on glycerol medium.
RT-PCR Analysis.
Yeast cells (QBY320) that had lost the maintenance plasmid pQB223, but contained pC3-Mtb (wild type or mutant) or pQB184 were grown in medium containing 0.05% glucose, 1% glycerol, and 1% ethanol to an optical density (600 nM) of 1.5. Total RNA was isolated from the yeast mitochondria (29). The RNA was denatured for 1 min at 90°C, and primers targeting the B4 and B5 exons were annealed at 48°C for 45 min. RT was carried out by using avian myeloblastosis virus-RT (Promega) at 37°C for 1 h. The cDNA was denatured and amplified by PCR (30 cycles: 94°C, 1 min; 50°C, 1 min; 72°C, 2 min). Controls carried out in the absence of RT yielded no PCR products, thus indicating that amplification was not caused by DNA contaminants.
Results
Complementation of Disrupted Yeast Mitochondrial Strains by Human and M. tuberculosis LeuRS Genes.
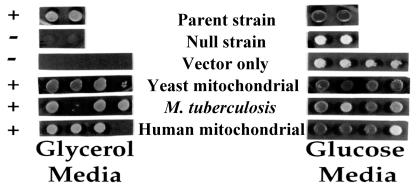
We first constructed a null allele (ΔMSL1) of the gene for S. cerevisiae mitochondrial LeuRS (MSL1). The null strain (QBY320; Table 1) then was used to investigate complementation by genes expressing LeuRS from human mitochondria (pC3-Hs) and M. tuberculosis (pC3-Mtb). Each LeuRS-encoding sequence was genetically fused at its N terminus to a DNA fragment coding for a yeast mitochondrial import sequence. Growth of the null strain QBY320 with each of the transgenes was tested on glycerol and glucose medium (Fig. 1). After 2 days at 30°C, strains containing pQB184, pC3-Mtb, or pC3-Hs grew on glycerol medium. Thus, M. tuberculosis LeuRS and human mitochondrial LeuRS can substitute for the yeast mitochondrial LeuRS.
Figure 1.
Genes encoding M. tuberculosis or human mitochondrial LeuRSs rescue a respiration-deficient yeast strain that contains a null allele of the gene encoding mitochondrial LeuRS. Strain QBY320 was transformed with plasmids expressing LeuRS originating from M. tuberculosis (pC3-Mtb), human mitochondria (pC3-Hs), and yeast mitochondria (pQB184), as well as a vector only control (pQB153). Four Leu+ transformants from each were purified on 5-fluoroorotic acid to remove the maintenance plasmid and then were tested for complementation of the MSL1 null strain on glycerol medium as described in Materials and Methods. Two sets of colonies for the parent (W303) and null (QBY276) strain also were tested as controls. Dark colonies reflect red pigmentation because of an ade2 mutation (see Materials and Methods).
The prokaryotic M. tuberculosis LeuRS presumably does not function in splicing. Likewise human mitochondria DNA completely lacks introns (30), suggesting that human mitochondrial LeuRS is not designed for splicing per se. The results in Fig. 1 are in sharp contrast to TyrRS-dependent RNA splicing in N. crassa mitochondria where tyrosine enzymes from E. coli and S. cerevisiae cannot compensate for a missing N. crassa mitochondrial TyrRS (10).
Mutational Analysis of M. tuberculosis LeuRS in Group I Intron Splicing.
Li et al. (11) previously constructed a series of yeast mitochondrial LeuRS mutants to dissect regions that impact splicing versus aminoacylation activity. In particular, they targeted a discrete domain called connective polypeptide 1 or CP1 [≈200 aa (31, 32)], which interrupts the primary sequence of the catalytic cores of class I tRNA synthetases. The CP1 domain within the closely related Val- and Ile-tRNA synthetases contains an active site for editing misactivated amino acids (33, 34). Determinants for binding to the tRNA acceptor helix also are encoded by CP1 (35–38). This inserted domain was the site of a suppressor mutation that was selected to compensate for a defective bI4 maturase expressed from the bI4 intron (3, 4, 6).
The previous work also identified mutations in yeast mitochondrial LeuRS that abolished splicing activity, but retained activity for aminoacylation. These included a point mutation in CP1 distinct from the aforementioned suppressor mutation. In addition, a deletion of 5 aa from the C terminus of the LeuRS suppressor mutant (3, 6) eliminated splicing activity. In contrast, this deletion protein complemented a yeast strain that contained an intronless mitochondrial genome. The latter result showed that the C-terminal deletion did not eliminate aminoacylation in vivo.
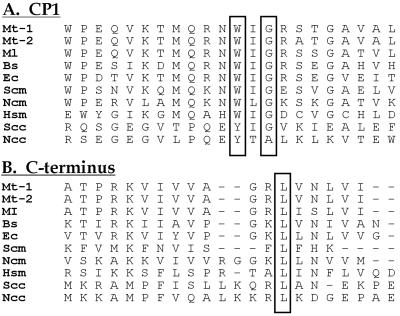
An alignment of sequences of leucine enzymes from yeast, Mycobacteria, E. coli, and human mitochondria show sequence similarities in the C-terminal region and also in the region of CP1 that is important for splicing. This latter region is marked by a conserved WIG tripeptide (Fig. 2). We introduced mutations into M. tuberculosis LeuRS that were analogous to those that selectively affected splicing activity in yeast LeuRS (11). These mutations included a W286C substitution (that abolishes splicing activity in yeast mitochondrial LeuRS) and the G288S suppressor mutation that compensated for the absence of a fully active maturase (3). Finally, the G288S suppressor substitution was combined with a 5-aa C-terminal deletion (ΔC5) in M. tuberculosis LeuRS that would (by analogy with the previous work on the yeast enzyme) be expected to eliminate splicing.
Figure 2.
Polypeptide sequence alignments of regions of LeuRS that contain splicing-sensitive elements. (A) CP1 region. The conserved boxed G is the location of the G288S splicing suppressor (3, 6). The conserved boxed W is the location of the W286C substitution that abolishes splicing activity (11). (B) C-terminal region. The two eukaryotic cytoplasmic enzymes contain a C-terminal extension of approximately 75 residues that is not shown. Abbreviations: Mt-1, M. tuberculosis (reported herein); Mt-2, GenBank accession no. 2501027; Ml, M. leprae; Bs, B. subtilis; Ec, E. coli; Scm, S. cerevisiae mitochondrial; Ncm, N. crassa mitochondrial; Hsm, Homo sapiens mitochondrial; Scc, S. cerevisiae-cytoplasmic; Ncc, N. crassa cytoplasmic.
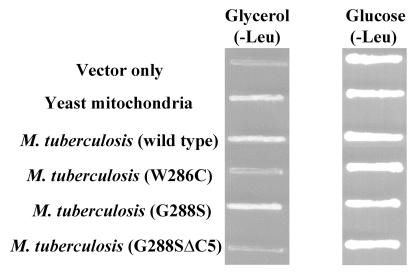
The M. tuberculosis wild type and G288S LeuRS complemented the yeast null strain on glycerol medium (Fig. 3). In contrast, the W286C LeuRS and G288SΔC5 variant did not complement. Thus, the phenotypes of mutations within the M. tuberculosis enzymes are consistent with those seen with the mutant yeast mitochondrial enzyme.
Figure 3.
A W286C mutant or G288SΔC5 deletion of M. tuberculosis LeuRS abolishes complementation. Mutations were introduced into M. tuberculosis LeuRS genes using pC3-Mtb and tested for complementation of null strain QBY320. The G288S mutant and wild-type M. tuberculosis gene complemented the null strain. Similar to previous studies using yeast mitochondrial LeuRS (11), a W286C and a G288SΔC5 mutation in the M. tuberculosis protein did not complement strain QBY320 on glycerol medium. A vector only control (pQB153) also was included. All strains grew on medium containing glucose that does not require mitochondrial function.
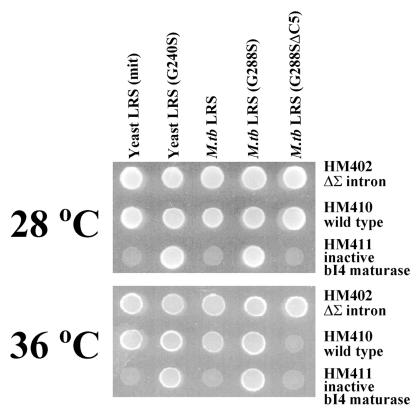
Complementation experiments using null strains with altered mitochondrial genomes, which originally were used to test yeast mitochondrial LeuRS mutants as described above (ref. 11; Table 1), were reproduced by using the M. tuberculosis LeuRS wild-type and mutant genes (Fig. 4). As found for the QBY320 null strain (Fig. 1), the wild-type M. tuberculosis LeuRS gene complemented the HM410 null strain (contains a wild-type mitochondrial genome). However, and similar to the yeast mitochondrial LeuRS gene (11), the M. tuberculosis gene could not compensate for a mitochondrial mutation that yields an inactive maturase (HM411). The maturase mutation in the yeast null strain HM411 was suppressed by an M. tuberculosis LeuRS mutant (G288S), which is homologous to the G240S mutant yeast mitochondrial LeuRS NAM2–1 suppressor.
Figure 4.
Complementation by the M. tuberculosis wild-type LeuRS, G288S “suppressor,” and G288SΔC5 deletion mutant of null strains that contain mitochondrial genome variations. Null strains tested for complementation included wild type that contained all 13 mitochondrial introns (HM410), an intronless mitochondrial genome (HM402), and a single site mutation (V328; HM411) that inactivates the bI4 maturase (ref. 11; Table 1). Each null strain contained a deletion in the nam2 allele. The plates were incubated on glycerol medium at either 28°C for 3 days or 36°C for 5 days exactly as described for previous experiments with yeast mitochondrial LeuRS mutants (11).
The M. tuberculosis deletion mutant G288SΔC5 complemented the null strain HM410, but in a temperature-sensitive manner at 28°C and not at 36°C (Fig. 4), as found with the corresponding yeast mitochondrial LeuRS mutant (11). Complementation activity by the M. tuberculosis LeuRS G288SΔC5 mutant was further investigated to determine whether it was specifically deficient in the splicing function. A null strain that completely lacked mitochondrial introns (HM402) was complemented by M. tuberculosis G288SΔC5 LeuRS at 36°C. Thus, the M. tuberculosis LeuRS C-terminal truncation mutant sustained translation, but not splicing at higher temperatures.
Complementation experiments also determined that the M. tuberculosis mutant W286C did not rescue strains HM402, HM410, or HM411 (data not shown). The same results were found previously with the corresponding yeast mitochondrial LeuRS mutant (11). The explanation for this result remains unclear.
HM402 containing the M. tuberculosis mutant W286C or G288SΔC5 was further tested by crossing the null strain with a rhoo strain [S912/50 (ref. 11; Table 1)] that lacked a mitochondrial genome, but contained a wild-type yeast mitochondrial LeuRS. Progeny from the mating test grew on glycerol medium, supporting the conclusion that the mitochondrial genome was intact in these progeny and therefore in HM402. Thus, the mutations placed in the M. tuberculosis enzyme do not affect the integrity of the mitochondrial genome. Instead, they affect its ability to create functional mitochondria.
Analysis of Products of bI4 Intron Splicing.
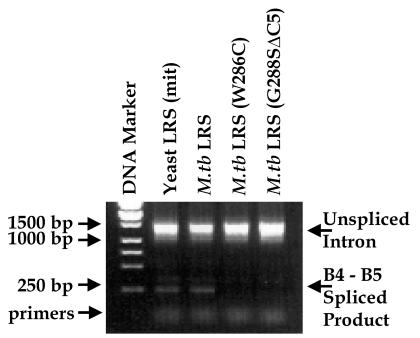
Primers targeting the B4 and B5 exons that flank the bI4 intron as well as RNA isolated from the yeast strains (QBY320) used in the complementation assays were incorporated into RT-PCRs to test for spliced products. Strains complemented by the wild-type yeast and M. tuberculosis LeuRS contained a spliced B4–B5 product of about 250 bp as expected (Fig. 5). Consistent with previous results with the yeast mitochondrial enzyme (11), strains that expressed the W286C and G288SΔC5 mutant M. tuberculosis leucine enzymes failed to yield a spliced B4–B5 product. This result shows that the mutant M. tuberculosis enzymes fail to support a specific RNA splicing event. This, in turn, is what appears to prevent formation of functional mitochondria.
Figure 5.
RT-PCR amplification of spliced B4-B5 exons. The first lane contains a DNA ladder of markers (Stratagene). The second and third lanes, respectively, represent RNA isolated from the yeast null strain (QBY320) that contained plasmids expressing wild-type LeuRS from yeast and M. tuberculosis. The unspliced and spliced B4-B5 exon products are indicated by respective bands at about 1.5 kbp and 250 bp. The fourth and fifth lanes, respectively, show that the intron remains unspliced when genes containing either the W286C mutant or G288SΔC5 deletion of M. tuberculosis LeuRS are used in attempts to complement the yeast null strain.
Analysis of M. tuberculosis LeuRS Aminoacylation.
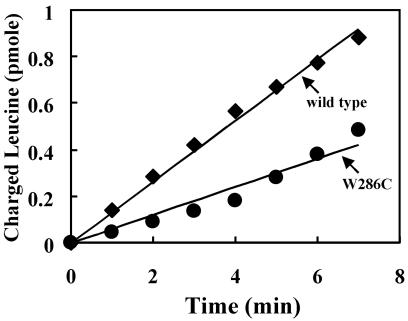
We also expressed in E. coli the M. tuberculosis wild-type and W286C mutant LeuRS as N-terminal fusions with glutathione S-transferase (GST). Using glutathione affinity chromatography, GST-LeuRS was purified to homogeneity. Although the W286C mutant enzyme has no complementation or splicing activity in vivo, it retained activity for aminoacylation (Fig. 6).
Figure 6.
Aminoacylation activity of M. tuberculosis wild-type and W286C glutathione S-transferase-LeuRS. The aminoacylation assay was carried out by using 50 nM LeuRS and 10 μM tRNALeu (E. coli), which was prepared by in vitro transcription using T7 RNA polymerase (47). M. tuberculosis LeuRS wild type (■); M. tuberculosis LeuRS W286C mutant (●).
Discussion
Leucine enzymes from diverse origins can substitute for the yeast mitochondrial LeuRS intron-specific splicing function. In contrast, N. crassa mitochondrial TyrRS, which contains a critical peptide that is idiosyncratic to N. crassa and P. anserina (10), promotes splicing of a diverse set of introns (39, 40). Aminoacylation of tRNATyr in N. crassa also depends on this unique peptide element, suggesting that it may have properties that promote specific protein–RNA interactions with both the group I intron and N. crassa tRNATyr (10, 41). RNA footprinting analysis combined with tertiary structure models supports that TyrRS interacts with a tRNA-like moiety in the group I intron (42, 43).
Neither M. tuberculosis nor human mitochondrial LeuRSs would be expected to have specifically adapted for a role in splicing because both the prokaryote and human mitochondria genome lack group I introns. Because the LeuRSs from widely separated organisms can facilitate splicing in the mitochondria of S. cerevisiae, it is likely that broadly conserved determinants required for aminoacylation of tRNALeu have been recruited to bind to the group I intron and enable splicing of the bI4 and aI4α introns within yeast mitochondria. Although it is possible that LeuRS binds to the group I intron via a tRNA-like feature, it is clear that interactions with the leucine enzyme have required minimal, if any, special adaptations beyond its natural tRNA binding properties.
The differences between LeuRS- and TyrRS-dependent splicing functions are further highlighted when considering that the two tRNA synthetases interact with group I introns having different structural features. CYT-18p interacts with group I introns from all structural classes if they do not normally contain the P5abc RNA domain or if the naturally occurring P5abc domain is removed. The P5abc domain nucleates folding of the group I intron RNA, but blocks TyrRS binding (45, 46). Although CYT-18p substitutes for the P5abc RNA domain in some introns, this may be only a part of the TyrRS function because it contacts a much larger region of the catalytic core (45). The major role of CYT-18p is to stabilize the overall group I fold [presumably tRNA-like (42)], and it does this in essentially all group I introns, even those that lack a P5abc domain. Significantly, the bI4 and aI4α introns whose splicing is aided by the LeuRS contain at least part of the essential P5abc RNA domain (44). Although it is clear that TyrRS and LeuRS interactions with the group I intron differ, it is possible that similar to CYT-18p, LeuRS also may hold a tRNA-like element in place to help stabilize folding of the group I intron.
An appended domain is important for the splicing function of N. crassa TyrRS. The absence of an apparent splicing-specific domain within the leucine enzymes (6) may be compensated by the P5abc RNA domain in the bI4 and aI4α introns and, additionally or alternatively, by the required maturase protein in S. cerevisiae. Although it remains unclear whether the maturase and LeuRS interact via direct protein–protein interactions, we have obtained evidence via yeast three-hybrid and RNA-dependent two-hybrid analyses that LeuRS and bI4 maturase each can bind directly and also simultaneously to the bI4 intron (48). Thus, in a ternary complex with the RNA, these two protein splicing partners would be in position to collaborate and regulate the ribozyme's inherent self-splicing activity.
Acknowledgments
We are grateful to Professor A. Lambowitz for reviewing this manuscript and for numerous helpful discussions. We thank Dr. C. Herbert and Professor P. Perlman for feedback and advice and also Drs. D. Rose, L. Ribas de Pouplana and K. Shiba for assistance with sequence comparisons and secondary structure predictions. We also thank Professors P. Brennan, R. Young, C. Holm, M. Rose, E. Elion, A. Tzagaloff, and Dr. S. Ferrington and Dr. C. Herbert for providing essential reagents. This work was supported by grants to S.A.M. from the National Institutes of Health (1R431I36615) and the Texas Advanced Research Program (003652–0017-1999), National Institutes of Health Grant GM23562 (P.S.), and a Fellowship from the National Foundation for Cancer Research.
Abbreviations
- LeuRS
leucyl-tRNA synthetase
- TyrRS or CYT-18p
tyrosyl-tRNA synthetase
- RT
reverse transcription
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240465597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240465597
References
- 1.Martinis S A, Schimmel P. In: Escherichia coli and Salmonella Cellular and Molecular Biology. 2nd Ed. Neidhardt F C, editor. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 887–901. [Google Scholar]
- 2.Martinis S A, Plateau P, Cavarelli J, Florentz C. EMBO J. 1999;18:4591–4596. doi: 10.1093/emboj/18.17.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Labouesse M, Dujardin G, Slonimski P P. Cell. 1985;41:133–143. doi: 10.1016/0092-8674(85)90068-6. [DOI] [PubMed] [Google Scholar]
- 4.Labouesse M, Herbert C J, Dujardin G, Slonimski P P. EMBO J. 1987;6:713–721. doi: 10.1002/j.1460-2075.1987.tb04812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labouesse M. Mol Gen Genet. 1990;224:209–221. doi: 10.1007/BF00271554. [DOI] [PubMed] [Google Scholar]
- 6.Herbert C J, Labouesse M, Dujardin G, Slonimski P P. EMBO J. 1988;7:473–483. doi: 10.1002/j.1460-2075.1988.tb02835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akins R A, Lambowitz A M. Cell. 1987;50:331–345. doi: 10.1016/0092-8674(87)90488-0. [DOI] [PubMed] [Google Scholar]
- 8.Kämper U, Kück U, Cherniack A D, Lambowitz A M. Mol Cell Biol. 1992;12:499–511. doi: 10.1128/mcb.12.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kittle J D, Jr, Mohr G, Gianelos J A, Wang H, Lambowitz A M. Genes Dev. 1991;5:1009–1021. doi: 10.1101/gad.5.6.1009. [DOI] [PubMed] [Google Scholar]
- 10.Cherniak A D, Garriga G, Kittle J D, Jr, Akins R A, Lambowitz A M. Cell. 1990;62:745–755. doi: 10.1016/0092-8674(90)90119-y. [DOI] [PubMed] [Google Scholar]
- 11.Li G-Y, Becam A-M, Slonimski P P, Herbert C J. Mol Gen Genet. 1996;252:667–675. doi: 10.1007/BF02173972. [DOI] [PubMed] [Google Scholar]
- 12.Young R A, Bloom B R, Grosskinsky C M, Ivanyl J, Thomas D, Davis R W. Proc Natl Acad Sci USA. 1985;82:2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinis S A, Fox G E. Nucleic Acids Symp Ser. 1997;36:125–128. [PubMed] [Google Scholar]
- 14.Broach J R, Strathern J N, Hicks J B. Gene. 1979;8:121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- 15.Tzagoloff A, Akai A, Kurkulos M, Repetto B. J Biol Chem. 1988;263:850–856. [PubMed] [Google Scholar]
- 16.Berben G, Dumont J, Gilliquet V, Bolle P A, Hilger F. Yeast. 1991;7:475–477. doi: 10.1002/yea.320070506. [DOI] [PubMed] [Google Scholar]
- 17.Sugino A, Gietz R D. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 18.Pinkham J, Dudley A M, Mason T L. Mol Cell Biol. 1994;101:202–211. doi: 10.1128/mcb.14.7.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurt E C, Presold-Hurt B, Schatz G. EMBO J. 1984;3:3149–3156. doi: 10.1002/j.1460-2075.1984.tb02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair S, Ribas de Pouplana L, Houman F, Avruch A, Shen X, Schimmel P. J Mol Biol. 1997;269:1–9. doi: 10.1006/jmbi.1997.1025. [DOI] [PubMed] [Google Scholar]
- 21.Kim S, Jo Y J, Lee S H, Motegi H, Shiba K, Sassanfar M, Martinis S A. FEBS Lett. 1998;427:259–262. doi: 10.1016/s0014-5793(98)00417-7. [DOI] [PubMed] [Google Scholar]
- 22.Nomura N, Miyajima N, Kawarabayasi Y, Tabata S. DNA Res. 1994;1:251–262. doi: 10.1093/dnares/1.5.251. [DOI] [PubMed] [Google Scholar]
- 23.Rose A B, Broach J R. Methods Enzymol. 1990;185:234–279. doi: 10.1016/0076-6879(90)85024-i. [DOI] [PubMed] [Google Scholar]
- 24.Elion E A, Brill J A, Fink G R. Proc Natl Acad Sci USA. 1991;88:9392–9396. doi: 10.1073/pnas.88.21.9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldring E S, Grossman L I, Krupnick D, Cryer D R, Marmur J. J Mol Biol. 1970;52:323–335. doi: 10.1016/0022-2836(70)90033-1. [DOI] [PubMed] [Google Scholar]
- 26.Vallen E A, Hiller M A, Scherson T Y, Rose M D. J Cell Biol. 1992;117:1277–1287. doi: 10.1083/jcb.117.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothstein J. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- 28.Strathern J N, editor. The Molecular Biology of the Yeast Saccharomyces: Metabolism and Gene Expression. Plainview, NY: Cold Spring Harbor Lab. Press; 1982. [Google Scholar]
- 29.Lundblad V. In: Short Protocols in Molecular Biology. 3rd Ed. Ausubel F, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. New York: Wiley; 1995. pp. 13–51. [Google Scholar]
- 30.Anderson S, Bankier A T, Barrell B G, de Bruijn M H, Coulson A R, Drouin J, Eperon I C, Nierlich D P, Roe B A, Sanger F, et al. Nature (London) 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 31.Starzyk R M, Webster T A, Schimmel P. Science. 1987;237:1614–1618. doi: 10.1126/science.3306924. [DOI] [PubMed] [Google Scholar]
- 32.Hou Y-M, Shiba K, Mottes C, Schimmel P. Proc Natl Acad Sci USA. 1991;88:976–980. doi: 10.1073/pnas.88.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin L, Hale S P, Schimmel P. Nature (London) 1996;384:33–34. doi: 10.1038/384033b0. [DOI] [PubMed] [Google Scholar]
- 34.Nureki O, Vassylyev D G, Tateno M, Shimada A, Nakama T, Fukai S, Konno M, Hendrickson T L, Schimmel P, Yokoyama S. Science. 1998;280:578–582. doi: 10.1126/science.280.5363.578. [DOI] [PubMed] [Google Scholar]
- 35.Silvian L F, Wang J, Steitz T A. Science. 1999;285:1074–1077. [PubMed] [Google Scholar]
- 36.Wakasugi K, Quinn C L, Tao N, Schimmel P. EMBO J. 1998;17:297–305. doi: 10.1093/emboj/17.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rould M A, Perona J J, Söll D, Steitz T A. Science. 1989;246:1135–1142. doi: 10.1126/science.2479982. [DOI] [PubMed] [Google Scholar]
- 38.Burbaum J J, Schimmel P. J Biol Chem. 1991;266:16965–16968. [PubMed] [Google Scholar]
- 39.Guo Q, Lambowitz A M. Genes Dev. 1992;6:1357–1372. doi: 10.1101/gad.6.8.1357. [DOI] [PubMed] [Google Scholar]
- 40.Mohr G, Zhang A, Gianelos J A, Belfort M, Lambowitz A M. Cell. 1992;69:483–492. doi: 10.1016/0092-8674(92)90449-m. [DOI] [PubMed] [Google Scholar]
- 41.Lambowitz A M, Caprara M G, Zimmerly S, Perlman P S. In: The RNA World. 2nd Ed. Gesteland R F, Cech T R, Atkins J F, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1999. pp. 451–485. [Google Scholar]
- 42.Caprara M G, Lehnert V, Lambowitz A M, Westhof E. Cell. 1996;87:1135–1145. doi: 10.1016/s0092-8674(00)81807-3. [DOI] [PubMed] [Google Scholar]
- 43.Caprara M G, Mohr G, Lambowitz A M. J Mol Biol. 1996;257:512–531. doi: 10.1006/jmbi.1996.0182. [DOI] [PubMed] [Google Scholar]
- 44.Michel F, Westhof E. J Mol Biol. 1990;216:585–610. doi: 10.1016/0022-2836(90)90386-Z. [DOI] [PubMed] [Google Scholar]
- 45.Mohr G, Caprara M G, Guo Q, Lambowitz A M. Nature (London) 1994;370:147–150. doi: 10.1038/370147a0. [DOI] [PubMed] [Google Scholar]
- 46.Wallweber G J, Mohr S, Rennard R, Caprara M G, Lambowitz A M. RNA. 1997;3:114–131. [PMC free article] [PubMed] [Google Scholar]
- 47.Sampson J R, Uhlenbeck O C. Proc Natl Acad Sci USA. 1988;85:1033–1037. doi: 10.1073/pnas.85.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rho, S. B. & Martinis, S. A. (2000) RNA, in press. [DOI] [PMC free article] [PubMed]
- 49.de la Salle H, Jacq C, Slonimsky P P. Cell. 1982;28:721–732. doi: 10.1016/0092-8674(82)90051-4. [DOI] [PubMed] [Google Scholar]
- 50.Groudinsky O, Dujardin G, Slonimsky P P. Mol Gen Genet. 1981;184:493–503. doi: 10.1007/BF00352529. [DOI] [PubMed] [Google Scholar]