Abstract
Background
Hemorrhagic shock (HS) with conventional resuscitation (CR) (HSCR) primes neurophils and modulates leukocyte (WBC)–endothelium interaction as part of an exaggerated systemic inflammatory response. We hypothesize that topical application of clinical peritoneal dialysis solutions (PD) modulates such interaction.
Methods
Intestinal intravital microscopy was used to measure WBC rolling in terminal ileum post capillary venules (V2 and V3) in sham-operated animals, and in animals that underwent fixed pressure hemorrhage (50% mean arterial pressure for 60 minutes), followed by conventional resuscitation with the return of the shed blood and 2 vol of saline. Number of rolling WBCs per thirty seconds in selected V2 and V3, bathed in either Kreb’s solution or a 2.5% clinical peritoneal dialysis solution (PD) was quantified. Diameters were measured for the in-flow arterioles (A1), and out-flow venules (V1), for calculation of local blood flow with optical Doppler velocimetry.
Results
The PD solution significantly (P < .05, n = 11) attenuated WBC–endothelium interaction in sham-operated animals while no significant difference was elicited in HSCR (P < .05, n = 9 Kreb’s, n = 7 PD). In addition, the PD solution produced an instantaneous dilation at all levels of the intestinal arterioles in both sham and HSCR. While intestinal venular blood outflow was increased by the PD solution, venular diameters changed very little.
Conclusion
Superfusion of the gut with glucose-based peritoneal dialysis solutions decreases the concentration of rolling leukocytes along the venular vascular endothelium by a vasodilation-mediated increase in arteriolar inflow and venous outflow mechanism. Hemorrhagic shock and conventional resuscitation enhance the concentration of rolling leukocytes presumably by mechanisms related to upregulation of the adhesion molecules and the low-flow state. Hemorrhage and resuscitation-enhanced leukocytes rolling was not reversed by adjunctive DPR despite the associated marked increase in arterial inflow and venous outflow. The status of the endothelium and the level of leukocyte priming in low-flow states are stronger predictors of leukocyte–endothelium interaction than rheology factors.
Keywords: Hemorrhagic shock, Peritoneal dialysis solution, Neutrophils, Vascular endothelium
Morbidity from trauma and blood loss with subsequent conventional resuscitation remains the leading cause of death in the surgical intensive care unit [1]. Visceral organs experience a persistent and progressive deterioration of mucosal blood flow in hemorrhage with conventional intravenous resuscitation (CR), despite restoration and maintenance of central hemodynamics by aggressive fluid therapy [2–6]. Three major alterations of end-organ microcirculatory perfusion, tissue metabolism associated with the genesis of a gut-derived exaggerated systemic inflammatory response syndrome (SIRS) and a massive fluid shift persist with conventional resuscitation from hemorrhagic shock (HSCR). The mechanisms of these alterations after HS and CR are unclear as are the variety of sequelae that are responsible for the domino effect ultimately leading to multiple organ dysfunction syndrome (MODS). Identification of central effectors in the cascade leading to MODS could have great pathophysiologic and therapeutic implications. Numerous interventions were designed to protect organ systems and cellular viability from the lethal injury accompanying ischemia/reperfusion (IR). Whereas others have attempted to enhance the metabolic processes or have used specific antagonists or synthesis inhibitors to modify the state of shock [7–9]. None of these interventions have successfully halted or reversed the main course of the pathophysiology associated with HSCR. An overall therapy that modifies the pathophysiological processes in HSCR has proven to be elusive.
Recently we have shown that the persistent and progressive post-resuscitation intestinal vasoconstriction and hypoperfusion can be prevented or reversed to an instantaneous and sustained vasodilation and hyperperfusion with direct peritoneal resuscitation (DPR), which utilizes intraperitoneal instillation of a clinical peritoneal dialysis solution as an adjunct to conventional intravascular fluid resuscitation [10,11]. Our studies have demonstrated that DPR-mediated enhancement of blood flow is not restricted to the gut, but that whole organ blood flow increases by greater than 50% in spleen and pancreas and greater than 100% in lung, psoas muscle, and diaphragm compared to conventional resuscitation alone [12]. Thus, the major problem of the no reflow phenomenon or a decrement in flow as a result of HS resuscitation was circumvented. This DPR-mediated splanchnic and distal end-organ hyperperfusion occurs without adverse effects on hemodynamics.
A better outcome is certain with the use of adjunctive DPR. This was demonstrated in recent studies, which show 40% mortality within the first 24 hours in animals with HSCR, while administration of DPR as adjunct to CR leads to a 100% survival for 72 hours [13]. This improved survival with DPR was associated with a marked upregulation in the anti-inflammatory interleukin (IL)-10 and a downregulation of the pro-inflammatory mediators IL-6 and tumor necrosis factor-α (TNF-α) in the liver and gut [13]. It is tempting to hypothesize DPR may then do more than restore perfusion in HSCR rats but may also ameliorate SIRS. It is presumable that partial prevention of neutrophil and or endothelial inflammatory activation may be an outcome of these changes. This study was designed to find the effect of DPR upon the white blood cell (WBC)–endothelial interaction.
Materials and Methods
Animals were maintained in a facility approved by the American Association for Accreditation of Laboratory Animal Care. The Institutional Animal Care and Use Committee and Biohazard Safety Committee at the Louisville Veterans Affairs Medical Center approved the research protocol. Male Sprague–Dawley rats (200 to 220 g) were used in the experiments. Animals were acclimated for 1 to 2 weeks before experimental use, during which time each animal received 15 g of rat food per diem and water ad libitum, except for the night before the experiment. All animal and experimental interventions were performed under standard aseptic conditions. Anesthesia was induced with intraperitoneal pentobarbital (1.2 mL/kg), and supplemental subcutaneous injections (20% the original dose) were given as needed to maintain a surgical plane of anesthesia throughout the experimental protocol. Before the surgical procedure, 2 mL of normal saline was injected subcutaneously to maintain body fluid homeostasis during surgery and equilibration periods. Body temperature was maintained at 37 ± 0.5°C with a rectal probe and a servo-controlled heating pad. Surgery was performed after loss of blink and withdrawal reflexes. The right carotid was cannulated with PE-50 tubing for blood pressure monitoring with a pressure transducer. The femoral artery was cannulated for blood withdrawal and the femoral vein for resuscitation fluid replacement.
Hemorrhagic shock and resuscitation model
Hemorrhagic shock was achieved with blood withdrawal a maximum rate of 1 mL/min, from the femoral artery in a syringe pretreated with 8 units of heparin in 0.8 mL saline. This was continued until 30 mm Hg arterial blood pressure is obtained. Blood pressure was allowed to recover to 50% of mean arterial pressure. Blood was withdrawn/reinfused as needed to maintain a nominal 50% mean arterial pressure for 60 minutes. On average, the blood volume withdrawn was of the order of 5 mL. CR was achieved with the return of the shed blood over 5 minutes, followed by intravenous infusion of 2 vol of normal saline from an infusion pump over the subsequent 25 minutes. In experiments designed for adjunctive DPR, the Kreb’s solution in the tissue bath was replaced with a glucose-based (2.5%) clinical peritoneal dialysis solution simultaneous with the start of CR.
Experimental groups
Group I (n = 11) consited of sham operation with Kreb’s solution in bath; group II (n = 11), sham operation with peritoneal dialysis solution in bath; group III (n = 9), sham operation and hemorrhagic shock and conventional resuscitation with Kreb’s in bath; and group IV (n = 7), sham operation and hemorrhagic shock and conventional resuscitation with PD solution in bath.
Tissue bath solutions
The intestinal segment was continuously suffused during tissue preparation and equilibration with a nonvasoactive modified Krebs’ solution that contained 6.92 g/L sodium chloride, 0.44 g/L potassium chloride, 0.37 g/L calcium chloride, and 2.1 g/L sodium bicarbonate at a pH of 7.4 and osmolality of 285 mOsm/L. A conventional 2.25% dextrose-based dialysis solution (Delflex, Fresenius USA, Ogden, UT) that contained 5.67 g/L sodium chloride, 3.92 g/L sodium lactate, 0.257 g/L calcium chloride, 0.152 g/L magnesium chloride at a pH of 5.5, and an initial osmolality of 398 mOsm/L was used in the tissue bath to simulate DPR.
Intravital microscopy Experimental procedure
The peritoneal cavity was exposed through a midline abdominal incision of 1.5 cm, and a 2- to 3-cm segment of jejunum was gently withdrawn from the peritoneal cavity with its neurovascular supply intact. The segment was opened along the antimesenteric border by electrocautery. The enteric contents and mucus were gently removed from the mucosal surface. The animals were positioned on a specially designed polyurethane board. The opened jejunum was suspended, serosal side up, over a viewing port in a tissue bath with 4-0 silk sutures. The nonvasoactive bathing solution was maintained at 37°C, and bubbled with nitrogen and carbon dioxide to maintain the pH at 7.4. Isoproterenol was added to the bathing solution in a very dilute concentration (0.01 μg/mL) to retard peristalsis. This dose of isoproterenol is below the threshold that alters vascular smooth muscle tone [14].
The animal board was positioned on the stage of a trinocular microscope for direct in vivo intravital microscopy. Microvascular images were transmitted through the microscope to a photodiode array in an optical Doppler velocimeter (Microcirculation Research Institute, Texas A&M University, College Station, TX) to measure center-line red blood cell velocity for the calculation of blood flow in the intestinal A1 inflow arteriole. The microvascular image was then transmitted to a digital camera (Hitachi Denshi, Models K-P D51/D50; Hitachi-Denshi America, Ltd, Woodbury, NY), which provided 30 images per second to a computer. The digitalized microvascular images were stored as streamline video in the computer hard drive for later measurement of microvascular diameters with calipers.
Criteria for an acceptable microvascular preparation during intravital microscopy included a baseline mean arterial pressure greater than 90 mm Hg, a center-line red blood cell velocity in a first-order arteriole greater than 20 mm/s, and an active vasomotion in the intestinal premucosal A3 arterioles. We used a standard nomenclature for intestinal microvessels, as originally described by Bohlen and Gore [14]. Briefly, first-order arterioles (A1) arise from a mesenteric arcade artery to traverse the mesenteric border of the bowel wall and then penetrate through the muscle layers to the submucosal layer. In the submucosal layer, second-order arterioles (A2) arise from the first-order arterioles to run along the longitudinal axis of the bowel. First and second-order venules parallel the first and second-order arterioles. A2 arterioles give rise to branching second-order arcade vessels as well as to smaller third-order arterioles (A3). The A3 vessel branches at right angle from the A2 arteriole to form distal A3 (dA3), which terminates in the mucosa as a central villus arteriole. Along their course, A3 arterioles also give rise to smaller proximal A3 arterioles that supply the seromuscular layers of the bowel wall.
Experimental protocol and measurements
The intestinal segment was allowed to equilibrate for 40 minutes in the tissue bath. During this time, the segment was continuously suffused with the nonvasoactive Kreb’s solution. Blood pressure, heart rate, rectal and bath temperatures and bath pH were continuously monitored (Digi-Med Signal Analyzers, Louisville, KY) and recorded every 5 minutes. Microvascular data consisted of A1, V1 diameters; and center-line red cell velocity in the inflow A1 arteriole and out-flow V1 venule. Baseline measurements were considered valid when the variability in the measurement is less than 0.5%.
WBC–endothelial interaction
Previous studies have either studied the mesenteric circulation or the cremaster preparation for WBC analysis. The study of WBC–endothelium interaction in the intestinal is more relevant to the pathophysiology of hemorrhagic shock/resuscitation as the gut was considered a central organ for MODS. Post capillary venules (V3) were selected for intra-vital microscopy video. Cells that “rolled” by a chosen perpendicular line in said vessels were counted for a 30-second period every 20 minutes for a total of 80 minutes post resuscitation. This technique is generally accepted and regarded valid for blood flow velocities above 0.5 mm/s [15]. Such blood flow velocity was not observed during shock, and therefore no WBC–endothelium interaction was attempted during shock. Unexpectedly, firm adhesions or extravasations were not noted in our model.
Data analysis and statistics
All data are presented as the mean ± SEM unless stated otherwise. Percentage change of the vessel diameter from baseline was assessed with 1-way analysis of variance (ANOVA), and Dunnett multiple-range test to evaluate changes from the baseline within the same animal. Two-way ANOVA was used to assess the relationship between vascular reactivity, or time and WBC–endothelium interaction. Differences between groups were assessed with 2-way ANOVA and Bonferroni post-test. A result was considered to be significant if the probability of a type-1 error was P < .05.
Results
There were no significant differences in baseline hemodynamics among the 4 groups. Mobilization and surgical preparation of the gut for intravital microscopy is known to cause inevitable inflammation and enhance WBC–endothelium interaction. All animal groups were subjected to the same surgical procedure, and therefore the level of the local inflammation in the segment of gut under investigation was expected to be the same in all animals.
The first series of experiments (groups I and II) was designed to assess the effects of topical application of a clinical glucose-based peritoneal dialysis solution on the WBCs rolling along the vascular endothelium. Data from this series of experiments are summarized in Fig. 1. The absolute count of rolling WBCs was significantly greater in the V3 compared to V2. Surgical manipulation caused an apparent increase in the WBC rolling over time when the intestinal segment was bathed in a physiologic Kreb’s solution. The slope of the regression line was 2.70 ± 0.71, which is significantly different from 0 (n = 11, P < .001). However, exposure of the preparation to a hypertonic 2.5% peritoneal solution elicits a significant decrease in WBC rolling at 60 minutes. A linear regression analysis yielded a slope of 0.69 ± 0.37, which is not significant from 0 (n = 11, P < .05). A1 and V1 flow remained unchanged through the duration of the experiment when the intestine was exposed to the Kreb’s solution. Exposure to the hypertonic 2.5% peritoneal dialysis solution, produced a significant increase in both arterial inflow and venous outflow to the prepared region of ileum (middle and lower panels of Fig. 1).
Fig. 1.
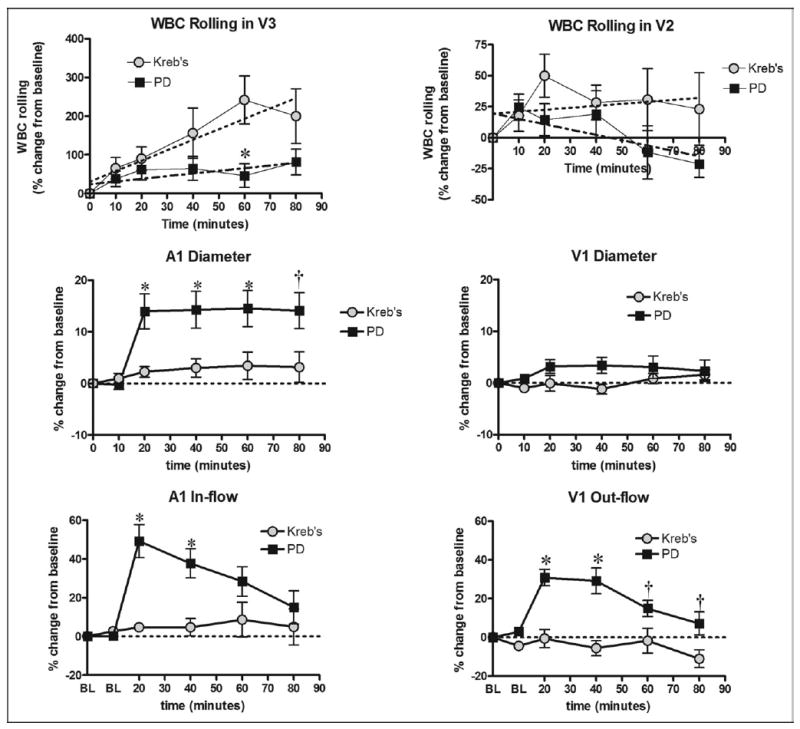
WBC–endothelial interaction coupled with diameter and flow data. Absolute count of rolling WBC was significantly greater in V3 than V2 venules. Relative WBC rolling was quantitatively lower with peritoneal dialysis suffusion (PD) and significantly lower after a 60-minute exposure compared to suffusion with Kreb’s (upper panel). PD caused significant arteriolar dilation and increased arteriolar (A1) and venular (V1) blood flow (middle and lower panels). Dotted lines depict the regression analysis. *P < .01, †P < .05, by 2-way ANOVA and Bonferroni post-test.
The second series of experiments (groups III and IV) was designed to assess the effects of topical application of a clinical glucose-based peritoneal dialysis solution-mediated vasodilation on the WBC rolling during conventional resuscitation from hemorrhagic shock. Data from this series of experiments are summarized in Fig. 1. As expected, hemorrhagic shock caused a drop in mean arterial pressure which was maintained for 60 minutes according to the experimental protocol. Mean arterial pressure was recovered to baseline with conventional resuscitation and with conventional resuscitation and adjunctive DPR (data not shown). Surgical manipulation plus HSCR causes a slight increase in the WBC–endothelial interaction over time in the ileum when bathed in a normal physiologic Kreb’s solution. However, exposure of the HSCR pre-prepared ileum to a hypertonic 2.5% peritoneal solution as a simulated adjunctive DPR elicits no significant change from that of Kreb’s in WBC–endothelial interaction (Fig. 2, upper panel). Similar magnitude of absolute count of rolling WBCs was observed in both V2 and V3, which is in contrast to the data in Fig. 1. Simulated DPR with a hypertonic 2.5% peritoneal dialysis solution (group IV) caused an instantaneous and sustained vasodilation in the A1 arteriole compared to a persistent A1 vasoconstriction in animals of group III that received conventional resuscitation alone. Subsequently, post-resuscitation arterial A1 and venous V1 flow decreased significantly with conventional resuscitation in group III, in contrast to an increase of A1 blood flow in the DPR group (group IV) (Fig. 2, middle and lower panels).
Fig. 2.
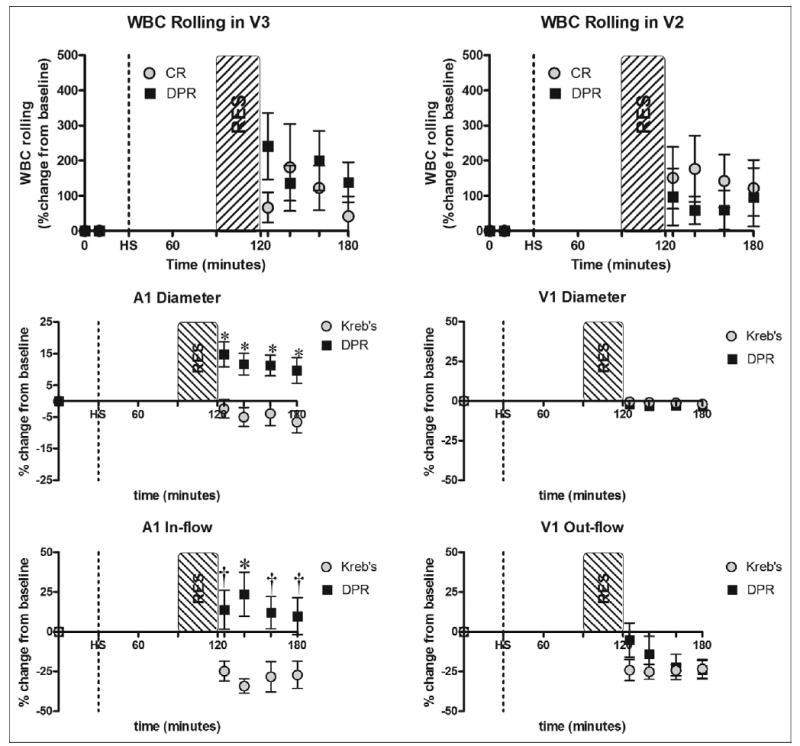
WBC–endothelial interaction coupled with diameter and flow data after hemorrhagic shock (HS) and conventional resuscitation (CR), or simulated adjunctive direct peritoneal resuscitation (DPR). RES = 30-minute resuscitation period. Relative WBC rolling was not modulated by resuscitation method after hemorrhagic shock (upper panel). DPR caused significant arteriolar dilation and increased arteriolar (A1) flow (middle and lower panels). *P < .01, †P < .05, by 2-way ANOVA and Bonferroni post-test.
Comments
Under normal physiologic conditions no WBC–endothelium interaction occurs. In recent years, response of the endothelial cell to blood loss has become central to our understanding of the pathophysiology of shock because of the hemorrhage and resuscitation-induced alteration of 4 critical functions: (1) anticoagulant/procoagulant balance, (2) microvascular smooth muscle tone, (3) microvascular permeability, and (4) WBCs activity. The same stimuli (ischemia, gut-derived inflammatory mediators and oxygen free radicals) that induce activation of the endothelial cell also activates WBCs and render both cells sensitive to adhesion through up-regulation of cell surface adhesion molecules and immunoglobulin-like receptors on both cells population. The primed WBC–endothelium system determines WBC activities, which are characterized by defined steps starting with margination followed by rolling, firm adhesion, emigration, and finally migration in the interstitial space. Theoretically, shear forces exerted by blood flow should influence rolling WBC concentration and explain the predominance of this interaction with the venular rather than arteriolar endothelium. Intravital microscopy studies of the mesocecum in rats have demonstrated a sustained increase in wall shear rate and a concomitant progressive decrease in WBC rolling concentration over 20 minutes of observation, when the mesentery was suffused with a conventional heat-sterilized peritoneal dialysis solution [16]. However, the peritoneal dialysis solution-mediated decrease in WBC rolling was only partially explained by an increase in blood flow, as a greater fraction of rolling WBCs remained after correction of changes in wall shear rate was applied [16]. These data are consistent with our present studies, which also suggest that the blood concentration of rolling WBCs is the parameter clearly affected by the dialysis solution as demonstrated in our current and previous studies [17].
As shown in the present studies, topical applications of clinical peritoneal dialysis solutions modulate WBC–endothelium interaction in part by the solution-mediated enhancement of arterial blood flow and venous outflow. Both A1 and V1 flow increase as a result of peritoneal dialysis solution administration, suggesting an increased delivery of WBCs to the venule under observation that also explains the greater data variability in the peritoneal dialysis group. Increased flow equals greater momentum and force to overcome the intermolecular tethering associated with WBC–endothelial interaction of rolling. As seen under the microscope, WBC rolling occurs at the postcapillary venule where red blood cells cause margination of the leukocytes. Anatomical differences exist in the venular tree not only from rat to rat, but venule to venule in the same preparation. Efforts to minimize variability were taken by choosing the first major branching (~15 to 20 μm) out of the postcapillary venule for WBC rolling counts. Increases in flow would likely have a greater effect at this location because it allows the WBCs an opportunity to release from the endothelium rather than in the immediate postcapillary venule where rolling first begins. Therefore, less WBC–endothelial rolling occurs in the intestinal ileum superinfused with the peritoneal dialysis solution. Phamacologic applications that increase blood flow may bring about the same result of a decrease in WBC–endothelial rolling.
Topical application of clinical peritoneal dialysis solutions (DPR) does not modulate WBC–endothelium rolling in the HSCR model rat. The lack of significant change in WBC–endothelial rolling in the intestinal ileum of the HSCR model rat was surprising. However, hemorrhagic shock and resuscitation add more compounding variables that can significantly affect WBC–endothelium interaction. Hemorrhagic shock with subsequent resuscitation will directly damage the endothelium while activation of the immune system upon reperfusion indirectly alters endothelial function through upregulation of endothelial surface adhesion molecules and neutrophil priming [4–6]. It has consistently been demonstrated that the status of the vascular endothelium, other than the local hemodynamics is the major determinant of WBC–endothelium interaction [18–20]. In addition, types of resuscitation fluid and method of infusion can influence neutrophil function [21–23]. In one study, animals resuscitated with Ringer’s lactate showed significant WBC oxidative burst capacity notably when a large volume was given at a fast rate. Resuscitation with 5% albumin (equal volume) or fresh whole blood (equal volume) resulted in no significant increase neutrophil activation [21–23]. Collectively, these data explain why adjunctive DPR did not reduce WBC–endothelium rolling in the present study, suggesting that DPR-mediated downregulation of the exaggerated systemic inflammatory response noted after conventional resuscitation must have other mechanistic explanations.
Shortcomings of the intravital microscopy study of the intestine are obviated by the absence of further WBC activation of sticking or extravasation. While sticking seems to occur during the low-flow state of hemorrhagic shock, downstream washout of stationary WBCs does characterize the resuscitation-induced reflow establishment. Perhaps the chronology of WBC activation limits the processes viewed within the first hour. Previous studies yielded times of 4 hours as the optimal time of neutrophil adherence after an initial small decline immediately following resuscitation [24]. Another study suggested 180 minutes for quantification of neutrophil oxidative burst activity in whole blood following HSCR using flow cytometry [25]. Compared to the thin mesentery, which allows for detailed studies at high resolution of intravascular phenomena, the intestinal wall (more relevant in shock research) is relatively thick, causing deterioration of the video images over time due to edema formation. Nonetheless, more studies on time-dependent measurements of tissue myeloperoxidase activity, as an index of neutrophil sequestration are needed, to validate the sequel outcomes of WBC–endothelium interaction.
In conclusion, superfusion of the gut with glucose-based peritoneal dialysis solutions decreases the concentration of rolling leukocytes along the venular vascular endothelium by a vasodilation-mediated increase in arteriolar inflow and venous outflow mechanism. Hemorrhagic shock and conventional resuscitation enhance the concentration of rolling leukocytes, presumably by mechanisms related to upregulation of the adhesion molecules and the low-flow state. Hemorrhage and resuscitation-enhanced leukocytes rolling was not reversed by adjunctive DPR despite the associated marked increase in arterial inflow and venous outflow. The status of the endothelium and the level of leukocyte priming in low-flow states, are stronger predictors of leukocyte–endothelium interaction than rheology factors.
Footnotes
Presented at the 30th Annual Surgical Symposium of the Association of VA Surgeons, Cincinnati, Ohio, May 7–9, 2006
Supported by a VA Merit Review grant and by NIH research Grant No. 5R01 HL076160-03, funded by the National Heart, Lung, and Blood Institute and the United States Army Medical Resources and Material Command.
References
- 1.Hassoun HT, Kone BC, Mercer DW, et al. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1–10. doi: 10.1097/00024382-200115010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Flynn WJ, Cryer HG, Garrison RN. Pentoxifylline but not saralasin restores hepatic blood flow after resuscitation from hemorrhagic shock. J Surg Res. 1991;50:616–21. doi: 10.1016/0022-4804(91)90051-m. [DOI] [PubMed] [Google Scholar]
- 3.Flynn WJ, Jr, Gosche JR, Garrison RN. Intestinal blood flow is restored with glutamine or glucose suffusion after hemorrhage. J Surg Res. 1992;52:499–504. doi: 10.1016/0022-4804(92)90318-t. [DOI] [PubMed] [Google Scholar]
- 4.Fruchterman TM, Spain DA, Wilson MA, et al. Selective microvascular endothelial cell dysfunction in the small intestine following resuscitated hemorrhagic shock. Shock. 1998;10:417–22. doi: 10.1097/00024382-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Zakaria ER, Spain DA, Harris PD, et al. Resuscitation regimens for hemorrhagic shock must contain blood. Shock. 2002;18:567–73. doi: 10.1097/00024382-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Zakaria ER, Garrison RN, Spain DA, Harris PD. Impairment of endothelium-dependent dilation response after resuscitation from hemorrhagic shock involved postreceptor mechanisms. Shock. 2004;21:175–81. doi: 10.1097/00024382-200402000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Childs EW, Smalley DM, Moncure M, et al. Effect of LFA-1beta antibody on leukocyte adherence in response to hemorrhagic shock in rats. Shock. 2000;14:49–52. doi: 10.1097/00024382-200014010-00009. [DOI] [PubMed] [Google Scholar]
- 8.Flynn WJ, Cryer HG, Garrison RN. Pentoxifylline restores intestinal microvascular blood flow during resuscitated hemorrhagic shock. Surgery. 1991;110:350–6. [PubMed] [Google Scholar]
- 9.Fruchterman TM, Spain DA, Wilson MA, et al. Complement inhibition prevents gut ischemia and endothelial cell dysfunction after hemorrhage/resuscitation. Surgery. 1998;124:782–91. doi: 10.1067/msy.1998.91489. [DOI] [PubMed] [Google Scholar]
- 10.Zakaria ER, Garrison RN, Spain DA, et al. Intraperitoneal resuscitation improves intestinal blood flow following hemorrhagic shock. Ann Surg. 2003;237:704–13. doi: 10.1097/01.SLA.0000064660.10461.9D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zakaria ER, Garrison RN, Kawabe T, et al. Direct peritoneal resuscitation from hemorrhagic shock: effect of time delay in therapy initiation. J Trauma. 2005;58:499–506. doi: 10.1097/01.TA.0000152892.24841.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zakaria ER, Hurt RT, Matheson PJ, et al. A novel method of peritoneal resuscitation improves organ perfusion after hemorrhagic shock. Am J Surg. 2003;186:443–8. doi: 10.1016/j.amjsurg.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Garrison RN, Conn AA, Harris PD, et al. Direct peritoneal resuscitation as adjunct to conventional resuscitation from hemorrhagic shock: a better outcome. Surgery. 2004;136:900–8. doi: 10.1016/j.surg.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 14.Bohlen HG, Gore RW. Preparation of rat intestinal muscle and mucosa for quantitative microcirculatory studies. Microvasc Res. 1976;11:103–10. doi: 10.1016/0026-2862(76)90081-9. [DOI] [PubMed] [Google Scholar]
- 15.Ley K, Linnemann G, Meinen M, et al. Fucoidin, but not yeast polyphosphomannan PPME, inhibits leukocyte rolling in venules of the rat mesentery. Blood. 1993;81:177–85. [PubMed] [Google Scholar]
- 16.Jonasson P, Bagge U, Wieslander A, Braide M. Heat-sterilized PD fluid blocks leukocyte adhesion and increases flow velocity in rat peritoneal venules. Perit Dial Int. 1996;16:S137–40. [PubMed] [Google Scholar]
- 17.Zakaria ER, Garrison RN, Kawabe T, et al. Role of neutrophils on shock/resuscitation-mediated intestinal arteriolar derangements. Shock. 2004;21:248–53. doi: 10.1097/01.shk.0000111824.07309.19. [DOI] [PubMed] [Google Scholar]
- 18.Ley K, Gaehtgens P. Endothelial, not hemodynamic, differences are responsible for preferential leukocyte rolling in rat mesenteric venules. Circ Res. 1991;69:1034–41. doi: 10.1161/01.res.69.4.1034. [DOI] [PubMed] [Google Scholar]
- 19.Ley K. Leukocyte adhesion to vascular endothelium. J Reconstr Microsurg. 1992;8:495–503. doi: 10.1055/s-2007-1006736. [DOI] [PubMed] [Google Scholar]
- 20.Ley K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc Res. 1996;32:733–42. [PubMed] [Google Scholar]
- 21.Alam HB, Sun L, Ruff P, et al. E- and P-selectin expression depends on the resuscitation fluid used in hemorrhaged rats. J Surg Res. 2000;94:145–52. doi: 10.1006/jsre.2000.6011. [DOI] [PubMed] [Google Scholar]
- 22.Alam HB, Stanton K, Koustova E, et al. Effect of different resuscitation strategies on neutrophil activation in a swine model of hemorrhagic shock. Resuscitation. 2004;60:91–9. doi: 10.1016/j.resuscitation.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Angle N, Hoyt DB, Coimbra R, et al. Hypertonic saline resuscitation diminishes lung injury by suppressing neutrophil activation after hemorrhagic shock. Shock. 1998;9:164–70. doi: 10.1097/00024382-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Davis JM, Stevens JM, Peitzman A, et al. Neutrophil migratory activity in severe hemorrhagic shock. Circ Shock. 1983;10:199–204. [PubMed] [Google Scholar]
- 25.Scalia R, Armstead VE, Minchenko AG, Lefer AM. Essential role of P-selectin in the initiation of the inflammatory response induced by hemorrhage and reinfusion. J Exp Med. 1999;189:931–8. doi: 10.1084/jem.189.6.931. [DOI] [PMC free article] [PubMed] [Google Scholar]


