Abstract
Calponin is an actin filament-associated regulatory protein and its h2 isoform is expressed in lung alveolar epithelial cells under postnatal up-regulation during lung development corresponding to the commencement of respiratory expansion. Consistent with this correlation to mechanical tension, the expression of h2-calponin in alveolar cells is dependent on substrate stiffness and cytoskeleton tension. The function of h2-calponin in the stability of actin cytoskeleton implicates a role in balancing the strength and compliance of alveoli. An interesting finding is a rapid degradation of h2-calponin in lung after prolonged deflation, which is prevented by inflation of the lung to the in situ expanded volume. Decreasing mechanical tension in cultured alveolar cells by reducing the dimension of culture matrix reproduced the degradation of h2-calponin. Inhibition of myosin II ATPase also resulted in the degradation of h2-calponin in alveolar cells, showing a determining role of the tension in the actin cytoskeleton. Alveolar cells statically cultured on silicon rubber membrane build high tension in the cytoskeleton corresponding to a high expression of h2-calponin. Chronic cyclic stretching of cells on the membrane did not increase but decreased the expression of h2-calponin. This finding suggests that when cellular structure adapted to the stretched dimension, cyclic relaxations periodically release cytoskeleton tension and lower the total amount of tension that the cell senses over time. Therefore, the isometric tension, other than tension dynamics, determines the expression of h2-calponin. The tension regulation of h2-calponin synthesis and degradation demonstrate a novel mechanical regulation of cellular biochemistry.
Living cells respond to mechanical forces by changes in cellular structure and function through gene regulation and posttranslational protein modification (1–5). Reorganization of the cytoskeleton is a key component of the cellular response to mechanical stimuli (3, 6). The actin cytoskeleton is a dynamic filamentous network that determines cell shape and strength and plays an important role in cellular response to mechanical tension (7). Calponin is an actin filament-associated regulatory protein (8) and has been extensively studied for its role in the contractility of smooth muscle (9, 10). Biochemical activities of calponin have been documented in details mainly from experiments using the h1 isoform in smooth muscles (11). Through high affinity binding to F-actin, calponin inhibits the actin-activated smooth muscle myosin MgATPase and the production of force (12). Despite the extensive investigations, the physiological function of calponin in living cells remains to be established.
The h2 isoform of calponin (11) is found in smooth muscle and non-muscle cells. Calponin's association with actin stress fibers and function in regulating actin-myosin interaction suggest a role in cytoskeleton activities. Forced expression of calponin in smooth muscle cells and fibroblasts inhibited cell proliferation (13, 14). Providing a novel lead for the function of calponin, we recently demonstrated a mechanical tension-regulated expression of h2-calponin in fibroblasts and epidermal keratinocytes with a role in stabilizing the actin filaments (15).
In the responses of h2-calponin function to mechanical tension, gene regulation represents a chronic and sustained control. On the other hand, proteolysis may provide rapid structural and functional modifications during adaptations to environmental changes. Selective proteolysis can remove regulatory proteins when they are not needed, while transforming others from the dormant into the biological active state (16). Therefore, proteolytic regulation of calponin may also play a role in cytoskeleton responses to mechanical stimuli.
In the present study, we examined the expression of h2-calponin in multiple representative tissues and found that it is abundant in lung alveolar epithelial cells. The lung undergoes dynamic mechanical tension changes from alternating distension and collapse that modulate the phenotypes of alveolar epithelial cells (17), and so is an informative system to study the tension-responsive regulation of cytoskeletal proteins. The expression of h2-calponin is rapidly up-regulated during postnatal lung development corresponding to respiratory expansion. In addition to the mechanical tension dependent expression and role in stabilizing actin cytoskeleton, a novel finding in the present study is that h2-calponin is regulated in alveolar cells by mechanical tension dependent proteolysis. A rapid degradation of h2-calponin occurs in lung tissues after prolonged deflation, which is effectively prevented at the inflated state. Decreasing mechanical tension in cultured alveolar cells by reducing the matrix dimension reproduced the degradation of h2-calponin. The myosin II ATPase-based tension in the actin cytoskeleton is required to prevent h2-calponin degradation. Another interesting finding is that continuous cyclic stretching of cells did not increase but decreased the expression of h2-calponin. Therefore, after the cellular structure is remodeled to fit the stretched dimension, cyclic relaxations would periodically release cytoskeleton tension and lower the total amounts of tension over time, which determines the expression of h2-calponin. The tension regulation of h2-calponin synthesis and degradation provides new insight into the mechanical function of lung alveolar cells that are physiologically under distension-relaxation stimuli and demonstrates a novel mechanical regulation of cellular biochemistry.
MATERIALS AND METHODS
Anti-h2-calponin antibodies
A rabbit polyclonal antiserum, RAH2, raised against mouse h2-calponin with a weak cross-reaction to h1-calponin was published in 1998 (18). A mouse anti-h1-calponin monoclonal antibody (mAb)1 CP3 was published in 1996 (19). Mouse anti-h2-calponin mAbs CP21 and 1D2 were published in 2003 (13) and 2005 (15).
A new mAb 1D11 (CP22, IgG2bk) against h2-calponin was developed as described previously (13) using mouse h2-calponin (20) as immunogen. Spleen cells of an immunized Balb/c mouse were harvested for fusion with SP2/0-Ag14 mouse myeloma cells. Hybridoma clones were screened by indirect enzyme-linked immunosorbant assay (ELISA) against the immunogen and subcloned three times to establish stable cell lines. The mAb was produced in the forms of hybridoma cultural supernatant and mouse ascites fluids. The specificity of CP22 was determined by Western blotting on purified h1- and h2-calponins (20).
Cell cultures
Human alveolar carcinoma cell line SW1573 (American Type Culture Collection, CRL-2170) (21) was cultured in Leibovitz’s L-15 medium containing 10% fetal bovine serum, 2 mM L-glutamine, penicillin (100 i.u./ml) and streptomycin (50 i.u./ml) at 37 °C in a humidified incubator without added CO2. Rat alveolar type II epithelial cell line RLE-6TN (American Type Culture Collection CRL-2300) derived by spontaneous immortalization (22) was cultured in Ham's F12 containing 10% fetal bovine serum and 10 μg/ml bovine pituitary extract, 5 μg/ml insulin, 2.5 ng/ml recombinant human insulin growth factor I, 25 μg/ml transferrin, 2.5 ng/ml epidermal growth factor, and 2 mM L-glutamine at 37 °C in a humidified incubator in the presence of 5% CO2. H2-calponin expression is found in the two alveolar cell lines at similar levels and with similar patterns of regulation. Therefore, they are studied together as ex vivo experimental systems to investigate the regulation and function of h2-calponin in lung alveolar cells.
SDS-PAGE and Western blotting
Representative tissues were obtained from adult (4–5 months old, 25–30 gm) C57B/L6 mice. Developing and adult lung samples were obtained from C57B/L6 mice and Sprague-Dawley rats at a series of time points from late embryonic stage to 6 months after birth (Figure 3). Immediately after euthanasia of the animal, the tissues were rapidly dissected and briefly rinsed in cold PBS (phosphate buffered saline). The lung samples analyzed were dissected from the outer edge and free of major bronchi. Total proteins were immediately extracted from the tissues by homogenization in SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer containing 2% SDS (to inactivate proteases) using a Polytron-like high speed mechanical tissue homogenizer and heated at 80 °C for 5 minutes. Total protein extracts from cultured alveolar cells were prepared by lysing PBS-washed monolayer cells in SDS-PAGE sample buffer and heating at 80 °C for 5 minutes. The protein extracts were examined by SDS-PAGE using 12% gel with an acrylamide:bisacrylamide ratio of 29:1 in the Laemmli buffer system. Western blotting was performed using the anti-calponin antibodies followed by alkaline phosphatase-labeled anti-rabbit IgG or anti-mouse IgG second antibody (Sigma) and 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium chromogenic substrate reaction (13, 15). The contents and integrity of the protein samples were determined by staining the gel with Coomassie Blue R250. Quantification of the Western blots was done by normalization to the amount of actin, total cellular protein, or histones determined in the parallel gels. Purified h2- and h1-calponins (20) were used as control.
Figure 3.
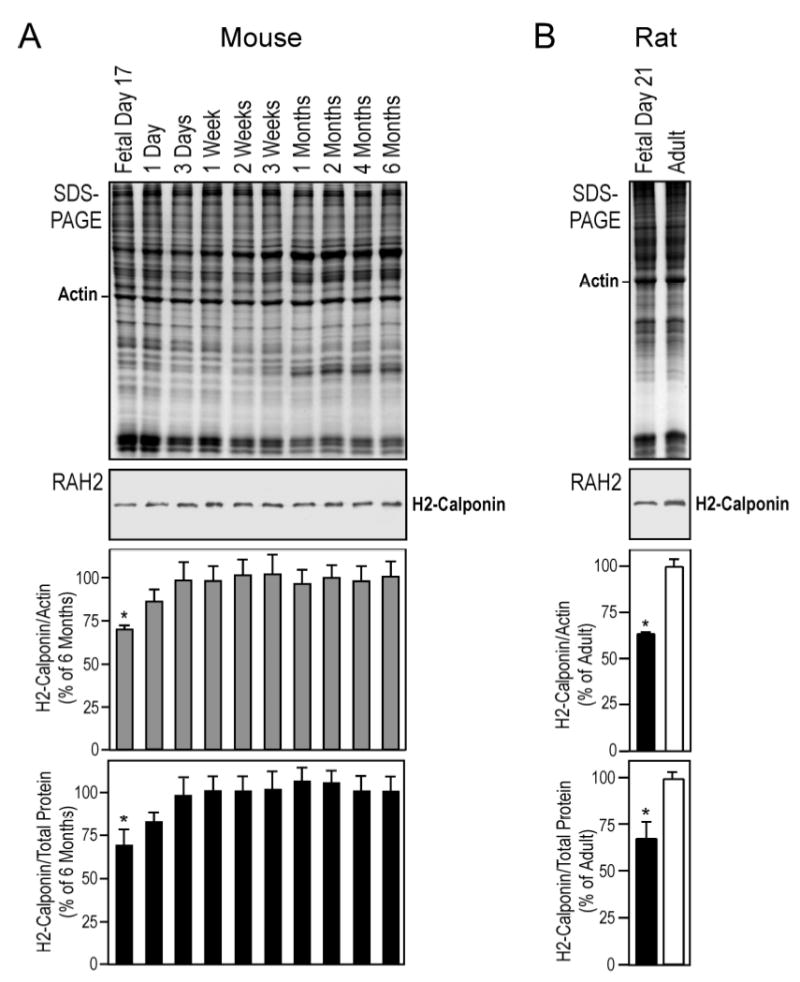
Postnatal up-regulation of h2-calponin in the lung. (A) Mouse lung tissues were obtained at a series of developmental time points and examined by Western blots using anti-h2-calponin antibody RAH2. Normalized by the level of actin or total protein, densitometry analysis demonstrated a rapid postnatal up-regulation of h2-calponin in the developing lung with a correlation to the commencement of respiratory activity. (B) Fetal versus adult rat lungs were examined by Western blot using RAH2 antibody. Densitometry quantification against actin or total cellular protein showed significantly higher levels of h2-calponin expressed in the adult versus fetal lung. *P<0.001 as compared with the plateau levels.
Immunohistochemistry
To examine the expression and distribution of h2-calponin in the lung, thin frozen sections of mouse lung were stained with anti-h2-calponin mAb CP22 (hybridoma cultural supernatant was used to avoid potential autoimmune reactivity of sera or ascites fluid against cytokeratins, unpublished results) followed by horseradish peroxidase-labeled anti-mouse IgG second antibody (Sigma) and H2O2-daminobenzidin substrate reaction using standard immunohistochemical method (15). SP2/0 myeloma cultural supernatant was used as negative control. Counterstaining with 0.6% hematoxylin for 20 sec was used to outline the morphology of the tissue sections. The results were observed under a Zeiss Axiovert 100H microscope.
Examination of substrate anchorage-dependence of h2-calponin expression
To examine the effects of substrate anchorage on the expression of h2-calponin in alveolar cells, the cell culture dishes were placed on an orbital shaker (Bel-Art Products, Pequannock, NJ) driven by a magnetic stirrer at 80 rpm in a tissue culture incubator at 37 °C. The continuous vibration prevented cell attachment to the culture dish (23). The cells were harvested after 3 days of vibrating culture to examine the levels of h2-calponin by Western blotting as described previously (15). The floating cell aggregates were re-seeded on tissue culture dishes and incubated without vibration to confirm their viability. Parallel steady monolayer cultures were examined as controls.
Examination of substrate stiffness-dependence of h2-calponin expression
Thin layers of polyacrylamide gel were used to provide cell culture substrates with different stiffness that produces corresponding traction force in the cytoskeleton (24–26). Hard (10% gel with an acrylamide:bisacrylamide ratio of 40:1) and soft (3% gel with an acrylamide:bisacrylamide ratio of 74:1) gels were prepared and covalently coated with Type I collagen as described previously (15). Alveolar cells were cultured on either the hard and soft gel or rigid plastic dish (control) to examine the effect of substrate stiffness-generated cytoskeletal tension on the expression of h2-calponin. The cells were harvested after 3 days of culture by direct lysis in SDS-gel sample buffer after PBS washes. The level of h2-calponin was examined by Western blot as above.
Cytochalasin B treatment of alveolar cell cultures
To investigate the potential role of h2-calponin in lung alveolar structure and mechanical function, SW1573 human alveolar cells were plated on collagen-coated polyacrylamide gel substrate of high or low stiffness as above. After 3 days of culture, the cells were treated with 0.5, 0.75 and 1.0 μg/ml of cytochalasin B (Sigma) at 37 °C for 30 min. Cells treated with 0.2% dimethyl sulfoxide (DMSO) were used as the cytochalasin B solvent control. The treated cells were washed with PBS and fixed with cold acetone. The actin stress fibers was examined by rhodamine-labeled phalloidin staining as described previously (15) and examined by fluorescence microscopy for the resistance to cytochalasin B destruction.
Examination of h2-calponin degradation in deflated lung
Neonatal and adult human lung autopsy tissue samples were obtained from the Rainbow Babies and Children’s Hospital, University Hospitals of Cleveland in full compliance with IRB regulations. The autopsies were performed from hours to days postmortem and the isolated tissue samples were stored at −80 °C until SDS-PAGE and Western blot examination using the RAH2 antibody as above.
Adult (~250 gm) rat lung was examined immediately after euthanasia or after incubation on ice for 24 hrs by Western blot for h2-calponin contents.
Adult (25–30 gm) mouse and rat lungs were isolated immediately after euthanasia and incubated in the naturally collapsed state at 37 °C in a humidified container. Tissue samples were taken at a series of time points up to 5h during the incubation. The tissues were dissected free of major bronchi and homogenized in SDS-PAGE sample buffer. The level of h2-calponin versus the total protein contents was examined by SDS-PAGE and Western blot as above.
To examine the role of mechanical tension in h2-calponin stability in lung alveolar cells, postmortem mouse and rat lungs were inflated with air to remain at the in situ inflated volume while remained in the thoracic cavity and incubated at 37 °C together with the collapsed controls. Samples were taken after 6h of incubation and examined by SDS-PAGE and Western blots as above for the effect of distension on the degradation of h2-calponin.
H2-calponin degradation and function in relaxed alveolar cells
Using a Flexercell Tension Plus system, Model FX-4000T (Flexcell International, Hillsborough, NC), RLE-6TN rat alveolar cells were seeded in Bioflex 6-well culture plates (Bioflex 3000C) with Type I collagen-coated elastic silicon rubber membrane bottoms at 1 x 105 cells/well and incubated in a tissue culture incubator as above. After letting the cells to attach to the plates for 6 hours, the rubber substrate was constantly stretched by continuously applying vacuum to result in an 8% elongation of the membrane. The use of a 25 mm diameter round Bioflex loading station produced equal biaxial extension of the rubber membrane. After three days of culture on the stretched membrane, the vacuum was turned off to relax the rubber membrane to the original dimension. The cells were continuously cultured on the unstretched membrane and collected at a series of post relaxation time points to examine the degradation of h2-calponin by Western blot as above.
After 6h of continuing culture in the relaxed state, treatment with 0.75, 1.00, and 1.25 μg/ml of cytochalasin B for 30 min and examination of actin stress fibers were performed as above. The fluorescence microscopy on cells cultured on the silicon rubber membrane was carried out by fixing the cells on the membrane with cold acetone, staining with rhodamine-labeled phalloidin, and mounting the membrane under a No. 1 cover slip.
Inhibition of myosin II ATPase
To examine the effect of reducing the tension built in the actin cytoskeleton on the content of h2-calponin in alveolar cells, RLE-6TN cells were seeded on either plastic dishes or gelatin-coated cover slips (3 x 104 cells/35 mm dish). After 3 days of culture, the cells were treated with 100 μM blebbistatin, a myosin II ATPase inhibitor that decreases the myosin motor-generated tension in cytoskeleton (27), as described previously (15, 28) for either 6 hours or 3 days. Cells were cultured in normal media or media containing 0.2% DMSO (solvent of the blebbistatin stock) as controls. The cells were harvested by lysis in SDS-PAGE sample buffer after PBS washes and the levels of h2-calponin were examined by Western blot analysis as above. The cells on the cover slips were fixed with cold acetone and the actin stress fibers were visualized by stained with rhodamine-conjugated phalloidin (15). The fluorescence and phase contrast images were examined under a Zeiss Axiovert 100H epifluorescence microscope.
Northern blotting analysis
As described previously (18), Total RNA was extracted from blebbistatin-treated and control NIH 3T3 mouse fibroblasts with the TRIzol reagent (Invitrogen) according to the manufacturer's protocol. Ten μg of each RNA sample was separated on 1.0% agarose gel in the presence of formaldehyde and capillarily transferred to Hybond N+ blotting nylon membrane (Amersham) in 10X SSPE (1.8 M NaCl, 0.1 M Na-phosphate, pH7.4, 10 mM EDTA). The blot was incubated in prehybridization solution (0.5 M Na-phosphate/1mM EDTA, pH 7.2 containing 1% BSA, 7% SDS and 30% formamide) at 55 °C for 6 hr and then hybridized (in the prehybridization solution plus 5% Dextran sulfate) at 55 °C overnight with mouse h2-calponin cDNA probe (20) labeled with 32P by random priming method as described previously (18). The blot was washed at 68 °C with 40 mM Na-phosphate, pH 7.2, 1 mM EDTA and 5% SDS. The detection of h2-calponin mRNA was documented by autoradiography at −80 °C with an intensifying screen.
Cell culture under cyclic stretching
RLE-6TN rat alveolar cells were seeded in Bioflex 6-well culture plates (Bioflex 3000C) with Type I collagen-coated silicon rubber membrane bottoms and incubated at 37 °C as described above. After letting the cells to attach to the membranes for 6 hours, the rubber substrate was cyclically stretched for 2 sec by applying vacuum to produce 1.25% biaxial equal extension followed by 2 sec of relaxation using the Flexercell system as described above. After 3 days of culture under continuously cyclic stretching, the cells were washed with PBS and harvested by lysis in SDS sample buffer. Total cellular protein extracts were analyzed by SDS-PAGE and Western blots to examine the levels of h2-calponin as described above. Cells cultured in parallel on rubber membrane without stretching were examined as static controls.
Data analysis
Densitometry analysis of SDS-gels and Western blots were done on digital images scanned at 600 dpi using the NIH Image program version 1.61. The quantitative data are presented as mean ± SD unless SEM is noted in the figure legends. Statistical analysis was done using the Microsoft Excel computer program.
RESULTS
Expression of h2-calponin in representative tissues
Total protein extracts from major organs of the mouse were analyzed by Western blots using the anti-h2-calponin polyclonal antibody RAH2, anti-h2-calponin mAb CP21 and anti-h1-calponin mAb CP3. The sample loading was normalized by the actin contents. The blots show that h2-calponin is found in smooth muscle as demonstrated previously (13) and in multiple non-muscle tissues with a higher level of expression in the spleen and lung (Figure 1). Neither h1- nor h2-calponin was detected in adult heart nor mature slow and fast skeletal muscles (soleus and extensor digitorum longus, respectively). The levels of h2-calponin in the adult mouse smooth muscle organs (trachea, stomach, intestine, urinary bladder and uterus) are lower than that of h1-calponin, consistent with the previous observation that h1-calponin is the predominant calponin isoform expressed in smooth muscle (13). H1-calponin is not detectable in the non-muscle tissues examined (brain, lung, liver, spleen, kidney, testis, ovary and skin), consistent with the current consensus that h1-calponin is smooth muscle-specific. The high level h2-calponin found in the spleen may reflect that in the endothelial cells (29, 30). Trace amount of h2-calponin was detected in the brain homogenate, possibly also from endothelial cells. The lung tissue analyzed was free of trachea and major bronchi as shown by the absence of h1-calponin that is a specific indicator of smooth muscle tissue. Therefore, the strikingly high level of h2-calonin detected in the lung is from non-muscle tissues, such as the alveolar epithelium that is the predominant tissue type in the lung.
Figure 1.
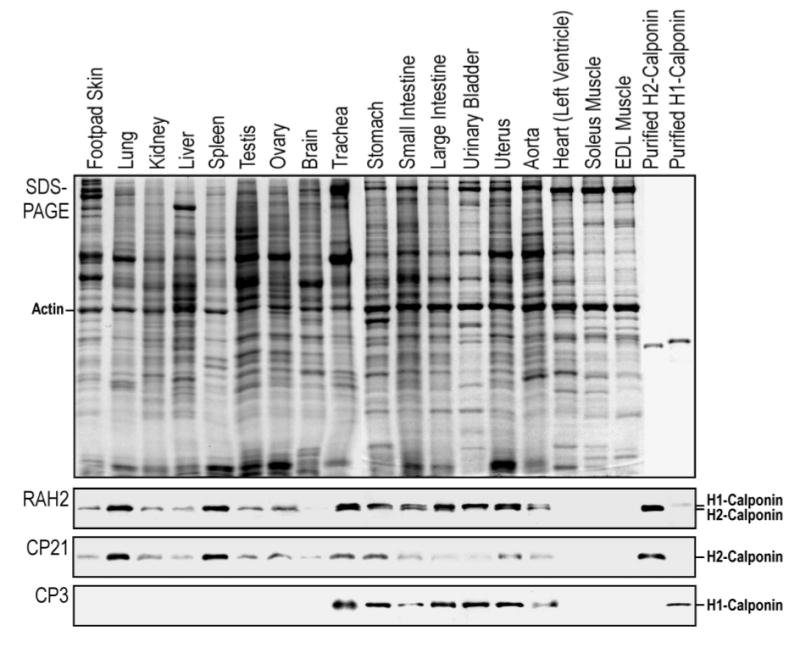
Expression of h2-calponin in representative tissues. Total protein extracts from major organs of the mouse were analyzed by Western blots using the anti-h2-calponin polyclonal antibody RAH2 and mAb CP21 together with the anti-h1-calponin mAb CP3. Purified mouse h1- and h2-calponin proteins were used as control. The sample loading was normalized by the level of actin (separately in the non-muscle and muscle tissue groups). The blots detected h2-calponin in smooth muscle and several non-muscle organs with high levels of expression in spleen and lung.
High level h2-calponin in lung alveolar epithelial cells
To confirm the expression of h2-calponin in lung alveolar epithelia, frozen sections of adult mouse lung were examined by immunohistochemistry using anti-h2-calponin mAb CP22. The results in Figure 2A showed a strong h2-calponin staining of alveolar epithelia. The high level expression of h2-calponin in alveolar epithelial cells was further confirmed by Western blots on protein extracts from both rat and human lung alveolar cell lines. In addition to showing the specificity of mAb CP22 against h2-calponin, the Western blots in Figure 2B demonstrate that SW1573 human lung alveolar cell, as the example, expresses significant amounts of h2-calponin while no h1-calponin is detectable.
Figure 2.
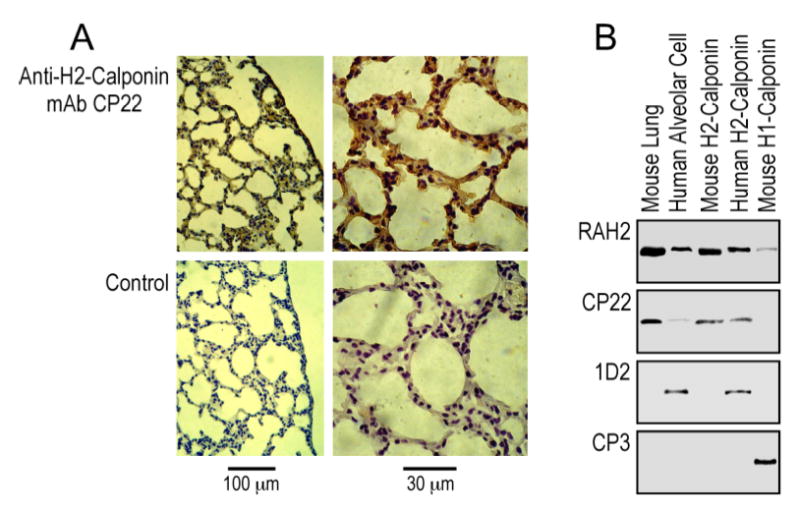
Expression of h2-calponin in lung alveolar epithelial cells. (A) Frozen sections of adult mouse lung were incubated with of anti-h2-calponin mAb CP22 hybridoma cultural supernatant or SP2/0 myeloma control supernatant followed by horseradish peroxidase-labeled anti-mouse IgG second antibody and H2O2-daminobenzidin substrate reaction. Significant amounts of h2-calponin were detected in alveolar epithelial cells. (B) Total protein extracts from SW1573 human alveolar cells were analyzed by SDS-PAGE and Western blots using anti-h2 calponin polyclonal antibody RAH2, mAb CP22 and mAb 1D2 (raised against cloned human h2-calponin, 15) together with the anti-h1-calponin mAb CP3. Purified mouse h1- and h2-calponin proteins were used as controls. The results show that the alveolar cells express h2-calponin and no h1-calponin was detected.
Rapid postnatal up-regulation of h2-calponin in the lung
A finding in the study of h2-calponin in the lung was an increase of expression during postnatal development. As shown in Figure 3A, Western blots using anti-h2-calponin antibody RAH2 on mouse lung samples obtained from a series of fetal and postnatal developmental time points detected a rapid postnatal up-regulation of h2-calponin. Normalizing the h2-calponin levels with actin or total cellular protein yielded similar trends. The lower level of h2-calponin in the fetal lung (absence of significant tissue distension) compared to the fully functional adult lung was also seen in the rat (Figure 3B). A major change in the lung structure and function at birth is the commencement of respiration activity that subjects the alveolar cells to distension. Together with many other developmental changes occur in the lung during the first a few days after birth (17), the rapid postnatal up-regulation of h2-calponin, by either genetic or functional adaptation, shows a correlation to the change of mechanical tension in the alveolar cells.
Cytoskeletal tension-dependent expression of h2-calponin in alveolar cells
We previously observed that h2-calponin expression in fibroblasts and epidermal keratinocytes is dependent on anchorage to the cultural substrate (15). Similar results were found with the lung alveolar epithelial cells. As shown in Figure 4, A and B, while monolayer cultures of human alveolar cells on plastic dish express a high level of h2-calponin, the floating cell aggregates in vibration culture had significantly decreased expression of h2-calponin. When the floating cells were allowed to attach to the dish in the absence of vibration, the expression of h2-calponin was up-regulated to reach the control level. This full potential of recovery indicates the viability of the floating-cultured cells. Normalization of the h2-calponin level against the levels of actin, total cellular protein, or histones (representing the amount of chromosomal DNA and, therefore, the number of cells) demonstrated similar changes. The results demonstrate that, like what in keratinocytes and fibroblast, the expression of h2-calponin in lung alveolar epithelial cells is dependent on substrate anchorage but not the cell-cell contact in the aggregates. When the alveolar cells grew as aggregates in non-compatible cultural dish, the decrease in h2-calponin expression also occurred (data not shown), excluding the effect of fluid shearing in the vibrating culture model.
Figure 4.
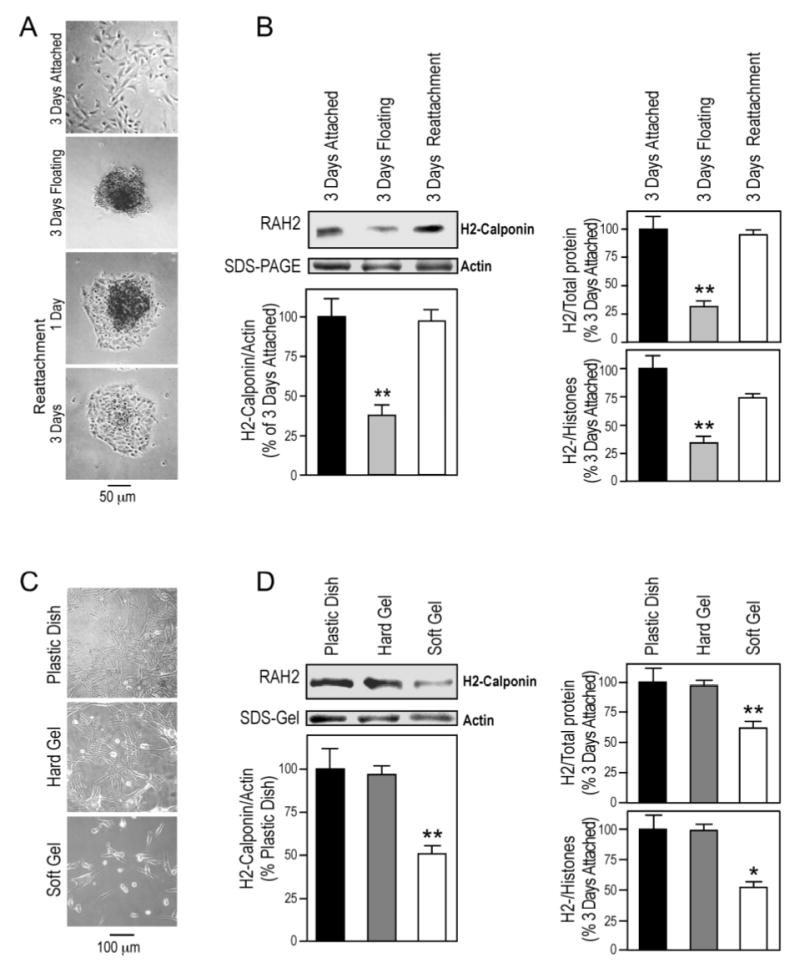
Substrate anchorage- and stiffness-dependent expression of h2-calponin in alveolar cells. (A) SW1573 human alveolar cells were cultured on plastic dishes steadily or with vibration. Phase contrast microscopic images show that in contrast to the monolayer formed in the steady culture, continuous vibration prevented the cells from anchoring to the cultural dish and produced cell aggregates that could attach to the dish when vibration was stopped. (B) Total protein extracts from the cells were examined by SDS-PAGE and Western blotting using anti-h2-calponin polyclonal antibody RAH2. Normalized by the level of actin, total cellular protein, or histones (representing the number of cells), the results show that H2-calponin expression in alveolar cells decreased significantly when growing as floating aggregates in comparison with that in monolayer cells attached to plastic dish (*P<0.001). The expression of h2-calponin was up-regulated to resume the control level after the floating cells attached to the plastic substrate to form a monolayer. The results were summarized from 4 individual experiments. (C) SW1573 human alveolar cells were cultured on plastic dish or polyacrylamide gels of high or low stiffness for 3 days. (D) The levels of h2-calponin were determined by Western blot analysis using anti-h2-calponin antibody RAH2. Normalized by the amount of actin, total cellular protein, or histones, densitometry quantification shows a significant decrease in the expression of h2-calponin in alveolar cells grown on soft polyacrylamide gel in comparison with that in the hard gel and plastic dish cultures (*P<0.005, **P<0.001). The results were summarized from 3 individual experiments.
To investigate the regulation of h2-calponin expression by traction force generated in the cytoskeleton against substrate stiffness, human alveolar cells were cultured on substrates of different rigidity. The Western blots in Figure 4, C and D showed that the cells cultured on soft gel substrate that produces less isometric tension (prestress) in the cytoskeleton (31) had a significantly less h2-calponin in comparison to that of the hard gel or plastic substrate controls corresponding to higher prestress conditions. Normalization of the h2-calponin level against actin, total cellular protein, or histone levels showed similar changes. The result that the hard gel substrate produced high levels of h2-calponin expression similar to that in cells cultured on plastic substrate indicates a determining role of the physical, but not chemical, nature of the cultural substrates. Together with the previous observations in keratinocytes and fibroblasts (15), the results demonstrate a conserved regulation of h2-calponin gene expression by cellular tension.
H2-calponin increases the stability of actin cytoskeleton in alveolar cells
To investigate the functional significance of the tension regulation of h2-calponin in alveolar cells, we examined the correlation between its level and the stability of actin filaments. SW1573 human alveolar cells cultured on hard or soft gel substrates was examined for the resistance of actin stress fibers to cytochalasin B. Previous studies have shown that cells build up less obvious stress fibers when cultured on gel versus rigid substrates (26). This effect is also seen between lung alveolar cells cultured on gel substrates. The results in Figure 5A show that the low stiffness gel substrate-produced decrease in h2-calponin expression (Figure 4, C and D) correlates with a decreased resistance of the actin filaments to cytochalasin B treatment. Although the cells cultured on gel substrates formed only very fine stress fibers (26), the disruption of actin filaments can be quantitatively evaluated by the collapsing of cytoskeleton at a lower concentration of cytochalasin B in the low h2-calponin cells than the high h2-calponin controls as reflected by the decreases in cell spreading areas (Figure 5B). The results support the hypothesis that calponin may play a role in stabilizing actin filaments by binding to F-actin and decreasing the rate of depolymeryzation against cytocholasin B treatment. The results suggest that the tension regulation of h2-calponin may affect the structure and function of cytoskeleton to modulate the mechanical strength as well as compliance of the alveoli.
Figure 5.
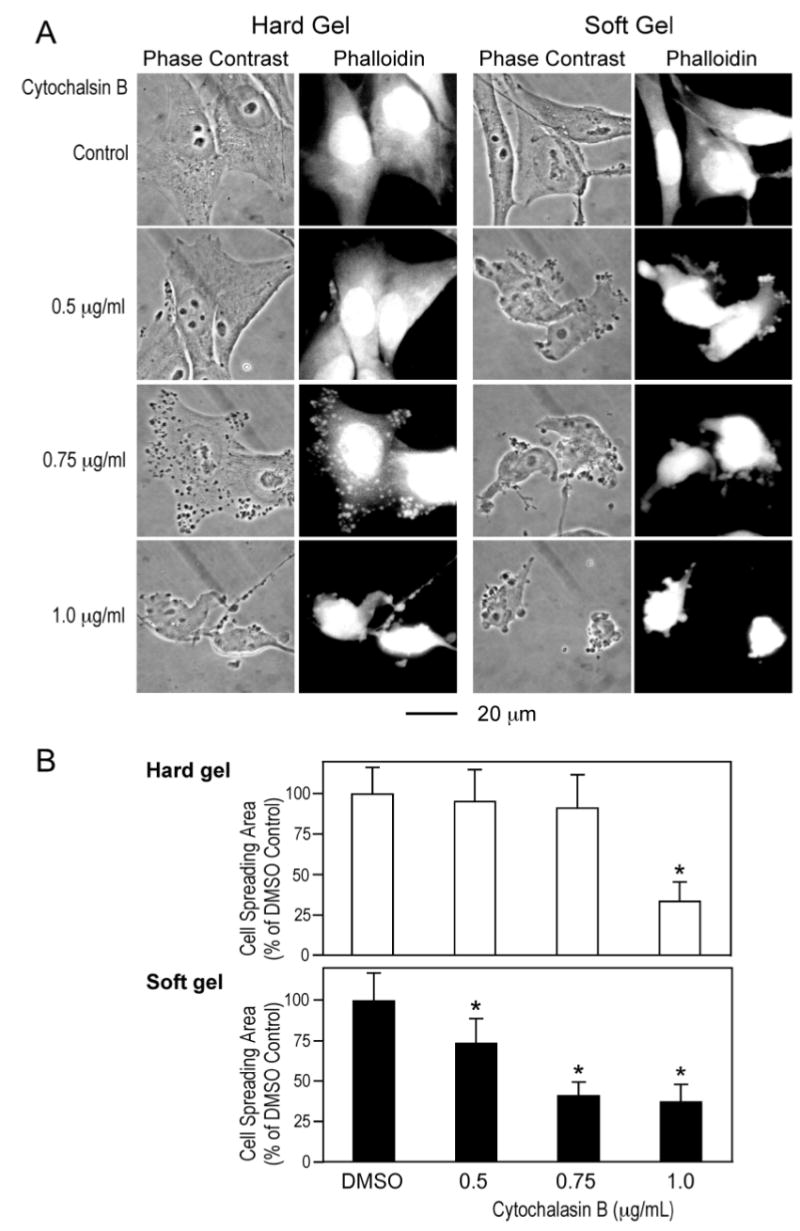
Role of h2-calponin in the stability of actin cytoskeleton in alveolar cells. SW1573 human alveolar cells cultured on hard or soft gel substrate were treated with cytochalasin B. (A) The effects on cellular structure were examined by phase contrast microscopy and the actin cytoskeleton was stained with rhodamine-labeled phalloidin. The results demonstrate that cytochalasin B treatment disrupted the actin cytoskeleton to result in slack appearance of the cell in a concentration dependent manner. Cells cultured on the gel substrates contained very fine actin stress fibers compared with that in cells cultured on plastic substrate. The cells cultured on soft gel showed collapses of actin cytoskeleton at lower concentrations of cytochalasin B in comparison to the hard gel controls, indicating a lower stability of the actin filaments in correspondence to the decreased h2-calponin content (Figure 4, C and D). (B) Cell spreading area was measured to quantitatively compare the destructing effects of cytochalasin B on cytoskeleton structure. The results are consistent with a decreased stability of the actin filaments in cells cultured on soft gel substrate as compared with the hard gel control. *P<0.001. The data are summarized from measurements of 30 cells in each group.
Tension reduction-induced degradation of h2-calponin in lung alveolar cells
A novel finding in the present study was a rapid degradation of h2-calponinin in collapsed lung. This was first observed in the Western blots on several neonatal and adult human lung autopsy tissue samples (Figure 6A). Distinct from freshly prepared animal lung samples, many of the human autopsy lung tissues showed reduced amounts of h2-calponin when normalized by the actin or total cellular protein levels. Since the SDS-gel showed no proportional degradation of other proteins in the samples, the results suggest a selective degradation of h2-calponin in the postmortem lungs and the variable amounts of remaining h2-calponin are likely due to the unknown durations between death and autopsy. Supporting a proteolytic degradation of h2-calponin in the postmortem human lung, the Western blots also detected a low molecular weight protein band that increased intensity in inverse relationship to the amount of intact h2-calponin in the sample (Figure 6A). To confirm this observation in a controlled experimental setting, we examined the levels of h2-calponin in isolated rat lungs after incubation on ice for 24h. The results in Figure 6B showed a decrease in the amounts of intact h2-calponin in postmortem rat lung although no specific degradation product was present. Figure 6C further shows that incubation of deflated mouse lung at 37 °C for 6 hours produced h2-calponin degradation with a detectable low molecular weight fragment similar to that in the postmortem human lung samples.
Figure 6.
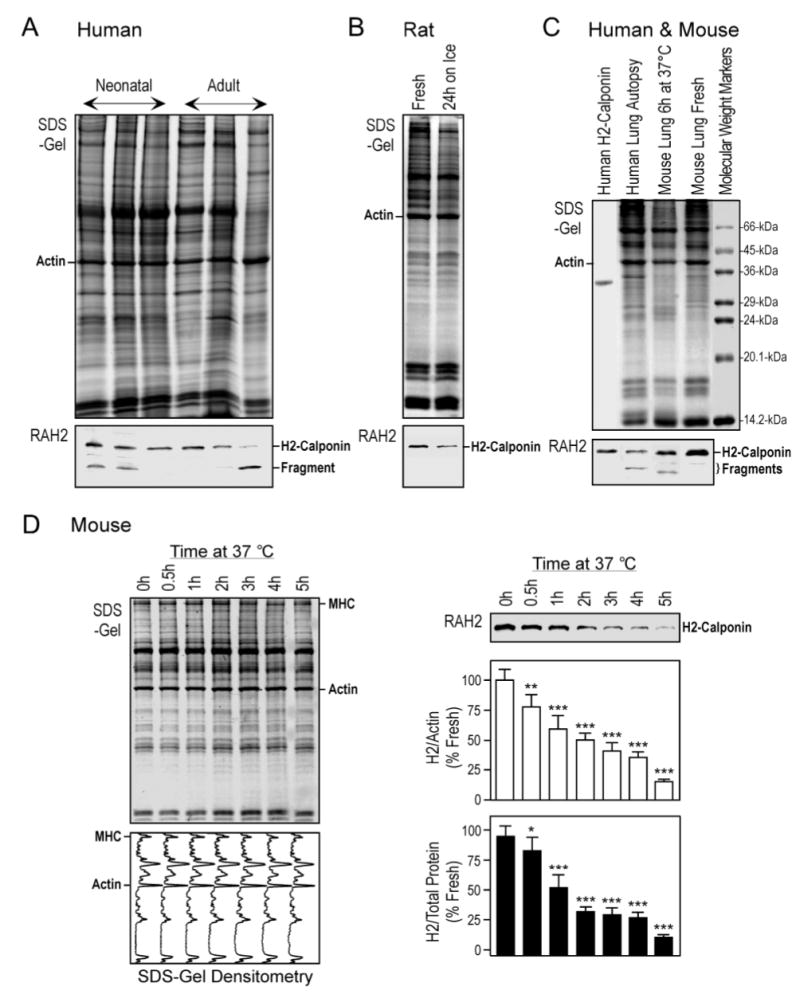
Rapid degradation of h2-calponin in lung tissue after prolonged relaxation. (A) Total protein extracts from infant and adult human lung autopsy tissues were analyzed by Western blot using the anti-h2-calponin antibody RAH2. The results revealed various degrees of h2-calponin degradation in the postmortem lung samples. (B) Adult rat lung tissue samples, freshly prepared or stored on ice for 24h, were examined by Western blotting using RAH2 antibody. The results showed a decrease in h2-calponin after the storage. (C) Deflated adult mouse lung was incubated at 37 °C for 6 hours and analyzed by 15% high cross linker SDS-gel and Western blotting using the RAH2 antibody. H2-calponin degradation with a fragment similar to that found in the human autopsy lung samples was detected. (D) Deflated adult mouse lung were incubated at 37°C and tissue samples were harvested at a series of time points for SDS-PAGE and RAH2 Western blot analysis. Normalized by the levels of actin or total protein, densitometry of the Coomassie Brilliant Blue R250-stained SDS-gels and Western blots demonstrated a rapid selective degradation of h2-calponin. Actin and other major proteins (MHC, myosin heavy chain) did not show significant changes in the SDS-gel and densitometry scans. *P<0.05; **P<0.005; ***P<0.001. The results are summarized from 4 individual experiments.
The lung tissue was under a reduced mechanical tension after it collapses due to the loss of pleural negative pressure. To examine the time course of h2-calponin proteolytic degradation in collapsed lung, the SDS-PAGE and Western blot analysis in Figure 6D demonstrates that staying at the collapsed state resulted in a rapid decrease in the amount of intact h2-calponin in adult mouse lungs, detectable within one hour at physiological body temperature (37 °C). Normalized to the level of actin or total cellular protein, densitometry analysis of the Western blots showed that more than 80% of h2-calponin was degraded in the collapsed lungs after 5 h. In contrast, densitometry profiles of the Coomassie Blue R250-stained SDS-gel of the samples showed no significant degradation of other major protein bands. Similar results were obtained in adult rat lung experiments (data not shown). The results suggest that in contrast to the extension-relaxation cycles physiologically occurring in the normal lung, prolonged periods at low tension state would result in a selective degradation of h2-calponin in lung alveolar cells. The h2-calponin partial degradation fragments seen in the postmortem human lung samples (Figure 6A) and deflated mouse lung (Figure 6C) might represent an intermediate proteolytic product that promotes the rapid degradation of h2-calponin under reduced cytoskeleton tension.
To demonstrate that the tension-related proteolysis of h2-calponin in the lung resulted from the decreased tension in alveolar cells, we performed an ex vivo tension release experiment in cultured RLE-6TN rat alveolar cells. The cells were cultured as a monolayer on silicon rubber membrane that was constantly vacuum-stretched and maintained at 108% of the original dimension for three days to constitute a static high tension culture condition (15). The cellular tension was then reduced by release of vacuum to decrease the dimension of the cultural matrix. The Western blots in Figure 7A demonstrated that the level of h2-calponin in the alveolar cells decreased within 3–6 hours after tension reduction. It has been shown that actin filament associated proteins have an in vivo half-life of 4–5 days (32). Therefore, the rapid degradation of h2-calponin indicates an active proteolytic regulation mechanism instead of a part of general protein turnover. Similar to that observed in the lung tissue, the degradation of h2-calponin protein in the alveolar cell cultures after the tension reduction was highly selective as no apparent change in other major proteins was seen in the SDS-PAGE gel and no degradation of tropomyosin, an actin-binding protein that is functionally related to h2-calponin (15) was detected (Figure 7A and B). The results confirmed that degradation of h2-calponin in alveolar cells is induced by the decrease in cellular tension even outside of the organ environment.
Figure 7.
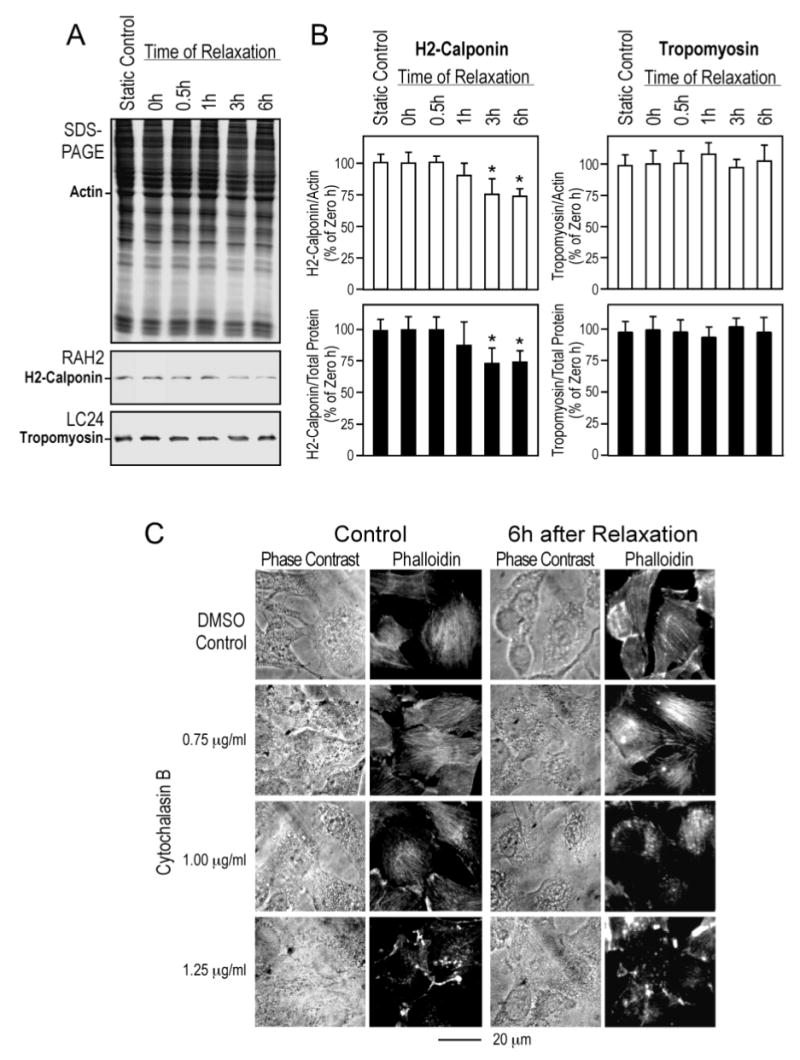
Tension release in cultured alveolar cells resulted in h2-calponin degradation. RLE-6TN rat alveolar cells were cultured on rubber membrane that was bi-axially stretched and held at 108% of its original surface size for three days before the stretching was released to relax the attached cells. SDS-PAGE and Western blot analysis using RAH2 antibody (A) and densitometry quantification against the levels of actin or total cellular protein (B) detected decreases in h2-calponin after the reduction of cellular tension. In contrast, the levels of tropomyosin did change as shown by the Western blot quantification using mAb LC24 (54). The data are summarized as SEM from six sets of samples, *P<0.05. (C) The phase contrast micrographs and rhodamine-phalloidin fluorescence stains of the actin cytoskeleton demonstrate a decreased resistance of actin stress fibers to cytochalasin B treatment in alveolar cells after the reduction of tension. This is shown by the collapses of actin stress fibers at lower concentrations of cytochalasin B than that needed for the high tension control, indicating a lower stability of the actin filaments corresponding to the decreased level of h2-calponin.
We also examined the correlation between h2-calponin degradation and the stability of actin filaments in ex vivo alveolar cells. RLE-6TN rat alveolar cells cultured on constantly stretched rubber membrane were examined 6 h after release tension (Figure 7C) for resistance of actin stress fibers to cytochalasin B. The results in Figure 7C demonstrate that the tension release-induced degradation of h2-calponin (Figure 6) correlated with collapses of actin cytoskeleton structure at lower concentration of cytochalasin B in comparison to the controls, consistent with a decreased stability of the actin filaments.
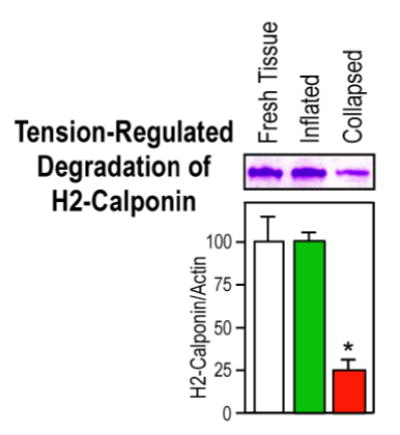
Distension prevents h2-calponin degradation in the lung
We carried out a tension-protection experiment to investigate the role of mechanical tension in regulating h2-calponin degradation in lung alveolar cells and the results are shown in Figure 8. Postmortem adult mouse and rat lungs were maintained at either collapsed or inflated state at 37 °C for 6 h. The SDS-PAGE and Western blot analysis demonstrated a highly effective prevention of h2-calponin proteolysis in the continuously inflated lungs versus the collapsed control (P<0.001). Although the inflation of postmortem lung by air could introduce a higher tissue oxygenation than that in the deflated lung (Figure 6D), the effect of hypoxia on h2-calponin degradation can be ruled out in the cell culture unloading experiments that were done under identical oxygenation conditions (Figure 7). Together with the respiration-related postnatal up-regulation of h2-calponin in the lung, the results suggest that mechanical distension is critical to high level expression as well as posttranslational stability of h2-calponin in alveolar cells.
Figure 8.
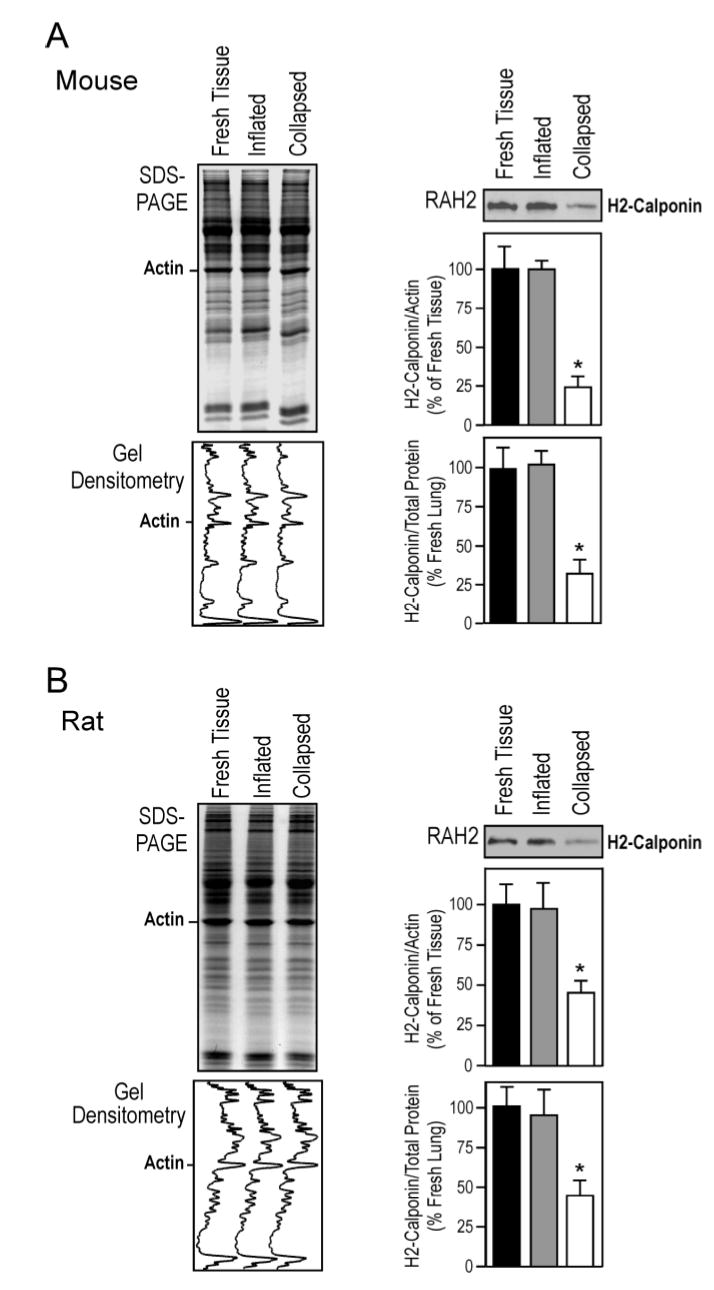
Inflation-distension of the lung prevents the degradation h2-calponin. Mouse (A) and rat (B) lungs were inflated with air and incubated at 37°C for 6 hours together with collapsed control lungs. Total protein extracts were examined by Western blot with the RAH2 antibody. The densitometry quantification of Western blots and Coomassie blue stained SDS-gel showed a effective prevention of h2-calponin proteolysis in the inflated lung (normalized by the levels of actin or total protein, *P<0.001). The results are summarized from 4 individual experiments.
Myosin motor-based cytoskeleton tension regulates both expression and degradation of h2-calponin in alveolar cells
To investigate the role of cytoskeleton tension in the regulation of h2-calponin in alveolar cells, we used blebbistatin to inhibit non-muscle myosin II ATPase activity and, therefore, reduce mechanical tension built in the actin cytoskeleton (33). When monolayer cultures of RLE-6TN rat alveolar cells were treated with blebbistatin, the morphology of the cells started to change within 1 h to show a “slack” appearance (Figure 9A), consistent with the specific effect of blebbistatin on reducing cytoskeletal tension. The slack state was maintained for three days and the SDS-PAGE and Western blots in Figure 9B detected a decrease of h2-calponin in alveolar cells after 6h of blebbistatin treatment. This rapid reduction of h2-calponin protein upon blebbistatin inhibition of non-muscle myosin II motor function suggests that the loss of tension in the actin cytoskeleton induces h2-calponin degradation, in agreement with the mechanical tension release experiments (Figure 7). Figure 9B also shows that the level of h2-calponin, normalized against actin, total cellular protein, or histones, decreased further after 3 days of blebbistatin treatment. Consistent with the reduced expression of h2-calponin in alveolar cells cultured on soft gel substrates (Figure 4, C and D), the result suggests that myosin II motor-based cytoskeleton tension regulates also the expression of h2-calponin gene. Together with blebbistatin’s effect on down-regulating h2-calponin protein in NIH 3T3 fibroblasts (Figure 10C and ref. 15), the decrease in h2-calponin gene expression by lowering cytoskeleton tension was confirmed by Northern blot showing a decreased amount of the h2-calponin mRNA (Figure 10B). The results support a hypothesis that tension in the actin filaments regulates both expression and degradation of h2-calponin.
Figure 9.

Regulation of h2-calponin in alveolar cells by myosin motor-based cytoskeleton tension. (A) Monolayer cultures of RLE-6TN rat alveolar cells were treated with 100 μM blebbistatin or DMSO solvent control for 6 hours or 3 days. Morphology changes of the cells can be seen in the lower magnification phase contrast images taken from the culture dish. The cells on cover slips were fixed with cold acetone and the actin stress fibers were visualized by staining with rhodamine-conjugated phalloidin under higher magnification. The phase contrast and fluorescence images showed that the blebbistatin-treated cells became slack, indicating a reduced cytoskeleton tension. (B) Parallel cultures were analyzed by SDS-PAGE and RAH2 Western blot. The densitometry analysis of the h2-calponin blots against the level of actin, total cellular protein or histones showed significant decreases of h2-calponin after short or long time blebbistatin treatments, suggesting that myosin II-based cytoskeleton tension affects both proteolytic and transcriptional regulations of h2-calponin in alveolar cells. The data are summarized from two sets of the experiment. *P<0.05; **P<0.001.
Figure 10.

Inhibition of myosin motor decreases h2-calponin gene expression in NIH 3T3 cells. NIH 3T3 mouse fibroblasts cultured on plastic substrate were treated with 100 μM blebbistatin for 3 days. The control cells were treated with 0.2% DMSO solvent control. (A) Phase contrast images show the slack morphology of the blebbistatin-treated cells. (B) Northern blotting was carried out with 32P-labeled h2-calponin specific cDNA probe on total RNA extracted from the cells. Normalized by the ribosomal RNA levels, the autoradiograph shows decreased expression of h2-calponin mRNA in the blebbistatin-treated cells. (C) The corresponding decrease in h2-calponin protein is demonstrated by Western blot using the anti-h2-calponin antibody RAH2.
Effect of cyclic stretching on h2-calponin expression in lung alveolar cells
Normal lung tissue continuously undergoes cyclic inflation and deflation during respiration. To investigate the effect of cyclic tension changes on the regulation of h2-calponin in the lung, RLE-6TN rat alveolar cells were cultured on rubber membrane with continuously cyclic stretch for 3 days to mimic the mechanical stimuli in the lung in situ and examined for the effect on h2-calponin expression. Figure 11A demonstrates that the cells cultured on plastic dish and static silicon rubber membrane had similar protein contents and levels of h2-calponin expression. This result is in agreement with the notion that the two types of materials are both non-deformable substrates for the adhesion alveolar cells. Therefore, the cells cultured on these substrates would develop high tension in the cytoskeleton (31). Densitometry quantification of the h2-calponin Western blots against the levels of actin, total cellular protein, or histones (Figure 11B) shows that cyclic stretching did not increase but significantly decreased h2-calponin expression by nearly 40%.
Figure 11.
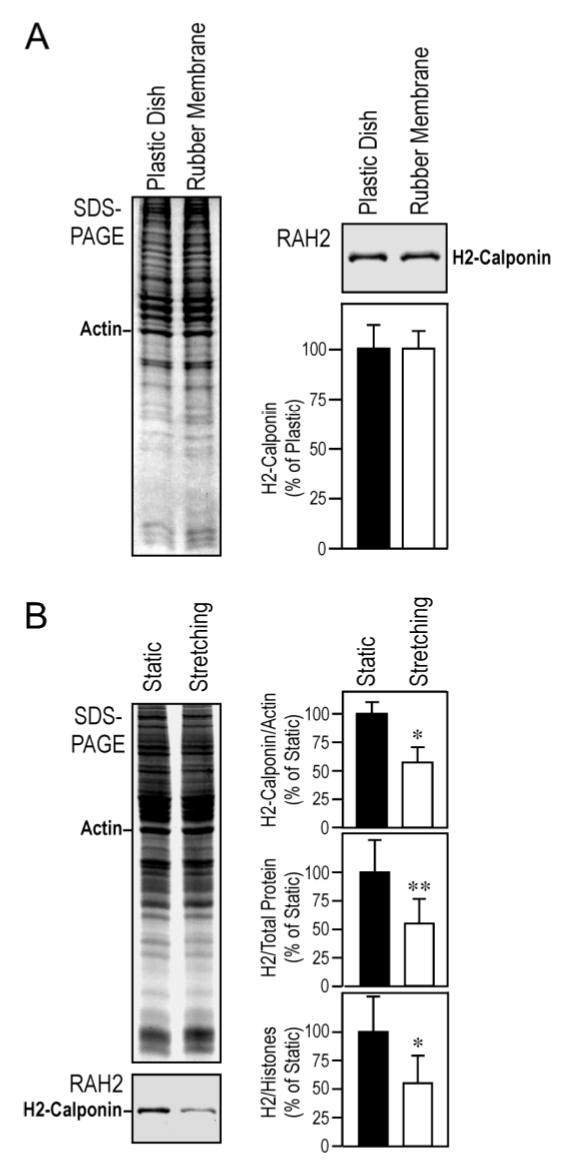
Cyclic stretching decreases h2-calponin expression in alveolar cells. (A) SW1573 human alveolar cells were cultured on plastic dish or rubber membranes without stretching for 3 days. Total protein extracts were examined by SDS-PAGE and RAH2 antibody Western blot. The SDS-gel and Western blots densitometry quantifications against the level of actin show that alveolar cells statically cultured on plastic dish and rubber membrane had similar protein contents and high h2-calponin expression, indicating that they both provide cultural substrates non-deformable by the force generated in alveolar cells. (B) RLE-6TN rat alveolar cells cultured on rubber membranes were treated with cyclic stretching for 3 days. Total protein extracts were examined as above and SDS-gel and Western blots densitometry quantifications against the level of actin, total cellular protein, or histones showed that cyclic stretch significantly decreased the level of h2-calponin expression in the cells (*P<0.005, **P<0.001). The results are summarized from 6 individual experiments.
This intriguing finding is actually consistent with the observation that h2-calponin expression is regulated by the tension generated in the cytoskeleton against the stiffness of cultural substrate (15). The cells cultured on the rubber membrane of the cyclic stretch plate have built up near maximum tension in the cytoskeleton close to that in cells cultured on a rigid (plastic) substrate since the silicon rubber membrane, same as the plastic dish, is non-deformable by the cellular contractile force. The stretched phases of the cyclic stretching culture would induce the adhesion cells to grow in corresponding dimensions to avoid breakage. After stabilized at the increased cell dimension, the cellular structure would be slack during the relaxed phase of each cycle. As a result, the cells cultured under cyclic stretching would sense a lower amount of tension in the cytoskeleton over time in comparison to that in cells steady cultured on non-deformable substrate, resulting in a decreased h2-calponin expression.
DISCUSION
The present study found that h2-calponin is present at high levels in lung alveolar cells under postnatal up-regulation during lung development and the beginning of respiratory movement. Together with tension-regulated gene expression and function in stabilizing actin cytoskeleton, we observed a novel tension-regulated degradation of h2-calponin. Cyclic stretching experiments demonstrated that the amount of tension over time, other than tension dynamics, determines the expression of h2-calponin. The new findings and their potential physiological and pathological significance are discussed below.
H2-calponin is found in tissues under mechanical tension
H2-calponin was first identified in smooth muscle by cDNA cloning (11) and then reported in several non-muscle tissue and cell types including endothelial cells (29), epidermal keratinocytes (34), and fibroblast (15). By specific anti-h2-calponin mAbs and high resolution SDS-PAGE that can distinguish mouse h1- and h2-calponins (20), our examination of representative mouse tissues confirmed the h2-calponin expression in smooth muscle organs and skin (Figure 1). It is also detected in many non-muscle organs examined. The low level h2-calponin in the solid organs (e.g., brain, testis, ovary, kidney and liver) may reflect its expression in vascular endothelial cells. This notion is supported by the observation that spleen contains a high level of h2-calponin (Figure 1) even after washing off all blood cell contents (data not shown). The high level of h2-calponin expressed in lung alveolar epithelial cells is a novel finding. We previously demonstrated that h2-calponin expression is dependent on mechanical tension (15). An interesting common feature of lung alveolar cells, smooth muscle cells, vascular endothelial cells and epidermal keratinocytes is that they are all physiologically under significant levels of mechanical tension. Therefore, the tissue distribution of h2-calponin supports its role in regulating the function of actin cytoskeleton in responses to mechanical tension.
For the critical role of actin cytoskeleton in cell structure and function, the high level of h2-calponin in lung alveolar cells may have physiological and pathological implications. The biochemical activity of h1-calponin in inhibiting smooth muscle actomyosin ATPase has been extensively investigated (9, 12). Structural conservation suggests that h1- and h2-calponins may function via similar mechanisms in the regulation of actin-myosin-based cell motility. The tension-regulation and function of h2-calponinin in lung alveolar cells provide a new informative experimental system to investigate the physiological function of calponin.
Mechanical tension regulates h2-calponin by controlling both gene expression and protein degradation
Cells respond to mechanical forces exerted through the extracellular environment (35–38). Our previous study demonstrated that increases in substrate stiffness-generated mechanical tension in the cytoskeleton increase the expression of h2-calponin in keratinocytes and fibroblasts (15). The present study of lung alveolar cells further demonstrated that while mechanical distension in postnatal lung may correlate to the up-regulation h2-calponin expression, prolonged decreases in cytoskeletal tension in collapsed lungs cause a rapid degradation of h2-calponin. The degradation of h2-calponin can be reproduced in alveolar cells ex vivo when cellular tension was lowered by reducing the dimension of the cultural substratum. Inflating the postmortem lung effectively prevented the degradation of h2-calponin. The gene expression and proteolytic degradation of h2-calponin are both responsive to the tension built in the actin cytoskeleton by myosin motor function as shown by the acute and chronic effects of blebbistatin treatment (Figures 9 and 10). Therefore, selective h2-calponin proteolysis suggests a novel tension-responsive posttranslational mechanism to rapidly regulate the cytoskeleton function.
Reduced tension may result in dissociation of h2-calponin from the actin filament that promotes degradation. We previously showed that phosphoryltation of calponin decreases the binding affinity for F-actin (39). On the other hand, the observation that phosphorylated calponin was hardly detectable in living cells (40) may implicate that phosphorylation-induced calponin dissociation from the actin filaments is coupled with rapid degradation. The biochemical roles of intracellular proteases are diverse, ranging from the removal of damaged proteins to protein maturation and precise processing of regulatory proteins (16). It has been reported that h1-calponin (41, 42) and acidic calponin (43) are cleaved by mu-calpain, indicating a direction for investigating the proteolytic mechanism for h2-calponin regulation in alveolar cells.
Comparing with the chronic effect of gene regulation, the proteolytic regulation provides a rapid short-term control for h2-calponin’s function. The tension-dependent degradation of h2-calponin may affect the stability of lung alveolar cytoskeleton with physiological and pathological significances. For example, the prolonged collapse of lung tissue during pneumothorax, open chest surgery, and lung transplantation may result in h2-calponin degradation and decreased stability of alveolar cytoskeleton, which may contribute to alveolar dysfunction after resuming respiratory activity (44).
Regulation of h2-calponin expression by the amount of cytoskeleton tension over time
It has been demonstrated that the expression of h2-calponin is positively related to the static tension built in the cytoskeleton (15; Figure 4, C and D). The observation that fluid shearing force did not stimulate the expression of h2-calponin in the vibrating cell cultures (Figure 4, A and B) indicates that dynamic force stimuli are not the primary regulating factor for the expression of h2-calponin. In agreement with this notion, cyclic stretching did not increase h2-calponin expression in alveolar cells despite the dynamic tension stimuli (Figure 11).
On the other hand, the decreased expression of h2-calponin in cyclic stretched cells leads to a hypothesis that chronic cyclic stretching lowered the total amount of tension in the cytoskeleton over time than that in cells statically cultured on rigid substrates. It has been established that cells sense substrate stiffness and respond by adhesion force in which substrates non-deformable by cellular forces including the membranes of the cyclic stretching plates produce maximum static tension in the cytoskeleton (45–48). After the cells adapted to the stretched dimension of the substrate by increase in size, the cytoskeleton tension during the relaxing phase of each cycle would be lower than maximum. Therefore, the outcome of cyclic stretching is that the total amount of cytoskeleton tension over time will be lower than that in cells cultured statically on non-deformable substrates. The observation that cells build up the maximum isometric tension (prestress, 31) when attached to a rigid substrate suggests a reference point for investigating the tension regulation of cytoskeletal protein gene expression and the mechanisms for cells to sense static tension.
Tension-regulated function of h2-calponin may contribute to the mechanical performance of lung alveoli
After the commencement of respiratory activity, alveolar cells in the lung are continuously under cyclic tension stresses throughout life. Both tonic distension and dynamic volume changes affect lung development, growth, compliance, differentiation and metabolism (17). Mechanical strain was found as a major signal for regenerative lung growth (49). The positive correlations between tension, h2-calponin level, and actin filament stability suggest that by stabilizing actin cytoskeleton (15; Figure 5 and Figure 7), h2-calponin may play a role in strengthening the alveolar structure to prevent distension injuries. In vitro cyclic stretches are known to induce reorganization of actin cytoskeleton (50, 51). The effect of cyclic stretching on h2-calponin expression discussed above suggests that not only the magnitude but also the duration of distension determines the function of h2-calponin in lung alveolar cells.
Lung alveolar cells undergo distension during periodic deep inspirations, which is considered to be a critical factor in normal lung development and function (17, 52, 53). Since the alveoli in normal lung are only under maximum distension at the peak of deep inspirations, the cells are under intermediate cytoskeletal tensions most of the time, which may be critical to maintaining a proper level of h2-calponin, a normal stability of actin cytoskeleton, and the balance between mechanical strength and compliance of the alveoli. Further investigations on the regulation and function of h2-calponin in alveolar cells may help understanding distension and deflation related lung injuries.
Acknowledgments
We thank Dr. Jim J.-C. Lin, University of Iowa, for the LC24 anti-tropomyosin mAb and helpful discussions.
Footnotes
This study was supported by grants from the National Institutes of Health (AR048816 and HL078773).
Abbreviations: DMSO, dimethyl sulfoxide; mAb, monoclonal antibody; PAGE, polyacrylamide gel electrophoresis; PBS, phosphate buffered saline.
References
- 1.Chicurel ME, Chen CS, Ingber DE. Cellular control lies in the balance of forces. Curr Opin Cell Biol. 1998;10:232–239. doi: 10.1016/s0955-0674(98)80145-2. [DOI] [PubMed] [Google Scholar]
- 2.Eckes B, Krieg T. Regulation of connective tissue homeostasis in the skin by mechanical forces. Clin Exp Rheumatol. 2004;22:S73–S76. [PubMed] [Google Scholar]
- 3.Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 4.Lehoux S, Tedgui A. Cellular mechanics and gene expression in blood vessels. J Biomech. 2003;36:631–643. doi: 10.1016/s0021-9290(02)00441-4. [DOI] [PubMed] [Google Scholar]
- 5.Walker JL, Fournier AK, Assoian RK. Regulation of growth factor signaling and cell cycle progression by cell adhesion and adhesion-dependent changes in cellular tension. Cytokine Growth Factor Rev. 2005;16:395–405. doi: 10.1016/j.cytogfr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91:877–887. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- 7.Olson MF. Contraction reaction: mechanical regulation of Rho GTPase. Trends Cell Biol. 2004;14:111–114. doi: 10.1016/j.tcb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Hiwada K, Kokubu T. Isolation and characterization of a 34,000-dalton calmodulin- and F-actin-binding protein from chicken gizzard smooth muscle. Biochem Biophys Res Commun. 1986;141:20–26. doi: 10.1016/s0006-291x(86)80328-x. [DOI] [PubMed] [Google Scholar]
- 9.Morgan KG, Gangopadhyay SS. Invited review: cross-bridge regulation by thin filament-associated proteins. J Appl Physiol. 2001;91:953–962. doi: 10.1152/jappl.2001.91.2.953. [DOI] [PubMed] [Google Scholar]
- 10.Szymanski PT. Calponin (CaP) as a latch-bridge protein--a new concept in regulation of contractility in smooth muscles. J Muscle Res Cell Motil. 2004;25:7–19. doi: 10.1023/b:jure.0000021349.47697.bf. [DOI] [PubMed] [Google Scholar]
- 11.Strasser P, Gimona M, Moessler H, Herzog M, Small JV. Mammalian calponin. Identification and expression of genetic variants. FEBS Lett. 1993;330:13–18. doi: 10.1016/0014-5793(93)80909-e. [DOI] [PubMed] [Google Scholar]
- 12.Winder SJ, Walsh MP. Calponin. Curr Top Cell Regul. 1996;34:33–61. doi: 10.1016/s0070-2137(96)80002-1. [DOI] [PubMed] [Google Scholar]
- 13.Hossain MM, Hwang DY, Huang QQ, Sasaki Y, Jin JP. Developmentally regulated expression of calponin isoforms and the effect of h2-calponin on cell proliferation. Am J Physiol Cell Physiol. 2003;284:C156–C167. doi: 10.1152/ajpcell.00233.2002. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Z, Grange RW, Walsh MP, Kamm KE. Adenovirus-mediated transfer of the smooth muscle cell calponin gene inhibits proliferation of smooth muscle cells and fibroblasts. FEBS Lett. 1997;413:441–445. doi: 10.1016/s0014-5793(97)00944-7. [DOI] [PubMed] [Google Scholar]
- 15.Hossain MM, Crish JF, Eckert RL, Lin JC, Jin JP. H2-calponin is regulated by mechanical tension and modifies the function of actin cytoskeleton. J Biol Chem. 2005;280:42442–42453. doi: 10.1074/jbc.M509952200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrmann M, Clausen T. Proteolysis as a regulatory mechanism. Annu Rev Genet. 2004;38:709–724. doi: 10.1146/annurev.genet.38.072902.093416. [DOI] [PubMed] [Google Scholar]
- 17.Dobbs LG, Gutierrez JA. Mechanical forces modulate alveolar epithelial phenotypic expression. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:261–266. doi: 10.1016/s1095-6433(01)00322-1. [DOI] [PubMed] [Google Scholar]
- 18.Nigam R, Triggle CR, Jin JP. H1- and h2-calponins are not essential for norepinephrine- or sodium fluoride-induced contraction of rat aortic smooth muscle. J Muscle Res Cell Motil. 1998;19:695–703. doi: 10.1023/a:1005389300151. [DOI] [PubMed] [Google Scholar]
- 19.Jin JP, Walsh MP, Resek ME, McMartin GA. Expression and epitopic conservation of calponin in different smooth muscles and during development. Biochem Cell Biol. 1996;74:187–196. doi: 10.1139/o96-019. [DOI] [PubMed] [Google Scholar]
- 20.Jin JP, Wu D, Gao J, Nigam R, Kwong S. Expression and purification of the h1 and h2 isoforms of calponin. Protein Expr Purif. 2003;31:231–239. doi: 10.1016/s1046-5928(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 21.Wright WC, Daniels WP, Fogh J. Distinction of seventy-one cultured human tumor cell lines by polymorphic enzyme analysis. J Natl Cancer Inst. 1981;66:239–247. [PubMed] [Google Scholar]
- 22.Driscoll KE, Carter JM, Iype PT, Kumari HL, Crosby LL, Aardema MJ, Isfort RJ, Cody D, Chestnut MH, Burns JL, Leboeuf RA. Establishment of immortalized alveolar type II epithelial cell lines from adult rats. In Vitro Cell Dev Biol Anim. 1995;31:516–527. doi: 10.1007/BF02634029. [DOI] [PubMed] [Google Scholar]
- 23.Furukawa KS, Ushida T, Sakai Y, Suzuki M, Tanaka J, Tateishi T. Formation of human fibroblast aggregates (spheroids) by rotational culture. Cell Transplant. 2001;10:441–445. [PubMed] [Google Scholar]
- 24.Pelham RJ, Wang YL. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelham RJ, Wang YL. Cell locomotion and focal adhesions are regulated by the mechanical properties of the substrate. Biol Bull. 1998;194:348–350. doi: 10.2307/1543109. [DOI] [PubMed] [Google Scholar]
- 26.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 27.Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 28.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 29.Sakihara C, Nishimura J, Kobayashi S, Takahashi S, Kanaide H. Expression of calponin mRNA in porcine aortic endothelial cells. Biochem Biophys Res Commun. 1996;222:195–200. doi: 10.1006/bbrc.1996.0721. [DOI] [PubMed] [Google Scholar]
- 30.Tang J, Hu G, Hanai JI, Yadlapalli G, Lin Y, Zhang B, Galloway J, Bahary N, Sinha S, Thisse B, Thisse C, Jin JP, Zon LI, Sukhatme VP. A critical role for calponin 2 in vascular development. J Biol Chem. 2006;281:6664–6672. doi: 10.1074/jbc.M506991200. [DOI] [PubMed] [Google Scholar]
- 31.Polte TR, Eichler GS, Wang N, Ingber DE. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. Am J Physiol Cell Physiol. 2004;286:C518–528. doi: 10.1152/ajpcell.00280.2003. [DOI] [PubMed] [Google Scholar]
- 32.Martin AF. Turnover of cardiac troponin subunits. Kinetic evidence for a precursor pool of troponin-I. J Biol Chem. 1981;256:964–968. [PubMed] [Google Scholar]
- 33.Griffin MA, Sen S, Sweeney HL, Discher DE. Adhesion-contractile balance in myocyte differentiation. J Cell Sci. 2004;117:5855–5863. doi: 10.1242/jcs.01496. [DOI] [PubMed] [Google Scholar]
- 34.Fukui Y, Masuda H, Takagi M, Takahashi K, Kiyokane K. The presence of h2-calponin in human keratinocyte. J Dermatol Sci. 1997;14:29–36. doi: 10.1016/s0923-1811(96)00545-2. [DOI] [PubMed] [Google Scholar]
- 35.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 36.Discher DE, Jammey P, Wang Y. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 37.Ingber DE. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci. 1993;104:613–627. doi: 10.1242/jcs.104.3.613. [DOI] [PubMed] [Google Scholar]
- 38.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 39.Jin JP, Walsh MP, Sutherland C, Chen W. A role for serine-175 in modulating the molecular conformation of calponin. Biochem J. 2000;350:579–588. [PMC free article] [PubMed] [Google Scholar]
- 40.Gimona M, Sparrow MP, Strasser P, Herzog M, Small JV. Calponin and SM 22 isoforms in avian and mammalian smooth muscle. Absence of phosphorylation in vivo. Eur J Biochem. 1992;205:1067–1075. doi: 10.1111/j.1432-1033.1992.tb16875.x. [DOI] [PubMed] [Google Scholar]
- 41.Croall DE, Chacko S, Wang Z. Biochim Biophys Acta. Vol. 1298. 1996. Cleavage of caldesmon and calponin by calpain: substrate recognition is not dependent on calmodulin binding domains; pp. 276–284. [DOI] [PubMed] [Google Scholar]
- 42.Tsunekawa S, Takahashi K, Abe M, Hiwada K, Ozawa K, Murachi T. Calpain proteolysis of free and bound forms of calponin, a troponin T-like protein in smooth muscle. FEBS Lett. 1989;250:493–496. doi: 10.1016/0014-5793(89)80783-5. [DOI] [PubMed] [Google Scholar]
- 43.Yoshimoto R, Hori M, Ozaki H, Karaki H. Proteolysis of acidic calponin by mu-calpain. J Biochem (Tokyo) 2000;128:1045–1049. doi: 10.1093/oxfordjournals.jbchem.a022832. [DOI] [PubMed] [Google Scholar]
- 44.Kelly RF. Current strategies in lung preservation. J Lab Clin Med. 2000;136:427–440. doi: 10.1067/mlc.2000.110906. [DOI] [PubMed] [Google Scholar]
- 45.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 46.Deroanne CF, Lapiere CM, Nusgens BV. In vitro tubulogenesis of endothelial cells by relaxation of the coupling extracellular matrix-cytoskeleton. Cardiovasc Res. 2001;49:647–658. doi: 10.1016/s0008-6363(00)00233-9. [DOI] [PubMed] [Google Scholar]
- 47.Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86:617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang HB, Dembo M, Wang YL. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol. 2000;279:C1345–C1350. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- 49.Hsia CC, Wu EY, Wagner E, Weibel ER. Preventing mediastinal shift after pneumonectomy impairs regenerative alveolar tissue growth. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1279–L1287. doi: 10.1152/ajplung.2001.281.5.L1279. [DOI] [PubMed] [Google Scholar]
- 50.Endlich N, Kress KR, Reiser J, Uttenweiler D, Kriz W, Mundel P, Endlich K. Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol. 2001;12:413–422. doi: 10.1681/ASN.V123413. [DOI] [PubMed] [Google Scholar]
- 51.Smith PG, Deng L, Fredberg JJ, Maksym GN. Mechanical strain increases cell stiffness through cytoskeletal filament reorganization. Am J Physiol Lung Cell Mol Physiol. 2003;285:L456–L463. doi: 10.1152/ajplung.00329.2002. [DOI] [PubMed] [Google Scholar]
- 52.Wirtz HR, Dobbs LG. The effects of mechanical forces on lung functions. Respir Physiol. 2000;119:1–17. doi: 10.1016/s0034-5687(99)00092-4. [DOI] [PubMed] [Google Scholar]
- 53.Zhang S, Garbutt V, McBride JT. Strain-induced growth of the immature lung. J Appl Physiol. 1996;81:1471–1476. doi: 10.1152/jappl.1996.81.4.1471. [DOI] [PubMed] [Google Scholar]
- 54.Warren KS, Lin JL, McDermontt JP, Lin JJ. Forced expression of chimeric human fibroblast tropomyosin mutants affects cytokinesis. J Cell Biol. 1995;129:697–708. doi: 10.1083/jcb.129.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]


