Abstract
Arsenic exposure is associated with hypertension, diabetes and cancer. Some mammals methylate arsenic. Saccharomyces cerevisiae hexose permeases catalyze As(OH)3 uptake. Here we report that mammalian glucose transporter GLUT1 catalyzes As(OH)3 and CH3As(OH)2 uptake in yeast or in Xenopus laevis öocytes. Expression of GLUT1 in a yeast lacking other glucose transporters allows for growth on glucose. Yeast expressing yeast HXT1 or rat GLUT1 transport As(OH)3 and CH3As(OH)2. The Km of GLUT1 is to 1.2 mM for CH3As(OH)2, compared to a Km of 3 mM for glucose. Inhibition between glucose and CH3As(OH)2 is noncompetitive, suggesting differences between the translocation pathways of hexoses and arsenicals. Both human and rat GLUT1 catalyze uptake of both As(OH)3 and CH3As(OH)2 in öocytes. Thus GLUT1 may be a major pathway uptake of both inorganic and methylated arsenicals in erythrocytes or the epithelial cells of the blood-brain barrier, contributing to arsenic-related cardiovascular problems and neurotoxicity.
Arsenic ranks first on the United States Government's Comprehensive Environmental Response, Compensation, and Liability (Superfund) Act Priority List of Hazardous Substances <http://www.atsdr.cdc.gov/cercla/05list.html>. In response to the Safe Water Drinking Act, the United States Environmental Protection Agency has set the allowable level of arsenic in drinking water at 10 ppb <http://www.epa.gov/safewater/arsenic/regulations.html>, which is less than the level in the water supplies of many U.S. municipalities <http://water.usgs.gov/nawqa/trace/arsenic/>. In addition, the Environmental Protection Agency's Office of Pesticide Programs is concerned with exposure to the organic arsenicals methylarsenic acid and dimethylarsenic acid, pentavalent arsenicals used as pesticides and herbicides <http://www.epa.gov/sab/pdf/arsenic_review_panel_final_charge_7-25-05.pdf>.
Health effects associated with arsenic exposure include cardiovascular and peripheral vascular disease, neurological disorders, diabetes mellitus and various cancers, including liver, bladder, kidney and skin [1-3]. Arsenic trioxide, which is As2O3 in the solid, anhydrous form and As(OH)3 in solution at physiological pH [4], is used clinically as a chemotherapeutic drug for treatment of acute promyelocytic leukemia [5]. Although epidemiological studies demonstrated that arsenic exposure is associated with a high frequency of circulatory and neurological problems [2, 6, 7], how arsenic causes non-cancer-related diseases is far from clear.
Humans and some other mammals metabolize inorganic arsenic to a variety of methylated species that may be more toxic than their inorganic counterparts [8], These inorganic and organic species are found in many tissues and are excreted in urine and feces [9, 10]. In general, trivalent arsenicals are more toxic than penvalent, and methylated trivalent arsenicals are more cytotoxic than inorganic As(OH)3 [11].
We have previously shown that some aquaglyceroporins (AQPs) facilitate As(OH)3 movement, including mammalian AQP7 and AQP9 [12, 13]. AQP9 also conducts methylarsonous acid or CH3As(OH)2 [14], an intermediate in the pathway of arsenic methylation. AQP7 is expressed primarily in kidney, testis and adipose tissue [15, 16], and AQP9 is expressed primarily in liver and astrocytes [17]. How are As(OH)3 and/or CH3As(OH)2 transported into cells that do not have AQP7 or AQP9 such as erythrocytes and the epithelial cells that form the blood-brain barrier? S. cerevisiae hexose transporters, members of the major facilitator superfamily, catalyze uptake of As(OH)3 [18]. The mammalian homologue GLUT1, which is found in erythrocytes and the epithelial cells that form the blood-brain barrier, mediates the majority uptake of glucose into brain through the blood-brain barrier [19]. GLUT1-deficiency syndrome (GLUT1DS), which results in an inadequate energy supply to brain, manifests as delayed neurological development and other neurological problems [20, 21].
Here we report that GLUT1 facilitates uptake of As(OH)3 and CH3As(OH)2 using heterologous expression in S. cerevisiae and X. laevis öocytes. S. cerevisiae is a valuable model system for structure-function studies of mammalian membrane proteins such as GLUT1 [22, 23]. A yeast strain with low uptake of As(OH)3 and CH3As(OH)2 was constructed by deletion of all 18 hexose transporters, FPS1, which encodes an aquaglyceroporin that facilitates As(OH)3 influx, and ACR3, the gene for an As(OH)3 efflux carrier protein. This strain is unable to grow on glucose as a carbon source. Expression of the gene for a rat GLUT1 variant [24] allowed for growth on glucose. In this strain GLUT1 mediated low rates of As(OH)3 uptake and higher rates of CH3As(OH)2 uptake. Uptake of glucose was inhibited noncompetitively by CH3As(OH)2, and inhibition of CH3As(OH)2 uptake by glucose was noncompetitive. In öocytes expressing either human or rat GLUT1, As(OH)3 uptake was lower than CH3As(OH)2, consistent with GLUT1 catalyzing faster uptake of CH3As(OH)2 than As(OH)3. Since GLUT1 is more widely distributed than AQP7 and AQP9, transport of inorganic and organic arsenicals by GLUT1 may represent the major pathway for entry of arsenic into cells and organs such as erythrocytes and brain.
Experimental Procedures
Strains and plasmids
E. coli strain DH-5α (recA1 supE44 endA1 hsdR17 gyrA96 relA1 thi (lac-proAB) F′ (traD36 proAB+ lacIq lacZ.M15) was used for molecular cloning. S. cerevisiae strain EBY.VW1000, in which all 18 hexose permeases were deleted [25] was used to construct strain HD300 (fps1::leu acr3:his) by a step-wise replacement of 0.8 kbp of FPS1 with a LEU2 gene and 1.15 kbp of ACR3 with a HIS3 gene. Gene disruptions were verified by both polymerase chain reaction and phenotypic analysis of arsenite resistance. HD300 was transformed with pTHHXT series plasmids carrying HXT genes [18] or plasmid YEpH2-rGLUT1, which carries a V69M variant of GLUT1 under control of the yeast HXT2promoter [24]. The V69M mutation has no affect on glucose transport but allows for growth of yeast on glucose as a carbon source. Plasmid pL2-5-GLUT1 was constructed by amplification of the rat V69M GLUT1 gene using a pair of primers 5′-GAGATCTATGGAGCCCAGCAGCAAGAAG-3′ (BglII site underlined) and 5′-GAGATCTTCACACTTGGGAGTCAGCCC-3′ (BglII site underlined). The GLUT1 gene was cloned into pGEMT-easy (Promega, Madison, WI), and digested with BglII. The 1.5 kbp BglII fragment was cloned into BglII-digested pL2-5 [26], resulting in pL2-5-GLUT1. The sequence of the GLUT1 gene was verified by DNA sequencing. Human GLUT1 was cloned in a same strategy by using a pair of primers, 5′ GAGATCTATGGAGCCCAGCAGCAAGAAG (BglII site underlined) and 3′ GAGATCTTCACACTTGGGAATCAGC (BglII site underlined), generating pL2-5-hGLUT1.
Media
S. cerevisiae was grown at 30° C in minimal SD [27] medium with either 2% glucose or 2% maltose as carbon source supplemented with auxotrophic requirements. E. coli was grown in Luria-Bertani medium [28] supplemented when appropriate with 125 μg/ml ampicillin.
DNA manipulations
Molecular methods were carried out as described [28]. Transformation of yeast cells was carried out using a Geno easy-transform kit (Geno Technologies, St. Louis, MO). Yeast genomic DNA was isolated using QIAamp spin column (Qiagen Inc.).
Transport assays
Transport of As(OH)3 or CH3As(OH)2 in both yeast and öocytes was assayed as described previously [14]. Glucose uptake in yeast cells was assayed using D-[U-14C]glucose (PelkinElmer, Wellesley, MA), as described [29]. Uptake of [3H]2-deoxyglucose (DOG) (2 μCi/ml) (PelkinElmer, Wellesley, MA) in öocytes was measured as described [30].
Results
GLUT1 catalyzes uptake of both As(OH)3 and CH3As(OH)2
GLUT1 activity by rat GLUT1 was assayed in S. cerevisiae strain HD300. A rat V69M rGLUT1 variant under control of the yeast HXT2 promoter allows for better growth than wild type rGLUT1 with glucose as a carbon source, and yeast cells expressing this rGLUT1 take up glucose [24], so subsequent assays were conducted with V69M. Strain HD300 was constructed from strain EBY.VW1000, in which the genes for eighteen hexose transporters were deleted [24], by subsequent deletion of FPS1 [14] and ACR3 [31, 32].
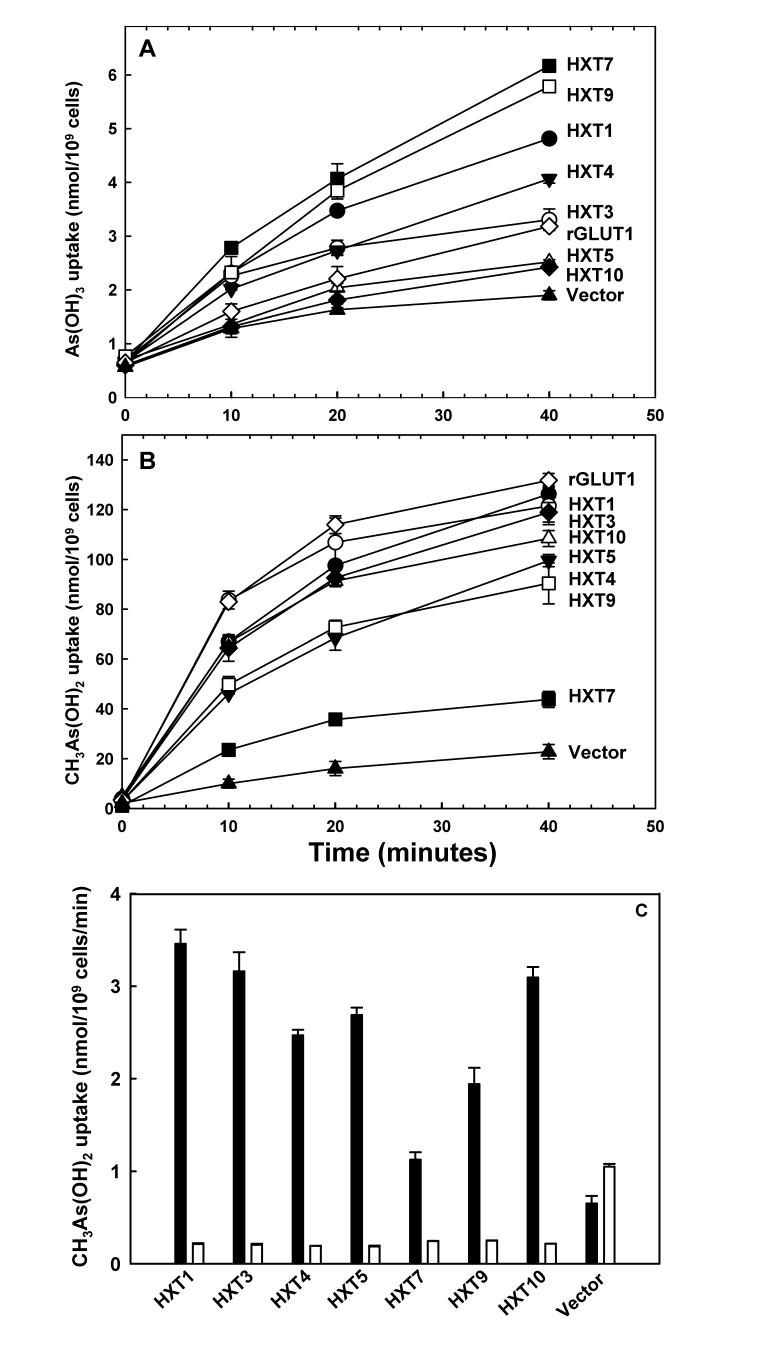
The ability of rGLUT1 to transport As(OH)3 and CH3As(OH)2 was compared with yeast permeases Hxt1p, Hxt3p, Hxt4p, Hxt5p, Hxt7p, Hxt9p and Hxt10p. Each catalyzed As(OH)3 transport (Fig. 1A). Hxt7p gave the highest rate of As(OH)3 uptake, while rGLUT1 exhibited low uptake at 0.1 mM sodium arsenite. Importantly, hexose transporters also catalyzed uptake of CH3As(OH)2 at 50 μM at rates up to 50-fold higher than As(OH)3. Their relative efficiencies were reversed compared with As(OH)3: rGLUT1 had the highest rate of uptake, while Hxt7p gave the lowest (Fig. 1B). Hexose permeases have different affinities, so the observed rank order may not apply at all concentrations of substrates. CH3As(OH)2 uptake by the yeast hexose transporters was inhibited by glucose (Fig. 1C).
Fig. 1.
HXTs and rGLUT1 facilitate uptake of As(OH)3 and CH3As(OH)2 in S. cerevisiae. Yeast cells expressing hexose transport genes ((▲), vector; (◇),YEpH2-rGLUT1; (●);pTHHXT1; (○),pTHHXT3; (▼),pTHHXT4; (△), pTHHXT5; (■),pTHHXT7; (□),pTHHXT9; (◆), pTHHXT10) were assayed for uptake of 0.1 mM As(OH)3 (A) or 50 μM CH3As(OH)2 (B), as described under Experimental Procedures. (C) Inhibition of 50 μM CH3As(OH)2 uptake by 50 mM glucose was assayed after 30 min. The values in each plot are the mean of three independent assays. The error bars represent the standard deviation of the mean calculated using SigmaPlot 9.0.
Kinetic analysis of rGLUT1
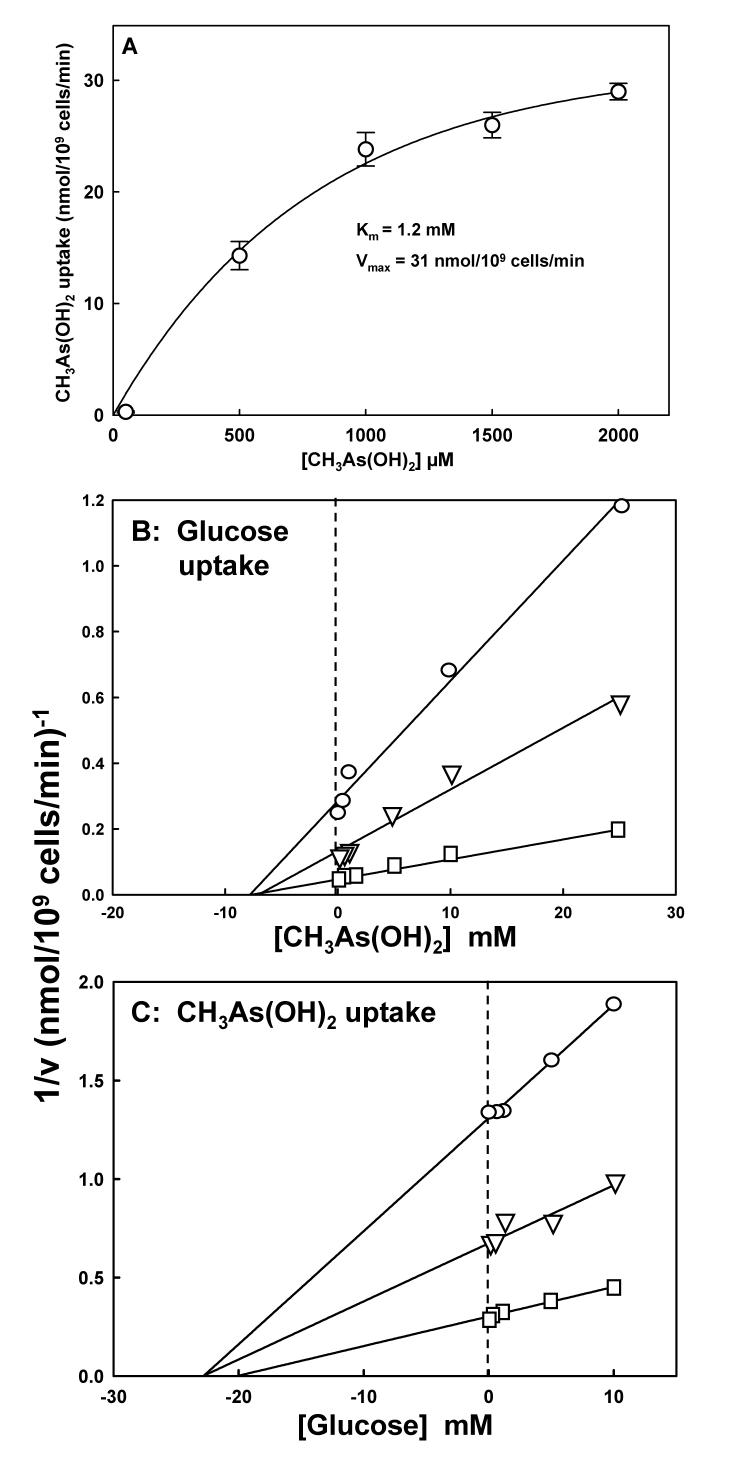
The affinity of rGLUT1 for CH3As(OH)2, with a Km of 1.2 mM (Fig. 2A), is similar to that for glucose, which is approximately 3 mM when assayed in yeast [24]. rGLUT1 appears to be selective for CH3As(OH)2; the rate of As(OH)3 uptake by rGLUT1 was so much lower than the methylated species that it was not possible to determine the kinetic constants for As(OH)3 uptake by rGLUT1. Uptake of CH3As(OH)2 by rGLUT1 is inhibited by glucose noncompetitively (Fig. 2B), and glucose uptake by rGLUT1 is inhibited by CH3As(OH)2 noncompetitively (Fig. 2C). These results suggest that the two substrates may utilize different initial binding sites or different translocation pathways.
Fig. 2.
Kinetic analysis of rGLUT1. A. Kinetics of CH3As(OH)2uptake. Kinetic parameters were calculated from the rates of uptake in yeast strain HD300 YEpH2-rGLUT1 at the indicated concentrations of CH3As(OH)2 after subtraction of the rates from strain HD300 alone, which lacks hexose transport genes. B. CH3As(OH)2 noncompetitively inhibits glucose uptake. Uptake of [14C]glucose in HD300 YEpH2-rGLUT1 was assayed for 30 sec with the indicated concentrations of CH3As(OH)2 at (□), 50 μM; (▽), 100 μM; or (○), 250 μM glucose. CH3As(OH)2 was added 3 min prior to the start of each assay at room temperature. [14C]glucose was added at a final concentration of 1mM. C. Glucose noncompetitively inhibits CH3As(OH)2 uptake. Uptake of CH3As(OH)2 in HD300 YEpH2-rGLUT1 was assayed for 30 sec with the indicated concentrations of glucose at (□), 50 μM; (▽), 100 μM; or (○), 250 μM CH3As(OH)2. Glucose was added 3 min prior to the start of each assay at room temperature. CH3As(OH)2 was added at a final concentration of 1mM. The values in each plot are the mean of three independent assays. The error bars represent the standard deviation of the mean calculated using SigmaPlot 9.0.
CH3As(OH)2 transport by rGLUT1 and HXT1 is inhibited by hexoses but not cytocholasin B or forskolin
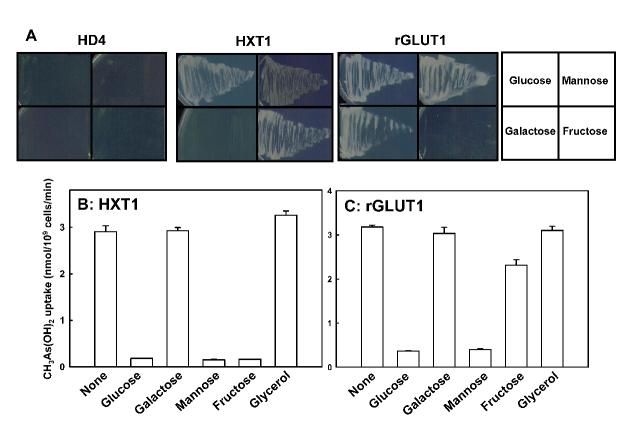
Substrates for rGLUT1 include glucose, galactose and mannose but not fructose [33]. Expression of rGLUT1 in yeast strain HD300 enables cells to use glucose, galactose and mannose as carbon sources, but cells cannot grow on fructose as sole carbon source (Fig. 3A), and CH3As(OH)2 uptake in those cells was inhibited by glucose, mannose but not galactose, fructose or glycerol (Fig. 3C). By way of comparison, yeast cells with Hxt1p transport glucose, mannose and fructose but not galactose [34] (Fig. 3B). Cells expressing HXT1 utilize glucose, mannose and fructose but not galactose as sole carbon sources (Fig. 3A), and CH3As(OH)2 uptake in those cells was inhibited by glucose, mannose and fructose but not galactose or glycerol (Fig. 3C). The lack of inhibition of GLUT1-mediated CH3As(OH)2 uptake by galactose suggests that the initial binding sites for arsenicals and hexoses might be different, consistent with the reciprocal noncompetitive inhibition between glucose and CH3As(OH)2. Cytochalasin B and forskolin, inhibitors of mammalian glucose permeases but not yeast hexose transporters [35, 36], inhibited rGLUT1-mediated uptake of glucose but not CH3As(OH)2 (data not shown), further indicating that there are differences in the way that hexoses and CH3As(OH)2 are recognized by rGLUT1.
Fig. 3.
CH3As(OH)2 transport by either rGLUT1 or HXT1 is inhibited by hexoses. A. Growth of yeast strain HD300 YEpH2-rGLUT1 (right), pTHHXT1 (middle) or no hexose permease genes (left) in SD minimal medium supplied with 2% of the glucose, mannose, galactose or fructose, as indicated. B. Inhibition of 50 μM CH3As(OH)2 transport in HD300 pTHHXT1 by the indicated hexoses, each at 50 mM. C. Inhibition of CH3As(OH)2 transport in strain HD300 YEpH2-rGLUT1 by the indicated hexoses, each at 50 mM. The values in each plot are the mean of three independent assays.
Both human and rat GLUT1 catalyze CH3As(OH)2 transport in öocytes
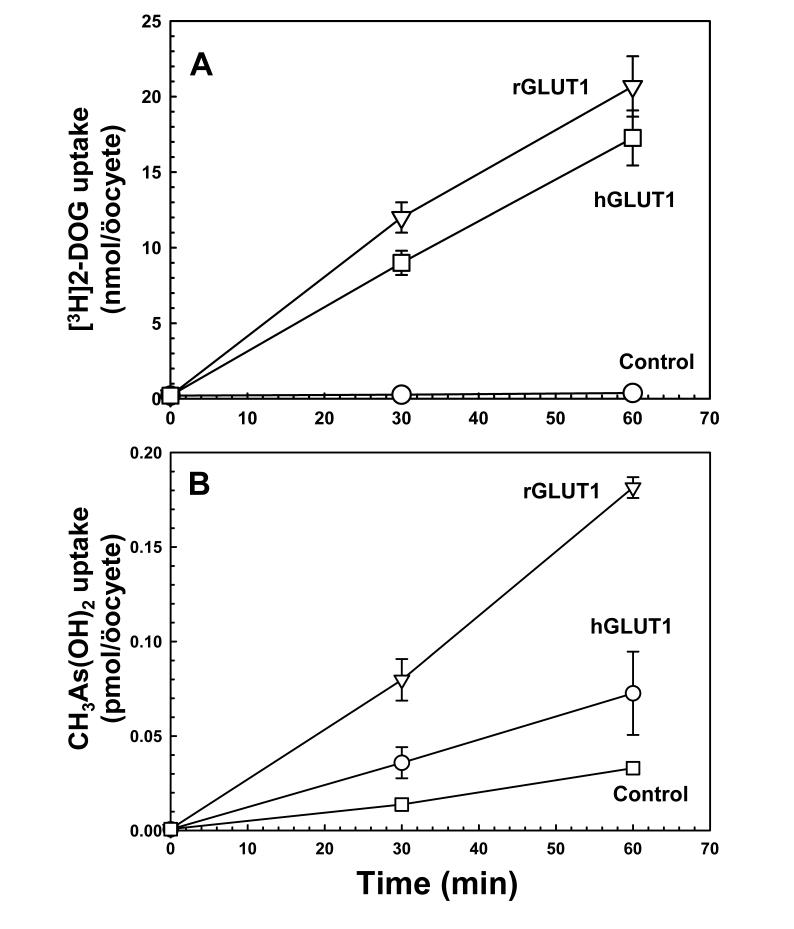
Although the rat V69M GLUT1 expresses in yeast, normal human GLUT1 is not expressed well enough to allow for characterization in yeast. To directly compare human and rat GLUT1, their ability to increase permeability to glucose and CH3As(OH)2 was examined in X. laevis öocytes (Fig. 4). Both human and rat V69M variant GLUT1 cRNAs were prepared in vitro and microinjected into öocytes. Expression of the two GLUT1s was estimated by immunoblotting using commercial antibody (Alpha Diagnosis), and both appear to be expressed at similar levels (data not shown). Human and rat GLUT1 transported 2-deoxyglucose at similar rates (Fig. 4A), but rGLUT1 transported CH3As(OH)2 at faster rates than hGLUT1 (Fig. 4B). Consistent with the results obtained with yeast expression, rat GLUT1 transported CH3As(OH)2 much faster than As(OH)3, which was barely above the rate of water-injected öocytes (data not shown), demonstrating that GLUT1 is a much better CH3As(OH)2 transporter than As(OH)3 transporter.
Fig. 4.
GLUT1 facilitates uptake of CH3As(OH)2 in X. laevis öocytes. A: Transport of 1 mM [3H]deoxyglucose in öocytes injected with rGLUT1 cRNA (▽), hGLUT1 cRNA (○) or water injected (□). B: Transport of 0.1 mM CH3As(OH)2 in öocytes injected with rGLUT1 cRNA (▽), hGLUT1 cRNA (○) or water injected (□). The values in each plot are the mean of three independent assays.
Discussion
We have shown previously that inorganic trivalent arsenic, As(OH)3, is transported by mammalian aquaglyceroporin channels such as human and rat AQP7 and AQP9 [12, 13]. AQP9 also facilitates movement of the monomethylate species, CH3As(OH)2 [14]. AQP7 is found predominately in kidney, testis and adiopose tissue [37, 38], and AQP9 is found mainly in liver, spleen and brain [39] but not in the epithelial cells the form the blood-brain barrier [40]. This raises the question of how tissues that do not have detectable aquaglyceroporins, including heart and the blood-brain barrier, take up trivalent arsenicals. Glucose transporter isoform 1 or GLUT1, a homologue of the yeast Hxt permeases, is widely distributed in mammalian tissues. It is the major pathway for glucose uptake in many cell types, including neonatal heart, erythrocytes and the endothelial cells that form the blood–brain barrier. Although adult heart does not depend on glucose for energy, in neonatal heart, glucose utilization via GLUT1 is a major source of energy [41]. Rat GLUT1 can be functionally expressed in yeast [24], which allowed analysis of its arsenic transport properties. Although human GLUT1 does not express well in yeast, it is reasonable to assume that its transport properties will be essentially the same as rGLUT1 since rGLUT1 and hGLU1 are 97% identical. Compared with a number of the yeast hexose transporters, rGLUT1 does not transport As(OH)3 efficiently (Fig. 1A). In contrast, rGLUT1 has the highest rate of CH3As(OH)2 uptake at 50 μM, followed yeast Hxt1p, Hxt3p, Hxt4p, Hxt5p, Hxt9p and Hxt10p (Fig. 1B). Importantly, for each transport protein, the rate of uptake of CH3As(OH)2 was at least an order of magnitude higher than for inorganic trivalent arsenic, demonstrating selectivity for the organic arsenical. These results suggest that uptake of CH3As(OH)2 might be common property of members of the eukaryotic hexose permease family. Transport could be a major contributor to the adverse effects of arsenic in mammalian cells, especially - as if often the case - uptake is the rate limiting step for subsequent intracellular reactions.
Glucose permeases undoubtedly transport As(OH)3 and CH3As(OH)2 adventitiously. It is not intuitively obvious why a trivalent metalloid and a sugar should be recognized by the same transporter but the observation of transport of both species suggests that they may have chemical or structural similarities. We have proposed that trimer forms a six-membered cyclic oxo-bridged ring which is sufficiently glucose-like to be recognized by yeast hexose transporters [18]. While there is no structural information on CH3As(OH)2, it is reasonable to assume that it can also form a trimeric cyclic glucose-like ring structure. These ring forms of As(OH)3 and CH3As(OH)2 may compete for the same binding site on the glucose permeases and share the translocation pathway. Another possibility is that they are bound as monomers of As(OH)3 or CH3As(OH)2, with the binding site on the glucose permeases filled with one, two or three molecules. GLUT1 has been shown to function as a water channel [42], and a T310I GLUT1 mutant, identified in a patient with GLUT1-deficiency syndrome, has reduced glucose transport and increased water permeation that has lost its inhibition by glucose, suggesting that water goes through a different pathway in GLUT1 than glucose [43]. Thus, just as aquaporin channels conduct As(OH)3 or CH3As(OH)2, these arsenicals might also move through a water channel in GLUT1. This is supported by two lines of evidence. First, CH3As(OH)2 inhibits glucose transport noncompetitively, and vice versa, which would be expected if they did not bind at the same site. Second, the classical inhibitors of glucose transport cytochalsin B and forskolin, do not inhibit CH3As(OH)2 uptake. Future experiments such as mutant analysis will be designed to test these possibilities.
Acknowledgments
We thank Dr. James Marsh, University of Arkansas College of Medicine, for advice and suggestions. The publication costs were supported by United States Public Health Service Grant GM55425 to BPR, American Heart Association Postdoctoral Fellowship 0520014Z to ZL, American Heart Association Beginning Grant-in-Aid 0560056Z to MAS, and NIH AI25920 to SML.
Footnotes
Abbreviations: deoxyglucose (DOG).
References
- 1.Abernathy CO, Thomas DJ, Calderon RL. Health effects and risk assessment of arsenic. The Journal of nutrition. 2003;133:1536S–1538S. doi: 10.1093/jn/133.5.1536S. [DOI] [PubMed] [Google Scholar]
- 2.Tchounwou PB, Patlolla AK, Centeno JA. Carcinogenic and systemic health effects associated with arsenic exposure--a critical review. Toxicol Pathol. 2003;31:575–588. doi: 10.1080/01926230390242007. [DOI] [PubMed] [Google Scholar]
- 3.Rahman M, Tondel M, Ahmad SA, Axelson O. Diabetes mellitus associated with arsenic exposure in Bangladesh. Am J Epidemiol. 1998;148:198–203. doi: 10.1093/oxfordjournals.aje.a009624. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez-Solis A, Mukopadhyay R, Rosen BP, Stemmler TL. Experimental and theoretical characterization of arsenite in water: insights into the coordination environment of As-O. Inorg Chem. 2004;43:2954–2959. doi: 10.1021/ic0351592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douer D, Tallman MS. Arsenic trioxide: new clinical experience with an old medication in hematologic malignancies. J Clin Oncol. 2005;23:2396–2410. doi: 10.1200/JCO.2005.10.217. [DOI] [PubMed] [Google Scholar]
- 6.Navas-Acien A, Sharrett AR, Silbergeld EK, Schwartz BS, Nachman KE, Burke TA, Guallar E. Arsenic exposure and cardiovascular disease: a systematic review of the epidemiologic evidence. Am J Epidemiol. 2005;162:1037–1049. doi: 10.1093/aje/kwi330. [DOI] [PubMed] [Google Scholar]
- 7.Zierold KM, Knobeloch L, Anderson H. Prevalence of chronic diseases in adults exposed to arsenic-contaminated drinking water. Am J Public Health. 2004;94:1936–1937. doi: 10.2105/ajph.94.11.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wildfang E, Radabaugh TR, Vasken Aposhian H. Enzymatic methylation of arsenic compounds. IX. Liver arsenite methyltransferase and arsenate reductase activities in primates. Toxicology. 2001;168:213–221. doi: 10.1016/s0300-483x(01)00481-4. [DOI] [PubMed] [Google Scholar]
- 9.Mandal BK, Ogra Y, Anzai K, Suzuki KT. Speciation of arsenic in biological samples. Toxicol Appl Pharmacol. 2004;198:307–318. doi: 10.1016/j.taap.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 10.Erry BV, Macnair MR, Meharg AA, Shore RF. The distribution of arsenic in the body tissues of wood mice and bank voles. Arch Environ Contam Toxicol. 2005;49:569–576. doi: 10.1007/s00244-004-0229-3. [DOI] [PubMed] [Google Scholar]
- 11.Thomas DJ, Waters SB, Styblo M. Elucidating the pathway for arsenic methylation. Toxicol Appl Pharmacol. 2004;198:319–326. doi: 10.1016/j.taap.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Shen J, Carbrey JM, Mukhopadhyay R, Agre P, Rosen BP. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci U S A. 2002;99:6053–6058. doi: 10.1073/pnas.092131899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z, Carbrey JM, Agre P, Rosen BP. Arsenic trioxide uptake by human and rat aquaglyceroporins. Biochemical and biophysical research communications. 2004;316:1178–1185. doi: 10.1016/j.bbrc.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Styblo M, Rosen BP. Methylarsonous Acid transport by aquaglyceroporins. Environ Health Perspect. 2006;114:527–531. doi: 10.1289/ehp.8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kageyama Y, Ishibashi K, Hayashi T, Xia G, Sasaki S, Kihara K. Expression of aquaporins 7 and 8 in the developing rat testis. Andrologia. 2001;33:165–169. doi: 10.1046/j.1439-0272.2001.00443.x. [DOI] [PubMed] [Google Scholar]
- 16.Ishibashi K, Kuwahara M, Gu Y, Kageyama Y, Tohsaka A, Suzuki F, Marumo F, Sasaki S. Cloning and functional expression of a new water channel abundantly expressed in the testis permeable to water, glycerol, and urea. J Biol Chem. 1997;272:20782–20786. doi: 10.1074/jbc.272.33.20782. [DOI] [PubMed] [Google Scholar]
- 17.Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA. Functional and molecular characterization of the human neutral solute channel aquaporin-9. Am J Physiol. 1999;277:F685–696. doi: 10.1152/ajprenal.1999.277.5.F685. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Boles E, Rosen BP. Arsenic trioxide uptake by hexose permeases in Saccharomyces cerevisiae. J Biol Chem. 2004;279:17312–17318. doi: 10.1074/jbc.M314006200. [DOI] [PubMed] [Google Scholar]
- 19.Vannucci SJ, Maher F, Simpson IA. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia. 1997;21:2–21. doi: 10.1002/(sici)1098-1136(199709)21:1<2::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 20.Klepper J, Voit T. Facilitated glucose transporter protein type 1 (GLUT1) deficiency syndrome: impaired glucose transport into brain-- a review. Eur J Pediatr. 2002;161:295–304. doi: 10.1007/s00431-002-0939-3. [DOI] [PubMed] [Google Scholar]
- 21.Takata K, Hirano H, Kasahara M. Transport of glucose across the blood-tissue barriers. Int Rev Cytol. 1997;172:1–53. doi: 10.1016/s0074-7696(08)62357-8. [DOI] [PubMed] [Google Scholar]
- 22.Kasahara T, Kasahara M. Expression of the rat GLUT1 glucose transporter in the yeast Saccharomyces cerevisiae. The Biochemical journal. 1996;315(Pt 1):177–182. doi: 10.1042/bj3150177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boles E. Yeast as a model system for studying glucose transport. In: MW Q, editor. Transmembrane transporters. Wiley, Inc.; New York: 2002. pp. 19–36. [Google Scholar]
- 24.Wieczorke R, Dlugai S, Krampe S, Boles E. Characterisation of mammalian GLUT glucose transporters in a heterologous yeast expression system. Cell Physiol Biochem. 2003;13:123–134. doi: 10.1159/000071863. [DOI] [PubMed] [Google Scholar]
- 25.Wieczorke R, Krampe S, Weierstall T, Freidel K, Hollenberg CP, Boles E. Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 1999;464:123–128. doi: 10.1016/s0014-5793(99)01698-1. [DOI] [PubMed] [Google Scholar]
- 26.Arriza JL, Kavanaugh MP, Fairman WA, Wu YN, Murdoch GH, North RA, Amara SG. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J Biol Chem. 1993;268:15329–15332. [PubMed] [Google Scholar]
- 27.Adams A, Gottschling DE, Kaiser C, Stearns T. Methods in Yeast Genetics: A Cold Spring Harbor laboratory course manual. Cold Spring Harbor laboratory; Cold Spring Harbor, NY.: 1998. [Google Scholar]
- 28.Sambrook R. Molecular Cloning, a laboratory manual. Third ed. Cold Spring Harbor Laboratory Press; New York: 2001. [Google Scholar]
- 29.Liu Z, Boles E, Rosen BP. Arsenic trioxide uptake by hexose permeases in Saccharomyces cerevisiae. J Biol Chem. 2004;279:17312–17318. doi: 10.1074/jbc.M314006200. [DOI] [PubMed] [Google Scholar]
- 30.Langford CK, Burchmore RJ, Hart DT, Wagner W, Landfear SM. Biochemistry and molecular genetics of Leishmania glucose transporters. Parasitology. 1994;108 doi: 10.1017/s0031182000075740. [DOI] [PubMed] [Google Scholar]
- 31.Wysocki R, Bobrowicz P, Ulaszewski S. The Saccharomyces cerevisiae ACR3 gene encodes a putative membrane protein involved in arsenite transport. J Biol Chem. 1997;272:30061–30066. doi: 10.1074/jbc.272.48.30061. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh M, Shen J, Rosen BP. Pathways of As(III) detoxification in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1999;96:5001–5006. doi: 10.1073/pnas.96.9.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994;219:713–725. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- 34.Reifenberger E, Boles E, Ciriacy M. Kinetic characterization of individual hexose transporters of Saccharomyces cerevisiae and their relation to the triggering mechanisms of glucose repression. Eur J Biochem. 1997;245:324–333. doi: 10.1111/j.1432-1033.1997.00324.x. [DOI] [PubMed] [Google Scholar]
- 35.Dan-Goor M, Sasson S, Davarashvili A, Almagor M. Expression of glucose transporter and glucose uptake in human oocytes and preimplantation embryos. Vol. 12. Human reproduction; Oxford, England: 1997. pp. 2508–2510. [DOI] [PubMed] [Google Scholar]
- 36.Doege H, Bocianski A, Scheepers A, Axer H, Eckel J, Joost HG, Schurmann A. Characterization of human glucose transporter (GLUT) 11 (encoded by SLC2A11), a novel sugar-transport facilitator specifically expressed in heart and skeletal muscle. The Biochemical journal. 2001;359:443–449. doi: 10.1042/0264-6021:3590443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishibashi K, Imai M, Sasaki S. Cellular localization of aquaporin 7 in the rat kidney. Experimental nephrology. 2000;8:252–257. doi: 10.1159/000020676. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki-Toyota F, Ishibashi K, Yuasa S. Immunohistochemical localization of a water channel, aquaporin 7 (AQP7), in the rat testis. Cell and tissue research. 1999;295:279–285. doi: 10.1007/s004410051234. [DOI] [PubMed] [Google Scholar]
- 39.Elkjaer M, Vajda Z, Nejsum LN, Kwon T, Jensen UB, Amiry-Moghaddam M, Frokiaer J, Nielsen S. Immunolocalization of AQP9 in liver, epididymis, testis, spleen, and brain. Biochemical and biophysical research communications. 2000;276:1118–1128. doi: 10.1006/bbrc.2000.3505. [DOI] [PubMed] [Google Scholar]
- 40.Lehmann GL, Gradilone SA, Marinelli RA. Aquaporin water channels in central nervous system. Current neurovascular research. 2004;1:293–303. doi: 10.2174/1567202043362081. [DOI] [PubMed] [Google Scholar]
- 41.Abel ED. Glucose transport in the heart. Front Biosci. 2004;9:201–215. doi: 10.2741/1216. [DOI] [PubMed] [Google Scholar]
- 42.Fischbarg J, Kuang KY, Vera JC, Arant S, Silverstein SC, Loike J, Rosen OM. Glucose transporters serve as water channels. Proc Natl Acad Sci U S A. 1990;87:3244–3247. doi: 10.1073/pnas.87.8.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iserovich P, Wang D, Ma L, Yang H, Zuniga FA, Pascual JM, Kuang K, De Vivo DC, Fischbarg J. Changes in glucose transport and water permeability resulting from the T310I pathogenic mutation in Glut1 are consistent with two transport channels per monomer. J Biol Chem. 2002;277:30991–30997. doi: 10.1074/jbc.M202763200. [DOI] [PubMed] [Google Scholar]