Abstract
Background:
Obesity and diabetes are established risk factors for colorectal cancer (CRC), but have mainly been assessed independently. There are few data regarding whether the metabolic syndrome, which refers to a clustering of cardiovascular disease risk factors thought to be related to insulin resistance, including obesity, type 2 diabetes, hyperlipidemia, and hypertension, is associated with CRC risk.
Methods:
During and after the randomized trial of aspirin and ß-carotene, 22,071 healthy male physicians initially aged 40-84 years reported overweight (body mass index ≥ 27 kg/m2), diabetes, elevated blood pressure (≥ 130/85 mmHg or use of antihypertensive medication), hypercholesterolemia (≥ 240mg/dl or use of lipid-lowering medication), and occurrence of cancer on annual questionnaires. Adjusted relative risks (RR) and 95% confidence intervals (CI) for time varying metabolic abnormalities and CRC were estimated using a multivariable proportional hazards model.
Results:
During 369,966 person-years of follow-up (median 19 years), 494 physicians developed CRC. With aging of the cohort, the prevalence of having two or more abnormalities increased from 13% to 35%. Overweight (RR=1.4;95%CI:1.1-1.7) and diabetes (RR=1.5;95%CI:1.1-2.0) were associated with increased risk for CRC, whereas elevated blood pressure (RR=1.1;95%CI:0.9-1.3) and hypercholesterolemia (RR=0.9;95%CI:0.7-1.1) were not. This model assessing metabolic abnormalities independently was more predictive for CRC than a model based on the number of abnormalities (each additional abnormality RR=1.16;95%CI:1.05-1.29).
Conclusions:
Out of the markers of the metabolic syndrome assessed, overweight and diabetes are risk factors for CRC, whereas, in contrast to their role in cardiovascular disease, elevated blood pressure and hypercholesterolemia are not.
Keywords: Colorectal Neoplasms, Epidemiology, Metabolic Syndrome X, Obesity, Diabetes Mellitus
INTRODUCTION
Colorectal cancer has been associated with markers of insulin or glucose control (1-3) and insulin resistance might be the unifying mechanism by which several risk factors affect colorectal carcionogenesis (4).
Insulin resistance is the cornerstone of the metabolic syndrome, which is a risk factor for the development of cardiovascular disease (myocardial infarction and stroke) and type 2 diabetes beyond the risk associated with obesity alone (5,6). Risk factors for insulin resistance include adiposity, (lack of) fitness, and genetics (7). The metabolic syndrome can be defined as the simultaneous presence of central adiposity, low levels of HDL cholesterol and elevated levels of triglycerides, impaired fasting glucose, and hypertension (5, 8, 9). Despite continued controversy regarding its definition and value (7, 10, 11), the metabolic syndrome may have the potential to capture more of the primary and secondary risk for cardiovascular disease and diabetes associated with the risk factors used to define it than when addressing each risk factor independently of the others.
Independently elevated risks for colorectal cancer associated with obesity (more clearly in men than in women, 12) and diabetes (e.g. 13) have been observed repeatedly and there have been some reports for hypertension (e.g. 14). In apparent contrast to cardiovascular disease, there have been several studies showing a decreased risk for colorectal cancer associated with hyperlipidemia (e.g. 15-17). Although some or all of this association has later been shown to be due to protopathic bias, i.e. the lowering of serum cholesterol levels due to preclinical disease (e.g. 18), there exist no data showing an increased risk for colorectal cancer in individuals with hyperlipidemia.
The aim of our study was to address the combined association between the presence of multiple metabolic abnormalities used to define the metabolic syndrome and risk for colorectal cancer, thus taking their frequent clustering in individuals into account. Our secondary aim was to address the possibility of a different role of hyperlipidemia in the prediction of risk for colorectal cancer compared with cardiovascular disease.
SUBJECTS AND METHODS
Population
The Physicians’ Health Study was designed to test the hypotheses that 325 mg of aspirin on alternate days reduces risk of cardiovascular disease and that 50 mg of beta-carotene on alternate days decreases the incidence of cancer. The population and methods have been described in detail in previous reports (19, 20). In brief, 33,223 US male physicians aged 40 to 84 years who did not regularly use aspirin or other NSAIDs (or were willing to give up use), without a history of myocardial infarction, stroke, cancer, liver or renal disease, gout, peptic ulcer, or other contraindications to aspirin were eligible and enrolled in a 3 month run-in phase of active aspirin. During the run-in period, 22,071 (66 percent) followed the study regimen, remained willing and eligible to participate, provided written informed consent, and were randomly assigned to the treatment regimen in 1982. The aspirin arm was terminated early after an average follow-up of five years in January 1988, primarily due to a 44% reduction in risk of a first myocardial infarction in the aspirin group (16). The beta-carotene component of the trial continued to its scheduled end in December 1995. Post trial observational follow-up continued after this date (21). The trial and continued follow-up of participants were approved by the Institutional Review Board of the Brigham and Women’s Hospital. For this analysis, we followed participants until death, the occurrence of cancer, or their last questionnaire returned until February 2003.
Assessment of metabolic abnormalities
Both during the randomized period as well as during the post-trial non-experimental period, participants completed annual questionnaires asking about life-style, co-morbidity, and the occurrence of relevant health outcomes. As of February 2003, 95 percent of surviving participants provided these data within the past two years.
According to the National Cholesterol Education Program’s (NCEP) third Adult Treatment Panel (ATP III), the metabolic syndrome in men is defined as the presence of 3 or more out of the following 5 items: waist circumference ≥ 40 inches, serum triglycerides > 150 mg/dl, high density lipoprotein (HDL) < 40 mg/dl, blood pressure ≥ 130/85 mmHg, and a fasting glucose ≥ 110 mg/dl (22, 23). In absence of data needed to define the metabolic syndrome according to NCEP throughout the 20 year follow-up period, we used a body mass index (BMI) of ≥ 27 kg/m2, a total cholesterol of ≥ 240mg/dl or use of lipid lowering drugs, blood pressure of ≥ 130/85 mmHg or use of anti-hypertensives, and a diagnosis of diabetes to characterize metabolic state. The cut-point for BMI was selected because it provided the highest sensitivity (68%) and specificity (90%) for a waist circumference of ≥ 40 inches in a cross-sectional validation sub-sample of 17,851 men who reported both measurements. The cut-point for self reported blood pressure and total cholesterol as well as the inclusion of information on the use of antihypertensives and lipid lowering drugs were chosen in accordance with ATP III (22, 23) and previous work (24). Over all questionnaires, 12% of elevated blood pressure and 30% of hypercholesterolemia were defined based on medication use only. The sensitivity and specificity of our definition of hypercholesterolemia for triglycerides > 150 mg/dl was again assessed in a cross-sectional validation sub-sample of 478 men (controls in a nested case-control analysis). As expected, the sensitivity for the item used to define the metabolic syndrome was low (20%), but the specificity was high (91%). No data on HDL were available for this analysis. A self reported diagnosis of diabetes can be assumed to have a low sensitivity but a high specificity for a fasting glucose of > 110 mg/dl.
All these variables were available at baseline and were updated whenever possible at various points throughout the follow-up. The BMI was calculated from self reported baseline height and self reported weight at baseline. It was updated using weight information after 96 months of follow-up and yearly thereafter. Self-reported total cholesterol values and use of lipid lowering drugs were available at baseline and were updated after 24, 84, and 216 months of follow-up. Systolic and diastolic blood pressure and use of anti-hypertensives were asked for at baseline and were updated 24, 84, 180, and 216 months into the study. A diagnosis of diabetes was asked for annually.
Information on Covariates
Current age was calculated at the time of every questionnaire. Information about the usual frequency of exercise vigorous enough to work up a sweat was assessed at baseline and updated at 36, 108, and 216 months. Smoking status and intensity (current: 20 or more cigarettes per day, current: less than 20 cigarettes per day, past, or never) was assessed at baseline and updated at 24, 60, 144, and 216 months. Usual frequency of alcohol consumption during the last 12 months was assessed at baseline and updated at 84 and 216 months. Multivitamin use was asked for at baseline and yearly starting with the 60 month questionnaire. Usual frequency of consumption of servings of vegetables and fruits was asked for at baseline and was updated at the 24, 48, 72, 96, 120, and 156 months questionnaires.
Assessment of Colorectal Cancer
All cancer reports (excluding non-melanoma skin cancers) were followed by a request to review medical records, including pathology reports. An endpoints committee of physicians blinded to the treatment assignment reviewed medical records. For this analysis, we used all confirmed cases of incident colorectal cancer (N=480) as well as cases reported and not yet confirmed (N=14) until February 2003. As of February 2003, 95 percent of surviving participants provided morbidity data within the past two years.
Statistical Analysis
Propensity for having 2 or more metabolic abnormalities
To assess variables independently associated with having 2 or more metabolic abnormalities at any given questionnaire, we used multivariable logistic regression models accounting for within person correlation in different time periods using generalized estimating equations (25). This analytic strategy was chosen in favor of one assessing predictors of first occurrence, because the time of onset of some of the metabolic abnormalities is unknown and some of the abnormalities are reversible. The analysis thus reflects predictors of incidence and persistence of having 2 or more metabolic abnormalities. Possible predictors were age, smoking, exercise, alcohol consumption, multivitamin use, and consumption of fruits and vegetables. All variables were assessed as of each questionnaire, i.e. taking into account changes over time (time-varying).
Risk for colorectal cancer
We excluded participants with a diagnosis of any cancer before randomization (N=21) or no follow-up (N=4) from all analyses. As of February 2003, mortality follow-up was 97 percent complete within the past 2 years and 4328 of the participants had died. Person-time was censored at the time of diagnosis for participants with any unrefuted cancer (except non-melanoma skin cancer). For all remaining participants, person-time was censored at the date of the last returned questionnaire or death. We calculated standardized morbidity ratios (SMRs) to compare the experience of the study group with that of the general U.S. population by using Surveillance, Epidemiology, and End Results (SEER) data for white men from 1994 to 2003, adjusting for age in 5-year categories and updating age every 5 years. We calculated person-time at risk and assigned colorectal cancer cases according to the number of metabolic abnormalities. We then estimated incidence rate ratios and their 95% confidence intervals (CIs) for colorectal cancer according to time-varying number of metabolic abnormalities for the total follow-up period using left and right censored Cox proportional hazards models (26). The model included updated, i.e. time-varying, covariates rather than the overall propensity score described above (27).
Risk for myocardial infarction
We conducted additional Cox proportional hazards models that were essentially identical to the ones described above but were based on 1423 first myocardial infarctions (reported by participants and confirmed by the endpoints committee according to WHO criteria based on chart review) as the outcome to compare the contribution of the individual metabolic abnormalities to the risk for colorectal cancer with the contribution of these abnormalities to the risk of myocardial infarction.
RESULTS
We present characteristics of the 22,046 male physicians as of baseline and as of the last questionnaire received in table 1 to illustrate the changes of the study population over a median follow-up period of 19.0 years (inter-quartile range: 16.1-19.6). From baseline to the last questionnaire, the mean age increased from 54 to 70 years. As expected, the most pronounced differences were observed for the metabolic abnormalities. The prevalence of a BMI greater or equal to 27 increased from 18% to 27%, of hypercholesterolemia from 11% to 30%, and of an elevated blood pressure from 34% to 53%. The prevalence of diabetes tripled from 3% at baseline to 9% as of the last questionnaire. At baseline, physicians most frequently (56%) reported exercising vigorously 1-4 times per week. On the last questionnaire, exercising never or less than once per week was reported as frequently (41%) as exercising 1-4 times per week (42%). Current smoking decreased from 11% at baseline to 5% as of the last questionnaire, with former smoking increasing accordingly. Both the proportions of abstainers and of those who reported drinking alcohol daily increased over time. We also observed strong increases over time for the use of multivitamins (20 to 47%), nonsteroidal anti-inflammatory drugs (0 to 12%), and arthritis (3 to 28%).
Table 1.
Characteristics of 22,046 male physicians as of first (baseline) and last questionnaire
| Baseline |
Last questionnaire* |
|||
| Age (years), mean (SD) | 53.8 | (9.5) | 69.6 | (8.7) |
| Metabolic abnormalities, N (%) | ||||
| BMI ≥ 27 kg/m2 | 3,905 | (17.7) | 5,937 | (26.9) |
| Hypercholesterolemia | 2,189 | (11.3) | 6,670 | (30.4) |
| Elevated blood pressure | 6,597 | (34.0) | 11,433 | (52.5) |
| Diabetes | 607 | (2.8) | 1,888 | (8.6) |
| Number of metabolic abnormalities, N (%) | ||||
| None | 11,494 | (53.5) | 6,027 | (27.4) |
| 1 | 7,199 | (33.5) | 8,375 | (38.0) |
| 2 | 2,455 | (11.4) | 5,579 | (25.3) |
| 3 or 4 | 357 | (1.7) | 2,050 | (9.3) |
| Vigorous exercise, N (%)‡ | ||||
| Less than once per week | 6,048 | (27.7) | 9,102 | (41.4) |
| 1-4 times per week | 12,202 | (56.0) | 9,248 | (42.0) |
| 5 or more times per week | 3,553 | (16.3) | 3,661 | (16.6) |
| Smoking, N (%) | ||||
| Never | 10,907 | (49.6) | 10,663 | (48.8) |
| Past | 8,663 | (39.4) | 10,157 | (46.4) |
| Current | 2,436 | (11.1) | 1,049 | (4.8) |
| Alcohol consumption, N (%) | ||||
| Never/rarely | 3,255 | (14.9) | 4,321 | (19.6) |
| 1-3 times per month | 2,440 | (11.2) | 2,548 | (11.6) |
| Once per week | 3,055 | (14.0) | 2,192 | (10.0) |
| 2-4 times per week | 4,896 | (22.4) | 4,029 | (18.3) |
| 5-6 times per week | 2,770 | (12.7) | 2,233 | (10.2) |
| Daily | 5,437 | (24.9) | 6,679 | (30.4) |
| Multivitamin use | 4,361 | (19.9) | 10,263 | (46.6) |
| Regular NSAIDs use§ | 32 | (0.2) | 2,728 | (12.4) |
| History of arthritis | 620 | (2.8) | 6,084 | (27.6) |
| Vegetables (servings per day), mean (SD) | 1.4 | (0.9) | 1.1 | (1.0) |
| Fruits (servings per day) , mean (SD) | 0.8 | (0.7) | 0.8 | (0.7) |
Last coded questionnaire from up to 20
Weight in kilograms divided by squared height in meters
Vigorous enough to work up a sweat
Nonsteroidal anti-inflammatory drug use on more than 60 days during last 12 months
In table 2, we present independent predictors of having 2 or more metabolic abnormalities at any given time during the follow-up. Odds ratios (OR) are adjusted for all variables presented in the table and values above 1 indicate a higher propensity of having 2 or more abnormalities. ORs are estimated based on 353,371 individual questionnaires and an overall prevalence of having 2 or more metabolic abnormalities of 19.3%.
Table 2.
Independent predictors of having 2 or more metabolic abnormalities in 22,046 male physicians
| OR* | 95% CI* | |
| Age (years) | ||
| 40-< 50 | 1.0 | reference |
| 50-< 60 | 2.0 | 1.8-2.1 |
| 60-< 70 | 3.1 | 2.9-3.4 |
| 70-< 80 | 3.6 | 3.3-4.0 |
| 80-< 90 | 3.1 | 2.8-3.5 |
| 90+ | 1.8 | 1.3-2.4 |
| Vigorous exercise† | ||
| Less than once per week | 1.0 | reference |
| 1-4 times per week | 0.7 | 0.7-0.8 |
| 5 or more times per week | 0.6 | 0.5-0.6 |
| Smoking | ||
| Never | 1.0 | reference |
| Past | 1.3 | 1.2-1.4 |
| Current | 1.1 | 1.0-1.3 |
| Alcohol consumption | ||
| Never/rarely | 1.0 | reference |
| 1-3 times per month | 1.1 | 1.0-1.2 |
| Once per week | 1.0 | 0.9-1.0 |
| 2-4 times per week | 0.9 | 0.8-0.9 |
| 5-6 times per week | 0.9 | 0.8-0.9 |
| Daily | 0.9 | 0.8 - 0.9 |
| Multivitamin use | 1.1 | 1.1-1.1 |
| Regular NSAIDs use (on > 60 days during last 12 months) | 1.5 | 1.4-1.5 |
| History of arthritis | 1.4 | 1.3-1.5 |
| Vegetables (per serving per day) | 1.00 | 0.97-1.02 |
| Fruits (per serving per day) | 0.94 | 0.91-0.97 |
Odds ratios and their 95% confidence intervals for having 2 or more metabolic abnormalities from generalized estimating equation model based on 353,371 individual questionnaires adjusting for intra-individual dependence of multiple (up to 20) questionnaires per person and all variables in the table
Vigorous enough to work up a sweat
The prevalence of having 2 or more metabolic abnormalities was highest in participants aged 70 to 80 years and seemed to decline thereafter. There was a strong inverse association between regular exercise and having 2 or more abnormalities with an OR of 0.6 (95% CI: 0.5-0.6) for those participants exercising 5 or more times a week compared with participants exercising less than once a week. We also observed inverse associations for an alcohol consumption of 2 or more times per week and for the number of servings of fruits consumed per day. Current (OR=1.1; 95% CI: 1.0-1.5) and especially past (OR=1.3; 95% CI: 1.2-1.4) smoking, use of nonsteroidal anti-inflammatory drugs (NSAIDs) on more than 60 days in the prior 12 months (OR=1.5; 95% CI: 1.4-1.5), and a history of arthritis (OR=1.4; 95% CI: 1.3-1.5) were all associated with having 2 or more metabolic abnormalities.
We observed 494 incident cases of colorectal cancer over a median follow-up period of 19 years and a total of 369,966 person-years. Study participants had a 5% reduced risk for colorectal cancer (SMR = 0.95; 95% CI: 0.87-1.04) compared with U.S. white men of the same age. Compared with an incidence rate of 100 per 100,000 person-years in physicians without any metabolic abnormality during follow-up, we found a monotonic increase in the rates of CRC over the number of metabolic abnormalities from 142 for 1 metabolic abnormality, to 192 and 194 per 100,000 for 3 and 4 metabolic abnormalities, respectively.
After adjusting for the increased prevalence of metabolic abnormalities with increasing age as well as after additional adjustment for all variables presented in tables 1 and 2, the hazard ratios or relative risks for 2 metabolic abnormalities and 3 or 4 metabolic abnormalities compared with no metabolic abnormality were both 1.4. After combining these 2 categories and allowing for up to 1 metabolic abnormality in the reference group, the hazard ratio for 2 or more abnormalities was 1.3 (95% CI: 1.1-1.6). Each additional metabolic abnormality was associated with an adjusted hazard ratio of 1.16 (95% CI: 1.05-1.29).
When we looked at each metabolic abnormality separately, a BMI of 27 kg/m2 or more and diabetes were independently associated with an increased risk for colorectal cancer with hazard ratios of 1.4 (95% CI: 1.1-1.7) and 1.5 (95% CI: 1.1-2.0), respectively. Elevated blood pressure was associated with an only slight increase in risk (hazard ratio = 1.1; 95% CI: 0.9-1.3) and hypercholesterolemia was associated with a slightly decreased risk for colorectal cancer with a hazard ratio of 0.9 (95% CI:0.7-1.1). Comparing this model with the model assessing the association of each additional metabolic abnormality with risk for colorectal cancer by likelihood ratio test, using individual metabolic abnormalities separately predicted colorectal cancer significantly better than just the number of metabolic abnormalities (p=0.02).
The results presented in table 3 were unchanged after stratification by randomized assignment to low-dose aspirin treatment. Secondary analyses including interaction terms between metabolic abnormalities and randomized aspirin assignment revealed generally stronger associations between metabolic abnormalities and colorectal cancer in participants randomized to aspirin compared with those randomized to placebo, but none of these interactions was statistically significant and we therefore have not presented the results from these post-hoc analyses.
Table 3.
Number of metabolic abnormalities, individual metabolic abnormalities, and incidence of colorectal cancer in the Physician’s Health Study
| Number of metabolic abnormalities | Person-years | Events | IR per 100,000 p-y | Age-adjusted | Fully-adjusted* | ||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||||
| None | 161,735 | 161 | 100 | 1.0 | reference | 1.0 | reference |
| 1 | 134,339 | 191 | 142 | 1.1 | 0.9-1.3 | 1.1 | 0.9-1.3 |
| 2 | 59,943 | 115 | 192 | 1.4 | 1.1-1.8 | 1.4 | 1.1-1.7 |
| 3 or 4 | 13,949 | 27 | 194 | 1.5 | 1.0-2.2 | 1.4 | 0.9-2.1 |
| 0 or 1 | 1.0 | reference | 1.0 | reference | |||
| 2 or more | 1.4 | 1.1-1.7 | 1.3 | 1.1-1.6 | |||
| Per abnormality | 1.19 | 1.07-1.32 | 1.17 | 1.06-1.30 | |||
| BMI≥27kg/m2 | 1.4 | 1.1-1.7 | 1.4 | 1.1-1.7 | |||
| Diabetes | 1.4 | 1.1-2.0 | 1.5 | 1.1-2.0 | |||
| Elevated blood pressure | 1.1 | 0.9-1.4 | 1.1 | 0.9-1.3 | |||
| Hypercholesterolemia | 0.9 | 0.7-1.2 | 0.9 | 0.7-1.1 | |||
Hazard ratios and their 95% confidence intervals from time-varying Cox proportional hazards model controlling for all variables presented in table 2: age (continuous, linear and squared), vigorous exercise (5 categories), smoking (never, former, current: 20 or more cigarettes/day, current: less than 20 cigarettes/day), alcohol consumption (7 categories), multivitamin use, regular NSAID use, history of arthritis, and consumption of fruits and vegetables (servings per day, continuous) ; all variables (metabolic abnormalities and covariates) were updated during follow-up whenever possible
Secondary analyses by sub-site revealed a somewhat stronger association of diabetes with colon cancer and a weaker association between diabetes and rectal cancer leading to an adjusted hazard ratio for each additional metabolic abnormality of 1.02 with a 95% CI of 0.80-1.29, which includes the point estimate for colorectal cancer, based on 103 events. Within the colon, results were similar for proximal (N=192) and distal (N=151) cancers.
We compare adjusted hazard ratios for incident colorectal cancer and myocardial infarction associated with the individual metabolic abnormalities and per abnormality in figure 1. A BMI of 27 or above and diabetes were associated with both an increased risk for colorectal cancer and myocardial infarctionand the confidence intervals of the estimates for colorectal cancer included the estimate for myocardial infarction. In contrast, elevated blood pressure and hypercholesterolemia were clearly associated with an increased risk for myocardial infarction, but not for colorectal cancer. Accordingly, the risk per metabolic abnormality was stronger for myocardial infarction than for colorectal cancer.
Figure 1.
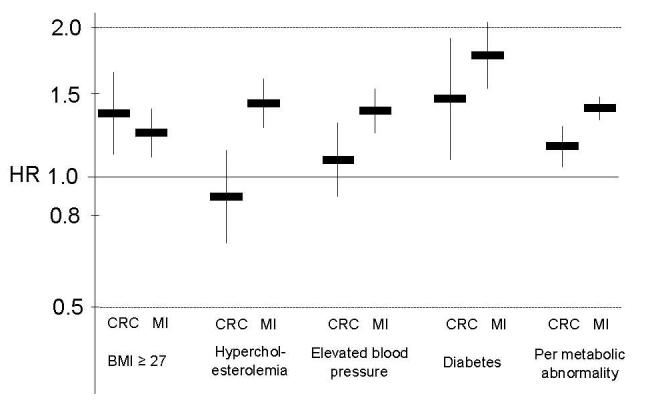
Similarities and discrepancies of hazard ratios for incident colorectal cancer and myocardial infarction associated with the individual metabolic abnormalities and per abnormality in the Physicians’ Health Study
DISCUSSION
In a large cohort study of men aged between 40 and 84 at baseline, the number of metabolic abnormalities used as indicators for the metabolic syndrome was associated with an increased risk for colorectal cancer over a follow-up period of 18 years. Elevated blood pressure and hypercholesterolemia were only marginally associated with colorectal cancer, however, and a model including each metabolic abnormality separately was more predictive for the incidence of colorectal cancer. Our results are in agreement with those in men in a recent cohort study (28) and the evidence base for these metabolic abnormalities and colorectal cancer. Overweight (at least in men) and diabetes are risk factors for colorectal cancer whereas an elevated blood pressure and hypercholesterolemia are not.
Diabetes has been repeatedly shown to be associated with colorectal cancer (e.g. 4, 13, 29, 30). Diabetes was for example associated with an increased risk for proximal colon cancer in the Iowa Women’s Health Study, but not distal colon or rectal cancer in women aged between 55 to 69 years at baseline over 14 years of follow-up (4). Similar findings were reported from the Nurses’ Health Study (30). Diabetes was also associated with an increased risk for colorectal cancer in the Cohort Study of Swedish Men over a mean follow-up of 6 years in men aged 45-79 years at baseline (29) and results were essentially the same for all sub-sites, including rectal cancer. In accordance with previous studies we observed diabetes to be a moderate risk factor for colorectal cancer.
Overweight, obesity, or body mass index have been consistently associated with increased risk for colorectal cancer incidence and mortality, at least in men and premenopausal women (12, 31-33). Body mass index was strongly associated with risk for colorectal cancer in the Physicians’ Health Study, despite the fact that we dichotomized this continuous variable at a body mass index of 27 kg/m2. We used this cut-point since it was associated with the highest sensitivity (68%) and specificity (90%) for a waist circumference of 40 inches or more, which is the measurement used to define the metabolic syndrome according to the NCEP guidelines (22,23). The moderate sensitivity indicates that a considerable proportion of physicians with a body mass index below 27 kg/m2 has a waist circumference of 40 inches or more which would tend to bias results towards observing no association. Any cut-point of inherently continuous traits is, however, arbitrary and waist circumference might not be the gold-standard to define the metabolic syndrome. The WHO definition of the metabolic syndrome, for example, allows the use of a body mass index of at least 30 kg/m2 instead of waist-circumference or waist-to-hip ratio (34). In accordance with previous studies in men, overweight was a moderate risk factor for colorectal cancer in the Physicians’ Health Study.
Elevated blood pressure or hypertension have not been consistently associated with cancer incidence or mortality (35, 36). Calcium and vitamin D deficiencies might lead to both elevated blood pressure and colorectal cancer (37-39) resulting in a positive association, although not necessarily a causal one. Interest in the hypertension-cancer association surged with the report of an association between calcium channel blockers and various cancers, including colorectal cancer, from the EPESE cohort studies (40). A recent meta-analysis did not report on colorectal cancer, however, because data were too limited to do so (41) and two recent reports found no association with colorectal cancer (42, 43). Based on self-reported blood-pressure values and self-reported use of antihypertensive medication, we observed no increased in risk for colorectal cancer associated with elevated blood pressure.
The association between hypercholesterolemia or blood lipids and colorectal cancer has raised considerable controversy over the past decades. Following an early report of an inverse association between (total) cholesterol levels and colon cancer mortality based on data from several cohort studies (e.g. 44), an increased colorectal cancer incidence associated with lower levels of serum cholesterol was observed in the Framingham cohort (15) and the Honolulu Heart Program (16). McMichael and Potter hypothesized that individuals with low serum cholesterol tend to have a more efficient hepatic clearance of cholesterol and, consequently, a raised biliary concentration of bile acids that may reach the colon and increase risk for colorectal cancer due to a range of adverse effects on the colonic mucosa (45). The inverse association between serum cholesterol and risk for colorectal cancer was observed to be strongest 6 or more years after cholesterol measurements in the NHANES I study, which seemed to make a preclinical cancer effect unlikely (17). Longer follow-up of these and other cohorts showed, however, that the association was more complex (18, 46) or even inexistent (e.g. 47). Nevertheless, low cholesterol values may be considered as an indicator of an increased risk for colorectal cancer over intermediate periods of time (48). This is in accordance with our results based on time-varying hypercholesterolemia, i.e. addressing short- and intermediate term effects rather than long-term effects.
Following early case-control studies on diet and colorectal cancer (e.g., 49, 50), recent work more specifically addressed the role of glycemic index (51), glycemic load (52), and markers of insulin or glucose control (1-3, 53-55) with respect to risk for colorectal cancer. Hyperinsulinemia might be the unifying mechanism by which several risk factors affect colorectal carcinogenesis (4). Following these considerations, we also addressed factors leading to or associated with hyperinsulinemia taking their clustering into account. We combined several metabolic abnormalities that are part of the metabolic syndrome into a single covariate assuming that these would have similar effects on the risk for colorectal cancer. This model worked well to detect an increased risk for colorectal cancer associated with increasing number of metabolic abnormalities, but because elevated blood pressure and hypercholesterolemia were not associated with risk for colorectal cancer, a model including diabetes and overweight as unique, independent covariates was more predictive.
There is considerable discussion with respect to the definition of the metabolic syndrome (7-11). A recent joint statement of the American Diabetes Association and the European Association for the Study of Diabetes concluded that the metabolic syndrome has not been well defined, that the pathophysiology is uncertain, and that its value as a marker for cardiovascular risk is not well established (10). Without entering the discussion with respect to cardiovascular risk, our results indicate that there might be similarities and differences in the clustering of risk factors for cardiovascular disease and colorectal cancer. Overweight and type 2 diabetes are both strongly associated with insulin resistance and the metabolic syndrome, overweight as a risk factor and diabetes as a marker (5, 56). Overweight and diabetes seem similarly related to cardiovascular disease than to colorectal cancer, at least in men (12). In contrast, elevated blood pressure and hypercholesterolemia may act differently indicating a lesser role of insulin resistance for colorectal cancer beyond the effects of obesity and type 2 diabetes alone. This discrepancy could also be due to a longer latency period necessary to affect colorectal cancer risk, the reduction of serum cholesterol due to preexisting disease long before the clinical manifestation of colorectal cancer (protopathic bias), or, more likely, to the specifics of the different organ systems. The cardiovascular system is directly exposed to blood pressure whereas the colon and rectum are directly exposed to the bowel contents.
Our study has to be interpreted taking its limitations into account. We did not have information on all items used to define the metabolic syndrome over the whole follow-up period and thus had to rely on metabolic abnormalities rather then these items themselves. In general, this would tend to bias results towards finding no association. Colorectal cancer was assessed by self-report and individuals with metabolic abnormalities might be in closer contact with the health care system and thus more likely to be diagnosed with colorectal cancer. In this highly screened population of physicians, however, these considerations might be less relevant. Any increased risk for colorectal cancer is likely to be the consequence of an increased risk of adenoma development or promotion (57) and might be attenuated with identification and removal of adenomas. Because the SMR for colorectal cancer in the PHS is very close to 1, these considerations seem to have no major impact on the incidence of colorectal cancer and results are therefore likely to be generalizable, at least to white men. Due to the time-varying analysis, we might have unduly adjusted for factors that are sequelae rather than predictors of metabolic abnormalities.
We cannot exclude residual confounding, e.g. by intensity of smoking beyond the categories used here and in prior work (58), physical activity, and by crude measures of diet. Because neither aspirin (59) nor non-aspirin NSAID use (60) were associated with risk for colorectal cancer in the PHS, residual confounding by NSAID use is unlikely despite its strong association with metabolic abnormalities. We assumed the items used to define metabolic abnormalities to be constant until they could be updated and analyzed short- and intermediate term effects of these metabolic abnormalities on risk for colorectal cancer. Both would tend to obscure associations due to long-term effects. Our assessment of hypercholesterolemia was mainly based on self-reports and the assumption that physicians that did not know or report their cholesterol values actually had normal values, unless they were taking lipid lowering drugs. Our secondary analyses indicate, however, that both our definitions of hypercholesterolemia and elevated blood pressure have predictive validity for incident myocardial infarction. Our results pertain to men and the metabolic syndrome might be a stronger risk factor for colorectal cancer in men than in women (28) due to sex differences in the effect of obesity and in the composition of the metabolic syndrome.
We conclude that only two metabolic abnormalities used to define the metabolic syndrome for risk prediction of cardiovascular disease, diabetes and overweight, are also associated with an increased risk for colorectal cancer in men, whereas elevated blood pressure and hypercholesterolemia are not. While generally similar, the clustering of metabolic risk factors for colorectal cancer might not be identical to the clustering of metabolic risk factors for cardiovascular disease.
Acknowledgments
Acknowledgements: We are indebted to the 22,071 study physicians for their dedicated and conscientious collaboration; to the staff of the Physicians’ Health Study, particularly Vadim Bubes, Cheryl Beseler, Rimma Dushkes, and Martin Van Denburgh; and to the BASF Corporation and Bristol-Myers Products for their logistic support.
Footnotes
Financial support: In part by research grants (CA-34944, CA-40360, HL-26490, and HL-34595) from the National Institutes of Health and AG-23178 from the National Institute on Aging, Bethesda, Maryland.
References
- 1.Schoen RE, Tangen CM, Kuller LH, et al. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999;91:1147–54. doi: 10.1093/jnci/91.13.1147. [DOI] [PubMed] [Google Scholar]
- 2.Kaaks R, Toniolo P, Akhmedkhanov A, et al. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000;92:1592–600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 3.Saydah SH, Platz EA, Rifai N, Pollak MN, Brancati FL, Helzlsouer KJ. Association of markers of insulin and glucose control with subsequent colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2003;12:412–8. [PubMed] [Google Scholar]
- 4.Limburg PJ, Anderson KE, Johnson TW, et al. Diabetes mellitus and subsite-specific colorectal cancer risks in the Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 2005;14:133–7. [PubMed] [Google Scholar]
- 5.Meigs JB, Wilson PWF, Fox CS, Vasan RS, Nathan DM, Sullivan L, D’Agostino RB. Body mass index, metabolic syndrome and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrin Metab. 2006 doi: 10.1210/jc.2006-0594. (epub) [DOI] [PubMed] [Google Scholar]
- 6.Bacha F, Saad R, Gungor N, Arslanian SA. Are Obesity-Related Metabolic Risk Factors Modulated by the Degree of Insulin Resistance in Adolescents? Diabetes Care. 2006;29:1599–1604. doi: 10.2337/dc06-0581. [DOI] [PubMed] [Google Scholar]
- 7.Reaven GM. The metabolic syndrome: is this diagnosis necessary? Am J Clin Nutr. 2006;83:1237–47. doi: 10.1093/ajcn/83.6.1237. [DOI] [PubMed] [Google Scholar]
- 8.Eckel RH, Grundy SM, Zimmet PZ. The metabolid syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 9.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 10.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal. Diab Care. 2005;28:2289–304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 11.Greenland P. Critical questions about the metabolic syndrome. Circulation. 2005;112:3675–3676. doi: 10.1161/CIRCULATIONAHA.105.583310. [DOI] [PubMed] [Google Scholar]
- 12.Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut. 2006;55:285–291. doi: 10.1136/gut.2005.073163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang CK, Ulrich CM. Hyperinsulinaemia and hyperglycaemia: possible risk factors of colorectal cancer among diabetic patients. Diabetologica. 2003;46:595–607. doi: 10.1007/s00125-003-1109-5. [DOI] [PubMed] [Google Scholar]
- 14.Brauer PM, McKeown-Eyssen GE, Jazmaji V, et al. Familial aggregation of diabetes and hypertension in a case-control study of colorectal neoplasia. Am J Epidemiol. 2002;156:702–13. doi: 10.1093/aje/kwf112. [DOI] [PubMed] [Google Scholar]
- 15.Williams RR, Sorlie PD, Feinleib M, McNamara PM, Kannel WB, Dawber TR. Cancer incidence by levels of cholesterol. JAMA. 1981;245:247–52. [PubMed] [Google Scholar]
- 16.Stemmermann GN, Nomura AM, Heilbrun LK, Pollack ES, Kagan A. Serum cholesterol and colon cancer incidence in Hawaiian Japanese men. J Natl Cancer Inst. 1981;67:1179–82. [PubMed] [Google Scholar]
- 17.Schatzkin A, Hoover RN, Taylor PR, et al. Site-specific analysis of total serum cholesterol and incident cancer in the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Cancer Res. 1988;48:452–8. [PubMed] [Google Scholar]
- 18.Nomura AM, Stemmermann GN, Chyou PH. Prospective study of serum cholesterol levels and large-bowel cancer. J Natl Cancer Inst. 1991;83:1403–7. doi: 10.1093/jnci/83.19.1403. [DOI] [PubMed] [Google Scholar]
- 19.Steering Committee of the Physicians’ Health Study Research Group Findings from the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129–35. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 20.Manson JE, Buring JE, Satterfield S, Hennekens CH. Baseline characteristics of participants in the Physicians’ Health Study: a randomized trial of aspirin and beta-carotene in U.S. physicians. Am J Prev Med. 1991;7:150–4. [PubMed] [Google Scholar]
- 21.Christen WG, Gaziano JM, Hennekens CH, for the steering committee of physicians’ health study II Design of Physicians’ Health Study II-a randomized trial of beta-carotene, vitamin E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 2000;10:125–34. doi: 10.1016/s1047-2797(99)00042-3. [DOI] [PubMed] [Google Scholar]
- 22.National Institutes of Health . Third report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): executive summary. National Institutes of Health; Bethesda, MD: 2001. NIH publ. no. 01-3670. [Google Scholar]
- 23.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 24.Scranton RE, Sesso HD, Glynn RJ, et al. Characteristics associated with differences in reported versus measured total cholesterol among male physicians. J Prim Prev. 2005;26:51–61. doi: 10.1007/s10935-004-0991-z. [DOI] [PubMed] [Google Scholar]
- 25.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–60. [PubMed] [Google Scholar]
- 26.Anderson PK, Gill RD. Cox’s regression model for counting processes: a large sample study. Ann Stat. 1992;10:1100–20. [Google Scholar]
- 27.Stürmer T, Schneeweiss S, Brookhart MA, Rothman KJ, Avorn J, Glynn RJ. Analytic strategies to adjust confounding using exposure propensity scores and disease risk scores: Nonsteroidal antiinflammatory drugs and short-term mortality in the elderly. Am J Epidemiol. 2005;161:891–898. doi: 10.1093/aje/kwi106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed RL, Schmitz KH, Anderson KE, Rosamond WD, Folsom AR. The metabolic syndrome and risk of incident colorectal cancer. Cancer. 2006;107:28–36. doi: 10.1002/cncr.21950. [DOI] [PubMed] [Google Scholar]
- 29.Larsson SC, Giovannucci E, Wolk A. Diabetes and colorectal cancer incidence in the cohort of Swedish men. Diabetes Care. 2005;28:1805–9. doi: 10.2337/diacare.28.7.1805. [DOI] [PubMed] [Google Scholar]
- 30.Hu FB, Manson JE, Liu S, et al. Prospective study of adult onset diabetes mellitus (type 2) and risk of colorectal cancer in women. J Natl Cancer Inst. 1999;91:542–7. doi: 10.1093/jnci/91.6.542. [DOI] [PubMed] [Google Scholar]
- 31.Slattery ML, Ballard-Barbash R, Edwards S, Caan BJ, Potter JD. Body mass index and colon cancer: an evaluation of the modifying effects of estrogen (United States) Cancer Causes Control. 2003;14:75–84. doi: 10.1023/a:1022545017867. [DOI] [PubMed] [Google Scholar]
- 32.Giovannucci E. diet, body weight, and colorectal cancer: a summary of the epidemiologic evidence. J Womens Health. 2003;12:173–82. doi: 10.1089/154099903321576574. [DOI] [PubMed] [Google Scholar]
- 33.El-Serag HB. Obesity and disease of the oesophagus and colon. Gastroenterol Clin North Am. 2005;34:63–82. doi: 10.1016/j.gtc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization . Definition, diagnosis, and classification of diabetes mellitus and its complications: report of a WHO consultation. World Health Org; Geneva: 1999. [Google Scholar]
- 35.Dyer AR, Berkson DM, Stamler J, Lindberg HA, Stevens E. High blood-pressure-risk factor for cancer mortality. Lancet. 1975;1:1051–6. doi: 10.1016/s0140-6736(75)91826-7. [DOI] [PubMed] [Google Scholar]
- 36.Gillis GR, Hole D, MacLean DS, Hawthorne VM, Watt HD, Watkinson G. Letter: High blood-pressure and cancer? Lancet. 1975;2(7935):612. doi: 10.1016/s0140-6736(75)90208-1. [DOI] [PubMed] [Google Scholar]
- 37.Grant WB. Epidemiology of disease risk in relation to vitamin D insufficiency. Prog Biophys Molecular Biol. 2006;92:65–79. doi: 10.1016/j.pbiomolbio.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Giovannucci E. Calcium plus vitamin D and the risk of colorectal cancer [letter] N Engl J Med. 2006;354:2287–8. [PubMed] [Google Scholar]
- 39.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentration of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 40.Pahor M, Guralnik JM, Ferrucci L, et al. Calcium-channel blockade and incidence of cancer in aged populations. Lancet. 1996;348:493–7. doi: 10.1016/S0140-6736(96)04277-8. [DOI] [PubMed] [Google Scholar]
- 41.Grossman E, Messerli FH, Boyko V, Goldbourt U. Is there an association between hypertension and cancer mortality? Am J Med. 2002;112:479–86. doi: 10.1016/s0002-9343(02)01049-5. [DOI] [PubMed] [Google Scholar]
- 42.Batty GD, Shipley MJ, Marmot MG, Smith GD. Blood pressure and site-specific cancer mortality: evidence from the original Whitehall study. Br J Cancer. 2003;89:1243–7. doi: 10.1038/sj.bjc.6601255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindgren AM, Nissinen AM, Tuomilehto JO, Pukkala E. Cancer pattern among hypertensive patients in North Karelia, Finland. J Hum Hypertension. 2005;19:373–9. doi: 10.1038/sj.jhh.1001834. [DOI] [PubMed] [Google Scholar]
- 44.Rose G, Blackburn H, Keys A, et al. Colon cancer and blood-cholesterol. Lancet. 1974;1(7850):181–3. doi: 10.1016/s0140-6736(74)92492-1. [DOI] [PubMed] [Google Scholar]
- 45.McMichael AJ, Potter JD. Host factors in carcinogenesis: certain bile-acid metabolic profiles that selectively increase the risk of proximal colon cancer. J Natl Cancer Inst. 1985;75:185–91. [PubMed] [Google Scholar]
- 46.Smith GD, Shipley MJ, Marmot MG, Rose G. Plasma cholesterol concentration and mortality. The Whitehall Study. JAMA. 1992;267:70–6. [PubMed] [Google Scholar]
- 47.Pekkanen J, Nissinen A, Punsar S, Karvonen MJ. Short- and long-term association of serum cholesterol with mortality. The 25-year follow-up of the Finnish cohorts of the seven countries study. Am J Epidemiol. 1992;135:1251–8. doi: 10.1093/oxfordjournals.aje.a116231. [DOI] [PubMed] [Google Scholar]
- 48.Song YM, Sung J, Kim JS. Which cholesterol level is related to the lowest mortality in a population with low mean cholesterol level: A 6.4-year follow-up study of 482,472 Korean men. Am J Epidemiol. 2000;151:739–47. doi: 10.1093/oxfordjournals.aje.a010272. [DOI] [PubMed] [Google Scholar]
- 49.Potter JD, McMichael AJ. Diet and cancer of the colon and rectum: a case-control study. J Natl Cancer Inst. 1986;76:557–69. doi: 10.1093/jnci/76.4.557. [DOI] [PubMed] [Google Scholar]
- 50.Trichopoulou A, Tzonou A, Hsieh CC, Toupadaki N, Manousos O, Trichopoulos D. High protein, saturated fat and cholesterol diet, and low levels of serum lipids in colorectal cancer. Int J Cancer. 1992;51:386–9. doi: 10.1002/ijc.2910510309. [DOI] [PubMed] [Google Scholar]
- 51.Giovannucci E. Glycemic index and colorectal carcinogenesis. Eur J Epidemiol. 2004;19:405–7. doi: 10.1023/b:ejep.0000027379.98449.d9. [DOI] [PubMed] [Google Scholar]
- 52.Michaud DS, Fuchs CS, Liu S, Willett WC, Colditz GA, Giovannucci E. Dietary glycemic load, carbohydrate, sugar, and colorectal cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2005;14:138–47. [PubMed] [Google Scholar]
- 53.Trevisan M, Liu J, Muti P, Misciagna G, Menotti A, Fucci F, Risk Factors and Life Expectancy Research Group Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol Biomarkers Prev. 2001;10:937–41. [PubMed] [Google Scholar]
- 54.Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:385–91. [PubMed] [Google Scholar]
- 55.Durai R, Yang W, Gupta S, Seifalian AM, Winslet MC. The role of the insulin-like growth factor system in colorectal cancer: review of current knowledge. Int J Colorectal Dis. 2005;20:203–20. doi: 10.1007/s00384-004-0675-4. [DOI] [PubMed] [Google Scholar]
- 56.Asao K, Kao WH, Baptiste-Roberts K, Bandeen-Roche K, Erlinger TP, Brancati FL. Short Stature and the Risk of Adiposity, Insulin Resistance, and Type 2 Diabetes in Middle Age: The Third National Health and Nutrition Examination Survey (NHANES III), 1988-1994. Diabetes Care. 2006;29:1632–7. doi: 10.2337/dc05-1997. [DOI] [PubMed] [Google Scholar]
- 57.Morita T, Tabata S, Mineshita M, Mizoue T, Moore MA, Kono S. The metabolic syndrome is associated with increased risk of colorectal adenoma development: the Self-Defense Forces health study. Asian Pac J Cancer Prev. 2005;6:485–9. [PubMed] [Google Scholar]
- 58.Stürmer T, Glynn RJ, Lee I-M, Christen WG, Hennekens CH. Lifetime cigarette smoking and colorectal cancer incidence in the Physicians’ Health Study I. J Natl Cancer Inst. 2000;92:1178–81. doi: 10.1093/jnci/92.14.1178. [DOI] [PubMed] [Google Scholar]
- 59.Stürmer T, Glynn RJ, Lee I-M, Manson JE, Buring JE, Hennekens CH. Aspirin use and colorectal cancer: post-trial follow-up data from the Physicians’ Health Study. Ann Intern Med. 1998;128:713–20. doi: 10.7326/0003-4819-128-9-199805010-00003. [DOI] [PubMed] [Google Scholar]
- 60.Stürmer T, Buring JE, Lee IM, Kurth T, Gaziano JM, Glynn RJ. Colorectal Cancer After Start of Nonsteroidal Anti-Inflammatory Drug Use. Am J Med. 2006;119:494–502. doi: 10.1016/j.amjmed.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


