Abstract
Background
As advances in genetics further our ability to identify genes influencing psychiatric disorders, the next challenge facing psychiatric genetics is to characterize the risk associated with specific genetic variants in order to better understand how these susceptibility genes are involved in the pathways leading to illness.
Methods
To further this goal, findings from behavior genetic analyses about how genetic influences act can be used to guide hypothesis testing about the effects associated with specific genes.
Results
Using the phenotype of alcohol dependence as an example, this paper provides an overview of how the integration of behavioral and statistical genetics can advance our knowledge about the genetics of psychiatric disorders. Areas currently being investigated in behavior genetics include careful delineation of phenotypes, to examine the heritability of various aspects of normal and abnormal behavior; developmental changes in the nature and magnitude of genetic and environmental effects; the extent to which different behaviors are influenced by common genes; and different forms of gene-environment correlation and interaction.
Conclusions
Understanding how specific genes are involved in these processes has the potential to significantly enhance our understanding of the development of psychiatric disorders.
Keywords: behavior genetics, twin studies, genetics, gene-environment interaction
INTRODUCTION
Despite strong evidence that genetic effects contribute to psychiatric phenotypes, detecting the specific genes involved has proven difficult. Early studies were plagued by small sample sizes and the use of statistical methods developed for single gene disorders that did not take into account the complexities introduced by multifactorial, polygenic disorders [1, 2]. Many of the factors that contributed to the failure of early studies aimed at gene identification have now have been addressed. The sample sizes used in studies aimed at gene identification have dramatically increased, and nonparametric, allele-sharing methods more appropriate for the analysis of complex phenotypes have been developed [3]. In conjunction with advances in genotyping technology and knowledge acquired from the Human Genome Project, there is reason to be enthusiastic about emerging successful gene identification efforts in complex disorders. Large, systematic collections of affected families informative for linkage and association analyses aimed at gene identification are on-going for most major psychiatric problems, including schizophrenia [4], bipolar disorder [5], autism [6], major depression [7], attention deficit hyperactivity disorder [8], alcoholism [9], and nicotine and other drug dependence [10]. These large collaborative projects promise a new era of gene discovery in relation to psychiatric disorders. Several reports of specific genes influencing psychiatric disorders, including alcohol dependence [11] and schizophrenia [12] have recently been published. The next challenge is to characterize the risk associated with these genetic variants in order to better understand how these susceptibility genes are involved in the pathways leading to illness. To further this goal, findings from behavior genetic analyses about how genetic influences act can be used to guide hypothesis testing about the effects associated with particular genes.
Using the phenotype of alcohol dependence as an example, this paper provides an overview of how the integration of behavioral and statistical genetics can advance our knowledge about the genetics of psychiatric disorders. Alcohol dependence provides a particularly relevant example, as this disorder embodies many of the complexities entailed in psychiatric disorders: multiple genes are thought to be involved, each of small effect; the environment plays an important role in the development of the disorder, as does gene-environment interaction [13]; genetic heterogeneity is thought to be involved; and there are multiple systems that have been suggested for defining the phenotype of alcohol dependence and related subtypes [14]. Despite these complications, this year has been witness to the publication of several reports of genes associated with alcoholism (GABRA2 [11], GABRG3 [15], CHRM2 [16]), accompanied by preliminary reports of replication for GABRA2 [17, 18], providing a timely context to discuss further characterization of risk associated with specific genes.
BEHAVIOR GENETIC CONTRIBUTIONS TO THE GENETICS OF SUBSTANCE USE
Behavior genetic (BG) research has convincingly demonstrated that genetic variation contributes to individual differences in virtually all behavioral domains [19–21]. Traditional twin analyses now have expanded to address questions about how genetic influences act [22]. Areas currently being investigated include careful delineation of phenotypes, to examine the heritability of various aspects of normal and abnormal behavior; developmental changes in the nature and magnitude of genetic and environmental effects; the extent to which different behaviors are influenced by common genes; and different forms of gene-environment correlation and interaction. Accordingly, findings emerging from behavior genetics can be used to develop and test hypotheses about the risk associated with specific genes identified through statistical and molecular genetic studies.
Refining Phenotypes
In relation to substance use, twin studies provide unambiguous evidence that genes play an important role in the development of alcohol dependence [23]. Genetic influences account for approximately 50–60% of the population variance in alcohol dependence [23]. Twin studies have also demonstrated that dimensions of alcohol use, such as quantity of alcohol consumed on a typical drinking occasion, frequency of use, frequency of intoxication, and alcohol metabolism measures, including time to peak blood alcohol concentration and rate of elimination, are under substantial genetic influence [24]. Although genes are known to influence both dependence and related substance use phenotypes, it is not clear to what extent these genes overlap [24]. As specific genes influencing alcohol dependence are identified, researchers will need to more explicitly demarcate the relationship between identified susceptibility genes and outcome. One question that needs to be addressed is the extent to which the effects of susceptibility genes influencing substance dependence also impact population variation in related behavioral phenotypes, such as indices of frequency/quantity of alcohol use among both affected and unaffected individuals: Do susceptibility genes contributing to clinically diagnosable dependence also contribute to problematic use at sub-clinical levels, to continuous variation in substance use across the population, and to the initiation and cessation of use?
In addition, alcohol dependence is a clinically heterogeneous disorder, with diagnoses based upon numerous behavioral components and symptoms that can vary between individuals. More complete understanding of the underlying processes contributing to dependence will arise from determining how susceptibility genes influence the characteristics that lead to a dependence diagnosis. For example, some susceptibility genes may be more related to loss of control when drinking, whereas others may be more closely related to the development of tolerance or withdrawal. It will be important to identify phenotypic subtypes that are related to susceptibility genes. Some susceptibility genes may be more closely linked to drinking that is accompanied by antisociality and criminality, whereas other susceptibility genes may be more involved in alcohol abuse related to anxiety and depression.
Evidence exists suggesting that genes may be more important in certain subtypes of alcoholism [25]. One classification system proposes two types of alcoholism: one characterized by early onset and comorbid antisociality and poor impulse control, and a second characterized by a later age of onset, and enhanced guilt and anxiety about drinking problems [26]. Preliminary genetic analyses support the hypothesis that susceptibility genes may differ for these subtypes of alcohol dependence. Genetic variation associated with the serotonin system appears to be particularly important in early-onset alcoholism accompanied by antisocial behavior and poor impulse control [27, 28]. An increased frequency of the short allele of the serotonin transporter gene was found among habitually violent, early onset alcoholics, compared to later onset alcoholics and controls [29]. Another serotonin receptor gene, HTR1B, has also been associated with antisocial alcohol dependence [30]. Other candidate genes for alcohol dependence, such as neuropeptide Y (NPY), also show more significant association with early onset alcohol dependence [31].
Developmental Genetic Analyses
Behavior genetic analyses have demonstrated that the effect of genes can vary dramatically across development. For example, there is evidence of genetic effects on patterns of alcohol use as early as adolescence, and these effects appear to increase over time [32]. Figure 1 combines data from two population-based longitudinal Finnish twin studies to show the relative importance of genetic and common environmental influences at ages 14, 16, 17, and 18.5. This figure demonstrates the significant increase in the importance of genetic influences across adolescence, and the corresponding decline in the importance of common environmental influences on drinking patterns across this developmental period. Genetic influences accounted for only 18% of the variance in drinking initiation at age 14, and this was significant only in girls [33]. There was no evidence of genetic influence on drinking patterns in boys yet at this early age. However, there is a steady increase in the relevance of genetic factors across adolescence, with genes accounting for a third of the variation in drinking patterns in both sexes by age 16, and half of the variation by age 18 [32]. Thus, as drinking patterns develop, differentiate, and stabilize across adolescence, genetic factors assume increasing importance on drinking patterns; however, alcohol use early in adolescence appears to be almost entirely influenced by family, school, and neighborhood influences [34].
Figure 1.
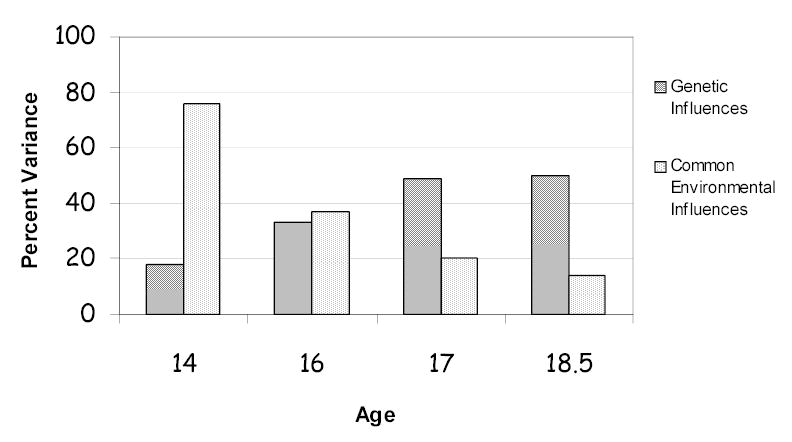
Data from the Finnish Twin Studies demonstrate the changing importance of genetic and common environmental influences across adolescence.
This is also true of alcohol dependence symptoms. Although alcohol dependence in adults is largely influenced by genetic factors, alcohol dependence symptoms in early adolescence appear to be largely influenced by environmental factors. In the same Finnish twin sample, 12% of the adolescents showed alcohol problems by age 14 (as indicated by the endorsement of DSM alcohol dependence symptoms); however, genetic analyses of alcohol dependence symptoms found no evidence of genetic effects in either males or females [35]. Alcohol dependence symptoms were entirely environmentally influenced at this age. Lack of evidence for genetic influence on alcohol dependence in early adolescence has been reported in other samples as well [36].
Thus, one developmental change that can occur is that the magnitude of genetic influence on a trait may vary across time. Another developmental change involves genetic influences being expressed as different phenotypes at different developmental stages. For example, childhood conduct disorder shows significant evidence of genetic influence across multiple samples, regardless of whether it is assessed retrospectively in adulthood [37, 38], prospectively in adolescence [35], or by child or parent report [39]. This finding is of particular interest because conduct disorder is a robust predictor of both concurrent and future alcohol problems, as demonstrated in both school-based and clinically-ascertained samples [36, 40–43]. However, the overlap between conduct disorder and adolescent alcohol problems appears to be entirely environmentally mediated (as no genetic effects are evident on alcohol dependence symptoms in early adolescence) [35], while the overlap between conduct disorder and adult alcohol dependence is largely attributed to shared genes [44]. A genome scan of retrospectively reported childhood conduct disorder identified linkage to a chromosomal region (2p) that also shows linkage to adult alcohol dependence [45]. These findings suggest that conduct disorder may be an adolescent manifestation of genes that later predispose to adult alcohol dependence. And further, these studies suggest that genes impacting adult alcohol dependence may be more closely related to conduct disorder in adolescence than to early adolescent problems and symptoms diagnosed as alcohol dependence, which appears to be largely caused by environmental factors. Only a small fraction of those who will receive a lifetime diagnosis of alcohol dependence fulfill criteria for alcohol dependence in early adolescence (and some do not even drink regularly). In short, this early onset form of alcohol dependence appears to have different determinants.
Interestingly, preliminary analyses of a subset of children who have been genotyped as part of the Collaborative Study on the Genetics of Alcoholism (COGA) sample, in which the GABRA2 association with adult alcohol dependence was first established [11], suggest that there are developmental changes in the risk associated with this gene. Although GABRA2 is associated with alcohol dependence in the adult sample, it does not appear to be significantly associated with alcohol dependence symptoms in the child sample. Rather, there was a significant association between GABRA2 and conduct disorder symptoms in the child COGA sample. These analyses support the suggestion from twin studies that conduct disorder may be an early adolescent manifestation of the genes that later influence adult alcohol dependence. Follow-up survival analyses examining the association between alcohol dependence and the GABRA2 genotype suggest that this relationship does not begin to emerge until later in adolescence, with the survival curves for alcohol dependence for individuals with different genotypes at GABRA2 not diverging until after age 15 (Figure 2) [46]. These analyses support twin studies finding that genetic influences on drinking behavior are not significant in early adolescence, but increase across adolescence and into adulthood.
Figure 2.
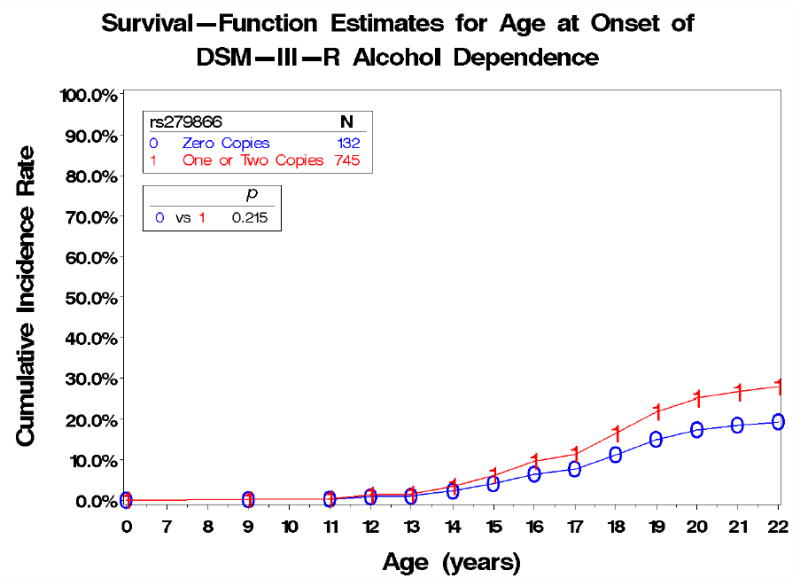
Survival–Function Estimates for Age at Onset of DSM–III–R Alcohol Dependence
Comorbidity Among Disorders
It is well known that many psychiatric disorders tend to co-occur. This is particularly true of alcohol dependence, which shows significant overlap with many other problems, including nicotine dependence [47], childhood externalizing disorders [42, 48], anxiety and mood disorders [49], and antisociality [50]. Behavior genetic models have investigated the degree to which covariation of different disorders or behaviors is due to common genetic influences, common environmental influences, or both. As an example, data from the Virginia Twin Registry was used to investigate genetic and environmental risk factors contributing to the pattern of comorbidity among 10 lifetime psychiatric disorders, including major depression, generalized anxiety disorder, phobia, alcohol dependence, drug abuse/dependence, adult antisocial behavior, and conduct disorder. It was concluded that “the pattern of lifetime comorbidity of common psychiatric and substance use disorders results largely from the effects of genetic risk factors” [51]. These findings suggest that some genes that are identified will contribute to multiple behavioral problems, whereas others will be more specific to a particular outcome. The term “co-morbidity” in itself may be misleading, as it implies the co-occurrence of independent disorders, whereas many psychiatric phenotypes, such as alcohol dependence, conduct disorder, and antisocial personality disorder, may represent alternative manifestations of behavioral disinhibitory processes [52, 53].
Data from specific gene studies are also accumulating to support these findings of significant genetic overlap suggested by twin data. As an example, the neurotransmitter dopamine is believed to play an important role in reward behavior [54]. An association between a particular dopamine receptor, DRD2, and alcoholism was first reported in 1990 [55], and subsequently has been replicated by several groups [56–64], although not others [65–84]. It has been suggested that DRD2 may contribute to a “reward deficiency syndrome”, a collection of addictive, impulsive, or compulsive behaviors, including alcoholism, polysubstance abuse, smoking, obesity, attention-deficit disorder, and gambling [85]. In addition, the CHRM2 gene, recently investigated in the COGA sample under a linkage peak, shows significant association with both alcohol dependence and major depressive disorder [16]. Many genes are likely to be involved in multiple psychiatric disorders, contributing to their co-occurrence. Once specific genes are identified, twin results suggesting that particular disorders/phenotypes overlap due to shared genes can be used to guide tests of the effects associated with any particular gene.
Gene-Environment Interaction and Correlation
Both genetic and environmental risk factors are known to contribute to the development of alcohol use and dependence, and more recent studies have examined the degree to which genetic and environmental influences may vary according to the environmental context. Data from the Australian twin registry provided early evidence of gene-environment interaction: genetic influences on alcohol use were attenuated among women who were married [86]. Religiosity also has been found to have a moderating effect on alcohol use among females [87]: in subjects without a religious upbringing, genetic effects played a large role, dwarfing the influence of shared environment; however, in individuals with a religious upbringing, there was no evidence of genetic influence, with the environment playing a large role instead. In adolescent data from the Finnish twin studies we have demonstrated dramatic changes in the importance of genetic influences in different environments. Genetic influences were found to have a stronger impact on alcohol use at age 16 among adolescents residing in urban environments, as compared to adolescents from rural environments [32]. This finding has been replicated in Minnesota twin data [88]. When we expanded our gene-environment interaction models to incorporate more continuous, descriptive measures of the environment, such as indices of neighborhood stability and regional alcohol sales, we found dramatic moderating effects, with more than five-fold differences in the degree of genetic influence evident across environmental extremes [13]. Adoption studies have also been particularly informative in delineating gene-environment interaction processes [89].
Genetic and environmental influences can also be related by gene-environment correlation. While gene-environment interaction can be conceptualized as genetic control of sensitivity to the environment (or environmental control of genetic expression), gene-environment correlation involves genetic control of exposure to the environment. An individual’s genotype can alter the probability that an individual will experience various environmental events. As an example, certain types of stressful life events have been shown to be moderately heritable [90]. Some individuals are more prone to experiencing stressful life events than others, and to the extent that individual dispositions influence an individuals’ exposure to certain events, these events show a degree of genetic influence. This represents gene-environment correlation: an individuals’ genotype is correlated with the likelihood of exposure to a particular environment. One could imagine that these processes may be particularly relevant to alcohol use, as individuals actively select peer groups [91] and other environmental settings that can differ in the availability of alcohol and/or acceptance of substance use. To the extent that genes influence individual predispositions and personality characteristics, these genes could be associated with the likelihood that individuals will select high risk environments that impact the subsequent development of problems.
Once specific genes begin to be identified we will be able to test specific gene-environment interaction and correlation processes. For example, preliminary analyses of the COGA sample suggest both gene-environment correlation and interaction between the GABRA2 genotype, marital status, and alcohol dependence. Marital status has consistently been related to alcohol use in the epidemiological literature, with married individuals consuming less alcohol than single or divorced individuals [92–94]. In the COGA sample, individuals who carry the high risk genotype at GABRA2 are more likely to be unmarried, providing an example of gene-environment correlation. Interestingly, this is not due simply to the association between GABRA2 and alcohol dependence, as the association between GABRA2 and marital status is more significant among nonalcoholic individuals. Instead, it appears to be mediated in part by an association between GABRA2 and reduced reward dependence and increased rates of antisocial personality disorder among individuals who carry the high risk genotype but are not alcoholic.
There is also evidence that marital status moderates the importance of the GABRA2 genotype (Figure 3). Among individuals who are not married, there is no difference in rates of alcohol dependence according to genotype; however, among individuals who are married, the effect of GABRA2 is magnified (an OR of 1.7 compared to 1.4 in the full sample). This interaction remains significant after controlling for age and gender. These analyses provide evidence of gene-environment interaction. The data suggest that being unmarried is a sufficiently potent environmental risk factor for individuals from these high risk families that the effect of the GABRA2 gene on alcohol dependence becomes negligible. The environment alone (or perhaps the cumulative consequences of a lifestyle) appears to be sufficient to drive individuals across the threshold for alcohol dependence. However, among individuals in the lower risk environment of being stably married, genetic factors become more apparent in the susceptibility for alcohol dependence; accordingly, the importance of GABRA2 on alcohol dependence is magnified in this environment [95].
Figure 3.
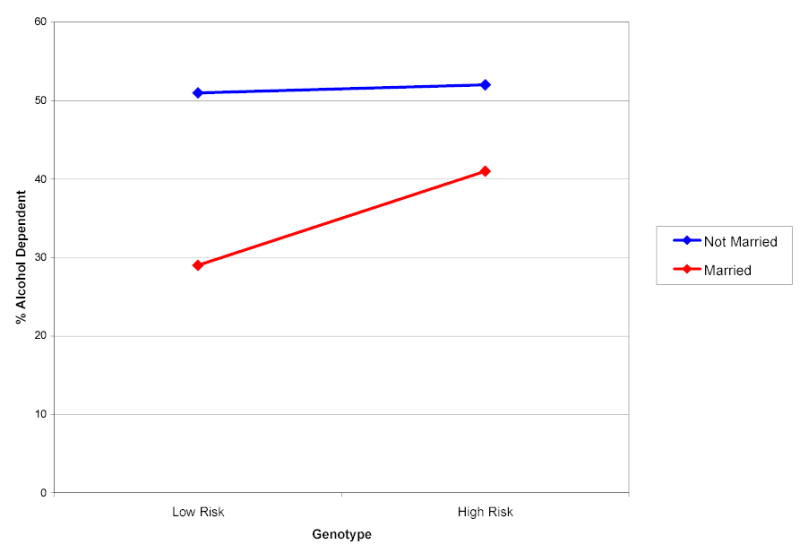
Rates of Alcohol Dependence as a Function of GABRA2 Genotype and Current Marital Status
CONCLUSIONS
Behavior genetic studies have been pivotal in demonstrating genetic influence on a variety of behavioral dimensions and in advancing our understanding of the dynamic nature of genetic influence. But traditional studies have modeled genetic influence latently, inferring genetic effects most often via comparisons of monozygotic and dizygotic twins, but also through family and adoption designs. Statistical and molecular genetic studies are making rapid progress in identifying genes involved in psychiatric disorders. The next step in advancing our understanding of genetic contributions to behavioral development must be to integrate these research traditions, incorporating knowledge from behavior genetics about the various heritable dimensions of behavior and how genetic influences act, in order to develop hypotheses to further delineate the risk associated with specific genes. For research on alcohol dependence, this will involve studying how genes identified as contributing to alcoholism also influence related behavioral traits, such as quantitative dimensions of alcohol use and smoking; what aspects of substance dependence these genes are most directly influencing; what related behavioral phenotypes these genes may also be involved in; how the influence of these specific genes changes across development; whether these genes are involved in different behaviors at different developmental stages; and how these genes are correlated with environmental risk factors and/or how the risk associated with these genetic variants may change in the presence or absence of particular environments.
More careful characterization of the risk associated with specific genes may also help resolve inconsistencies in association findings reported by different studies. Failure to consistently replicate genetic effects may result, in part, from differences in sample compositions between studies. Alcohol dependence is a heterogeneous disorder, diagnosed in the DSM-IV by the presence of any three of seven possible symptoms [96]. Accordingly, by definition, alcoholic individuals often have varying symptomatic profiles. If, for example, a particular gene is related most directly to alcohol withdrawal, study of a sample characterized by many alcoholics with withdrawal symptoms may detect an association between the gene and alcohol dependence, but a second sample with a smaller number of alcoholics who experienced withdrawal may fail to detect an association. More accurate characterization of the phenotypes most closely related to the gene could help clarify inconsistent findings. Similarly, if the effect of a particular gene is magnified or diminished in the presence of other environmental risk factors, studies that fail to measure and take into account the relevant environmental factors may differ in their ability to detect an association with the gene due to differences in the presence of the relevant environment among their study participants. Better characterization of the risk associated with specific genes would help reduce these problems as studies could be designed taking into account these other important variables.
In summary, delineating the risk specifically tied to particular genes across development, and in conjunction with environmental risk factors, has the potential to significantly enhance our understanding of the development of psychiatric disorders. The integration of behavioral and statistical genetics provides a promising framework within which to elucidate pathways of risk associated with genetic influences on psychiatric phenotypes.
Acknowledgments
The younger Finnish Twin Studies have been supported by NIAAA grants AA-08315, AA-09203, AA-12502, and by the Academy of Finland. The authors would like to thank Dr. Henri Begleiter for his helpful comments on an earlier version of this manuscript.
The Collaborative Study on the Genetics of Alcoholism (COGA) (Principal Investigator: H. Begleiter; Co-Principal Investigators: L. Bierut, H. Edenberg, V. Hesselbrock, B. Porjesz) includes nine different centers where data collection, analysis, and storage take place. The nine sites and Principal Investigators and Co-Investigators are: University of Connecticut (V. Hesselbrock); Indiana University (H. Edenberg,J. Nurnberger Jr., P.M. Conneally, T. Foroud); University of Iowa (S. Kuperman, R. Crowe); SUNY HSCB (B. Porjesz, H. Begleiter); Washington University in St. Louis (L. Bierut, A. Goate, J. Rice); University of California at San Diego (M. Schuckit); Howard University (R. Taylor); Rutgers University (J. Tischfield); Southwest Foundation (L. Almasy). Zhaoxia Ren serves as the NIAAA Staff Collaborator. This national collaborative study is supported by the NIH Grant U10AA08401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA). In memory of Theodore Reich, M.D., Co-Principal Investigator of COGA since its inception and one of the founders of modern psychiatric genetics, we acknowledge his immeasurable and fundamental scientific contributions to COGA and the field.
Footnotes
In press, the Annals of Clinical Psychiatry, special issue honoring Dr. Remi Cadoret
References
- 1.Egeland JA, Gerhard DS, Pauls DL, Sussex JN, Kidd KK, Allen CR, Hostetter AM, Housman DE. Bipolar affective disorders linked to DNA markers on chromosome 11. Nature. 1987;325(6107):p. 783–787. doi: 10.1038/325783a0. [DOI] [PubMed] [Google Scholar]
- 2.Kelsoe JR, Ginns EI, Egeland JA, Gerhard DS, Goldstein AM, Bale SJ, Pauls DL, Long RT, Kidd KK, Conte G, et al. Re-evaluation of the linkage relationship between chromosome 11p loci and the gene for bipolar affective disorder in the Old Order Amish. Nature. 1989;342(6247):p. 238–243. doi: 10.1038/342238a0. [DOI] [PubMed] [Google Scholar]
- 3.Risch N. Linkage strategies for genetically complex traits. II. The power of affected relative pairs. American Journal of Human Genetics. 1990;46:p. 229–251. [PMC free article] [PubMed] [Google Scholar]
- 4.Levinson DF, Holmans P, Straub RE, Owen MJ, Wildenauer DB, Gejman PV, Pulver AE, Laurent C, Kendler KS, Walsh D, Norton N, Williams NM, Schwab S, Lerer B, Mowry BJ, Sander A, Antonarakis SE, Blouin JL, DeLeuze JF, Mallet J. Multicenter linkage study of schizophrenia candidate regions on chromosomes 5q, 6q, 10p, and 13q: schizophrenia linkage collaborative group III. American Journal of Human Genetics. 2000;67:p. 652–663. doi: 10.1086/303041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nurnberger J, DePaulo JIJR, Gershon ES, Reich T, Blehar MC, Edenberg HJ, Foroud T, Miller M, Bowman E, Mayeda A, Rau NL, Smiley C, Conneally PM, McMahon F, Meyers D, Simpson S, McInnis M, Stine OC, Detera-Wadleigh S, Goldin L, Guroff J, Maxwell E, Kazuba D, Gejman PV, Badner J, Sanders A, Rice J, Bierut L, Goate A. Genomic survey of bipolar illness in the NIMH Genetics Initiative pedigrees: A preliminary report. American Journal of Medical Genetics. 1997;74:p. 227–237. doi: 10.1002/(sici)1096-8628(19970531)74:3<227::aid-ajmg1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 6.Hutcheson HB, Bradford Y, Folstein SE, Gardiner MB, Santangelo SL, Sutcliffe JS, Haines JL. Defining the autism minimum candidate gene region on chromosome 7. American Journal of Medical Genetics. 2003;117B:p. 90–96. doi: 10.1002/ajmg.b.10033. [DOI] [PubMed] [Google Scholar]
- 7.Levinson DF, Zubenko GS, Crowe R, DePaulo JR, Scheftner WS, Weissman MM, Holmans P, Zubenko WN, Boutelle S, Murphy-Eberenz K, MacKinnon DF, McInnis MG, Marta DH, Adams PB, Sassoon S, Knowles JA, Thomas J, Chellis J. Genetics of recurrent early-onset depression (Gen RED): design and preliminary clinical characteristics of a repository sample for genetic linkage studies. American Journal of Medical Genetics. 2003;119B:p. 118–130. doi: 10.1002/ajmg.b.20009. [DOI] [PubMed] [Google Scholar]
- 8.Faraone SV. Report from the 4th international meeting of the attention deficit hyperactivity disorder molecular genetics network. American Journal of Medical Genetics. 2003;121B:p. 55–59. doi: 10.1002/ajmg.b.20047. [DOI] [PubMed] [Google Scholar]
- 9.Begleiter H, Reich T, Hesselbrock V, Porjesz B, Li TK, Schuckit M, Ednberg HJ, Rice J. The Collaborative Study on the Genetics of Alcoholism. Alcohol Health & Research World. 1995;19:p. 228–236. [PMC free article] [PubMed] [Google Scholar]
- 10.http://www.drugabuse.gov/about/organization/Genetics/consortium/
- 11.Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe R, Goate A, Hesselbrock V, Jones KA, Kwon J, Li TK, Nurnberger JI, Jr, O’Connor SJ, Reich T, Rice J, Schuckit M, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the α2 subunit of the GABA-A receptor are associated with alcohol dependence and with brain oscillations. American Journal of Human Genetics. 2004;74:p. 705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Donovan MC, Williams NM, Owen MJ. Recent advances in the genetics of schizophrenia. Human Molecular Genetics. 2003;12(Spec No 2):R125–133. doi: 10.1093/hmg/ddg302. [DOI] [PubMed] [Google Scholar]
- 13.Dick DM, Rose RJ, Viken RJ, Kaprio J, Koskenvuo M. Exploring gene-environment interactions: Socioregional moderation of alcohol use. Journal of Abnormal Psychology. 2001;110:p. 625–632. doi: 10.1037//0021-843x.110.4.625. [DOI] [PubMed] [Google Scholar]
- 14.Hesselbrock, V., Genetic determinants of alcoholic subtypes, in The Genetics of Alcoholism, H. Begleiter and B. Kissen, Editors. 1995, Oxford Press: New York. p. 40–69.
- 15.Dick DM, Edenberg HJ, Xuei X, Goate A, Kuperman S, Schuckit M, Crowe R, Smith TL, Porjesz B, Begleiter H, Foroud T. Association of GABRG3 with alcohol dependence. Alcoholism: Clinical and Experimental Research. 2004;28:p. 4–9. doi: 10.1097/01.ALC.0000108645.54345.98. [DOI] [PubMed] [Google Scholar]
- 16.Wang JC, Hinrichs AL, Stock H, Budde J, Allen R, Bertelsen S, Kwon JM, Wu W, Dick DM, Jones K, Nurnberger JI, Jr, Tischfield JA, Porjesz B, Edenberg HJ, Hesselbrock V, Crowe R, Schuckit M, Begleiter H, Reich T, Goate A, Bierut L. Evidence of common and specific genetic effects: Association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Human Molecular Genetics. 2004;13:p. 1903–1911. doi: 10.1093/hmg/ddh194. [DOI] [PubMed] [Google Scholar]
- 17.Kranzler HR, Covault J, Gelernter J, Nellissery M. Allelic and haplotypic association of GABA alpha-2 gene with alcohol dependence. Alcoholism: Clinical and Experimental Research. 2004;28:p. 49A. [Google Scholar]
- 18.Xu K, Westly E, Taubman J, astor W, Lipsky RH, Goldman D. Linkage disequilibrium relationships among GABRA cluster genes located on chromosome 4 with alcohol dependence in two populations. Alcoholism: Clinical and Experimental Research. 2004;28:p. 48A. [Google Scholar]
- 19.McGuffin P, Riley B, Plomin R. Toward behavioral genomics. Science. 2001;291:p. 1232–1249. doi: 10.1126/science.1057264. [DOI] [PubMed] [Google Scholar]
- 20.McGue M, Bouchard TJJ. Genetic and environmental influences on human behavioral differences. Annual Review of Neuroscience. 1998;21:p. 1–24. doi: 10.1146/annurev.neuro.21.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Rose RJ. Genes and human behavior. Annual Review of Psychology. 1995;46:p. 625–654. doi: 10.1146/annurev.ps.46.020195.003205. [DOI] [PubMed] [Google Scholar]
- 22.Dick DM, Rose RJ. Behavior Genetics: What’s New? What’s Next? Current Directions in Psychological Science. 2002;11:p. 70–74. [Google Scholar]
- 23.McGue M. The behavioral genetics of alcoholism. Current Directions in Psychological Science. 1999;8:p. 109–115. [Google Scholar]
- 24.Heath, A.C., Genetic influences on drinking behavior in humans, in The Genetics of Alcoholism, H. Begleiter and B. Kissin, Editors. 1995, Oxford University Press: New York. p. 82–121.
- 25.Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse: Cross-fostering analysis of adopted men. Archives of General Psychiatry. 1981;38:p. 861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- 26.Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:p. 410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- 27.Virkkunen M, Linnoila M. Serotonin in early-onset alcoholism. Recent Development in Alcoholism. 1997;13:p. 173–189. doi: 10.1007/0-306-47141-8_10. [DOI] [PubMed] [Google Scholar]
- 28.Virkkunen M, Goldman D, Linnoila M. Serotonin in alcoholic violent offenders. Ciba Found Symp. 1996;194:p. 168–177. doi: 10.1002/9780470514825.ch10. [DOI] [PubMed] [Google Scholar]
- 29.Hallikainen T, Saito T, Lachman HM, Volavka J, Pohjalainen T, Ryynanen OP, Kauhanen J, Syvalahti E, Hietala J, Tiihonen J. Association between low activity serotonin transporter promoter genotype and early onset alcoholism with habitual impulsive violent behavior. Molecular Psychiatry. 1999;4:p. 385–388. doi: 10.1038/sj.mp.4000526. [DOI] [PubMed] [Google Scholar]
- 30.Lappalainen J, Long JC, Eggert M, Ozaki N, Robin RW, Brown GL, Naukkarinen H, Virkkunen M, Linnoila M, Goldman D. Linkage of antisocial alcoholism to the serotonin 5-HT1B receptor gene in 2 populations. Archives of General Psychiatry. 1998;55:p. 989–994. doi: 10.1001/archpsyc.55.11.989. [DOI] [PubMed] [Google Scholar]
- 31.Ilveskoski E, Kajander OA, Lehtimaki T, Kunnas T, Karhunen PJ, Heinala P, Virkkunen M, Alho H. Association of neuropeptide Y polymporphism with the occurrence of type 1 and type 2 alcoholism. Alcoholism: Clinical and Experimental Research. 2001;25:p. 1420–1422. doi: 10.1097/00000374-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Rose RJ, Dick DM, Viken RJ, Kaprio J. Gene-environment interaction in patterns of adolescent drinking: Regional residency moderates longitudinal influences on alcohol use. Alcoholism: Clinical and Experimental Research. 2001;25:p. 637–643. [PubMed] [Google Scholar]
- 33.Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Drinking or abstaining at age 14: A genetic epidemiological study. Alcoholism: Clinical and Experimental Research. 2001;25:p. 1594–1604. [PubMed] [Google Scholar]
- 34.Rose RJ, Viken RJ, Dick DM, Bates J, Pulkkinen L, Kaprio J. It does take a village: Nonfamilial environments and children’s behavior. Psychological Science. 2003;14:p. 273–277. doi: 10.1111/1529-1006.03434. [DOI] [PubMed] [Google Scholar]
- 35.Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Nurnberger JI, Jr, Kaprio J. Genetic and environmental effects on conduct disorder, alcohol dependence symptoms, and their covariation at age 14. Alcoholism: Clinical and Experimental Research. 2004;28:p. 1541–1548. doi: 10.1097/01.alc.0000141822.36776.55. [DOI] [PubMed] [Google Scholar]
- 36.Kuperman S, Schlosser SS, Kramer JR, Bucholz KK, Hesselbrock V, Reich T, Reich W. Risk domains associated with an adolescent alcohol dependence diagnosis. Addiction. 2001;96:p. 629–636. doi: 10.1046/j.1360-0443.2001.96462911.x. [DOI] [PubMed] [Google Scholar]
- 37.Slutske WS, Heath AC, Dinwiddie SH, Madden PAF, Bucholz KK, Dunne MP, Statham DJ, Martin NG. Modeling genetic and environmental influences in the etiology of conduct disorder: A study of 2,682 adult twin pairs. Journal of Abnormal Psychology. 1997;106:p. 266–279. doi: 10.1037//0021-843x.106.2.266. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein RB, Prescott C, Kendler KS. Genetic and environmental factors in conduct problems and adult antisocial behavior among adult female twins. Journal of Nervous and Mental Disorders. 2001;189:p. 201–209. doi: 10.1097/00005053-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Simonoff E, Pickles A, Meyer JM, Silberg JL, Maes HH. Genetic and environmental influences on subtypes of conduct disorder behavior in boys. Journal of Abnormal Child Psychology. 1998;26:p. 495–509. [PubMed] [Google Scholar]
- 40.White HR, Zie M, Thompson W, Loeber R, Stouthamer-Loeber M. Psychopathology as a predictor of adolescent drug use trajectories. Psychology of Addictive Behaviors. 2001;15:p. 210–218. [PubMed] [Google Scholar]
- 41.Moss HB, Lynch KG. Comorbid disruptive behavior disorder symptoms and their relationship to adolescent alcohol use disorders. Drug & Alcohol Dependence. 2001;64:p. 75–83. doi: 10.1016/s0376-8716(00)00233-7. [DOI] [PubMed] [Google Scholar]
- 42.Molina BSG, Bukstein OG, Lynch KG. Attention-deficit/hyperactivity disorder and conduct disorder symptomatology in adolescents with alcohol use disorder. Psychology of Addictive Behaviors. 2002;16:p. 161–164. doi: 10.1037//0893-164x.16.2.161. [DOI] [PubMed] [Google Scholar]
- 43.Kuperman S, Schlosser SS, Kramer JR, Bucholz KK, Hesselbrock V, Reich T, Reich W. Developmental sequence from disruptive behavior diagnosis to adolescent alcohol dependence. American Journal of Psychiatry. 2001;158:p. 2022–2026. doi: 10.1176/appi.ajp.158.12.2022. [DOI] [PubMed] [Google Scholar]
- 44.Slutske WS, Heath AC, Dinwiddle SH, Madden PAF, Bucholz KK, Dunne MP, Statham DJ, Martin NG. Common genetic risk factors for conduct disorder and alcohol dependence. Journal of Abnormal Psychology. 1998;107:p. 363–374. doi: 10.1037//0021-843x.107.3.363. [DOI] [PubMed] [Google Scholar]
- 45.Dick DM, Li TK, Edenberg HJ, Hesselbrock V, Kramer JR, Foroud T. A genome-wide screen for genes influencing conduct disorder. Molecular Psychiatry. 2003;9:p. 81–86. doi: 10.1038/sj.mp.4001368. [DOI] [PubMed] [Google Scholar]
- 46.Dick DM, Bierut L, Fox L, Bucholz KK, Kramer JR, Kuperman S, Hesselbrock V, Schuckit M, Porjesz B, Nurnberger JI, Jr, Xuei X, Foroud T, Edenberg HJ. The involvement of GABA-A receptor genes in conduct disorder and other behavioral phenotypes in children. American Journal of Medical Genetics. 2004;130B:p. 96. [Google Scholar]
- 47.Meyer JM, Rumpf HJ, Schumann A, Thyrian JR, Hapke U. Strength of the relationship between tobacco smoking, nicotine dependence and the severity of alcohol dependence syndrome criteria in a population-based sample. Alcohol and Alcoholism. 2003;38:p. 606–612. doi: 10.1093/alcalc/agg122. [DOI] [PubMed] [Google Scholar]
- 48.Clark DB, Pollock N, Bukstein OG, Mezzich AC, Bromberger JT, Donovan JE. Gender and comorbid psychopathology in adolescents with alcohol dependence. Journal of the American Acedemy of Child and Adolescent Psychiatry. 1997;36:p. 1195–1203. doi: 10.1097/00004583-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: Results from the National Epidemiological Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2004;61:p. 807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 50.Grant BF, Stinson FS, Dawson DA, Chou SP, Ruan WJ, Pickering RP. Co-occurrence of 12-month alcohol and drug use disorders and personaltiy disorders in the Unites States: restuls from the National Epidemiological Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2004;61:p. 361–368. doi: 10.1001/archpsyc.61.4.361. [DOI] [PubMed] [Google Scholar]
- 51.Kendler KS, Prescott C, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:p. 929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 52.Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. American Journal of Medical Genetics. 2000;96:p. 684–695. [PubMed] [Google Scholar]
- 53.Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: a twin-family study. Archives of General Psychiatry. 2004;61:p. 922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- 54.Wise RA, Rompre PP. Brain dopamine and reward. Annual Review of Psychology. 1989;40:p. 191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 55.Blum K, Noble EP, Sheridan PJ, Montgomery A, Ritchie T, Jagadeeswaran P, Nogami H, Briggs AH, Cohn JB. Allelic association of human dopamine D2 receptor gene in alcoholism. Journal of the American Medical Association. 1990;263:p. 2055–2060. [PubMed] [Google Scholar]
- 56.Blum K, Noble EP, Sheridan PJ, Finley O, Montgomery A, Ritchie T, Ozkaragoz T, Fitch RJ, Sadlack F, Sheffield D. Association of the A1 allele of the D2 dopamine receptor gene with severe alcoholism. Archives of General Psychiatry. 1991;48:p. 409–416. doi: 10.1016/0741-8329(91)90693-q. [DOI] [PubMed] [Google Scholar]
- 57.Parsian A, Todd RD, Devor EJ, O’Malley KL, Suarex BK, Reich T, Cloninger CR. Alcoholism and alleles of th human D2 dopamine receptor locus. Archives of General Psychiatry. 1991;48:p. 655–663. doi: 10.1001/archpsyc.1991.01810310073013. [DOI] [PubMed] [Google Scholar]
- 58.Comings DE, Comings BG, Muhleman D, Dietz G, Shahbahrami B, Tast D, Knell E, Kocsis P, Baumgarten R, Kovacs BW. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorders. JAMA. 1991;266:p. 1793–1800. [PubMed] [Google Scholar]
- 59.Amadeo S, Abbar M, Fourcade ML, Waksman G, Leroux MG, Madex A, Selin M, Champiat JC, Brethome A, Leclaire Y, Castelnau D, Venisse JL, Mallet J. D2 dopamine receptor gene and alcoholism. Journal of Psychiatry Research. 1993;27:p. 173–179. doi: 10.1016/0022-3956(93)90005-m. [DOI] [PubMed] [Google Scholar]
- 60.Noble EP, Syndulko K, Fitch RJ, Ritchie T, Bohlman MC, Guth P, Sheridan PJ, Montgomery A, Heinzmann C, Sparkes RS. D2 dopamine receptor TawI A alleles in medically ill alcoholic and onalcoholic patients. Alcohol and Alcoholism. 1994;29:p. 729–744. [PubMed] [Google Scholar]
- 61.Neiswanger K, Hill SY, Kaplan BB. Association and linkage studies of the TAQI A1 allele at the dopamine D2 receptor gene in samples of female and male alcoholics. American Journal of Medical Genetics (Neuropsychiatric Genetics) 1995;60:p. 267–271. doi: 10.1002/ajmg.1320600402. [DOI] [PubMed] [Google Scholar]
- 62.Kono Y, Yoneda H, Sakai T, Nonomura Y, Inayama Y, Koh J, Sakai J, Inada Y, Imamichi H, Asaba H. Association between early-onset alcoholism and the dopamine D2 receptor gene. American Journal of Medical Genetics (Neuropsychiatric Genetics) 1997;74:p. 179–182. doi: 10.1002/(sici)1096-8628(19970418)74:2<179::aid-ajmg13>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 63.Ishiguro H, Arinami T, Akazawa S, Enomoto M, Mitushio H, Fujishiro H, Tada K, Akimoto Y, Mifune H, Shioduka S, Hamaguchi H, Toru M, Shibuya H. Association study between the -141C Ins/Del and TaqI A polymorphisms of the dopamine D2 receptor gene and alcoholism. Alcoholism: Clinical and Experimental Research. 1998;22:p. 845–848. [PubMed] [Google Scholar]
- 64.Higuchi S, Muramatsu T, Murayama M, Hayashida M. Association of structural polymorphism of the dopamine D2receptor gene and alcoholism. Biochemical and Biophysical Research Communications. 1994;204:p. 1199–1205. doi: 10.1006/bbrc.1994.2590. [DOI] [PubMed] [Google Scholar]
- 65.Bolos AM, Dean M, Lucase-Derse S, Ramsburg M, Brown GL, Goldman D. Population and pedigree studies reveal a lack of asociation between the D2 receptor gene and alcoholism. JAMA. 1990;264:p. 3156–3160. [PubMed] [Google Scholar]
- 66.Gelernter J, O’Malley S, Risch N, Kranzler HR, Krystal J, Merikangas K, Kennedy JL, Kidd KK. No association between an allele at the D2 dopamine receptor gene (DRD2) and alcoholism. JAMA. 1991;266(1801–1807) [PubMed] [Google Scholar]
- 67.Goldman D, Dean M, Brown GL, Bolos AM, Tokola R, Virkkunen M, Linnoila M. D2 dopamine receptor genotype and cerebrospinal fluid homovanillic acid, 5-hydroxyindoleacetic acid and 3-methoxy-4-hydroxyphenylglycol in alcoholics in Finland and the United States. Acta Psychiatrica Scandinavia. 1992;86:p. 351–357. doi: 10.1111/j.1600-0447.1992.tb03279.x. [DOI] [PubMed] [Google Scholar]
- 68.Schwab S, Soyka M, Niederecker M, SAckenheil M, Scherer J, Wilderauer DB. Allelic association of human dopamine D2-receptor DNA polymorphism ruled out in 45 alcoholics. American Journal of Human Genetics. 1991;49(Suppl):p. 203. [Google Scholar]
- 69.Turner E, Ewing J, Shilling P, Smith TL, Irwin M, Schuckit M, Kelsoe JR. Lack of association between an RFLP near the D2 dopamine receptor gene and severe alcoholism. Biological Psychiatry. 1992;31:p. 285–290. doi: 10.1016/0006-3223(92)90052-2. [DOI] [PubMed] [Google Scholar]
- 70.Cook BL, Wang ZW, Crowe RR, hauser R, Freimer M. Alcoholism and the D2 receptor gene. Alcoholism: Clinical and Experimental Research. 1992;16:p. 806–809. doi: 10.1111/j.1530-0277.1992.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 71.Suarez BK, Parsian A, Hampe CL, Todd RD, Reich T, Cloninger CR. Linkage disequilibria at the D2 dopmaine receptor locus (DRD2) in alcoholics and controls. Genomics. 1994;19:p. 12–20. doi: 10.1006/geno.1994.1005. [DOI] [PubMed] [Google Scholar]
- 72.Sander T, Harms H, Podschus J, Finckh U, Nickel B, Rolfs A, Rommelspacher H, Schmidt LG. Dopamine D1, D2, and D3 receptor genes in alcohol dependence. Psychiatric Genetics. 1995;5:p. 171–176. doi: 10.1097/00041444-199524000-00004. [DOI] [PubMed] [Google Scholar]
- 73.Lu RB, Ko HC, Chang FM, Castiglione CM, Schoolfield G, Pakstis AJ, Kidd JR, Kidd KK. No association between alcoholism and multiple polymorphisms at the dopamine D2 receptor gene (DRD2) in three distinct Taiwanese populations. Biological Psychiatry. 1996;39:p. 419–429. doi: 10.1016/0006-3223(95)00182-4. [DOI] [PubMed] [Google Scholar]
- 74.Chen CH, Chien SH, Hwu HG. Lack of association between TawI A1 allele of dopamine D2 receptor gene and alcohol-use disorders in atayal natives of Taiwan. American Journal of Medical Genetics (Neuropsychiatric Genetics) 1996;67:p. 488–490. doi: 10.1002/(SICI)1096-8628(19960920)67:5<488::AID-AJMG10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 75.Arinami T, Itokawa M, Komiyama T, Mitsushio H, Mori H, Mifune H, Hamaguchi H, Toru M. Association between severity of alcoholism and the A1 allele of the dopamine D2 receptor gene TaqI A RFLP in Japanese. Biological Psychiatry. 1993;33:p. 108–114. doi: 10.1016/0006-3223(93)90309-2. [DOI] [PubMed] [Google Scholar]
- 76.Parsian A, Cloninger CR, Zhang ZH. Functional variant in the DRD2 receptor promoter region and subtypes of alcoholism. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2000;96:p. 407–411. doi: 10.1002/1096-8628(20000612)96:3<407::aid-ajmg32>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 77.Lee JF, Lu RB, Ko HC, Chang FM, Yin SJ, Pakstis AJ, Kidd KK. No association between DRD2 locus and alcoholism after controlling the ADH and ALDH genotypes in Chinese Han population. Alcoholism: Clinical and Experimental Research. 1999;23:p. 592–599. [PubMed] [Google Scholar]
- 78.Sander T, Ladehoff M, Samochowiec J, Finckh U, Rommelspacher H, Schmidt LG. Lack of an allelic associatin between polymorphisms of the dopamine D2 receptor gene and alcohol dependence in the German population. Alcoholism: Clinical and Experimental Research. 1999;23:p. 578–581. [PubMed] [Google Scholar]
- 79.Gelernter J, Kranzler HR. D2 dopamine receptor gene (DRD2) allele and haplotype frequencies in alcohol dependent and control subjects: No association with phenotype or severity of phenotype. Neuropsychopharmacology. 1999;20:p. 640–649. doi: 10.1016/S0893-133X(98)00110-9. [DOI] [PubMed] [Google Scholar]
- 80.Waldman ID, Robinson BF, Rhee SH. A logistic regression extension of the transmission disequilibrium test for continuous traits: Application to linkage disequilibrium between alcoholism and the candidate genes DRD2 and ADH3. Genetic Epidemiology. 1999;17(Suppl 1):p. S379–S384. doi: 10.1002/gepi.1370170764. [DOI] [PubMed] [Google Scholar]
- 81.Lobos EA, Todd RD. Association analysis in an evolutionary context: Cladistic analysis of the DRD2 locus to test for association with alcoholism. American Journal of Medical Genetics (Neuropsychiatric Genetics) 1998;81:p. 411–419. [PubMed] [Google Scholar]
- 82.Goldman D, Urbanek M, Guenther D, Robin R, Long JC. Linkage and association of a functional DRD2 variant (Ser311Cys) and DRD2 markers to alcoholism, substance abuse and schizophrenia in Southwestern American Indians. American Journal of Medical Genetics (Neuropsychiatric Genetics) 1997;74:p. 386–394. doi: 10.1002/(sici)1096-8628(19970725)74:4<386::aid-ajmg9>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 83.Cruz C, Camarena B, Mejia JM, Paez F, Eroza V, de la Fuente JR, Kershenobich D, Nicolini H. The dopamine D2 receptor gene TaqI A1 polymorphism and alcoholism in a Mexican population. Archives of Medical Research. 1995;26:p. 421–426. [PubMed] [Google Scholar]
- 84.Chen WJ, Chen CH, Huang J, Hsu YPP, Seow SV, Chen CC, Cheng ATA. Genetic polymorphisms of the promoter region of dopamine D2 receptor and dopamine transporter genes and alcoholism among four aboriginal groups and Han Chinese in Taiwan. Psychiatric Genetics. 2001;11:p. 187–105. doi: 10.1097/00041444-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 85.Blum K, Cull JG, Braverman ER, Comings DE. Reward deficiency syndrome. American Scientist. 1996;84:p. 132–145. [Google Scholar]
- 86.Heath AC, Eaves LJ, Martin NG. Interaction of marital status and genetic risk for symptoms of depression. Twin Research. 1998;1:p. 119–122. doi: 10.1375/136905298320566249. [DOI] [PubMed] [Google Scholar]
- 87.Koopmans JR, Slutske WS, van Baal GCM, Boomsma DI. The influence of religion on alcohol use initiation: Evidence for genotype x environment interaction. Behavior Genetics. 1999;29:p. 445–453. doi: 10.1023/a:1021679005623. [DOI] [PubMed] [Google Scholar]
- 88.Legrand L, McGue M, Keyes M, Malone S, Iacono WG. Do rural environments constrain genetic expression of externalizing psychopathology. Behavior Genetics. 2003;33:p. 710. [Google Scholar]
- 89.Cadoret RJ, Winokur G, Langbehn D, Troughton E, Yates WR, Stewart MA. Depression spectrum disease, I: The role of gene-environment interaction. American Journal of Psychiatry. 1996;153:p. 892–899. doi: 10.1176/ajp.153.7.892. [DOI] [PubMed] [Google Scholar]
- 90.Kendler KS. Adversity, stress and psychopathology: A psychiatirc genetic perspective. International Journal of Methods in Psychiatric Research. 1995;5:p. 163–170. [Google Scholar]
- 91.Rose, R.J., How do adolescents select their friends? A behavior-genetic perspective, in Personality in the life course: Paths to successful development, L. Pulkkinen and A. Caspi, Editors. 2001.
- 92.Power C, Rodgers B, Hope S. Heavy alcohol consumption and marital status: Disentangling the relationship in a national study of young adults. Addiction. 1999;94:p. 1477–1487. doi: 10.1046/j.1360-0443.1999.941014774.x. [DOI] [PubMed] [Google Scholar]
- 93.Temple MT, Fillmore KM, Hartka E, Johnstone B, Leino EV, Motoyosh M. A meta-analysis of change in marital and employment status as predictors of alcohol consumption on a typical occasion. British Journal of Addiction. 1991;86:p. 1269–1281. doi: 10.1111/j.1360-0443.1991.tb01703.x. [DOI] [PubMed] [Google Scholar]
- 94.Wang J, El-Guebaly N. Sociodemographic factors associated with comorbid major depressive episodes and alcohol dependence in the general population. Canadian Journal of Psychiatry. 2004;49:p. 37–44. doi: 10.1177/070674370404900106. [DOI] [PubMed] [Google Scholar]
- 95.Dick, D.M., M. Schuckit, L. Bierut, L. Fox, J. Mullaney, V. Hesselbrock, J.I. Nurnberger Jr., L. Almasy, T. Foroud, H.J. Edenberg, and H. Begleiter, Marital status, alcohol dependence and GABRA2: Evidence for gene-environment correlation and interaction. 2004, under review. [DOI] [PubMed]
- 96.Diagnostic and Statistical Manual of Mental Disorders: Fourth Edition. 1994, American Psychiatric Association: Washington, DC.


