Abstract
Colloidal microgels have recently received attention as environmentally responsive systems and now are increasingly used in applications as carriers for therapeutic drugs and diagnostic agents. Synthetic microgels consist of a crosslinked polymer network that provides a depot for loaded drugs, protection against environmental hazards and template for post-synthetic modification or vectorization of the drug carriers. The aim of this manuscript is to review recent attempts to develop new microgel formulations for oral drug delivery, to design metal-containing microgels for diagnostic and therapeutic applications, and to advance approaches including the systemic administration of microgels. Novel nanogel drug delivery systems developed in the authors’ laboratory are discussed in details including aspects of their synthesis, vectorization and recent applications for encapsulation of low molecular weight drugs or formulation of biological macromolecules. The findings reviewed here are encouraging for further development of the nanogels as intelligent drug carriers with such features as targeted delivery and triggered drug release.
APPLICATIONS OF MICROGELS
Microscopic particles of hydrogels (microgels) are presently under intense investigation for drug delivery and controlled release of bioactive molecules. Hydrogels can be defined as cross-linked or interwoven polymeric networks, which absorb and retain large amounts of water. The water content in a hydrogel and crosslinking density can determine the drug transfer in and out of the gel. Microgels can be classified as neutral, anionic or cationic. Electrostatic repulsion of charged groups dispersed in the microgel will result in additional swelling of the elastic polymer network in aqueous media. Interactions with protonated network can be restricted by the pore size that biomolecules having net charge opposite to that of the microgel will accumulate only on the microgel surface without diffusion into the internal volume. Nevertheless, encapsulating of biomolecules, for example, plasmid DNA, into the flexible polymer network of microgels can provide another opportunity for encapsulation and protection of biomolecules. Complementary hydrophobic, hydrogen or coordination bonding may significantly affect release of a drug from a hydrogel. Hydrogels have the unique property of undergoing abrupt volume changes in response to environmental factors, such as temperature, ionic strength and pH [1]. Thus, controlled release of a drug depends on properties of the drug and polymer design of the microgel. Many new applications of microgels for drug delivery have been proposed and experimentally evaluated during the last few years [2,3].
Mucoadhesive microgels
Microgels composed of mucoadhesive polymers may be used to design new types of carriers for oral and non-oral drug delivery [4,5,6]. Understanding of the surface interactions between hydrophilic polymer surfaces and mucins could lead to improved adhesion. Alternatively, decoration of a microgel surface with tethers of linear or block copolymers containing anionic or thiolated moieties could provide interpenetration and anchoring within the mucus. Encapsulation of anticancer drug bleomycin in microgels was only possible during the microgel synthesis - via UV-initiated radical polymerization from metacrylic acid and ethylene glycol. When taken orally, these microgels were shown to preferentially release the drug at higher pH levels, such as in the small intestine [7]. This type of hydrogels could be promising carriers in attempts to expand the number of chemotherapeutics capable of being administered orally. A novel oral drug delivery system (DDS) based on polymer microgels of polyacrylic acid (PAA) and Pluronics has been recently discussed [8]. Nine Pluronic® copolymers ranging in nominal molecular weight and poly(propylene oxide) (PPO) and poly(ethylene oxide) (PEO) content were grafted to PAA with or without the use of a crosslinker, ethylene glycol dimethacrylate (EGDMA). The hydrogel elasticity increased with the PPO content in the copolymers, as well as in the presence of EGDMA. It was demonstrated that PAA bonded to the longest Pluronic® copolymers resulted in copolymeric gels with strongest mucoadhesive properties. An approach including thiomers in the design of a microparticulate delivery system for peptide drugs was recently developed [9]. Cysteine-modified PAA polymers were able to form disulfide bonds and intermolecular crosslinks with mucus material that significantly (14-fold) improved mucoadhesive properties of the thiolated microgels compared to particles comprising unmodified PAA.
Metal-containing nanocarriers
Metal-containing microgels have been recently developed and tested in a wide range of therapeutic and diagnostic applications including magnetic, photoactive or quantum dot nanogels [10,11,12]. The major approach employed polymer microgels as templates for the synthesis of metal nanoparticles impregnated into the polymer network. In the field of diagnosis, magnetic resonance imaging was one of the first and most developed applications of metal nanoparticles. Very new biosensors based on the optical properties of colloidal gold and fluorescent nanocrystals, called quantum dots, seem to be ready to be implemented in both diagnosis and in situ medical imaging [13,14]. For therapeutic applications, the metal-containing microgels could aid in treatment by delivering and releasing drugs in a controlled manner. Magnetically guided carriers or thermal responsive matrices, in which drug release is triggered by the heating of metal nanoparticles have provided effective examples of drug delivery applications, while more recently efforts to develop metal-containing vectors for transfection and vaccination have been multiplied. Microgel templates were particularly useful in the synthesis of polymer nanoparticles heavily loaded with monodisperse superparamagnetic iron(III) oxide. One of the most interesting challenges for the future could be the use of metal-containing nanoparticles for an innovative, effective and selective physical treatment of solid tumors via targeted intracellular hyperthermia.
Systemic drug delivery
Finally, hydrophilic and biocompatible microgels could provide a unique mode for targeted delivery of encapsulated drugs via blood circulation. In this paper, several recent examples of microgel polymeric carriers with an effective diameter <100 nm (nanogels) will be discussed. For earlier reviews on nanogels developed in our laboratory for systemic delivery of different classes of drugs, see [15]. Biological and pharmaceutical applications of microgels and structurally similar PEGylated nanoparticles have been recently comprehensively reviewed [16,17].
Nanocarriers are smaller than typical blood cells, such as erythrocytes or lymphocytes (ca. 7–10 μm). After intravenous injection, they can freely float in the bloodstream into the smallest vessels and capillaries and achieve site- or tissue-specific delivery by exploiting physiological clearance mechanisms. Release of the drug then occurs after uptake of the nanocarriers inside the cells of the targeted tissue. Unfortunately, most particulate DDSs are eliminated by the reticuloendothelial system (RES) within minutes after intravenous injection. These particles accumulate in the macrophages of liver and spleen by phagocytosis. Uptake of nanocarriers is caused by the adsorption of blood proteins to the particle surface. These proteins, such as immunoglobulins or components of the complement system are well-known opsonins promoting phagocytosis. To achieve long circulation time in blood and localization in non-RES tissues, surface modifications of nanocarriers using PEO polymers have been used extensively. In particular, nanocarriers with PEO polymer loops on their surface demonstrated a better protection from protein binding (opsonisation) and avoidance of RES than PEO chains in brush conformation because, compared to PEO polymer loops, the PEO brush was too soft for efficient shielding [18,19].
Polymeric nanogels
Microgels can be rendered sensitive to physiological conditions. A responsive drug release system is recognized as one of the most important technologies necessary for an intelligent drug delivery. It must be able to regulate drug release in response to external chemical, physical, or biological stimuli. Biocompatible PAA was commonly incorporated in hydrogels to introduce pH sensitivity. The PAA-based microgels can protect protein or other sensitive drugs against digestion by proteolytic/nucleolytic enzymes in the stomach, due to their low swelling ratio at a low pH. However, when the hydrogels pass through the GI tract they swell due to ionization of carboxylic acid groups at higher pH and become able to release drug from the polymer network. A novel type of nanosized polymeric microgel (nanogels) consisting of a crosslinked polymer network of polyionic segments, such as polyethylenimine (PEI) or PAA, and neutral segments, such as polyethylene glycol (PEG) or Pluronic®, was recently developed in our laboratory [20,21]. Some applications of these nanogels for drug delivery have been later demonstrated [15]. This structure offered many advanced features as a DDS including simplicity of formulation, exceptional dispersion stability, and storage in the freeze-dried form, to name a few. Freeze-dried nanogels can be stored at ambient temperature and easily resuspended in aqueous media forming particulate dispersions with the hydrodynamic diameter of 120–350 nm. Initially, cationic nanogels were designed for encapsulation of therapeutic antisense oligonucleotides (SODN). These polyanionic compounds could be loaded into nanogels by simply mixing the nanogel dispersion with SODN solution in aqueous media. Polyionic complexes of negatively charged SODN with protonated PEI backbone of nanogels formed spontaneously in a matter of minutes and this process was accompanied by a significant compaction of the nanogel network leading to an 8–10-fold volume reduction [22]. While swelling degree of nanogels depended on the number of crosslinks in polymer network (or PEG/PEI molar ratio), an average 100% reduction in diameter was observed in nanogels loaded with oppositely charged drugs. We have found that the minimal number of six crosslinks per PEI molecule was required to obtain nanogels with desirable mechanical properties. At this PEG/PEI molar ratio homologous macrogels demonstrated the highest swelling degree in the range of 30–40-fold [23]. Drug loading capacity of nanogels was as high as 30% of their dry weight. Thus, even compacted drug-loaded nanogel particles will retain water in a quantity about 2/3 of their weight in swollen form, which added significantly to the dispersion stability of nanogels.
SYNTHESIS OF NANOGELS
Chemical crosslinking is a highly versatile method of creating polymeric microgels with large pore sizes [24]. Crosslinks have to be present in a hydrogel in order to prevent dissolution of the hydrophilic polymer chains in aqueous media. Since it is advantageous for drug delivery applications that hydrogels be biodegradable, labile bonds are frequently introduced into the gels [25]. These bonds can be present either in the polymer backbone or in the crosslinks used to prepare the gel. In cationic nanogels, PEI and PEG polymer chains were crosslinked via urethane bonds, usually considered as stable links. However, due to the presence of highly protonated PEI, hydrolysis of these bonds was significantly accelerated, and the polymer network of nanogels could rapidly degrade in aqueous solution at the physiological pH during a period of about two weeks.
According to the previously described method, synthesis of nanogels was conducted in a dichloromethane (dichloroethane)-in-water emulsion, followed by evaporation of the solvent in vacuo and maturation of the nanogel network in aqueous solution [22]. This procedure was convenient, except it used organic solvents, a vacuum evaporation step, and produced particles with a wide size distribution. Another synthetic approach was recently employed to obtain nanogels using a simple procedure and without organic solvents. The general outline of the method is illustrated in Fig. (1). Initially, a Pluronic® block copolymer, both ends of which were activated by 1,1′-carbonyldiimidazole (A), was dissolved in water at concentration above its critical micellization concentration and thoroughly stirred so that a micellar solution had formed (B). A diluted aqueous solution of PEI was then added dropwise to the micellar solution and stirred overnight. During this procedure a covalently linked cationic polymer layer was formed on the Pluronic® micelles (C). The last step of the one-pot procedure consisted of the efficient crosslinking of the PEI layer by carbonylimidazole-activated PEG molecules to produce nanogels with narrow size distribution covered with PEO loops (D). Purification of nanogels from all reactants could be achieved in one dialysis step and after that products were lyophilized. Using this procedure, nanogels based on Pluronic® P85/PEG and F127/PEG have been obtained with final yields of 55 and 70% by weight and an average hydrodynamic diameter of 100 and 180 nm, respectively. A similar approach based on micellar properties of Pluronic® block copolymers was recently used in the synthesis of Pluronic®-PAA crosslinked micelles [26].
Fig. (1).
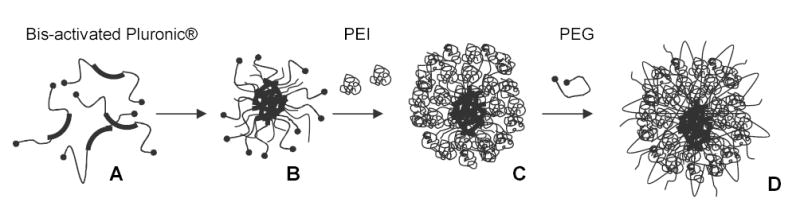
Scheme of nanogel synthesis using micellar approach. The carbonyldiimidazole-activated Pluronic® (A) formed micelles in aqueous solution (B) that were sequentially covered by a layer of branched PEI molecules (C) and crosslinked using carbonyldiimidazole-activated PEG (D).
To probe the structure of PEI-based nanogels, we have recently tagged them with heavy metals such as copper(II) that form coordination complexes with amino groups of PEI [23]. Copper (II) formed stable blue complexes with nanogels having absorbance at 280 nm. These complexes retained all drug-binding properties and did not affect particle size of nanogels. These tagged nanogels could be studied by transmission electron microscopy (TEM) as illustrated in Fig.(2). Initially, nanogels obtained by the emulsification-evaporation method showed particles with a well-developed surface and snowflake-like (PEG-cl-PEI) (Fig.2A) or sponge-like (Pluronic® P85-cl-PEI) (Fig.2C) structures. The white area were characteristic for locations of the PEG segments and grey area - for locations of the copper(II)-bound PEI segments. Drug-loading (ATP) has resulted in a compaction of nanogels into much smaller particles having only their dark cores visible (Fig.2B and Fig.2D). The neutral polymer envelope surrounding drug-PEI complexes was not observable at these conditions. In the case of nanogels obtained by the micellar approach, another type of structure was frequently detected (Fig.2E and Fig.2F). These donut-like structures could reflect an existence of a neutral hydrophobic Pluronic micellar core surrounded by a well-contrasted PEI layer.
Fig. (2).
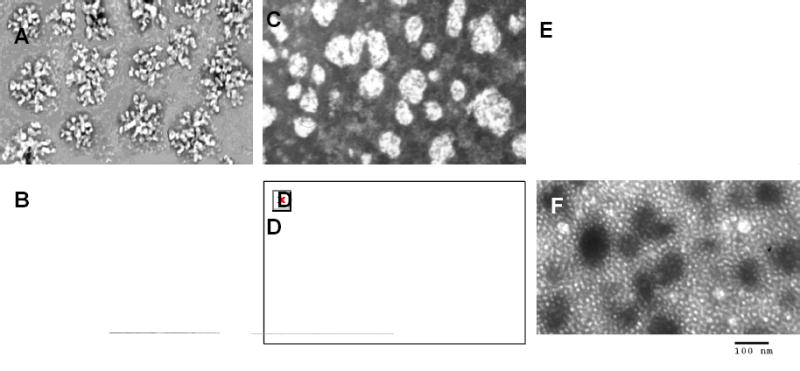
TEM pictures of nanogels: (A) PEG-cl-PEI, (B) ATP-loaded PEG-cl-PEI, (C) Pluronic® P85-cl-PEI, (D) ATP-loaded Pluronic® P85-cl-PEI, (E) Pluronic® F127-cl-PEI and (F) ATP-loaded Pluronic® F127-cl-PEI (the latter nanogel was obtained by micellar approach). All pictures are shown with the same magnification (× 53,000).
VECTORIZATION OF NANOGELS
Targeted DDS could be compared to microscopic pills delivered directly to the disease-affected site (organs, tumors or cancer metastases). The most effective method is to inject/infuse dispersion of submicron-sized drug-loaded particles into the blood stream. Floating nanoparticles, which are able to reach even smaller capillaries, would consequently accumulate in the sites where specific receptors are present to bind nanogel-targeting ligands. However, this strategy has posed various problems before developers of these DDS. These problems are very similar to those encountered by viruses soon after propagation in the blood. First, nanocarriers can interact with serum proteins forming complexes (opsonisation) or aggregates (agglutination). Next, the RES consisting mostly of macrophages is directed against the particles, and the complexes with serum proteins are efficiently seized from the circulation. Finally, nonspecific clearance of nanocarriers through kidney glomerules is observed in many circumstances [27]. Therefore, major requirements for an adequate systemic DDS include reducing the level of the carrier’s interaction with serum proteins, extended circulation time and small size, efficiently restricting them from renal clearance. All these requirements have once been met by PEG-covered (stealth) liposomes with the sizes between 100 and 200 nm [28]. Comparing nanogels with stealth liposomes, one can see many common properties. However, preliminary data on the in vivo properties of nanogels have shown that nanogels have a shorter plasma half-life than stealth liposomes, although both particles have longer half-lives than previously described nanoparticles. Nanogels can be additionally optimized for prolonged blood circulation in terms of size and PEG coverage, factors extensively studied previously for other cationic micellar systems [29,30,31]. A similar task of covering polymetacrylate microgels with tethered PEG chains possessing carboxylic groups was performed efficiently by using a α-vinylbenzyl-ω-carboxy-PEG in the emulsion polymerization procedure [32].
In previous research, we described biotinylated nanogels that could be vectorized via (strepta)avidin by biotinylated ligands (transferrin or insulin). Using these ligands, a significant increase of the blood-brain barrier (BBB) permeability for these SODN-loaded carriers was recently observed in the BBMEC monolayers, a cellular model of the BBB; transferrin and biotin were nearly equal in potency [33]. Although this system has demonstrated the targeting efficacy of nanogels, complications associated with slow aggregation of the nanogel-avidin constructs made the search for alternative targeting approaches a first priority. Recently, we have developed three novel methods for introduction of covalently linked targeting ligands in the nanogel structure. They included:
Attachment of folate moieties via water-soluble carbodiimide (Fig.3A). A simple modification of 1–5% of primary amino groups in nanogels with folic acid was performed using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) in aqueous media. To reduce problems associated with folate availability for corresponding cellular receptors, several authors recommended insertion of polymer linker between the folate moiety and drug carrier [34,35]. Recently obtained polymetacrylate microgels modified with folate demonstrated an increased and selective cellular uptake in cancer cell lines overexpressing folate receptors [36].
Modification with human transferrin (hTf) - a tumor-specific receptor target protein Fig.(3B). In this method, an amino group in the hTf protein is first reacted with a bifunctional reagent, sulfosuccinimidyl 4-(N-meleimidomethyl)-cyclohexane-1-carboxylate (SMCC), to obtain a maleimide derivative of hTf. Second, thiol groups are introduced into nanogels by reaction with 2-iminothiolane (Trout’s reagent). Finally, reaction between maleimide-hTf and thiol-nanogels yields hTf-nanogels with 4–12 hTf molecules per particle. Because of the protein size, most of hTf molecules are located on the surface of nanogels and allows for easy access to cellular transferrin receptors. Additionally, this procedure has also been applied to cancer-specific mAbs.
Introduction of protein/peptide ligands through a disulfide bridge using a PEG linker Fig.(3). First, a mono-N-acetylcystamine-PEG linker was prepared, activated by 1,1′-carbonyldiimidazole, and conjugated to a nanogel. Next, groups on the proteins/peptides were activated to form thiol-specific derivatives. These reactions included activation of amino groups with N-succinimidyl 3-(2-pyridyldithio)-propionate (SPDP), and thiol groups with 2, 2′-dipyridyldisulfide. Lastly, nanogels were treated with dithiothreitol (DTT), followed by thiol-specific protein/peptide derivatives to yield peptide-modified nanogels. Several short homing peptides and PSMA-specific mAbs have been conjugated to nanogels using this synthetic approach (data will be published elsewhere).
Fig. (3).
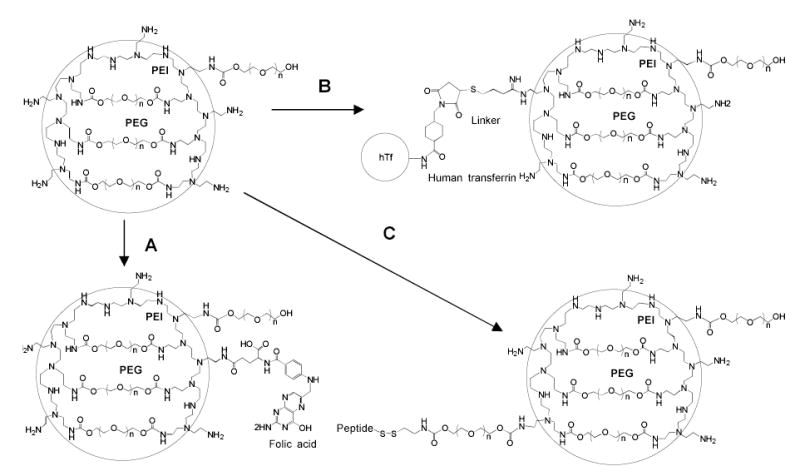
Scheme of the nanogel vectorization with targeting ligands. (A): Reaction of nanogel with folic acid and water-soluble carbodiimide (EDC) in aqueous medium, (B): Modification of (i) human transferrin (hTf) with a bifunctional reagent (SMCC), (ii) nanogel with 2-iminothiolane, and (iii) conjugation of thiolated-nanogel with maleimido-hTf, (C) Conjugation of (i) cystamine-PEG linker with nanogel, activation of thiolated peptide with 2,2′-dipyridinedisulfide, and (iii) nanogel with DTT, then (iv) preparation of the peptide conjugate.
DRUG DELIVERY OF SMALL MOLECULAR THERAPEUTICS
A significant progress was achieved in recent years in application of nanogels as DDS for small biologically active molecules. By contrast to SODN, these molecules usually contain only small number of ionic groups, which are able to interact with nanogels. It was evident that only ionic interactions between drug and nanogels could not provide complex stability at physiological conditions. Only if another force, like hydrophobic interactions between drug molecules, stabilized the complexes, could nanogels carry these small molecules. Initially, we successfully encapsulated retinoic acid into nanogels and obtained stable dispersions of the solubilized drug [37]. Retinoic acid was known to provide an anticancer effect and this formulation would be of interest for application in complex drug therapies [38]. Recently, a similar formulation of valproic acid (VA) in nanogels was prepared and studied in the cellular model of the BBB, BBMEC monolayers (Fig.4A). At the same low concentrations of tritium-labeled VA and VA-nanogel formulation, a more than 70% increase in the transcellular transport was observed when encapsulated drug was applied on the apical side of BBMEC monolayers. At chosen nanogel concentration no change in monolayer integrity was observed, therefore this effect could be explained only by a successful transcytosis of drug-loaded nanogels. We have already reported an increased transport of nanogels across BBMEC monolayers and an enhanced in vivo accumulation of the DDS in the brain [33]. Transfer of VA across the BBB was shown to be quite effective by means of a membrane-located carboxylic acid transporter (MCT1). However, at higher concentrations of VA in the blood, this transporter soon becomes saturated, and therapeutic doses in the brain do not accumulate [39,40]. Importantly, nanogel transport is evidently independent of this transporter; therefore, we could expect a significant amount of VA delivered to the brain using a nanogel formulation.
Fig. (4).
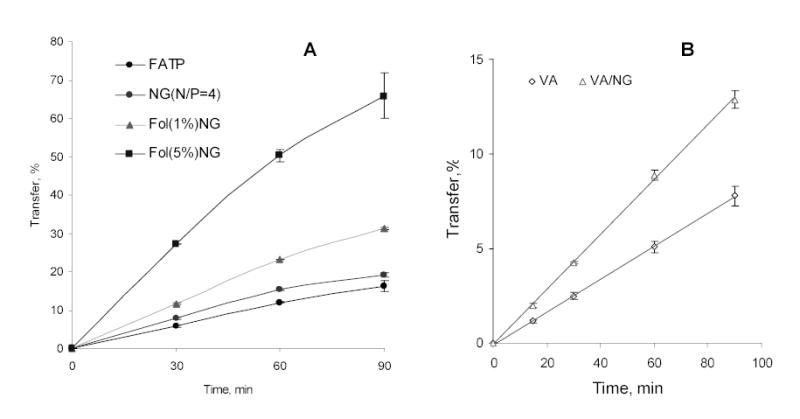
Study of drug delivery using drug-nanogel (NG) formulations in cellular models of the gastrointestinal tract (Caco-2) and of the blood-brain barrier (BBMEC): (A) Fludarabine 5′-triphosphate (FATP)/Caco-2 cells, and (B) valproic acid (VA)/BBMEC.
An anti-leishmaniasis drug, arjunglucoside I, was recently incorporated into nanogels composed of cross-linked random copolymers of N-isopropylacrylamide and N-vinyl pyrrolidone [41]. This formulation showed a two-fold enhanced therapeutic efficacy against parasites compared to free drug and a similar activity compared to the poly(lactic acid) (PLA) nanoparticles. Both DDS were found effective in reducing hepatotoxicity and nephrotoxicity of the drug to nearly the same extent. Similarly, PAA-Pluronic® microgels efficiently delivered encapsulated an anticancer drug doxorubicin overcoming the MDR-related efflux of this drug [42]. This type of microgels also demonstrated a low toxicity in cellular model of the gastrointestinal (GI) tract.
Another recently developed application of nanogels is delivery of nucleoside analogs. These anticancer drugs usually undergo complex transformation following administration to become an active compound. These drugs are first phosphorylated into nucleoside 5′-phosphates by intracellular nucleoside kinases. This initial phosphorylation is a critical step in anticancer treatment [43]. Further activation steps include formation of nucleoside 5′-diphosphates, conversion of ribonucleotides into deoxyribonucleotides by nucleoside reductases, and synthesis of nucleoside analog 5′-triphosphates (NTPs), which terminate cellular transcription and DNA replication events [44]. Previously, many of prospective nucleoside analogs were discarded in earlier preclinical studies, or withdrawn later from clinical studies, because their intracellular conversion into NTPs was insufficient in the acceptable dosage range. Thus, direct delivery of NTPs into cancer cells would be a promising strategy. Additionally, it can broaden considerably the choice of available efficient anticancer drugs. To test this hypothetical strategy, we prepared and studied nanogel formulations of cytotoxic NTPs. Several nucleoside analogs (Fludarabine, AZT and Cytarabine) were converted into NTPs and complexed with cationic nanogels. The cytotoxicity of these formulations tested in various cancer cell lines was about two orders of magnitude higher than that of the original drugs (Fig.5). This cytotoxic effect is independent of any an additional cytotoxicity of nanogels because similar ATP-nanogel complexes were shown to exhibit markedly lower cytotoxicity levels at higher concentrations. Besides, these complexes could be stored in dry form at 4°C without any trace of NTP decomposition at least for one year. Additionally, nanogels could deliver intact NTPs injected in the blood, providing sufficient protection of encapsulated drugs against phosphatases. As a proof of principle, folate-vectorized nanogels have shown clear advantages as a DDS for a fludarabine 5′-triphosphate in transport experiments using Caco-2 cell monolayers, a cellular model of GI tract (Fig.4B).
Fig. (5).
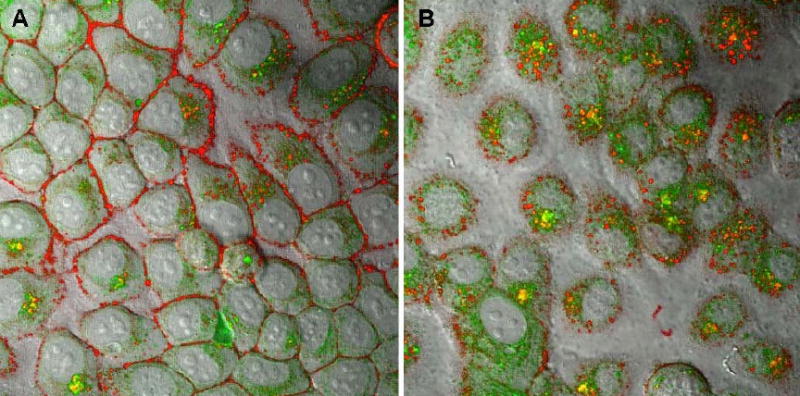
Trafficking of drug-loaded nanogel in human breast carcinoma MCF-7 cells. (A) 30 min, and (B) 60 min- treatment with ATP-BODIPY FL (green) encapsulated into Rhodamine-labeled nanogel (PEG-cl-PEI).
The mechanism of drug release from NTP-nanogel formulations is not yet fully understood. Evidently, a considerable amount of the drug was released in 5′-monophosphorylated form, which easily formed and dissociated from nanogels at the physiological conditions. However, a significant part (up to 50%) of the formulated drug could be untied only as the result of substitution of the bound NTP by competitive cellular (poly)anions. Previously, we suggested a mechanism of such substitution by interaction of nanogel network with phospholipids of the cellular membrane [15]. In the following experiment we traced the intracellular fate of rhodamine-labeled nanogels loaded with a fluorescein-labeled ATP (Fig.6). Initial accumulation of red nanogels on the cellular membrane was evident in these micrographs. This process was accompanied with a fast release of the green drug into cytosol. At a later time point, most of the drug-loaded nanogels, as well as membrane-bound unloaded nanogels, were taken inside by endocytosis (yellow dots), and accumulated in endosomes. However, the process of drug release continued inside the cells and a clearly higher level of green fluorescence was now observed in cytosol. This result was interpreted as an indication of the membrane-triggered drug release mechanism involved in the cellular dissociation of NTP-nanogel complexes. Additional studies should be performed to evaluate criteria that determine the relative importance of this mechanism in drug release from nanogel-based DDS.
Fig. (6).
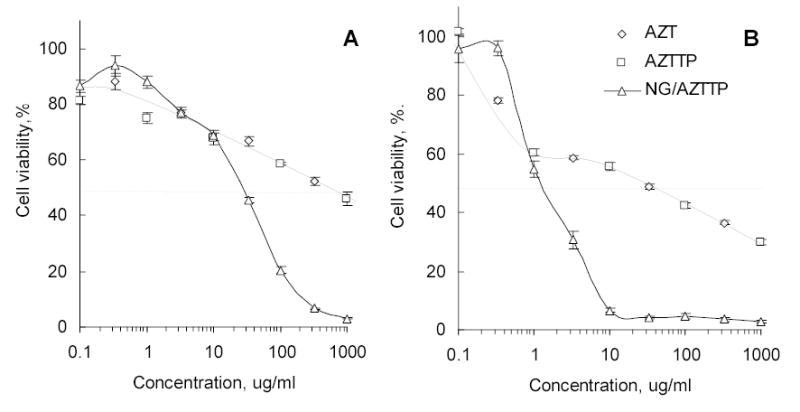
Cytotoxicity of 3′-azido-3′-deoxythymidine 5′-triphosphate (AZTTP) and its nanogel (PEG-cl-PEI) formulation in human breast carcinoma MCF-7 (A) and MDA-MB-231 (B) cells.
DELIVERY OF MACROMOLECULAR THERAPEUTICS
One of the most important features of a swollen nanogel particle with low number of crosslinks is the flexibility of its macroporous network in aqueous medium. Charged nanogel network can accommodate biomolecules penetrating the pores or form a kind of polymeric envelope surrounding larger biomolecules. Problems associated with the encapsulation of macromolecular drugs into microgels were critically reviewed recently [45]. One recent application of cationic nanogels is encapsulation of plasmid DNA (pDNA) for non-viral gene therapy or DNA vaccination. Our preliminary studies have demonstrated efficacy of nanogels for transfection of various cancer cells, although the observed level of transgene expression was significantly lower than that obtained with other transfection reagents, such as Lipofectine, Superfect, ExGene 500 or PEI 25kDa in the range of N/P ratios between 3 and 15. We first suggested an explanation related to the low surface charge of nanogels compared to those mostly highly cationic compounds. Since this charge was not increased significantly at high N/P ratios (>15), we did not expect an increase in transfection efficacy of pDNA-nanogel formulations at higher N/P ratio. However, we have found that transfection rose dramatically when N/P ratio reached to 25–30. This clearly meant a formation of some optimal complex between pDNA and nanogel network, confirmed also by dynamic light scattering data when size of the complexes stabilized at the lowest value for complexes prepared at N/P ratios ~ 25–30 (Fig.7A). We have calculated that at this N/P ratio an average swollen nanogel particle formed complex with only one molecule of supercoiled plasmid DNA. Thus, only at N/P ratio ≥ 25 could we obtain a population of pDNA-loaded nanogels with the smallest size, because at lower N/P ratios many nanogel particles had to accommodate two or more pDNAs molecules. This could also result in reduced stability of pDNA against nucleases, inactivation of the loaded pDNA and low levels of transfection (Fig.7B). Because the size of pDNA was estimated as about 110 nm from electron microscopy data, nanogel with an average diameter 150 nm could play the role of a soft pillow wrapping around anionic DNA strands. Additional modification of nanogels with human transferrin has helped to radically increase the transfection efficacy to the levels observed among the best known transfection reagents in the presence of serum (Fig.8A). Further optimization of the nanogel-based transfection system could be achieved by using efficient excipients or post-synthetic chemical derivatization. As an example, the partial complexation of pDNA with fish protamine (PA) before encapsulation into nanogels was shown to markedly increase expression level of transgene in human prostate carcinoma cells (Fig.8A). We hypothesized that a nuclear-localization signal sequence presented in PA, along with an additional nuclease protection of the PA-complexed pDNA in cytosol after release from nanogels, could increase chances for intact pDNA to reach the nucleus.
Fig. (7).
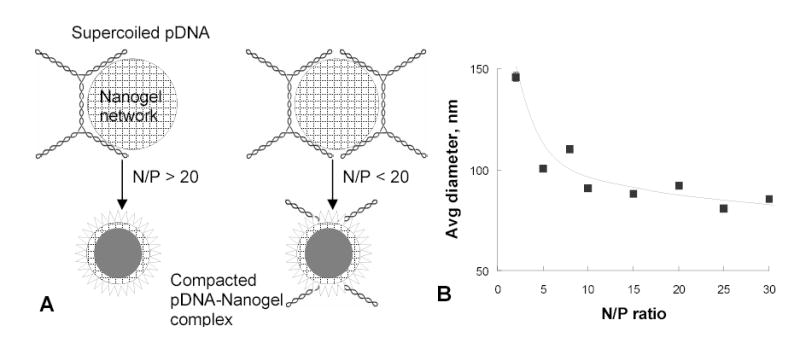
Formation of nanogel complexes with plasmid DNA (pDNA). (A) Schematic view of optimal (N/P>25) and non-optimal (N/P<25) formulations of pDNA with nanogels. (B) Size of the compacted pDNA-nanogel formulations as a function of N/P ratio at the complex formation.
Fig. (8).
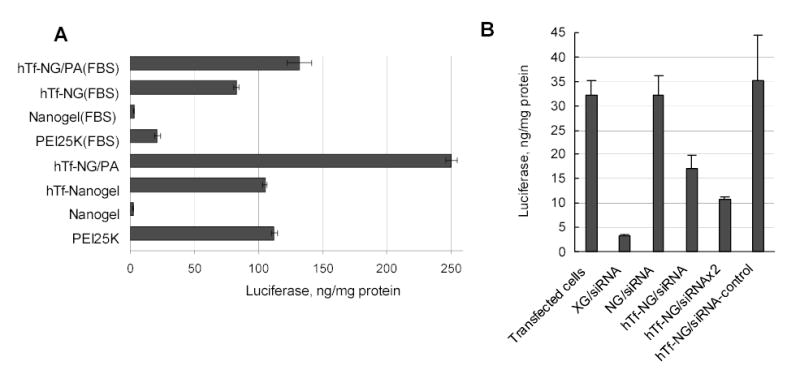
Transfection of human prostate carcinoma PC-3 cells with (A) luciferase-encoding pDNA in different nanogel formulations, and (B) siRNA-nanogel complexes for a suppression of the luciferase expression in transiently transfected PC-3 cells.
Vectorized nanogels could also be considered also as a prospective DDS for the novel class of potent gene inhibitors, small interfering RNAs (siRNAs). These double-stranded RNAs with a molecular mass less than 15kDa were able to penetrate into the large pores of nanogels and form polyionic complexes. We compared transfection efficacy of nanogels with commercial X-treme Gene reagent (La Roche) in prostate carcinoma PC-3 cell line and demonstrated the same level of inhibition (70–90%) in both cases at low concentrations of siRNA encapsulated in hTf-labeled nanogels (Fig.8B). This effect was sequence-specific, because no decrease in luciferase expression was detected in the case of nonspecific siRNA-loaded nanogels. We have previously confirmed nucleolytic protection of nanogel-loaded ASO. In the formulations with siRNA, not only was protection of RNA structure against degradation achieved, but also delivery of active double-stranded molecules of siRNAs into the cytosol of transfected cancer cells was also demonstrated. By contrast, another cationic carrier, modified polyacrylate microgel, which is among the most biocompatible and smallest particulate DDSs produced by reverse emulsion polymerization, was not capable of increased delivery of the biologically active free SODN into cytosol of treated cells [46].
CONCLUSIONS AND FUTURE PROSPECTS
Polymer microgels and nanogels as drug delivery vehicles have a unique set of properties that ideally fit conditions of drug transport via systemic routes of administration. They could be useful for the encapsulation of delicate compounds with low- or high molecular weights, significantly prolonging their activity in biological environments. Chemical engineering would further advance this drug delivery system by optimizing the properties of the nanogel carriers. Nanogels could be vector-driven carriers with high differential organs distribution ratios. Various mechanisms of drug release could be utilized by using microgels, from biodegradation to a stimuli-triggered release. Metal-microgel constructs could also provide additional ways to control drug release trace drug accumulation in targeted organs or provide new modalities of radioactive/cytotoxic cell treatment. Various fields of applications of these drug carriers are now extensively probed and moving closer to clinical practice.
Acknowledgments
Financial support was provided from the NIH grants (CA102791 and NS050660). Michael Jacobsen’s help in preparation of this manuscript is greatly appreciated.
References
- 1.Kaneko YS, K.; Okano, T. Temperature-responsive hydrogels as intelligent materials. In: Biorelated Polymers and Gels: Academic Press, 1998. pp. 29–66.
- 2.Kashyap N, Kumar N, Kumar MN. Hydrogels for pharmaceutical and biomedical applications. Crit Rev Ther Drug Carrier Syst. 2005;22 (2):107–49. doi: 10.1615/critrevtherdrugcarriersyst.v22.i2.10. [DOI] [PubMed] [Google Scholar]
- 3.Peppas NA, Wood KM, Blanchette JO. Hydrogels for oral delivery of therapeutic proteins. Expert Opin Biol Ther. 2004;4 (6):881–7. doi: 10.1517/14712598.4.6.881. [DOI] [PubMed] [Google Scholar]
- 4.Chowdary KP, Rao YS. Mucoadhesive microspheres for controlled drug delivery. Biol Pharm Bull. 2004;27 (11):1717–24. doi: 10.1248/bpb.27.1717. [DOI] [PubMed] [Google Scholar]
- 5.Alpar HO, Somavarapu S, Atuah KN, Bramwell VW. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv Drug Deliv Rev. 2005;57 (3):411–30. doi: 10.1016/j.addr.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Peppas NA, Huang Y. Nanoscale technology of mucoadhesive interactions. Adv Drug Deliv Rev. 2004;56 (11):1675–87. doi: 10.1016/j.addr.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Blanchette J, Peppas NA. Oral Chemotherapeutic Delivery: Design and Cellular Response. Annals of biomedical engineering. 2005;33 (2):142–9. doi: 10.1007/s10439-005-8973-8. [DOI] [PubMed] [Google Scholar]
- 8.Bromberg L, Temchenko M, Alakhov V, Hatton TA. Bioadhesive properties and rheology of polyether-modified poly(acrylic acid) hydrogels. Int J Pharm. 2004;282 (1–2):45–60. doi: 10.1016/j.ijpharm.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 9.Krauland AH, Bernkop-Schnurch A. Thiomers: development and in vitro evaluation of a peroral microparticulate peptide delivery system. Eur J Pharm Biopharm. 2004;57 (2):181–7. doi: 10.1016/j.ejpb.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Xu S, Kumacheva E. Polymer microgels: reactors for semiconductor, metal, and magnetic nanoparticles. J Am Chem Soc. 2004;126 (25):7908–14. doi: 10.1021/ja031523k. [DOI] [PubMed] [Google Scholar]
- 11.Sonvico F, Dubernet C, Colombo P, Couvreur P. Metallic colloid nanotechnology, applications in diagnosis and therapeutics. Curr Pharm Des. 2005;11 (16):2095–105. doi: 10.2174/1381612054065738. [DOI] [PubMed] [Google Scholar]
- 12.Arya H, Kaul Z, Wadhwa R, Taira K, Hirano T, Kaul SC. Quantum dots in bio-imaging: Revolution by the small. Biochem Biophys Res Commun. 2005;329 (4):1173–7. doi: 10.1016/j.bbrc.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa U, Nomura SM, Kaul SC, Hirano T, Akiyoshi K. Nanogel-quantum dot hybrid nanoparticles for live cell imaging. Biochem Biophys Res Commun. 2005;331 (4):917–21. doi: 10.1016/j.bbrc.2005.03.228. [DOI] [PubMed] [Google Scholar]
- 14.Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater. 2005;4 (6):435–46. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- 15.Vinogradov SV, Bronich TK, Kabanov AV. Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv Drug Deliv Rev. 2002;54 (1):135–47. doi: 10.1016/s0169-409x(01)00245-9. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54 (1):3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 17.Otsuka H, Nagasaki Y, Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliv Rev. 2003;55 (3):403–19. doi: 10.1016/s0169-409x(02)00226-0. [DOI] [PubMed] [Google Scholar]
- 18.De Jaeghere F, Allemann E, Feijen J, Kissel T, Doelker E, Gurny R. Cellular uptake of PEO surface-modified nanoparticles: evaluation of nanoparticles made of PLA:PEO diblock and triblock copolymers. J Drug Target. 2000;8 (3):143–53. doi: 10.3109/10611860008996860. [DOI] [PubMed] [Google Scholar]
- 19.Oupicky D, Ogris M, Howard KA, Dash PR, Ulbrich K, Seymour LW. Importance of lateral and steric stabilization of polyelectrolyte gene delivery vectors for extended systemic circulation. Mol Ther. 2002;5 (4):463–72. doi: 10.1006/mthe.2002.0568. [DOI] [PubMed] [Google Scholar]
- 20.Kabanov AV, Vinogradov SV, Alakhov YV. Nanogel networks and biological agent compositions. US Patent 6,333,051, 2001, Dec 25.
- 21.Kabanov AV, Vinogradov SV. Nanogel networks including polyion polymer fragments and biological agent compositions. US Patent 6,696,089, 2004, Febr 24.
- 22.Vinogradov S, Batrakova E, Kabanov A. Poly(ethylene glycol)-polyethylenimine NanoGel particles: novel drug delivery systems for antisense oligonucleotides. Colloids and Surfaces B: Biointerfaces. 1999;16 (4):291–304. [Google Scholar]
- 23.Vinogradov SV, Zeman AD, Batrakova EV, Kabanov AV. Polyplex Nanogel formulations for drug delivery of cytotoxic nucleoside analogs. J Controlled Release 2005;xx:xxx-xxx. [DOI] [PMC free article] [PubMed]
- 24.Hennink WE, van Nostrum CF. Novel crosslinking methods to design hydrogels. Adv Drug Deliv Rev. 2002;54 (1):13–36. doi: 10.1016/s0169-409x(01)00240-x. [DOI] [PubMed] [Google Scholar]
- 25.Park H, Temenoff JS, Holland TA, Tabata Y, Mikos AG. Delivery of TGF-beta1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials 2005. [DOI] [PubMed]
- 26.Bromberg L, Temchenko M, Alakhov V, Hatton TA. Kinetics of swelling of polyether-modified poly(acrylic acid) microgels with permanent and degradable cross-links. Langmuir. 2005;21 (4):1590–8. doi: 10.1021/la047893j. [DOI] [PubMed] [Google Scholar]
- 27.Mao HQ, Roy K, Troung-Le VL, Janes KA, Lin KY, Wang Y, August JT, Leong KW. Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Control Release. 2001;70 (3):399–421. doi: 10.1016/s0168-3659(00)00361-8. [DOI] [PubMed] [Google Scholar]
- 28.Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res. 2003;42 (6):463–78. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 29.Oupicky D, Ogris M, Seymour LW. Development of long-circulating polyelectrolyte complexes for systemic delivery of genes. J Drug Target. 2002;10 (2):93–8. doi: 10.1080/10611860290016685. [DOI] [PubMed] [Google Scholar]
- 30.Ogris M, Wagner E. Targeting tumors with non-viral gene delivery systems. Drug Discov Today. 2002;7 (8):479–85. doi: 10.1016/s1359-6446(02)02243-2. [DOI] [PubMed] [Google Scholar]
- 31.Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B. Immunomicelles: targeted pharmaceutical carriers for poorly soluble drugs. Proc Natl Acad Sci U S A. 2003;100 (10):6039–44. doi: 10.1073/pnas.0931428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi H, Iijima M, Kataoka K, Nagasaki Y. pH-Sensitive Nanogel Possessing Reactive PEG Tethered Chains on the Surface. Macromolecules. 2005;37 (14):5389–96. [Google Scholar]
- 33.Vinogradov SV, Batrakova EV, Kabanov AV. Nanogels for oligonucleotide delivery to the brain. Bioconjug Chem. 2004;15 (1):50–60. doi: 10.1021/bc034164r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leamon CP, Cooper SR, Hardee GE. Folate-liposome-mediated antisense oligodeoxynucleotide targeting to cancer cells: evaluation in vitro and in vivo. Bioconjug Chem. 2003;14 (4):738–47. doi: 10.1021/bc020089t. [DOI] [PubMed] [Google Scholar]
- 35.Shiokawa T, Hattori Y, Kawano K, Ohguchi Y, Kawakami H, Toma K, Maitani Y. Effect of polyethylene glycol linker chain length of folate-linked microemulsions loading aclacinomycin A on targeting ability and antitumor effect in vitro and in vivo. Clin Cancer Res. 2005;11 (5):2018–25. doi: 10.1158/1078-0432.CCR-04-1129. [DOI] [PubMed] [Google Scholar]
- 36.Nayak S, Lee H, Chmielewski J, Lyon LA. Folate-mediated cell targeting and cytotoxicity using thermoresponsive microgels. J Am Chem Soc. 2004;126 (33):10258–9. doi: 10.1021/ja0474143. [DOI] [PubMed] [Google Scholar]
- 37.Bronich TV, Vinogradov SV, Kabanov AV. Interaction of nanosized copolymer networks with oppositely charged amphiphilic molecules. NanoLetters. 2001;1 (10):535–40. [Google Scholar]
- 38.Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Annu Rev Nutr. 2004;24:201–21. doi: 10.1146/annurev.nutr.24.012003.132407. [DOI] [PubMed] [Google Scholar]
- 39.Bolanos JP, Medina JM. Effect of valproate on the metabolism of the central nervous system. Life Sci. 1997;60 (22):1933–42. doi: 10.1016/s0024-3205(96)00687-x. [DOI] [PubMed] [Google Scholar]
- 40.Fisher RS, Ho J. Potential new methods for antiepileptic drug delivery. CNS Drugs. 2002;16 (9):579–93. doi: 10.2165/00023210-200216090-00001. [DOI] [PubMed] [Google Scholar]
- 41.Tyagi R, Lala S, Verma AK, Nandy AK, Mahato SB, Maitra A, Basu MK. Targeted delivery of arjunglucoside I using surface hydrophilic and hydrophobic nanocarriers to combat experimental leishmaniasis. Journal of Drug Targeting. 2005;13 (3):161–71. doi: 10.1080/10611860500046732. [DOI] [PubMed] [Google Scholar]
- 42.Bromberg L, Alakhov V. Effects of polyether-modified poly(acrylic acid) microgels on doxorubicin transport in human intestinal epithelial Caco-2 cell layers. Journal of Controlled Release. 2003;88 (1):11–22. doi: 10.1016/s0168-3659(02)00419-4. [DOI] [PubMed] [Google Scholar]
- 43.Hatse S, De Clercq E, Balzarini J. Role of antimetabolites of purine and pyrimidine nucleotide metabolism in tumor cell differentiation. Biochem Pharmacol. 1999;58 (4):539–55. doi: 10.1016/s0006-2952(99)00035-0. [DOI] [PubMed] [Google Scholar]
- 44.Galmarini CM, Mackey JR, Dumontet C. Nucleoside analogues and nucleobases in cancer treatment. Lancet Oncol. 2002;3 (7):415–24. doi: 10.1016/s1470-2045(02)00788-x. [DOI] [PubMed] [Google Scholar]
- 45.Varde NK, Pack DW. Microspheres for controlled release drug delivery. Expert Opinion on Biological Therapy. 2004;4 (1):35–51. doi: 10.1517/14712598.4.1.35. [DOI] [PubMed] [Google Scholar]
- 46.McAllister K, Sazani P, Adam M, Cho MJ, Rubinstein M, Samulski RJ, DeSimone JM. Polymeric nanogels produced via inverse microemulsion polymerization as potential gene and antisense delivery agents. J Am Chem Soc. 2002;124 (51):15198–207. doi: 10.1021/ja027759q. [DOI] [PubMed] [Google Scholar]


