Abstract
The trillions of microbes that colonize our adult intestines function collectively as a metabolic organ that communicates with, and complements, our own human metabolic apparatus. Given the worldwide epidemic in obesity, there is interest in how interactions between human and microbial metabolomes may affect our energy balance. Here we report that, in contrast to mice with a gut microbiota, germ-free (GF) animals are protected against the obesity that develops after consuming a Western-style, high-fat, sugar-rich diet. Their persistently lean phenotype is associated with increased skeletal muscle and liver levels of phosphorylated AMP-activated protein kinase (AMPK) and its downstream targets involved in fatty acid oxidation (acetylCoA carboxylase; carnitine-palmitoyltransferase). Moreover, GF knockout mice lacking fasting-induced adipose factor (Fiaf), a circulating lipoprotein lipase inhibitor whose expression is normally selectively suppressed in the gut epithelium by the microbiota, are not protected from diet-induced obesity. Although GF Fiaf−/− animals exhibit similar levels of phosphorylated AMPK as their wild-type littermates in liver and gastrocnemius muscle, they have reduced expression of genes encoding the peroxisomal proliferator-activated receptor coactivator (Pgc-1α) and enzymes involved in fatty acid oxidation. Thus, GF animals are protected from diet-induced obesity by two complementary but independent mechanisms that result in increased fatty acid metabolism: (i) elevated levels of Fiaf, which induces Pgc-1α; and (ii) increased AMPK activity. Together, these findings support the notion that the gut microbiota can influence both sides of the energy balance equation, and underscore the importance of considering our metabolome in a supraorganismal context.
Keywords: AMP-activated protein kinase, fasting-induced adipose factor, fatty acid metabolism, gut microbiota, symbiosis
Although obesity stems from the interactions of genetic and environmental factors, its root cause is an excess of caloric intake over expenditure. The startling rise in the number of people who are obese, together with the inability of most individuals to comply with treatment regimens that require sustained lifestyle changes, has stimulated efforts to identify new therapeutic targets for the treatment and prevention of this pervasive disorder.
One potential target is our gut microbes. The distal human intestine can be viewed as an anaerobic bioreactor containing trillions of bacteria and archaea, programmed to perform metabolic functions that we have not been required to evolve on our own, including the ability to harvest otherwise inaccessible nutrients from our diet (1). By comparing germ-free (GF) and conventionally raised (CONV-R) mice, we have shown that the gut microbiota functions as an environmental factor that regulates fat storage (2). Colonization of adult GF C57BL/6J mice with a microbiota harvested from the distal intestine (cecum) of CONV-R animals (a process known as conventionalization) produces a significant increase in body-fat content, and relative insulin resistance within 14 days despite reduced food intake (2). This effect occurs in males and females belonging to several inbred strains of mice (2). Mechanistic studies revealed that the transplanted microbiota not only increases calorie harvest from dietary plant polysaccharides with glycosidic linkages that the host is ill-equipped to cleave with their own complement of glycoside hydrolases, but also modulates host genes that affect energy deposition in adipocytes. Colonization increases glucose uptake in the small intestine (2) as well as fermentation of carbohydrates to short-chain fatty acids (SCFAs) in the distal gut (3). SCFAs are absorbed with subsequent stimulation of de novo synthesis of triglycerides in the liver. In addition, the microbiota suppresses expression of fasting-induced adipose factor (Fiaf, also known as angiopoietin-like protein-4), a secreted lipoprotein lipase (LPL) inhibitor; this suppression is confined to the intestinal epithelium and does not occur at other sites where Fiaf is produced (liver and fat) (2). LPL functions in a number of cell lineages as the rate-limiting step for uptake of triglyceride-derived fatty acids (4, 5). By suppressing Fiaf, colonization increases LPL activity in adipocytes and enhances storage of liver-derived triglycerides (2). The physiologic importance of Fiaf was established by studying GF Fiaf−/− and wild-type littermates fed a standard low-fat polysaccharide-rich diet; GF knockout mice have the same degree of adiposity as their conventionalized counterparts, indicating that Fiaf is a key modulator of the microbiota-induced increase in fat storage (2).
Although LPL is the rate-limiting enzyme for import and subsequent storage of triglyceride-derived fatty acids in adipocytes, genetically engineered mice that express LPL only in their myocytes gain weight normally and have a normal body-mass composition. Instead of importing triglycerides from the circulation, they increase de novo fatty acid synthesis in adipose tissue (6). This finding raises the question of whether the lean phenotype of GF mice involves mechanisms beyond a Fiaf-mediated reduction in LPL activity.
AMP-activated protein kinase (AMPK) is a heterotrimeric enzyme that is conserved from yeast to humans and functions as a “fuel gauge” that monitors cellular energy status; it is activated in response to metabolic stresses that result in an increased intracellular ratio of AMP to ATP (e.g., exercise, hypoxia, and glucose deprivation; ref. 7). Adipocyte-derived leptin (8) and adiponectin (9), as well as an elevated NAD:NADH ratio (10), also increase AMPK activity. Activation of AMPK occurs by phosphorylation of Thr-172 in its catalytic α subunit (11, 12), leading to suppression of ATP-consuming anabolic pathways and induction of ATP-generating catabolic pathways (7).
Here, we show that GF mice are protected against obesity produced by consumption of a high-fat high-sugar Western diet. The mechanism involves AMPK and Fiaf operating through distinct pathways.
Results
GF Mice Are Protected Against Diet-Induced Obesity.
To determine whether GF mice are protected against diet-induced obesity, adult C57BL/6J males, maintained since weaning on an autoclaved low-fat chow diet (5% lipids; caloric density, 4.1 kcal/d), were conventionalized with an unfractionated cecal microbiota from a CONV-R donor that had also been fed low-fat chow. Three weeks later, half of the animals were switched to a “Western diet”, where 41% of the calories are in the form of fat; 41% as readily digested carbohydrates including simple sugars (sucrose); and 18% as protein (caloric density, 4.8 kcal/g). Chow consumption and weight gain were recorded weekly. After 8 weeks, conventionalized animals on the Western diet had gained significantly more weight than their GF counterparts (5.3 ± 0.8 vs. 2.1 ± 0.5 g; n = 5 mice per group; P < 0.05 according to Student's t test) (Fig. 1A). Weight gain in the GF group was not significantly different from the weight gain observed in GF mice that had been maintained on the standard low-fat polysaccharide-rich diet (data not shown). Epididymal fat-pad weights were also significantly greater in conventionalized mice fed the Western diet (37 ± 5 vs. 22 ± 1 mg/g of body weight; P < 0.05).
Fig. 1.
GF mice are protected against diet-induced obesity. (A) Adult male C57BL/6J mice were conventionalized 3 weeks before they were switched to a high-fat Western diet. Initial weight was recorded (25.5 ± 0.4 and 26.6 ± 0.7 g for GF and conventionalized mice, respectively). Weight gain was monitored weekly for 8 weeks and compared with GF mice (n = 5 per group). (B) Response to acute fat loading in GF and conventionalized mice maintained on a low-fat chow diet. Olive oil (400 μl) was administered by gavage to mice that had fasted overnight. Serum triglycerides levels were measured at the indicated time points (n = 5 per group). (C) Locomotor activity was recorded in low-fat chow-fed GF and conventionalized mice over a 3-day period and then again after they had been on a Western diet for 8 weeks (n = 4 per group). Mean values ± SE are plotted. ∗, P < 0.05; ∗∗, P < 0.01; and ∗∗∗, P < 0.001 compared with GF.
GF and conventionalized mice consumed similar amounts of the Western diet (2.61 ± 0.05 vs. 2.48 ± 0.07 g per mouse per day; n = 5 per group; P = 0.24), and there were no statistically significant differences in the energy content of their feces, as defined by bomb calorimetry (3.76 ± 0.01 vs. 3.81 ± 0.09 kcal/g; n = 5 per group; P = 0.53). Fatty acid absorption appeared to be similar in the two groups; when GF and conventionalized mice were given a single gavage of olive oil after a overnight fast, serum triglycerides rose rapidly over a 2-hr period, reaching equivalent levels in the two groups (Fig. 1B). However, although triglycerides were subsequently cleared from the circulation in conventionalized mice, they remained elevated in GF mice, a phenomenon that can be attributed to their reduced LPL activity (2). This decrease in LPL activity was also manifested by higher fasting serum triglyceride levels in GF compared with conventionalized mice on the Western diet (Table 1).
Table 1.
Biochemical and ELISA studies of sera obtained after a 4-hr fast from GF and conventionalized wild-type C57Bl/6J mice fed low- vs. high-fat diets
| Serum levels | Low-fat diet |
Western diet |
||||
|---|---|---|---|---|---|---|
| GF(n = 5) | Conventionalized(n = 5) | P value | GF(n = 8) | Conventionalized(n = 8) | P value | |
| Glucose, mM | 5.46 ± 0.16 | 7.28 ± 0.12 | 4E-05 | 10.4 ± 1.6 | 13.9 ± 1.2 | 0.04 |
| Insulin, ng/ml | 0.39 ± 0.03 | 0.85 ± 0.08 | 0.006 | 0.43 ± 0.07 | 0.75 ± 0.07 | 0.007 |
| Leptin, ng/ml | 1.65 ± 0.14 | 2.70 ± 0.02 | 0.023 | 2.01 ± 0.32 | 5.89 ± 0.60 | 0.004 |
| Triglycerides, mg/dl | 49 ± 4 | 39 ± 5 | 0.19 | 68.5 ± 3.8 | 50.4 ± 4.1 | 0.006 |
| Cholesterol, mg/dl | 110 ± 3 | 103 ± 18 | 0.17 | 173.5 ± 5.2 | 185.1 ± 7.9 | 0.51 |
| Free fatty acids, mM | 1.09 ± 0.12 | 1.01 ± 0.10 | 0.60 | 0.87 ± 0.08 | 1.07 ± 0.07 | 0.03 |
Mean values ± SE are shown.
GF Mice Have Increased Levels of Phosphorylated AMPK in Muscle and Liver.
To investigate whether AMPK is involved in mediating the resistance of GF mice to diet-induced obesity, we compared levels of phosphorylated (active) AMPK in gastrocnemius muscles harvested from GF and conventionalized animals on the Western diet. Immunoblots disclosed that phospho-AMPK concentrations are 40% higher in GF animals (n = 4 per group; P < 0.05; Fig. 2 A and B). There were no significant differences in the total level of immunoreactive AMPK α subunit (Fig. 2 A and B). Consistent with the elevations in phospho-AMPK, biochemical assays disclosed 50% higher levels of AMP in the gastrocnemius muscles of GF compared with conventionalized mice and no differences in ADP or ATP concentrations (Table 2).
Fig. 2.
The gut microbiota suppresses AMPK activity in the gastrocnemius muscle of mice consuming a Western diet. (A) Immunoblotting of protein lysates from gastrocnemius muscle harvested from 15-week-old male GF or conventionalized C57BL/6J mice fed a Western diet for 5 weeks before death. Representative results from two mice per group are shown. (B) Quantification of the results shown in A (n = 4 per group). Data are expressed relative to actin. (C) Effects of the gut microbiota on Cpt activity in freeze-clamped gastrocnemius muscle samples from the mice studied in A and B (n = 5 per group). ∗, P < 0.05 compared with GF; and ∗∗, P < 0.01.
Table 2.
Biochemical assays of various metabolites in gastrocnemius muscle and liver harvested from GF and conventionalized wild-type C57Bl/6J mice fed a Western diet
| Metabolite,μmol/g protein | Gastrocnemius muscle |
Liver |
||||
|---|---|---|---|---|---|---|
| GF | Conventionalized | P value | GF | Conventionalized | P value | |
| AMP | 6.14 ± 0.39 | 4.09 ± 0.42 | 0.01 | 20.08 ± 0.85 | 20.87 ± 1.60 | 0.68 |
| ADP | 6.36 ± 0.89 | 7.19 ± 0.18 | 0.35 | 4.25 ± 0.46 | 5.19 ± 0.52 | 0.22 |
| ATP | 26.78 ± 1.79 | 28.56 ± 3.29 | 0.40 | 3.41 ± 0.73 | 4.13 ± 1.04 | 0.62 |
| NAD+ | 1.99 ± 0.06 | 1.88 ± 0.12 | 0.86 | 1.98 ± 0.18 | 1.15 ± 0.21 | 0.02 |
| NADH | 0.06 ± 0.02 | 0.09 ± 0.03 | 0.27 | 0.29 ± 0.03 | 0.34 ± 0.06 | 0.46 |
Mean values ± SE are shown (n = 5).
Phosphorylated AMPK stimulates fatty acid oxidation in peripheral tissues by directly phosphorylating acetylCoA carboxylase (Acc; converts acetyl CoA to malonylCoA). Phosphorylation of Acc inhibits its activity, leading to decreased malonylCoA levels. Because malonylCoA inhibits carnitine:palmitoyl transferase-1 (Cpt1), which catalyzes the rate-limiting step for entry of long-chain fatty acylCoA into mitochondria, diminished malonylCoA concentrations result in increased Cpt1 activity and increased fatty acid oxidation (7).
We documented a 43% increase in the levels of phospho-Acc in the gastrocnemius muscle of GF animals by using an immunoblot assay (P < 0.01; Fig. 2 A and B) and a modest but statistically significant 17% increase in Cpt1 activity, as defined by a biochemical assay (Fig. 2C). In addition, we detected a 31 ± 4.5% increase in medium-chain acylCoA dehydrogenous (Mcad) expression in GF gastrocnemius by quantitative RT-PCR (qRT-PCR) (n = 4 per group; P < 0.05). Mcad is a mitochondrial enzyme that catalyzes the initial step in β oxidation of C8–C12 fatty acids. Together, these findings suggest that the presence of a gut microbiota suppresses skeletal muscle fatty acid oxidation through a metabolic pathway that may involve phosphorylation of AMPK.
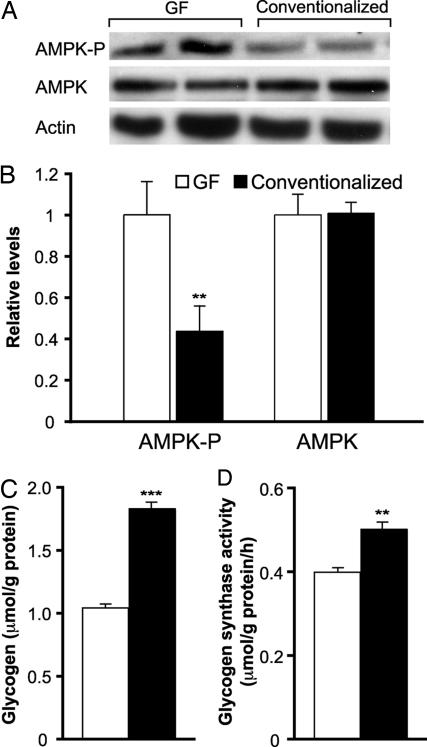
We found a similar increase in phosphorylated AMPK in the livers of these GF animals (Fig. 3 A and B). Foretz et al. (13) have shown that short-term adenoviral-mediated overexpression of a constitutively active form of AMPK in the livers of CONV-R mice produces mild hypoglycemia and reduced hepatic glycogen stores. GF mice fed a Western diet for 5 weeks also have significantly reduced hepatic glycogen levels and decreased glycogen synthase activity (Fig. 3 C and D). In addition, we observed significantly reduced serum glucose and insulin levels in GF compared with conventionalized mice on the Western diet (Table 1). Glucose and insulin tolerance tests confirmed their increased insulin sensitivity relative to their conventionalized obese counterparts (data not shown).
Fig. 3.
The gut microbiota suppresses AMPK activity in liver. (A) Immunoblotting of protein lysates from liver samples obtained from 15-week-old GF or conventionalized male mice fed a Western diet for 5 weeks before they were killed. Representative results from two mice per group are shown. (B) Quantification of the results shown in A (n = 4 per group). Data are expressed relative to actin. Effects of the gut microbiota on glycogen levels (C) and glycogen synthase activity (D) in freeze-clamped livers (n = 5 per group). ∗∗, P < 0.01 compared with GF; and ∗∗∗, P < 0.001.
Although phospho-AMPK is increased in the livers of GF mice (see above), there were no significant differences in hepatic AMP:ATP ratios between GF and conventionalized animals. However, biochemical assays disclosed that GF mice had 72% higher levels of NAD+ (Table 2), which also activates AMPK (10). Similar regulation of AMPK and its targets in both muscle and liver is consistent with recent reports that metabolic cross-talk exists among these distinct tissues (14, 15). Collectively, these findings suggest that insulin-sensitive GF mice are protected against diet-induced obesity at least in part because of increased AMPK activity and increased fatty acid oxidation in their peripheral tissues.
GF Fiaf-Deficient Mice Have Lost Their Resistance to Diet-Induced Obesity.
The obesity-resistant phenotype of GF mice can also be attributed to their increased intestinal expression of Fiaf (Fig. 4A; note that no differences in hepatic Fiaf expression were observed between the groups). When GF wild-type and Fiaf−/− mice were fed a Western diet by using the protocol described above, Fiaf-deficient animals gained significantly more weight than their wild-type littermates (6.2 ± 0.9 vs. 2.7 ± 1.0 g over a 5-week period; n = 5 per group; P < 0.05) (Fig. 4B) and had significantly greater epididymal fat-pad weights (Fig. 4C).
Fig. 4.
GF Fiaf−/− mice are not protected against diet-induced obesity and have lower expression of Pgc-1α and genes involved in fatty acid oxidation in their gastrocnemius muscles. (A) qRT-PCR assays of Fiaf expression in the distal small intestines and livers of GF and conventionalized wild-type male mice maintained on a low-fat diet since weaning or given a high-fat Western diet for 8 weeks before being killed. Mean values ± SE are plotted. n = 5 mice per group. ∗∗, P < 0.01, compared with GF mice on the chow diet; Ψ, P < 0.05 compared with GF mice on the Western diet. (B) GF Fiaf-deficient mice become obese on a Western diet. Eight- to 10-week-old GF male wild-type and Fiaf−/− mice were switched to the Western diet and their body weights monitored weekly for 5 weeks (n = 5 per group). (C) Epididymal fat-pad weights of the mice shown in B after 5 weeks on the Western diet. (D) qRT-PCR assays of gastrocnemius muscle RNAs prepared from GF Fiaf−/− mice and wild-type littermates on the Western diet (n = 6 per group). Mean values ± SE are plotted. ∗, P < 0.05 compared with wild-type animals; ∗∗, P < 0.01.
Consistent with their increased adiposity, GF Fiaf−/− mice on the Western diet had higher serum levels of leptin and insulin than their GF wild-type littermates (Table 3). Serum triglycerides but not free fatty acids were significantly reduced in GF Fiaf−/− mice on the Western diet (Table 3), consistent with the fact they lack this circulating inhibitor of LPL.
Table 3.
Biochemical and ELISA studies of sera, obtained after a 4-hr fast, from 15-week-old GF and conventionalized Fiaf−/− mice and their wild-type littermates maintained on a low-fat diet
| Serum levels | Fiaf+/+ (n = 8) | Fiaf−/− (n = 6) | P value |
|---|---|---|---|
| Glucose, mM | 6.4 ± 1.6 | 6.8 ± 0.8 | 0.49 |
| Insulin, ng/ml | 0.39 ± 0.05 | 0.54 ± 0.04 | 0.02 |
| Leptin, ng/ml | 2.90 ± 0.51 | 4.93 ± 0.77 | 0.04 |
| Triglycerides, mg/dl | 84.5 ± 16.3 | 54.7 ± 3.6 | 0.0005 |
| Cholesterol, mg/dl | 184.4 ± 8.4 | 137.8 ± 9.2 | 0.01 |
| Free fatty acids | 0.95 ± 0.04 | 0.99 ± 0.07 | 0.65 |
Mean values ± SE are shown.
Fiaf Regulates Pgc-1α Expression in Gastrocnemius Muscle.
Fiaf deficiency in GF animals fed a Western diet is associated with statistically significant 24–46% decreases in the expression of genes encoding key enzymes involved in fatty acid oxidation in muscle (Cpt1 and medium-chain acylCoA dehydrogenase; see Fig. 4D). This effect does not appear to involve AMPK; we found no statistically significant differences in phospho-AMPK, total AMPK, phospho-Acc, AMP, ADP, ATP, NAD+, or NADH levels in the gastrocnemius muscles and livers of GF Fiaf knockout compared with their wild-type littermates [n = 4 mice per group; supporting information (SI) Table 4; data not shown]. However, we did find a significant reduction in expression of the peroxisomal proliferator activated receptor coactivator 1α (Pgc-1α) in GF Fiaf−/− gastrocnemius muscle (24 ± 7% compared with GF Fiaf+/+ littermates; n = 6 mice per group; P < 0.05; Fig. 4D). Pgc-1α is capable of coactivating nearly all known nuclear receptors, as well as many other transcription factors; it is also known to increase expression of genes encoding regulators of mitochondrial fatty acid oxidation, including Cpt1 and medium-chain acylCoA dehydrogenous (16).
Locomotor Activity.
Using an implantable detector of locomotion (see Materials and Methods), we found that the absence of a microbiota is associated with significantly increased movement in wild-type mice, whether these age- and gender-matched animals were on a standard chow or a Western diet (n = 4 per group; Fig. 1C). Moreover, qRT-PCR assays revealed no statistically significant differences in the levels of expression of uncoupling protein 1 in the gastrocnemius muscles of these animals (n = 4 mice surveyed per treatment group; data not shown). Finally, a comparison of GF Fiaf+/+ and Fiaf−/− littermates on the standard chow diet revealed no statistically significant differences in their locomotor activity, despite significant differences in their adiposity. The mechanisms underlying the increased locomotor activity of wild-type GF vs. conventionalized mice are unknown, may reflect a heretofore unappreciated link between the metabolic activity of their microbiota, and behaviors which could contribute to the observed differences in their adiposity. Nonetheless, we cannot attribute the increased adiposity of GF Fiaf−/− vs. wild-type littermates to this phenomenon.
Discussion
Collectively, our data indicate that the gut microbiota is able to modulate energy balance through a number of intertwined pathways. A shift in gut microbial ecology occurs in genetically obese (ob/ob) mice consuming a standard chow diet: compared with their lean +/+ and ob/+ littermates, the representation of the Bacteroidetes diminishes by ≈50%, and the Firmicutes increase to a corresponding degree. Remarkably, these changes are division-wide and not due to a suppression of one or a few Bacteriodetes lineages or to bloom in one or a few members of the Firmicutes (17). A similar shift in the ratio of Bacteroidetes to Firmicutes occurs in obese compared to lean humans; moreover, as humans lose weight, there is a division-wide increase in the proportion of Bacteroidetes and reduction in Firmicutes (18). Comparative metagenomic studies of the distal gut microbial communities of ob/ob mice and their lean littermates fed a standard low-fat rodent chow diet indicate that the ob/ob community is enriched for genes that are able to harvest calories from complex plant-derived polysaccharides (19). Moreover, transplantation of the gut microbiota from ob/ob donors to adult GF +/+ recipients consuming a standard chow diet low in fat and rich in polysaccharides results in a greater increase in adiposity in the recipients over a 2-week period than does a transplantation of a microbiota from lean +/+ donors (19). Metagenomic studies of the gut microbial community of mice with obesity due to consumption of a high-fat simple-sugar diet, and microbiota transplant experiments analogous to those described above, are needed to identify the organismal and gene lineages present in their gut community and to characterize its energy-harvesting capacity.
Combined with the observations described here, these findings support an emerging view that gut microbes can affect both sides of the energy balance equation, as a factor that influences the harvest of energy from components of the diet, and as a factor that affects host genes that regulate how energy is expended and stored. The microbiota provides glycoside hydrolases and polysaccharide lyases required to cleave glycosidic linkages in plant glycans (1). The resulting monosaccharides are absorbed or metabolized to short-chain fatty acids, which are delivered to the liver and converted to triacylglycerols; these de novo synthesized lipids are then deposited in adipocytes through a process that involves, in part, microbial suppression of intestinal epithelial production of the circulating LPL inhibitor, Fiaf. Comparisons of GF and colonized mice on a fat- and sugar-rich diet indicate that the microbiota can also affect adiposity by producing a physiologic state where AMPK activity is reduced in muscle, leading to reduced phosphorylation of its downstream target, Acc; increases in malonylCoA production; greater inhibition of Cpt1; and diminished mitochondrial fatty acid oxidation. Reduced fatty acid oxidation is also mediated through suppression of Pgc-1α in skeletal muscle (20). Interestingly, we found that Pgc-1α is regulated by Fiaf, whereas AMPK activation (phosphorylation) in muscle (and liver) is independent of Fiaf signaling. The mechanism by which Fiaf induces Pgc-1α expression is still unclear. Unlike other angiopoietins, which are known to be potent stimulators of the Tie 2 receptor (21), a receptor for Fiaf (angiopoietin-like protein 4) that could initiate intracellular signaling has yet to be identified. In addition, the effects of the microbiota on bile acid metabolism and on the bioavailability of dietary lipids need to be further evaluated using methods such as mass spectrometry and NMR.
We have found that CONV-R C57BL/6J transgenic mice, where overexpression of Fiaf is achieved by using enterocyte-specific transcriptional regulatory elements that are not affected by the microbiota (nucleotides −1,178 to +28 of the rat intestinal fatty acid-binding protein gene; Fabpi; ref. 22), exhibit statistically significant reductions in their adiposity compared with nontransgenic littermates [8.3 ± 0.3% vs. 10.6 ± 0.4% total body fat as defined by dual energy x-ray absorptiometry; n = 6–8 animals fed a standard chow diet; P < 0.01 (F.B. and J.I.G., unpublished observations)]. In addition, CONV-R aP2-Fiaf transgenic mice with engineered forced expression of Fiaf in adipocytes exhibit significant reduction of adipose tissue weight by stimulating fatty acid oxidation and uncoupling (23).
Our studies in gnotobiotic mice have also identified microbial determinants of Fiaf expression. When GF mice are colonized with Bacteroides thetaiotaomicron, a prominent saccharolytic member of the normal human colonic microbiota, together with the dominant human colonic methanogen, Methanobrevibacter smithii, the efficiency of polysaccharide fermentation is markedly improved (24). De novo lipogenesis is augmented, and host adiposity is increased compared with animals colonized with either organism alone (24). This enhanced adiposity is associated with significantly increased suppression of intestinal Fiaf expression (81 ± 3% in cocolonized compared with GF mice vs. 65 ± 1% and 48 ± 5% in mice colonized with either B. thetaiotaomicron or M. smithii alone, respectively; n = 5 per group; P < 0.05; B. S. Samuel and J.I.G, unpublished observation).
Collectively, these findings suggest that the gut microbiota contributes to mammalian adiposity by regulating more than one node within the metabolic network that controls bioenergetics. Manipulating microbial characteristics in ways that impact calorie harvest from a diet, and/or Fiaf expression, or Fiaf-mediated control of Pgc-1α, may represent new strategies for modifying host energy balance to promote health.
Materials and Methods
Animals.
GF wild-type C57BL/6J animals were maintained in gnotobiotic isolators under a strict 12-h light cycle (lights on at 0600 h) and fed an autoclaved low-fat polysaccharide-rich chow diet (B & K Universal, East Yorkshire, U.K.) ad libitum Fiaf−/− mice on a mixed C57BL/J:129/Sv background were backcrossed one generation to C57BL/6J animals and rederived as GF, as described (2). Wild-type and Fiaf-deficient littermates were used in these studies.
GF mice were colonized at 6–10 weeks of age with cecal contents harvested from an adult CONV-R mouse and kept in their gnotobiotic isolators. Mice were either switched to an irradiated Western diet (TD96132; Harlan Teklad, Madison, WI) 2–3 weeks after conventionalization or maintained on their autoclaved low-fat chow diet. Body weight and food consumption were monitored weekly. Only male animals were used in this study, which was performed by using protocols approved by the Washington University Animal Studies Committee.
Locomotor Activity Measurements.
Mice were anesthetized before a transmitter (minimitter PDT-4000; Mini Mitter, Bend, OR) was implanted intraabdominally. Mice were allowed to recover for 7 days after implantation, and locomotor activity data were collected continuously during the next 3 days. To do so, the signal emitted by the transmitter was detected by receivers positioned underneath the plastic gnotobiotic isolators. Data were then converted into activity counts by VitalView software (Mini Mitter). Mice were subsequently switched to a Western diet, and at the end of 8 weeks, were monitored, continuously, over a 3-day interval.
Isolation and Initial Processing of Tissues.
After animals were killed, their small intestine was removed and divided into 16 equal-sized segments. Segments 13–14, liver and gastrocnemius muscle from each animal were snap-frozen, and total RNA was isolated [Qiagen (Valencia, CA) RNeasy kit] for real-time qRT-PCR assays.
Additional gastrocnemius and liver-tissue samples (≈50 mg each) were directly placed in 1 ml of lysis buffer [20 mM Tris (pH 7.5)/150 mM NaCl/1 mM EDTA/1% Triton X-100] containing Complete protease inhibitor mixture (Roche Diagnostics, Indianapolis, IN) and phosphatase inhibitor mixture 1 (Sigma, St. Louis, MO). After homogenization at 4°C, extracts were centrifuged for 10 min at 4°C at 15,000 × g to remove insoluble debris, and the protein concentration in the resulting supernatant fraction was determined (DC protein assay; Bio-Rad, Hercules, CA). Tissue samples used for assaying enzyme activities and metabolites were harvested after freeze clamping (2) and processed as described below.
Immunoblotting.
Soluble proteins from liver and gastrocnemius muscle were separated on 10% Bis-Tris gels (Invitrogen, Carlsbad, CA) and transferred to PVDF membranes. Membranes were placed in 5% BSA/0.1% Tween-20/PBS for 60 min at room temperature and then incubated overnight at 4°C in 1% BSA/0.1% Tween/PBS together with one of the following antibodies: rabbit anti-phospho-Acc, rabbit anti-phospho-AMPK, and rabbit anti-α-subunit of AMPK (all from Cell Signaling Technology, Beverly, MA; final dilution, 1:1,000), or rabbit antiactin (Sigma; 1:3,000).
qRT-PCR.
RNA prepared from each tissue sample was reverse-transcribed by using SuperScript II (Invitrogen) and a dT15 primer (Roche Diagnostics), as described in ref. 2. qRT-PCR assays were performed in 25-μl reactions containing gene-specific primers (900 nM; for a list, see SI Table 5) and SYBR green (Abgene, Epsom, U.K.). Data were normalized to L32 mRNA (ΔΔCT analysis) (see SI Text).
Assays of Sera.
Standard biochemical methods were used to assay sera for glucose, cholesterol, triglycerides, and nonesterified fatty acids (2). Insulin and leptin levels were determined by ELISA (Crystal Chemical, Downers Grove, IL). Glucose and insulin tolerance tests were performed as described in ref. 2.
Statistical Analysis.
Data were analyzed by using Student's t test or ANOVA with Tukey's post hoc analysis.
Supplementary Material
Acknowledgments
We thank Maria Karlsson, David O'Donnell, and Sabrina Wagoner for superb technical assistance; Tim Nagy for performing bomb calorimetry (P30DK56336); and Peter Crawford for helpful suggestions. This work was supported in part by National Institutes of Health Grants DK70977 and P30 DK56341. F.B. is the recipient of a postdoctoral fellowship from the Wenner-Gren Foundation.
Abbreviations
- AMPK
AMP-activated protein kinase
- GF
germ-free
- CONV-R
conventionally raised
- Fiaf
fasting-induced adipose factor
- LPL
lipoprotein lipase
- Acc
acetylCoA carboxylase
- Cpt1
carnitine:palmitoyl transferase-1
- Pgc-1α
peroxisomal proliferator-activated receptor coactivator 1α
- qRT-PCR
quantitative RT-PCR.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0605374104/DC1.
References
- 1.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 2.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolin M. Science. 1981;213:1463–1468. doi: 10.1126/science.7280665. [DOI] [PubMed] [Google Scholar]
- 4.Merkel M, Eckel RH, Goldberg IJ. J Lipid Res. 2002;43:1997–2006. doi: 10.1194/jlr.r200015-jlr200. [DOI] [PubMed] [Google Scholar]
- 5.Preiss-Landl K, Zimmermann R, Hammerle G, Zechner R. Curr Opin Lipidol. 2002;13:471–481. doi: 10.1097/00041433-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Weinstock PH, Levak-Frank S, Hudgins LC, Radner H, Friedman JM, Zechner R, Breslow JL. Proc Natl Acad Sci USA. 1997;94:10261–10266. doi: 10.1073/pnas.94.19.10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn BB, Alquier T, Carling D, Hardie DG. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 9.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 10.Rafaeloff-Phail R, Ding L, Conner L, Yeh WK, McClure D, Guo H, Emerson K, Brooks H. J Biol Chem. 2004;279:52934–52939. doi: 10.1074/jbc.M409574200. [DOI] [PubMed] [Google Scholar]
- 11.Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. J Biol Chem. 1998;273:35347–35354. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- 12.Stein SC, Woods A, Jones NA, Davison MD, Carling D. Biochem J. 2000;345:437–443. [PMC free article] [PubMed] [Google Scholar]
- 13.Foretz M, Ancellin N, Andreelli F, Saintillan Y, Grondin P, Kahn A, Thorens B, Vaulont S, Viollet B. Diabetes. 2005;54:1331–1339. doi: 10.2337/diabetes.54.5.1331. [DOI] [PubMed] [Google Scholar]
- 14.Ranalletta M, Jiang H, Li J, Tsao TS, Stenbit AE, Yokoyama M, Katz EB, Charron MJ. Diabetes. 2005;54:935–943. doi: 10.2337/diabetes.54.4.935. [DOI] [PubMed] [Google Scholar]
- 15.Lee CH, Olson P, Hevener A, Mehl I, Chong LW, Olefsky JM, Gonzalez FJ, Ham J, Kang H, Peters JM, Evans RM. Proc Natl Acad Sci USA. 2006;103:3444–3449. doi: 10.1073/pnas.0511253103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vega RB, Huss JM, Kelly DP. Mol Cell Biol. 2000;20:1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Proc Natl Acad Sci USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 19.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 20.Puigserver P, Spiegelman BM. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 21.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 22.Sweetser DA, Hauft SM, Hoppe PC, Birkenmeier EH, Gordon JI. Proc Natl Acad Sci USA. 1988;85:9611–9615. doi: 10.1073/pnas.85.24.9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandard S, Zandbergen F, van Straten E, Wahli W, Kuipers F, Muller M, Kersten S. J Biol Chem. 2006;281:934–944. doi: 10.1074/jbc.M506519200. [DOI] [PubMed] [Google Scholar]
- 24.Samuel BS, Gordon JI. Proc Natl Acad Sci USA. 2006;103:10011–10016. doi: 10.1073/pnas.0602187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.