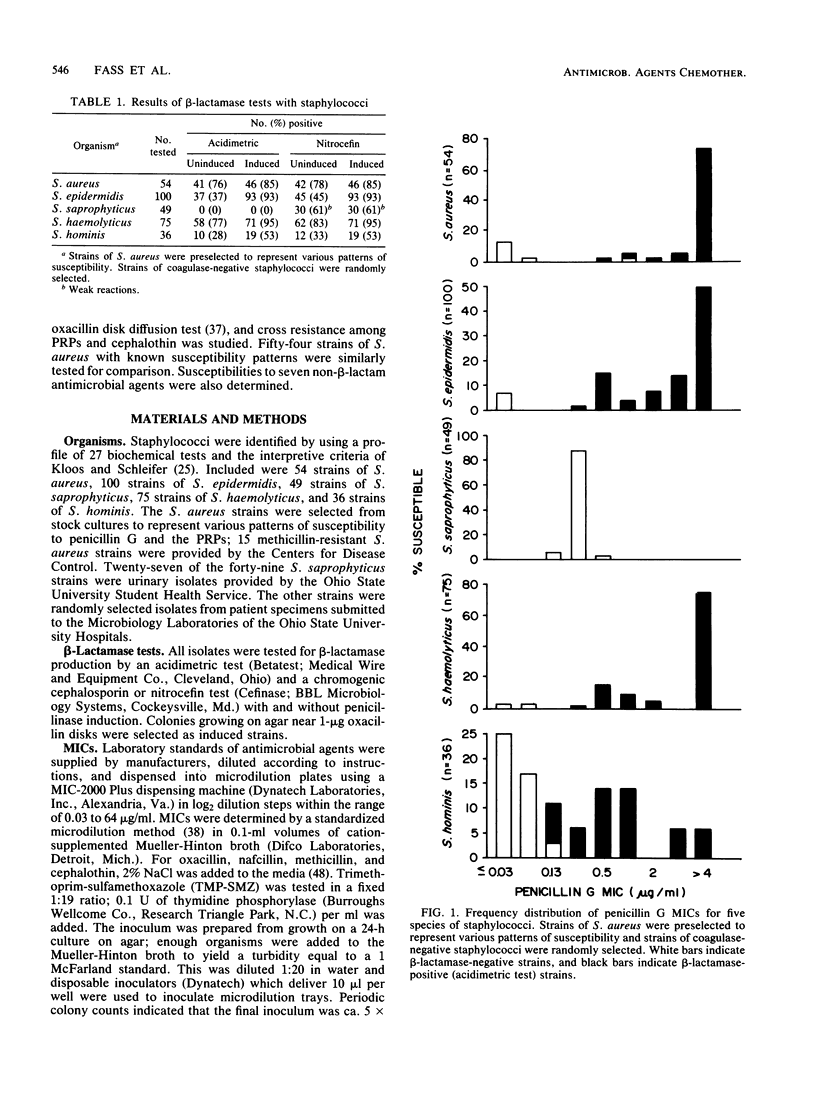
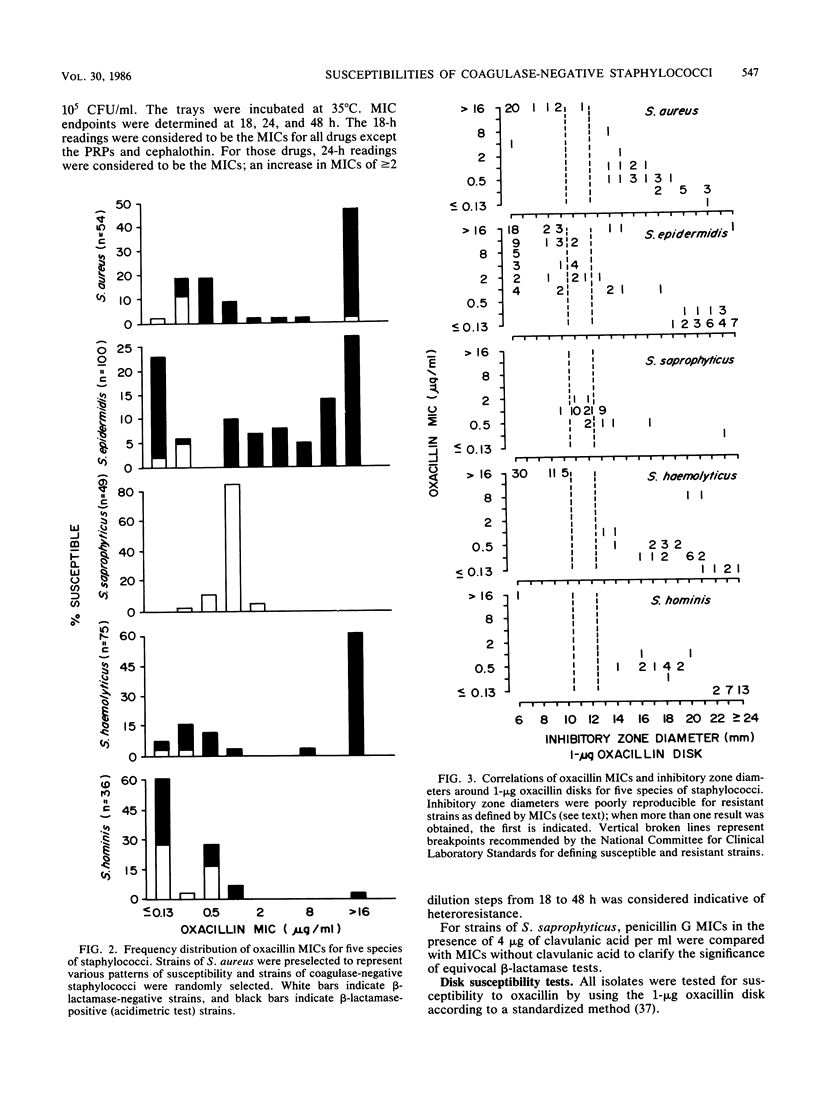
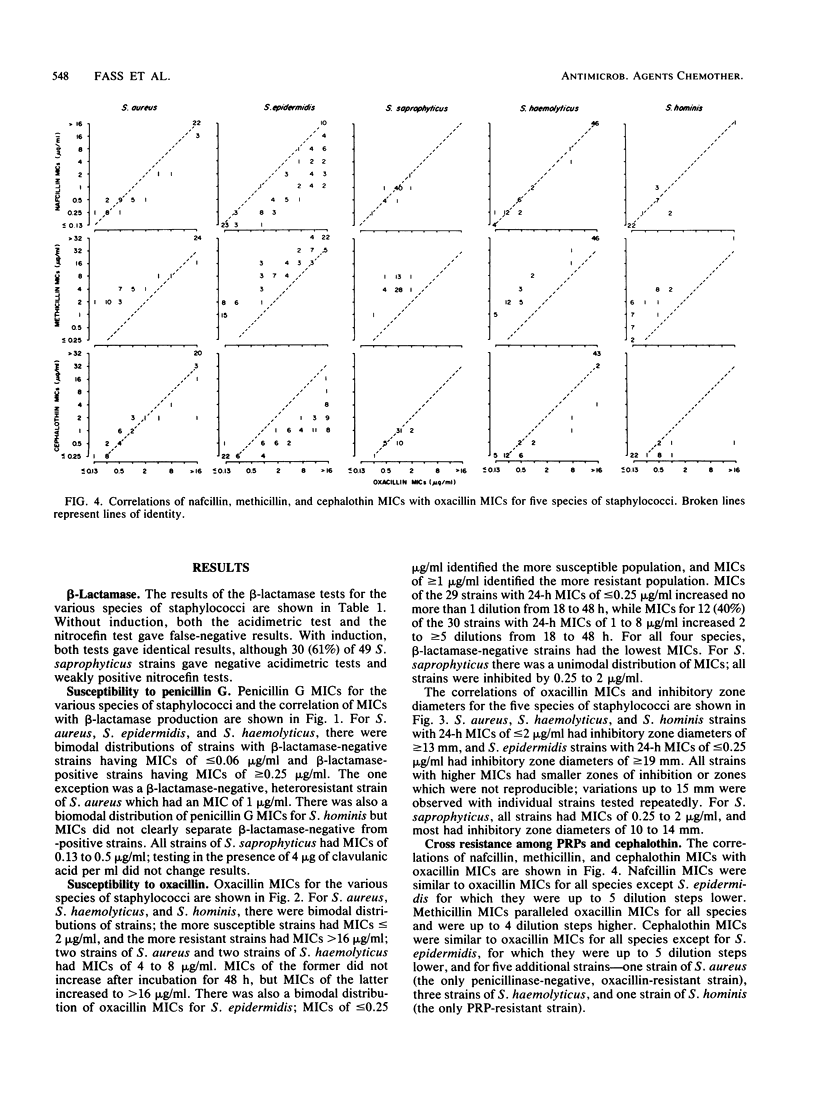
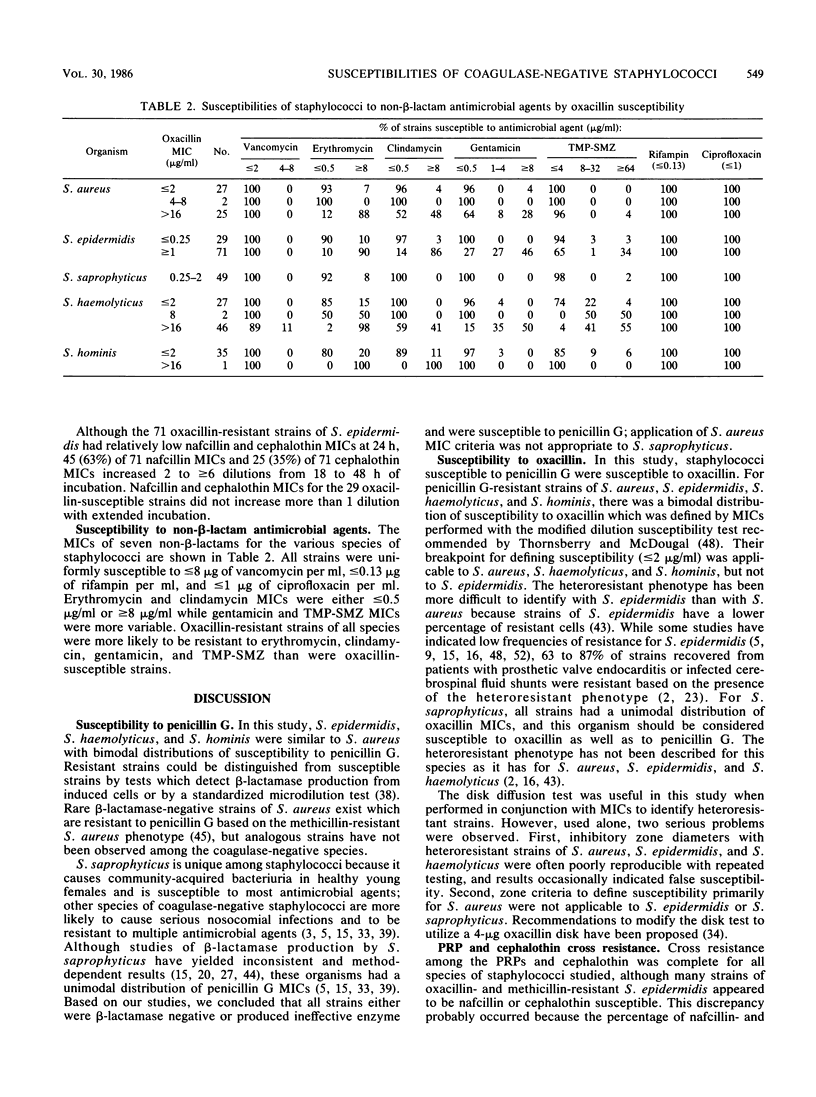
Abstract
The in vitro susceptibilities of 260 strains of coagulase-negative staphylococci to penicillin G, oxacillin, nafcillin, methicillin, cephalothin, and seven non-beta-lactam antimicrobial agents were determined and compared with the susceptibilities of 54 strains of Staphylococcus aureus with known patterns of susceptibility. Penicillin G susceptibility for S. aureus, Staphylococcus epidermidis, Staphylococcus haemolyticus, and Staphylococcus hominis was readily determined by using beta-lactamase tests with induced cells and with a standardized microdilution test. MIC criteria for susceptibility used for S. aureus were applicable to the coagulase-negative species. Percentages of organisms susceptible were as follows: S. epidermidis, 7%; S. haemolyticus, 5%; and S. hominis, 47%. Oxacillin susceptibility for these four species was readily determined by using a modification of the microdilution test. MIC criteria for susceptibility used for S. aureus were applicable to S. haemolyticus and S. hominis, but alternate criteria were necessary for S. epidermidis. Percentages of organisms susceptible were as follows: S. epidermidis, 29%; S. haemolyticus, 36%; and S. hominis, 97%. Staphylococcus saprophyticus differed from the other staphylococcal species; all strains were beta-lactamase negative and were penicillin susceptible but had higher penicillin G MICs than did susceptible strains of the other species. There was total cross resistance among the penicillinase-resistant penicillins and cephalothin for the coagulase-negative staphylococci as well as for S. aureus; oxacillin MICs were more reliable than MICs of the other drugs or a standardized disk diffusion test for distinguishing resistant from susceptible strains. Vancomycin, rifampin, and ciprofloxacin were consistently active against all staphylococci. Erythromycin, clindamycin, gentamicin, and trimethoprim-sulfamethoxazole were more active against oxacillin-susceptible staphylococci than against oxacillin-resistant staphylococci.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge K. E., Janney A., Sanders C. V. Comparison of the activities of coumermycin, ciprofloxacin, teicoplanin, and other non-beta-lactam antibiotics against clinical isolates of methicillin-resistant Staphylococcus aureus from various geographical locations. Antimicrob Agents Chemother. 1985 Nov;28(5):634–638. doi: 10.1128/aac.28.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G. L. Antimicrobial susceptibility and selection of resistance among Staphylococcus epidermidis isolates recovered from patients with infections of indwelling foreign devices. Antimicrob Agents Chemother. 1978 Sep;14(3):353–359. doi: 10.1128/aac.14.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G. L., Tenenbaum M. J., Haywood H. B., 3rd Rifampin therapy of Staphylococcus epidermidis. Use in infections from indwelling artificial devices. JAMA. 1978 Aug 25;240(8):751–753. [PubMed] [Google Scholar]
- Bourgault A. M., Gauvreau L. Antimicrobial susceptibilities of coagulase-negative staphylococci isolated from urinary infections. Antimicrob Agents Chemother. 1983 May;23(5):793–795. doi: 10.1128/aac.23.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S. W., Steigbigel R. T. Staphylococcal beta-lactamase and efficacy of beta-lactam antibiotics: in vitro and in vivo evaluation. J Infect Dis. 1983 Jun;147(6):1078–1089. doi: 10.1093/infdis/147.6.1078. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Bisno A. L., Parisi J. T., McLaughlin B., Hester M. G., Luther R. W. Nosocomial septicemia due to multiply antibiotic-resistant Staphylococcus epidermidis. Ann Intern Med. 1982 Jan;96(1):1–10. doi: 10.7326/0003-4819-96-1-1. [DOI] [PubMed] [Google Scholar]
- Craven D. E., Kollisch N. R., Hsieh C. R., Connolly M. G., Jr, McCabe W. R. Vancomycin treatment of bacteremia caused by oxacillin-resistant Staphylococcus aureus: comparison with beta-lactam antibiotic treatment of bacteremia caused by oxacillin-sensitive Staphylococcus aureus. J Infect Dis. 1983 Jan;147(1):137–143. doi: 10.1093/infdis/147.1.137. [DOI] [PubMed] [Google Scholar]
- Dillon L. K., Howe S. E. Early detection of oxacillin-resistant staphylococcal strains with hypertonic broth diluent for microdilution panels. J Clin Microbiol. 1984 Apr;19(4):473–476. doi: 10.1128/jcm.19.4.473-476.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ein M. E., Smith N. J., Aruffo J. F., Heerema M. S., Bradshaw M. W., Williams T. W., Jr Susceptibility and synergy studies of methicillin-resistant Staphylococcus epidermidis. Antimicrob Agents Chemother. 1979 Nov;16(5):655–659. doi: 10.1128/aac.16.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng R. H., Smith S. M., Tillem M., Cherubin C. Rifampin resistance. Development during the therapy of methicillin-resistant Staphylococcus aureus infection. Arch Intern Med. 1985 Jan;145(1):146–148. doi: 10.1001/archinte.145.1.146. [DOI] [PubMed] [Google Scholar]
- Faville R. J., Jr, Zaske D. E., Kaplan E. L., Crossley K., Sabath L. D., Quie P. G. Staphylococcus aureus endocarditis. Combined therapy with vancomycin and rifampin. JAMA. 1978 Oct 27;240(18):1963–1965. doi: 10.1001/jama.240.18.1963. [DOI] [PubMed] [Google Scholar]
- Fong I. W., Engelking E. R., Kirby W. M. Relative inactivation by Staphylococcus aureus of eight cephalosporin antibiotics. Antimicrob Agents Chemother. 1976 Jun;9(6):939–944. doi: 10.1128/aac.9.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frongillo R. F., Bianchi P., Moretti A., Pasticci M. B., Ripa S., Pauluzzi S. Cross-resistance between methicillin and cephalosporins for staphylococci: a general assumption not true for cefamandole. Antimicrob Agents Chemother. 1984 May;25(5):666–668. doi: 10.1128/aac.25.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill V. J., Selepak S. T., Williams E. C. Species identification and antibiotic susceptibilities of coagulase-negative staphylococci isolated from clinical specimens. J Clin Microbiol. 1983 Dec;18(6):1314–1319. doi: 10.1128/jcm.18.6.1314-1319.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton-Miller J. M., Iliffe A. Antimicrobial resistance in coagulase-negative staphylococci. J Med Microbiol. 1985 Apr;19(2):217–226. doi: 10.1099/00222615-19-2-217. [DOI] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Expression of methicillin resistance in heterogeneous strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Jan;29(1):85–92. doi: 10.1128/aac.29.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B., Tomasz A. Altered penicillin-binding proteins in methicillin-resistant strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1981 May;19(5):726–735. doi: 10.1128/aac.19.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschl A., Stanek G., Rotter M. Effectiveness of cefamandole against methicillin-resistant strains of Staphylococcus aureus in vitro and in experimental infections. J Antimicrob Chemother. 1984 May;13(5):429–435. doi: 10.1093/jac/13.5.429. [DOI] [PubMed] [Google Scholar]
- Hovelius B., Mårdh P. A. On the diagnosis of coagulase-negative staphylococci with emphasis on Staphylococcus saprophyticus. Acta Pathol Microbiol Scand B. 1977 Dec;85B(6):427–434. doi: 10.1111/j.1699-0463.1977.tb01998.x. [DOI] [PubMed] [Google Scholar]
- John J. F., Jr, McNeill W. F. Activity of cephalosporins against methicillin-susceptible and methicillin-resistant, coagulase-negative staphylococci: minimal effect of beta-lactamase. Antimicrob Agents Chemother. 1980 Feb;17(2):179–183. doi: 10.1128/aac.17.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Edson D. C. Special topics in antimicrobial susceptibility testing: test accuracy against methicillin-resistant Staphylococcus aureus, pneumococci, and the sensitivity of beta-lactamase methods. Am J Clin Pathol. 1983 Oct;80(4 Suppl):609–614. [PubMed] [Google Scholar]
- Karchmer A. W., Archer G. L., Dismukes W. E. Staphylococcus epidermidis causing prosthetic valve endocarditis: microbiologic and clinical observations as guides to therapy. Ann Intern Med. 1983 Apr;98(4):447–455. doi: 10.7326/0003-4819-98-4-447. [DOI] [PubMed] [Google Scholar]
- Kloos W. E., Schleifer K. H. Simplified scheme for routine identification of human Staphylococcus species. J Clin Microbiol. 1975 Jan;1(1):82–88. doi: 10.1128/jcm.1.1.82-88.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M., Sasatsu M., O'Hara K., Shiomi Y., Hayasaka T. Mechanism of resistance to some cephalosporins in Staphylococcus aureus. Antimicrob Agents Chemother. 1983 Jun;23(6):938–940. doi: 10.1128/aac.23.6.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham R. H., Zeleznik D., Minshew B. H., Schoenknecht F. D., Stamm W. E. Staphylococcus saprophyticus beta-lactamase production and disk diffusion susceptibility testing for three beta-lactam antimicrobial agents. Antimicrob Agents Chemother. 1984 Nov;26(5):670–672. doi: 10.1128/aac.26.5.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverdiere M., Peterson P., Verhoef J., Williams D. N., Sabath L. D. In vitro activity of cephalosporins against methicillin-resistant, coagulase-negative staphylococci. J Infect Dis. 1978 Mar;137(3):245–250. doi: 10.1093/infdis/137.3.245. [DOI] [PubMed] [Google Scholar]
- Lowy F. D., Chang D. S., Lash P. R. Synergy of combinations of vancomycin, gentamicin, and rifampin against methicillin-resistant, coagulase-negative staphylococci. Antimicrob Agents Chemother. 1983 Jun;23(6):932–934. doi: 10.1128/aac.23.6.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy F. D., Hammer S. M. Staphylococcus epidermidis infections. Ann Intern Med. 1983 Dec;99(6):834–839. doi: 10.7326/0003-4819-99-6-834. [DOI] [PubMed] [Google Scholar]
- Lowy F. D., Wexler M. A., Steigbigel N. H. Therapy of methicillin-resistant Staphylococcus epidermidis experimental endocarditis. J Lab Clin Med. 1982 Jul;100(1):94–104. [PubMed] [Google Scholar]
- Markowitz N., Pohlod D. J., Saravolatz L. D., Quinn E. L. In vitro susceptibility patterns of methicillin-resistant and-susceptible Staphylococcus auerues strains in a population of parenteral drug abusers from 1972 to 1981. Antimicrob Agents Chemother. 1983 Mar;23(3):450–457. doi: 10.1128/aac.23.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T. J., Kwan C. Antimicrobial susceptibility of Staphylococcus saprophyticus and urethral staphylococci. Antimicrob Agents Chemother. 1982 Sep;22(3):395–397. doi: 10.1128/aac.22.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal L. K., Thornsberry C. New recommendations for disk diffusion antimicrobial susceptibility tests for methicillin-resistant (heteroresistant) staphylococci. J Clin Microbiol. 1984 Apr;19(4):482–488. doi: 10.1128/jcm.19.4.482-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal L. K., Thornsberry C. The role of beta-lactamase in staphylococcal resistance to penicillinase-resistant penicillins and cephalosporins. J Clin Microbiol. 1986 May;23(5):832–839. doi: 10.1128/jcm.23.5.832-839.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton R. P., Lampe A. S., van Maanen D. B., van der Reijden T. J. Activity of cephalosporins against methicillin-resistant coagulase-negative staphylococci. Eur J Clin Microbiol. 1984 Apr;3(2):144–146. doi: 10.1007/BF02014333. [DOI] [PubMed] [Google Scholar]
- Nicolle L. E., Harding G. K. Susceptibility of clinical isolates of Staphylococcus saprophyticus to fifteen commonly used antimicrobial agents. Antimicrob Agents Chemother. 1982 Nov;22(5):895–896. doi: 10.1128/aac.22.5.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce de Leon S., Wenzel R. P. Hospital-acquired bloodstream infections with Staphylococcus epidermidis. Review of 100 cases. Am J Med. 1984 Oct;77(4):639–644. doi: 10.1016/0002-9343(84)90354-1. [DOI] [PubMed] [Google Scholar]
- Richardson J. F., Marples R. R. Changing resistance to antimicrobial drugs, and resistance typing in clinically significant strains of Staphylococcus epidermidis. J Med Microbiol. 1982 Nov;15(4):475–484. doi: 10.1099/00222615-15-4-475. [DOI] [PubMed] [Google Scholar]
- Sabath L. D. Mechanisms of resistance to beta-lactam antibiotics in strains of Staphylococcus aureus. Ann Intern Med. 1982 Sep;97(3):339–344. doi: 10.7326/0003-4819-97-3-339. [DOI] [PubMed] [Google Scholar]
- Selepak S. T., Witebsky F. G. beta-Lactamase detection in nine staphylococcal species. J Clin Microbiol. 1984 Dec;20(6):1200–1201. doi: 10.1128/jcm.20.6.1200-1201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman S. J. Penicillinase-negative variants of methicillin-resistant Staphylococcus aureus. Nature. 1966 Mar 5;209(5027):994–996. doi: 10.1038/209994a0. [DOI] [PubMed] [Google Scholar]
- Sjöström J. E., Löfdahl S., Philipson L. Transformation reveals a chromosomal locus of the gene(s) for methicillin resistance in Staphylococcus aureus. J Bacteriol. 1975 Sep;123(3):905–915. doi: 10.1128/jb.123.3.905-915.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Eng R. H. Activity of ciprofloxacin against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985 May;27(5):688–691. doi: 10.1128/aac.27.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., McDougal L. K. Successful use of broth microdilution in susceptibility tests for methicillin-resistant (heteroresistant) staphylococci. J Clin Microbiol. 1983 Nov;18(5):1084–1091. doi: 10.1128/jcm.18.5.1084-1091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuazon C. U., Miller H. Comparative in vitro activities of teichomycin and vancomycin alone and in combination with rifampin and aminoglycosides against staphylococci and enterococci. Antimicrob Agents Chemother. 1984 Apr;25(4):411–412. doi: 10.1128/aac.25.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varaldo P. E., Debbia E., Schito G. C. In vitro activity of teichomycin and vancomycin alone and in combination with rifampin. Antimicrob Agents Chemother. 1983 Mar;23(3):402–406. doi: 10.1128/aac.23.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J. C., Schimpff S. C., Newman K. A., Wiernik P. H. Staphylococcus epidermidis: an increasing cause of infection in patients with granulocytopenia. Ann Intern Med. 1982 Oct;97(4):503–508. doi: 10.7326/0003-4819-97-4-503. [DOI] [PubMed] [Google Scholar]
- Watanakunakorn C., Tisone J. C. Synergism between vancomycin and gentamicin or tobramycin for methicillin-susceptible and methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 1982 Nov;22(5):903–905. doi: 10.1128/aac.22.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston D. J., Dudnick D. V., Chapin M., Ho W. G., Gale R. P., Martin W. J. Coagulase-negative staphylococcal bacteremia in patients receiving immunosuppressive therapy. Arch Intern Med. 1983 Jan;143(1):32–36. [PubMed] [Google Scholar]


