Abstract
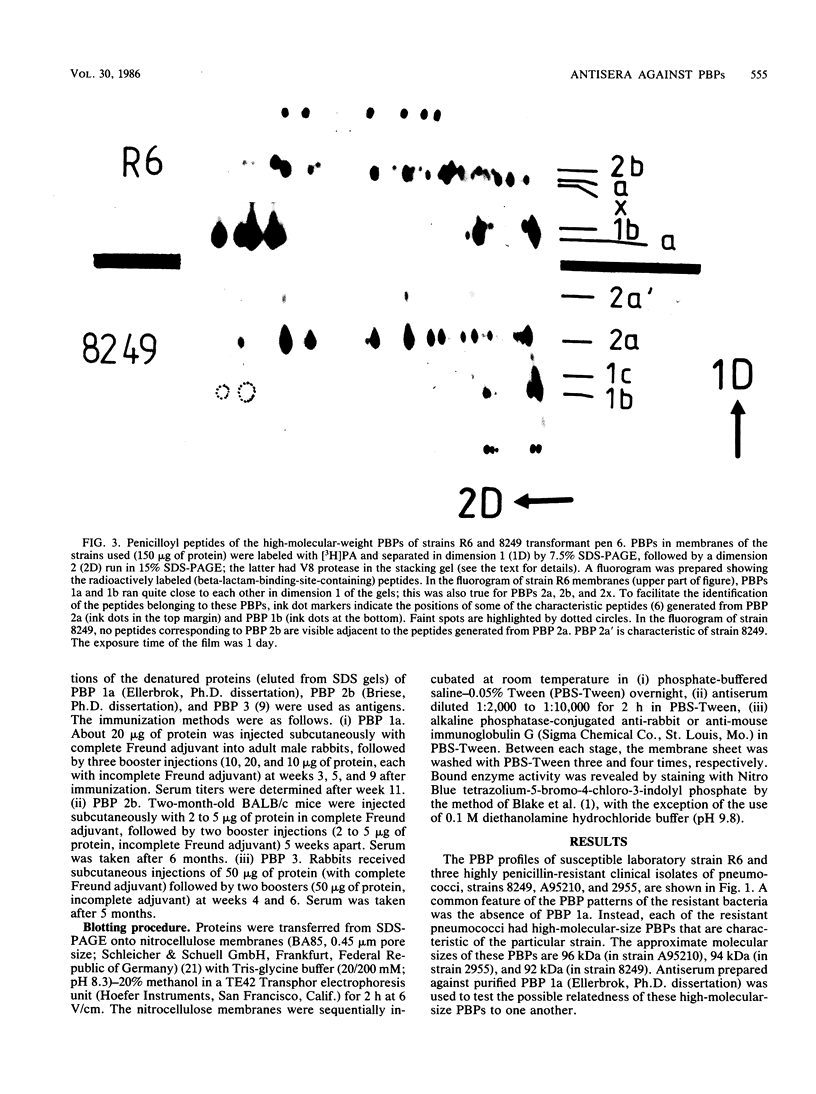
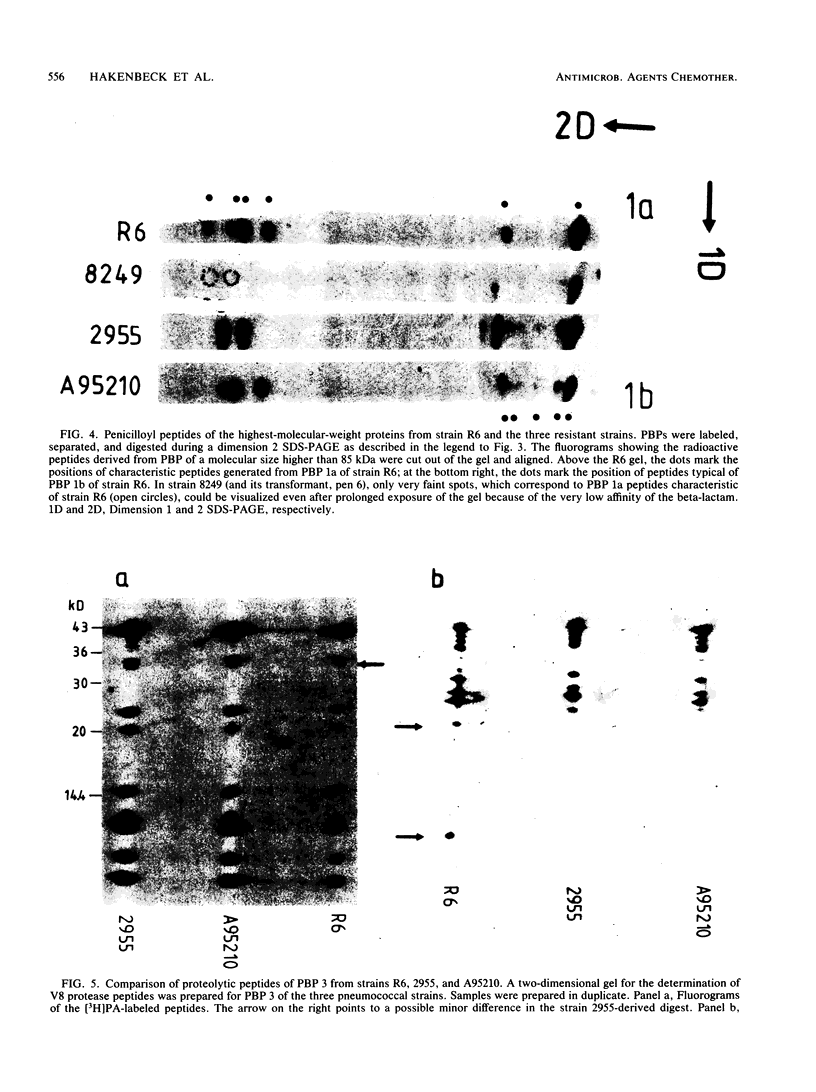
There are several major differences between the penicillin-binding proteins (PBPs) of highly penicillin-resistant and -susceptible strains of pneumococci. The highest-molecular-size PBP 1a (98 kilodaltons [kDa]) of susceptible pneumococci is not detectable in resistant bacteria. Instead, resistant strains contain a PBP of smaller size: 92 and 94 kDa in South African strains 8249 and A95210, respectively, and 96 kDa in New Guinea strain 2955 (S. Zighelboim and A. Tomasz, Antimicrob. Agents Chemother. 17:434-442, 1980). Using antibodies prepared against PBP 1a of penicillin-susceptible pneumococci, we demonstrated that these anomalous-sized proteins in the resistant strains are immunologically related to PBP 1a of penicillin-susceptible bacteria. A second difference between the PBP patterns of strain 8249 and the susceptible pneumococci is that the 78-kDa PBP 2b is not detectable by the radioactive penicillin binding assay in the resistant strain. Using antibodies prepared against PBP 2b of susceptible cells, we demonstrated the presence of PBP 2b in membrane preparations from strain 8249 cells. Thus, the poor detection of this PBP appears to be related to its greatly decreased affinity for the antibiotic molecule. We also compared the patterns of penicillin-labeled peptides derived from PBPs of resistant and susceptible cells during partial proteolysis by V8 protease. Several changes were observable in small peptides carrying the beta-lactam binding site generated from the high Mr (PBP 1a-related) binding proteins. In contrast, no differences in the pattern of penicillin-labeled peptides were seen when the pattern of PBP 2a of susceptible pneumococci was compared with the peptide pattern of PBP 2a from resistant strains. One of the resistant isolates (strain 2955) also had a PBP 3 with a higher-than-normal molecular weight. This protein gave strong positive reaction with antibodies against PBP 3 of susceptible cells. Examination of the pattern of penicilloyl peptides generated from the susceptible and resistant PBP 3s during partial proteolysis revealed only differences which seem to reside distant from the beta-lactam binding site.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Brown D. F., Reynolds P. E. Intrinsic resistance to beta-lactam antibiotics in Staphylococcus aureus. FEBS Lett. 1980 Dec 29;122(2):275–278. doi: 10.1016/0014-5793(80)80455-8. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dougherty T. J., Koller A. E., Tomasz A. Penicillin-binding proteins of penicillin-susceptible and intrinsically resistant Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1980 Nov;18(5):730–737. doi: 10.1128/aac.18.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerbrok H., Hakenbeck R. Penicillin-binding proteins of Streptococcus pneumoniae: characterization of tryptic peptides containing the beta-lactam-binding site. Eur J Biochem. 1984 Nov 2;144(3):637–641. doi: 10.1111/j.1432-1033.1984.tb08512.x. [DOI] [PubMed] [Google Scholar]
- Fontana R., Cerini R., Longoni P., Grossato A., Canepari P. Identification of a streptococcal penicillin-binding protein that reacts very slowly with penicillin. J Bacteriol. 1983 Sep;155(3):1343–1350. doi: 10.1128/jb.155.3.1343-1350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Smith S. A., Bonner D. P. Penicillin-binding proteins in a Staphylococcus aureus strain resistant to specific beta-lactam antibiotics. Antimicrob Agents Chemother. 1982 Jul;22(1):172–175. doi: 10.1128/aac.22.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakenbeck R., Kohiyama M. Purification of penicillin-binding protein 3 from Streptococcus pneumoniae. Eur J Biochem. 1982 Oct;127(2):231–236. doi: 10.1111/j.1432-1033.1982.tb06860.x. [DOI] [PubMed] [Google Scholar]
- Hakenbeck R., Tarpay M., Tomasz A. Multiple changes of penicillin-binding proteins in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):364–371. doi: 10.1128/aac.17.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerger S., Tomasz A. Alterations in kinetic properties of penicillin-binding proteins of penicillin-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother. 1986 Jul;30(1):57–63. doi: 10.1128/aac.30.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerger S., Tomasz A. Alterations in penicillin-binding proteins of clinical and laboratory isolates of pathogenic Streptococcus pneumoniae with low levels of penicillin resistance. J Infect Dis. 1986 Jan;153(1):83–89. doi: 10.1093/infdis/153.1.83. [DOI] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACKS S., HOTCHKISS R. D. A study of the genetic material determining an enzyme in Pneumococcus. Biochim Biophys Acta. 1960 Apr 22;39:508–518. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mendelman P. M., Chaffin D. O., Stull T. L., Rubens C. E., Mack K. D., Smith A. L. Characterization of non-beta-lactamase-mediated ampicillin resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1984 Aug;26(2):235–244. doi: 10.1128/aac.26.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percheson P. B., Bryan L. E. Penicillin-binding components of penicillin-susceptible and -resistant strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Sep;18(3):390–396. doi: 10.1128/aac.18.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- Williamson R., Hakenbeck R., Tomasz A. In vivo interaction of beta-lactam antibiotics with the penicillin-binding proteins of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Oct;18(4):629–637. doi: 10.1128/aac.18.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R., Tomasz A. Inhibition of cell wall synthesis and acylation of the penicillin binding proteins during prolonged exposure of growing Streptococcus pneumoniae to benzylpenicillin. Eur J Biochem. 1985 Sep 16;151(3):475–483. doi: 10.1111/j.1432-1033.1985.tb09126.x. [DOI] [PubMed] [Google Scholar]
- Zighelboim S., Tomasz A. Penicillin-binding proteins of multiply antibiotic-resistant South African strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):434–442. doi: 10.1128/aac.17.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]