Abstract
Follicle-stimulating hormone controls the maturation of mammalian ovarian follicles. In excess, it can increase ovulation (egg production). Reported here is a transgenic doxycycline-activated switch, tested in mice, that produced more FSHB subunit (therefore more FSH) and increased ovulation by the simple feeding of doxycycline (Dox). The transgenic switch was expressed selectively in pituitary gonadotropes and was designed to enhance normal expression of FSH when exposed to Dox, but to be regulated by all the hormones that normally control FSH production in vivo. Feeding maximally effective levels of Dox increased overall mRNA for FSHB and serum FSH by over half in males, and Dox treatment more than doubled the normal ovulation rate of female mice for up to 10 reproductive cycles. Lower levels of Dox increased the number of developing embryos by 30%. Ovarian structure and function appeared normal. In summary, gene switch technology and normal FSH regulation were combined to effectively enhance ovulation in mice. Theoretically, the same strategy can be used with any genetic switch to increase ovulation (or any highly conserved physiology) in any mammal.
Keywords: assisted reproductive technology, anterior pituitary, follicle-stimulating hormone, gene regulation, ovulation
INTRODUCTION
Transgenes are difficult to express appropriately in vivo. Most often they have been controlled by promoters that possess little or no natural regulation over them, so they are expressed at happenstance levels throughout the animal’s lifetime (embryo, neonate, adult) in many inappropriate tissues and without natural homeostatic control mechanisms to keep expression within normal physiological boundaries. A transgene for growth hormone, for instance, was used to accelerate growth and decrease body fat in pigs, but its unregulated high production caused a number of unwanted health problems [1]. A similar use of transgenes that expressed both subunits of FSH in many cells increased overall FSH expression in mice, but the increase overwhelmed and down-regulated the ovary causing infertility [2]. Because of problems associated with inappropriate expression of transgenes in vivo, an authoritative review on transgenic pigs concluded, ‘‘Regulatory sequence that will permit full control of gene expression must be developed before the full potential of gene transfer . . . can be realized’’ [1].
Gene switches like the one used in this report [3, 4] hold the promise of temporal control of transgenes so that their expression can be induced at the appropriate time in the embryo, neonate, or adult. Gene switches also act as rheostats that can gradually increase the level of transgene expression based on their level of activation. Nevertheless, gene switches are rarely used in vivo and are restricted almost entirely to applications involving cells in culture. A significant barrier to the use of transgenes in vivo to augment normal physiologies is the administration of gene switch activators at just the right times to mimic normal complex regulation, which is laboriously time-consuming and can never accurately duplicate normal regulation.
Presented here is a transgenic strategy that used a gene switch to effectively increase expression of FSH to reliably increase ovarian performance (increased ovulation rate) without the side effects associated with constitutive over-expression of FSH. This report details an achievement that can be used to increase understanding of germ cell production in females and males and also lead to increased reproductive efficiency in laboratory and farm animals. Of equal importance, the strategy can be used as a model for enhancing any highly conserved and important physiology that is central to the life of any eukaryote. Such genetic control can serve as a research tool to gain information about the basic regulation of important physiologies or it can be used in practice to control key physiologies to correct genetic defects, enhance health or improve phenotypic characteristics.
MATERIALS AND METHODS
Constructing Tetracycline-Sensitive Gene Switches
The Tg(FSHB-rtTA, tetO-FSHB) gene switch (Fig. 1) was made by cloning the promoter/intron (−4741 base pair [bp] to +759 bp) for the ovine FSHB subunit [5] into the tetracycline gene switch as it occurs in pKBMpMCR [6]. In this construct, the promoter for ovine FSHB controls expression of the reverse tetracycline-controlled transactivator, rtTA (see Fig. 1, left bold box). The promoter for FSHB is known to direct expression of genes selectively to pituitary gonadotropes in mice, where they are regulated just like the endogenous mouse gene for FSHB [7, 8]. The 5.5 kb Pst1 fragment of the ovine promoter/intron for FSHB was cloned into pKBMpMCR using Not1 and Bgl2 restriction sites added by pZero Blunt TOPO subcloning (Invitrogen Life Technologies, Carlsbad, CA). Then the structural gene for ovine FSHB (+1 bp to +3061 bp) [9] was cloned into the construct using polymerase chain reaction (PCR)-generated Mlu1 and Pvu1 sites. An analogous gene switch, Tg(CGA-rtTA, tetO-FSHB), was made using the human alpha-glycoprotein subunit promoter (−315 bp to +45 bp) [10] to drive expression of rtTA specifically in pituitary gonadotropes in mice. For this cloning, Not1 and Bgl2 restriction sites were created as above. The pKBMpMCR parent construct contains kanamycin resistance for growth on bacterial agar plates as well as neomycin (or G418) resistance for the stable transformation of eukaryotic cells.
FIG. 1.
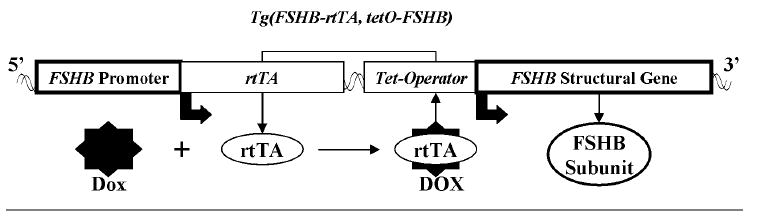
The ovine Tg(FSHB-rtTA, tetO-FSHB) gene switch. The promoter for ovine FSHB (left box) was used to control expression of the reverse tetracycline-sensitive transactivator (rtTA). This promoter has been shown to express other genes specifically in gonadotropes where they are regulated just like endogenous mouse FSHB subunit protein. Activation of rtTA by doxycycline (Dox), a tetracycline analog, was designed to induce expression of the structural gene for ovine FSHB (right box) by binding to the Tet operator DNA sequence. Because expression of rtTA is designed to mimic expression of endogenous mouse FSHB, Dox activation of rtTA should simply amplify normal regulation of FSHB expression leading to enhanced production of FSHB and FSH in a way that reflects the normal ebb and flow of endogenous FSH.
Expressing Tg(CGA-rtTA, tetO-FSHB) or Tg(FSHB-rtTA, tetO-FSHB) Gene Switches in LβT2 Cells
LβT2 cells were cultured as reported previously [11] and transiently transfected with constructs containing either Tg(CGA-rtTA, tetO-FSHB) or Tg(FSHB-rtTA, tetO-FSHB) in 24-well tissue culture plates (Falcon; Becton Dickinson Labware, Franklin Lakes, NJ) using Lipofectamine Plus (Invitrogen, Carlsbad, CA) and 1 μg of DNA/well. For stable incorporation of transgenes into LβT2 cells, cultures were treated after transfection with 500 μg/ml of geneticin (G418; Sigma, St Louis, MO). Surviving colonies were expanded and tested for their ability to produce FSH when treated with or without 1 μM Dox for 48 h, and the media were assayed for FSH by radioimmunoassay (RIA; see below). For dose-response studies, cultures were treated for 48 h with 0.01, 0.1, 1 or 10 μM Dox and then culture media were assayed by RIA for FSH.
Universal RIA for FSH (Pan-FSH RIA)
FSH was measured using a pan-FSH RIA distributed by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; Bethesda, MD) using a double antibody method [11]. All samples were assayed in duplicate from at least triplicate samples; the slope was −1.1 logit units per log unit of FSH, and intra-assay variation was ≤ 8%. Culture media and serum were stored at −20°C until analysis. Serum was obtained from clotted whole blood collected by heart puncture in killed mice. The RIA used rabbit anti-ovine FSH antiserum (AFP-C5288113) as first antibody, and purified mouse FSH (AFP-5308D) was used as tracer and reference protein. The NIDDK indicated that the RIA could not distinguish between FSH from different mammalian species, so it was considered a ‘‘universal’’ assay for FSH. The second antibody was sheep anti-rabbit antiserum prepared in our laboratory.
Generation and Screening of Transgenic Mice
All mouse experiments were approved by the Institutional Animal Care and Use Committee of North Carolina State University or the University of North Carolina at Chapel Hill. Constructs carrying Tg(FSHB-rtTA, tetO-FSHB) or Tg(CGA-rtTA, tetO-FSHB) were restricted with Srf1 and injected into zygotes from B6/SJL mice by the Animal Model Core Facility at the University of North Carolina, Chapel Hill, NC. Transgenic mice were identified by tail DNA as reported [7] using PCR oligonucleotides that detect sequences in the gene encoding rtTA of pKBMpMCR (435 bp to 1344 bp). The forward oligonucleotide was 5′-CAAGAGCTTC AGATGTGCCCTG-3′ and the reverse was 5′-GAATGCAATTGTTGTT GTTAAC-3′ (Sigma Genosys, The Woodlands, TX).
Measuring Mouse and Ovine mRNAs Encoding FSHB
Pituitaries were obtained within 5 min of death and homogenized in Tri Reagent (Molecular Research Center Inc., Cincinnati, OH) to obtain total RNA according to Tri Reagent instructions. Total RNA was quantified by absorbance at 260 ηm, and 2 μg from each pituitary sample was converted to cDNA using the iScript cDNA Synthesis kit (Bio-Rad, Inc., Hercules, CA). Portions of iScript cDNA (equivalent to 100 ηg of original RNA) were assayed in triplicate by either PCR or real-time PCR. Oligomers for all PCR products were designed to detect mRNAs encoding FSHB of either mouse (endogenous) or ovine (switch-made) origin, and their products spanned intron II of both genes. Samples assayed without the iScript conversion showed no product formation.
Initial screening of transgenic mice for pituitary-specific expression and Dox-mediated induction of mRNA for ovine FSHB employed semi-quantitative PCR with the following oligomer primers from Sigma Genosys: 5′-GACGTAGCTGTTTACTTCCCAG-3′ (forward) and 5′-CACAG CCAGGCAATCTTACGGTC-3′ (reverse) for mRNA encoding mouse FSHB and 5′-CAGGATGAAGTCCGTCCAG-3′ (forward) plus 5′-CTC TTTATTCTCTGATGTGACTGAAG-3′ (reverse) for mRNA encoding ovine FSHB. Fragment sizes obtained using these primers were 280 bp and 399 bp for mRNAs encoding mouse and ovine FSHB, respectively, as expected (data not shown).
Primers and probes for real-time reverse transcription-polymerase chain reaction (RT-PCR) were obtained from Integrated DNA Technologies, Inc, (Coralville, IA). Oligomers for mouse cDNA were 5′-AGAGA AGGAAGAGTGCCGTTTCTG-3′ (forward) and 5′-ACATACTTTCTG GGTATTGGGCCG-3′ (reverse), plus the Taqman probe (FAM) 5′-ATC AATACCACTTGGTGTGCGGGCTA-3′. Oligomers for ovine cDNA were 5′-ACCGTGGAGAAAGAGGAATGTAGC-3′ (forward) and 5′-TACATGCTTTCTGGATGTTGGGCCTT-3′ (reverse) plus the Taqman probe (FAM) 5′TGCATAAGCATCAACACCACGTGGTG-3′. Real-time PCR was performed in the iCycler (Bio-Rad) according to the manufacturer’s instructions.
Real-time RT-PCR was specific for the mRNAs encoding either mouse or ovine FSHB because of sequence differences. Absolute quantitation of these mRNAs was performed by comparing ‘‘ct’’ values of samples and standards; standards were made by producing cDNAs from total RNA extracts using the iScript procedure followed by cloning into pZero Blunt TOPO. The standard constructs were quantified using Hoefer Dyna Quant 200 fluorometer (Amersham, Pharmacia Biotech) and numbers of RNA molecules were calculated by comparing ct values of unknowns to ct values of either mouse or ovine standards. The standard assay lines for both mouse and ovine cDNA clones were superimposable with identical slopes and potencies. Variation of unknown values was ≤10% within or between assays.
Ovulation
Females were exposed to bedding from males for 1 wk before feeding Dox and were fed rodent chow with or without Dox (6 g/kg rodent chow; Bioserv, Frenchtown, NJ) starting 2 or 30 days before being placed with fertile males (one male per two females). Females were checked each morning at 0900 h for copulation plugs, the presence of which suggested that ovulation occurred during the previous 9 h. By 1200 h each day, females with plugs were killed, and were analyzed for ovulation by quantifying eggs in both ampullae. Rarely, ovulation did not occur or was extremely low, perhaps reflecting activity from only one ovary. This occurred equally for control and Dox-fed mice, so these data were not included.
Some mice were fed Dox at 0.2 g/kg rodent chow, which is the standard food routinely sold by Bioserv for inducing doxycycline-sensitive transgenes in vivo. These mice were treated just like those fed the higher-Dox diet (6 g/kg rodent chow). Ten days after the copulation plug appeared, mice were killed and the developing embryos were counted. In all cases, the embryos were ~5 mm in diameter, and all appeared healthy based on inspection through the uterine wall. In a follow-up experiment, mice were fed the high-Dox diet (6 g Dox/kg rodent chow) and pregnancies were carried to term. All mice were born vigorous and healthy with no apparent defects.
Analyzing Ovarian Follicles
The ovaries of mice treated with or without Dox were fixed in Bouin solution for ≥24 h, sectioned in wax, and stained with hemotoxylin and eosin under standard conditions. Each ovary was sectioned from beginning to end in groupings of four sections of 5 μm thickness each. These groupings were interspersed with 50 μm of tissue that was not sectioned. To avoid double counting of tertiary follicles that averaged 200 μm in diameter, every third grouping of four sections was analyzed carefully. Primary, secondary and tertiary follicles were defined as described previously [12] and counted. Care was taken not to double-count follicles. Tertiary follicles were counted and measured with an eyepiece micrometer in sections only where the cut bisected the developing oocyte. Primordial follicles were not analyzed because FSH has never been shown to significantly affect them. The data represent the mean ± SEM from females fed rodent chow with or without Dox for 30–39 days (six treated, six controls). Mice fed rodent chow with or without Dox for 2–11 days (five treated, five controls) were also analyzed with identical results (data not shown).
Statistics
Statistical calculations were performed using software from Prism 4 (GraphPad Software, Inc., San Diego, CA) and SAS (SAS Institute, Inc, Cary, NC). To determine differences between several means, analysis of variance (ANOVA) was used followed by a Tukey multiple comparison test for post hoc evaluation of differences between different treatment groups. Differences between two means were analyzed using a Student t-test. Differences between the slopes of lines (FSH RIA and real-time PCR analyses) were analyzed using the PROC REG procedure of SAS. All experiments were performed with triplicate replicates, with individual samples being assayed in duplicate. Means ± SEM are plotted in all figures except Figure 4, in which individual data points are displayed.
FIG. 4.
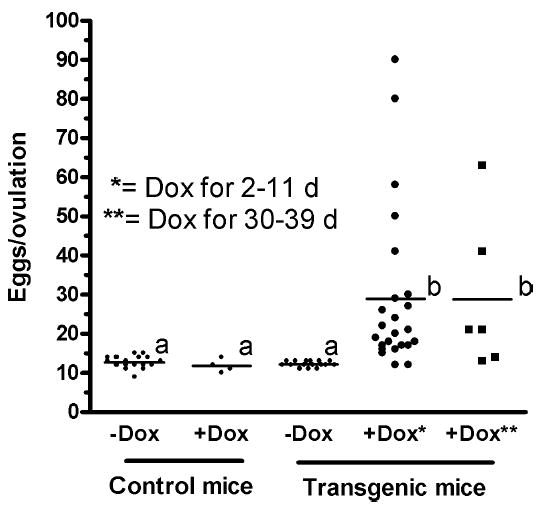
Tg(FSHB-rtTA, tetO-FSHB)1Wmil females increased ovulation rates by 240% ± 33% when fed Dox (6g/kg rodent chow) for 2–11 or 30–39 days. Three-month-old hemizygous mice were treated with Dox for 2 or 30 days and then exposed to males. All females showed evidence of copulation (copulation plug) suggesting ovulation within 9 days of exposure to males; >80% showed a plug within 4 days of male exposure. Homozygous mice were also tested at 3 mo of age and showed an ovulation rate of 29 ± 4. Moreover, hemizygous and homozygous mice were tested at age 7 mo with the same increase in ovulation for Dox-fed mice compared to controls. Means of treatment values are designated with a line horizontal to the x-axis. Means that are statistically the same (P < 0.05) have the same letter (a or b).
RESULTS
Testing FSH Production in LβT2 Cells Using Two Gene Switches
Figure 1 is a diagram of the Tg(FSHB-rtTA, tetO-FSHB) gene switch that functioned successfully in our studies to increase FSH and ovulation in transgenic mice. Expression of this switch was controlled by the promoter for ovine FSHB, a promoter known to target expression to pituitary gonadotropes [7, 8]. An analogous gene switch, Tg(CGA-rtTA, tetO-FSHB), was controlled by the promoter for the human alpha-glycoprotein subunit that is also known to target gene expression to gonadotropes [10]. Both gene switches were produced as described in Materials and Methods, and were shown to function well in LβT2 cells (Fig. 2).
FIG. 2.
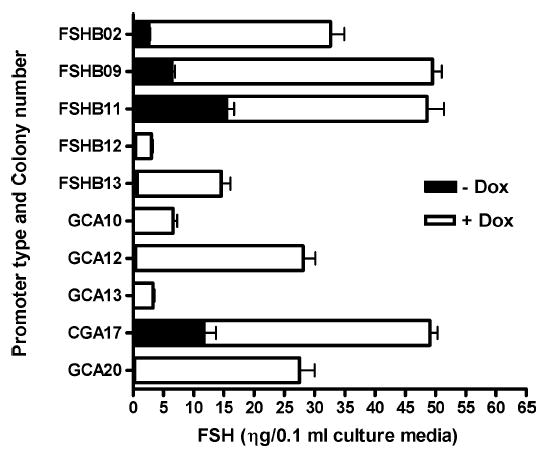
Induction of FSH expression by Dox in LβT2 cells harboring the Tg(CGA-rtTA, tetO-FSHB) or Tg(FSHB-rtTA, tetO-FSHB) gene switches. Five cell lines stably carrying the Tg(CGA-rtTA, tetO-FSHB) gene switch and five cell lines expressing Tg(FSHB-rtTA, tetO-FSHB) were cultured for 48 h with or without 10 μM Dox. The media were assayed by a pan-FSH RIA and the means ± SEMs are shown for triplicate replicates assayed in duplicate for all cell lines. Expression of FSH was significantly increased above control levels for all cell lines (P < 0.001).
Figure 2 shows Dox-induced expression of FSH in individual LβT2 colonies stably expressing either Tg(CGA-rtTA, tetO-FSHB) or Tg(FSHB-rtTA, tetO-FSHB). The LβT2 cells naturally express mouse alpha-glycoprotein subunit [13], but cannot produce measurable amounts of FSHB or FSH without activin treatment [13, 14]. Basal expression and induction of each transgene were measured by the ability of each colony to produce FSH when treated with Dox; FSH was quantitatively measured by the pan-FSH RIA. Maximal levels of Dox (10 μM) induced FSH production 3- to 20-fold with Tg(FSHB-rtTA, tetO-FSHB) and 4- to 100-fold with Tg(CGA-rtTA, tetO-FSHB) as shown in Figure 2. Dose-response data obtained with three colonies expressing Tg(CGA-rtTA, tetO-FSHB) showed that the ED50 for Dox was 0.3–1.0 μM, as expected [3, 4, 6]. These data proved that both of the FSHB-producing gene switches functioned as intended and that the endogenous mouse alpha-glycoprotein subunit effectively joined with ovine FSHB (Dox-induced FSHB) to produce a chimeric mouse/ ovine molecule of FSH.
Screening transgenic mice for FSH and mRNA encoding FSHB
Ten founder mice harboring the Tg(FSHB-rtTA, tetO-FSHB) gene switch [Tg(FSHB-rtTA, tetO-FSHB)1–10Wmil] and fourteen founders carrying the Tg(CGA-rtTA, tetO-FSHB) gene switch [Tg(CGA-rtTA, tetO-FSHB)1–14Wmil] were produced and mated with CD-1 mice. When the offspring were reproductively mature (>7 wk old), males were treated ± Dox for 2 wk and then killed, and blood was assayed for serum FSH by RIA; mRNA encoding mouse or ovine FSHB was quantified using semiquantitative PCR. Males were used to avoid the wide variation found in female FSH values, which is presumably caused by differences in reproductive cycle stage that are difficult to assess.
Initial RT-PCR screening found that founder lines Tg(FSHB-rtTA, tetO-FSHB)1–6Wmil expressed mRNA for FSHB specifically in the pituitary (luciferase activity [RLU/ mg protein] was 99 times higher in pituitary than in testis or liver) and Dox increased mRNA for FSHB 2- to 4-fold. Likewise, founder lines Tg(CGA-rtTA, tetO-FSHB)1–5Wmil showed preferential expression in pituitary tissue. In all cases, Dox increased levels of mRNA for FSHB in pituitary tissue and also appeared to increase overall FSH in mouse serum, but increases in serum FSH were not statistically significant because of high variation between mice, both male and female. Based on these initial RT-PCR data, two founder lines, Tg(FSHB-rtTA, tetO-FSHB)1&2Wmil, were expanded to determine if Dox treatment in vivo would increase ovulation rate in females. Measuring mRNA for FSHB by real-time RT-PCR was deemed the best way to determine the presence of a functional transgene, and real-time RT-PCR was used to accurately quantify mRNAs for endogenous and/or ovine FSHB subunits throughout this report.
Real-time RT-PCR was first used to accurately quantify tissue-specific expression of ovine FSHB mRNA in Tg(FSHB-rtTA, tetO-FSHB)1Wmil, which was the mouse line ultimately chosen for study in this report (see super-ovulation below and Fig. 3). The data in Figure 3 indicate that fully induced male expression of FSHB mRNA (2 wk of Dox at 6 g/kg rodent chow) was highly specific for pituitary gonadotropes because expression was <2% in fore-brain, heart, lung, liver, spleen, and gonads compared to pituitary expression.
FIG. 3.
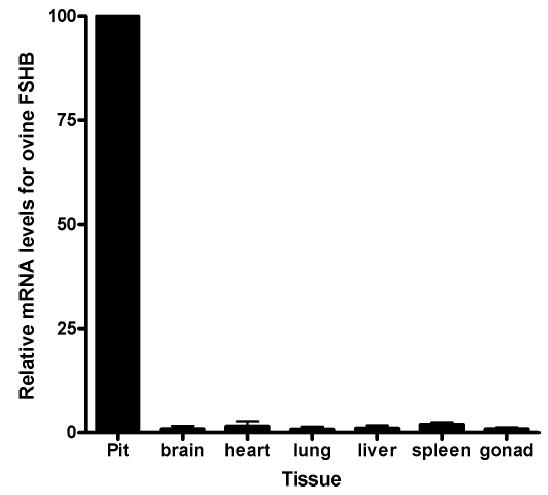
Pituitary-specific expression of mRNA for ovine FSHB in Tg(FSHB-rtTA, tetO-FSHB)1Wmil mice during Dox treatment. Male mice from Tg(FSHB-rtTA, tetO-FSHB)1Wmil founders were treated with Dox for two weeks, and then tissues were taken and assayed for mRNA of ovine FSHB using real-time RT-PCR. All samples from individual mice were assayed together and the results were normalized to pituitary expression of ‘‘100.’’ To determine differences between several means, analysis of variance was used, followed by the Tukey multiple comparison test for post hoc evaluation of differences between different treatment groups. Assay variation was ≤10%. Individual percentage expressions for brain, heart, lung, liver, spleen, and gonad were 0.9 ± 0.7, 1.5 ± 1.2, 0.7 ± 0.7, 1.0 ± 0.7, 1.9 ± 0.6, and 0.9 ± 0.4, respectively, compared to expression of mRNA for FSHB in the pituitary.
Superovulation by Dox
The ability of transgenes to increase ovulation was tested on 12 females each from three separate founder mouse lines: Tg(FSHB-rtTA, tetO-FSHB)1&2Wmil and Tg(CGA-rtTA, tetO-FSHB)1Wmil. Treatment of Tg(FSHB-rtTA, tetO-FSHB)1&2Wmil females with Dox (6 g/kg of rodent chow) equally increased ovulation in both founder lines from 12 oocytes up to 20–40 (or 90 in one case) per ovulatory cycle. The one founder line chosen from mice containing the Tg(CGA-rtTA, tetO-FSHB) gene switch did not exhibit increased ovulation when fed Dox (data not shown). Because both Tg(FSHB-rtTA, tetO-FSHB)1&2Wmil gave similar Dox-mediated increases in ovulation, only one line, Tg(FSHB-rtTA, tetO-FSHB)1Wmil, was expanded to produce mice for larger scale studies.
Figure 4 shows that females from Tg(FSHB-rtTA, tetO-FSHB)1Wmil increased ovulation by 240% ± 33% when fed Dox (6 g/kg rodent chow). Control CD-1 mice ovulated 12 ± 0.5 eggs with or without Dox treatment, as did transgenic female mice without Dox. With Dox, Tg(FSHB-rtTA, tetO-FSHB)1Wmil females produced 29 ± 4 eggs per ovulatory cycle. Moreover, it made no difference whether mice were 3 or 7 mo old (only data from 3 mo old mice are shown) or if the transgene was hemizygous or homozygous (only data from hemizygous mice are shown), and it made no difference if Dox was given for 2–11 days (1–3 estrous cycles) or 30–39 days (7–10 estrous cycles). These data show that exposure to Dox for up to 10 reproductive cycles increased ovulation an average of 240%, presumably at every ovulation (Fig. 4).
To investigate the viability of ovulated oocytes, transgenic hemizygous mice were treated as in Fig. 4 (see 2–11-day results), but with a lower dose (0.2 g/kg rodent chow) to avoid producing the 30–50 ovulations associated with feeding the higher level of Dox diet (6 g/kg rodent chow). The extremely high ovulation rates often resulted in uterine overcrowding, multiple embryo absorption, and no overall gain in birth rate (data not shown). This study used 38 Tg(FSHB-rtTA, tetO-FSHB)1Wmil females that were fed normal rodent chow (12 mice) or rodent chow containing Dox (26 mice) for 2 days before and during exposure to fertile males. Females were given normal rodent chow when plugs were observed, and mice were killed 10 days afterwards. Although embryos were not analyzed in detail, all embryos appeared uniform in size ~5 mm in diameter and looked as normal as the control embryos; there was no evidence of embryo absorption in any of the Dox-fed mice. Without feeding Dox there was no statistical difference between embryo number in transgenic mice (12.7 ± 6) and normal CD-1 birthing rate (11.5 ± 0.2; n = 41) or CD-1 ovulation rate (see Fig. 4). Females fed Dox carried 30% more embryos (16.5 ± 0.8) than control CD-1 mice fed Dox. To test possible deleterious effects of extremely high levels of Dox, Tg(FSHB-rtTA, tetO-FSHB)1Wmil females were fed the highest level of Dox (6 g/kg rodent chow) 2 days before mating and throughout the entire pregnancy. Birthing rate was 10.7 ± 1 per litter (not different from control mice, but unexpectedly low, presumably because of overcrowding of uterine space), and all offspring were vigorous and seemingly normal up through weaning, when the experiment was terminated.
Production of FSH and mRNA for FSHB in Tg(FSHB-rtTA, tetO-FSHB)1Wmil
Males were used for regulatory studies because cycle variation in females made these studies very difficult. Figure 5 shows changes in pituitary mRNA levels for mouse FSHB, ovine FSHB, and serum FSH in hemizygous or homozygous Tg(FSHB-rtTA, tetO-FSHB)1Wmil males following treatment with Dox. Hemizygous or homozygous Tg(FSHB-rtTA, tetO-FSHB)1Wmil males were analyzed by real-time RT-PCR for endogenous mouse and switch-made mRNA for ovine FSHB. Total mRNA for mouse and ovine FSHB was plotted by stacking the bar for switch-made ovine FSHB mRNA (white) on top of the bar representing endogenous mouse Fshb mRNA (black). The data show that Dox increased total mRNA for FSHB by ~50% and serum FSH by 56% ± 5% over untreated mice (Fig. 5; Hemi or Homo). When homozygous males were used, Dox also increased total mRNA for FSHB and serum FSH by ~50% (see Homo), but endogenous mouse Fshb mRNA decreased by ~50% showing the effects of feedback inhibition.
FIG. 5.
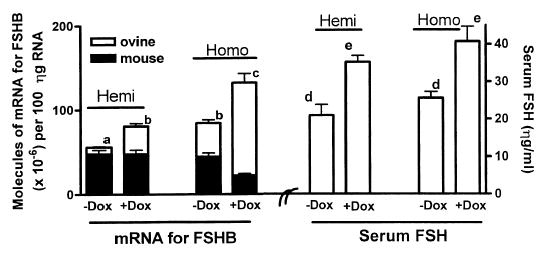
Dox increased total FSH and mRNA for FSHB by 50%–60% in male Tg(FSHB-rtTA, tetO-FSHB)1Wmil mice. No increase was observed in males lacking the fertility switch (not shown). Transgenic B6SJL founder mice were bred into CD-1 mice, giving them considerable CD-1 genetic makeup. Data from the fourth generation are presented in this report. Males were fed rodent chow ± 6g/kg of Dox for 14 days, and then their pituitaries and blood were analyzed. Total RNA was isolated, and mRNAs for ovine FSHB and mouse FSHB were quantified in 100 ηg of RNA using real-time RT-PCR. Standard curves for PCR analyses consisted of known amounts of cDNA for ovine or mouse FSHB in plasmids. Each data point represents the mean ± SEM for either four hemizygous or seven homozygous mice. Statistical analysis used analysis of variance plus the Tukey multiple comparison test. There are significant differences (P < 0.05) between means with different letters.
Basal expression of mRNA for ovine FSHB occurred even without Dox treatment in both hemi and homozygous mice. Basal expression was 4-fold greater in homozygous mice so that the basal mRNA level for ovine FSHB actually equaled the fully stimulated level of mRNA for ovine FSHB in Dox-treated hemizygous mice (compare hemi+Dox with homo-Dox).
Ovarian Morphology With or Without Dox Treatment
The ovaries for this study were taken from the same mice analyzed for ovulation in Figure 4 that had been treated with or without Dox for 30–39 days. Data in Figure 6 show that there was no evidence for changes in the numbers of primary, secondary, or tertiary follicles, nor was there any difference in the average size of tertiary follicles. Furthermore, careful inspection of the ovaries showed no evidence of damage to the ovaries such as cyst formation, obvious depletion of primordial follicles, or any malformation in transgenic animals that were fed Dox over a 30–39 day period. In fact, the ovaries of mice fed Dox for 30–39 days were not different from those of control mice (see Fig. 6) except they contained more corpora lutea (corpora lutea were not quantified).
FIG. 6.
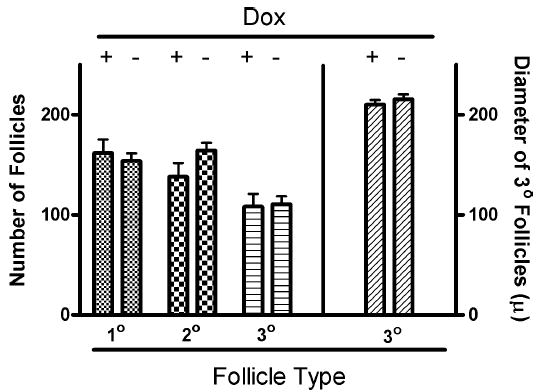
Treatment with Dox for 30–39 days did not alter the number of primary, secondary or tertiary follicles or the size of tertiary follicles (Student t-test; P < 0.05). The data represent the means ± SEM for results from six control and six Dox-treated transgenic mice. The diameter of tertiary follicles is reported in microns.
DISCUSSION
Reported here is a method for increasing ovulation rate using a doxycycline-sensitive gene switch to enhance production of FSH. There have been significant effort and resources expended in the past trying to achieve increased fertility in laboratory mice [15] and farm animals [16] by breeding for enhanced ovulation rate and/or uterine capacity. These traits segregate separately in complex ways that have permitted only limited success for increasing either the number of eggs ovulated or uterine capacity. The approach used here permitted reliable increases in ovulation that can be theoretically adjusted to any level between maximum enhancement and zero effect. Such a model system is likely to be useful for those studying oocyte maturation, ovulation and spermatogenesis or trying to breed for increased fertility, which may require increased uterine capacity.
The transgenic method used here differed significantly from others used in the past. This method was designed to use a gene switch as a simple amplifier of normal FSH expression so that all the normal homeostatic complexity would be preserved (regulation by activins, inhibins, follistatin, estradiol, progesterone, GnRH, and possibly others) [17, 18]. Preferential expression of the transgene (>98% in the pituitary; Fig. 3) demonstrated that gonadotrope-specific expression was achieved just as with two other transgenes driven by the promoter for ovine FSHB [7, 8]. Considering that gonadotropes comprise <5% of mouse pituitary cells [8], the specificity of ovine FSHB expression in gonadotropes was calculated to be more than 1000 times greater compared to the other cell types tested. Furthermore, gonadotrope-specific regulation of Tg(FSHB-rtTA, tetO-FSHB) was demonstrated by the feedback inhibition on mRNA for endogenous mouse FSHB in homozygous mice (see Fig. 5). Therefore, the strategy used in this transgenic switch seems to have achieved significant exogenous control (induction by Dox treatment) while preserving physiological regulation of FSHB produced by the transgene. This was the goal articulated in the introduction of this paper as the prerequisite for achieving the full potential of modern transgenic technology [1].
The transgenic strategy used here stands in contrast to tetracycline-activated switches that are expressed constitutively at high levels using strong viral or eukaryotic promoters. In these more traditional constructs, regulation is controlled entirely by Dox. In fact, use of the strong alpha-glycoprotein subunit promoter tested in Tg(CGA-rtTA, tetO-FSHB)1Wmil may have failed because it was too much like a constitutively active viral promoter. Based on PCR data, mRNA for ovine FSHB was expressed and induced in the pituitaries of these mice, but it failed to increase ovulation, perhaps because it lacked regulation by an authentic promoter for FSHB. Because only one transgenic line was tested, however, it is impossible to know what caused the failure, because failure could have resulted from a number of factors, including location of the transgene in chromatin.
Mice that expressed Tg(FSHB-rtTA, tetO-FSHB) showed an increased ovulation rate of 240% whether they were analyzed 2 or 39 days after eating Dox. In fact, acute treatment with Dox (2 days) caused some mice to ovulate 40–50 oocytes. This was as effective as acute treatments with pregnant mare’s serum gonadotropins and human chorionic gonadotropin [19]. Superovulation after this short time suggested that Dox rapidly increased FSH in mice that were in proestrus and almost ready to ovulate, which was apparently enough to rescue a significant number of mature follicles from apoptosis to more than double the ovulation rate. It was gratifying that superovulation occurred even after 39 days of continuous Dox treatment. These results suggested that expression of transgenic ovine FSHB mimicked the normal rhythm of endogenous mouse FSHB, enhancing it at just the right time before each ovulation of each estrous cycle. A related observation that reinforces the notion of enhanced but normal transgene regulation is the fact that 70–80% of mice ovulated within 4 days of exposure to males whether or not they were pretreated with Dox for 0, 2, or 30 days. This indicated that Dox had no effect on estrous cycle patterns in the mice, a fact that is consistent with transgenic and endogenous patterns of FSHB being similar.
It was of interest to determine whether expression and Dox activation of Tg(FSHB-rtTA, tetO-FSHB) had an effect on the number and/or nature of primary, secondary or tertiary follicles, because it is known that the latter are affected by FSH [20]. The data in Figure 6 indicate that Dox had no effect on the qualitative nature of any of the three types of follicles even after 30–39 days of treatment, suggesting that the transgene must have restricted its action primarily to rescuing follicles at the last minute before ovulation.
It is worth considering how the transgenic mice adjusted to the higher than normal levels of mRNA for FSHB produced by the ‘‘leaky’’ Tg(FSHB-rtTA, tetO-FSHB) gene switch even in the absence of Dox (Fig. 5). Presumably, this adjustment occurred as they developed in utero or neonatally. The data show that without Dox, pituitaries of hemizygous or homozygous males naturally produced ~10% or ~50%, respectively, more total mRNA for FSHB than pituitaries in normal CD-1 mice (Fig. 5). However, these transgenic mice did not have higher serum FSH or higher ovulation rates than normal CD-1 mice unless they were treated with Dox (Fig. 4). These data indicate that developmental mechanisms at the pituitary, and/or possibly the ovary, kept FSH levels and ovulation within boundaries associated with normal CD-1 mouse physiology [19]. These mechanisms are not presently understood but are clearly present.
Finally, the strategy used in this study to increase fertility should be useful for enhancing other physiologies in mammals. Growth hormone (GH), for example, shows peak secretion during the night in males [21] or throughout the day in females [22]. If a GH gene switch were designed like the Tg(FSHB-rtTA, tetO-FSHB) gene switch, using the GH locus control region and regulatory sequences in its proximal promoter [23], an appropriate amount of Dox (or nonantibiotic tetracycline derivative [24, 25]) could be administered during high growth periods to increase lean protein deposition in farm animals in a normal way that should not interfere with other physiologies. With reference to human medicine, the ability to control genetic diseases with genes expressed in somatic cells has made significant progress. It may be possible to control this type of gene therapy precisely using a gene switch arrangement like that of the Tg(FSHB-rtTA, tetO-FSHB) switch described here. An insulin gene switch, for example, could be used as a transgene targeted specifically to insulin-producing pancreatic cells [26], where it could be regulated by Dox along with its own cell-specific homeostatic control mechanisms. Likewise, any peptide hormone could be enhanced in a regulated way in other mammals (or plants).
In summary, a method has been devised to use a ligand-activated gene switch to enhance production of a hormone (in this case FSH) in a physiologically effective way in mammals. This approach has been seldom used for expressing useful transgenes in mammals, and therefore represents a useful strategic shift for regulating transgenes in vivo. The method has immediate value for those studying oocyte maturation, ovulation, uterine capacity, and spermatogenesis. It has potential application for increasing ovulation and birthing rates not only in mice, but in larger mammals as well. Finally, it may have broader application to the regulation of any transgene in any organism including humans.
Acknowledgments
We are grateful to Drs. Robert J. Wall and Kevin D. Wells for the generous gift of their plasmid, pKBMpMCR, which was used to make both gene switches (USDA-ARS, Beltsville MD 20705-2350). We also thank Dr. Pamela L. Mellon (University of California at San Diego, La Jolla, CA) for the gift of LβT2 cells, and Anastasia Doutova (undergraduate in our laboratory) for creating and testing LβT2 cell lines that stably expressed the Tg(CGA-rtTA, tetO-FSHB) or Tg(FSHB-rtTA, tetO-FSHB) transgenes. Dr. John Nilson (Pullman, WA) is thanked for his generous gift of the promoter for human CGA.
Footnotes
Supported in part by the College of Agriculture and Life Sciences, North Carolina State University, and by USDA grant 99-35203-7661.
References
- 1.Pursel VG, Bolt DJ, Miller KF, Pinkert CA, Hammer RE, Palmiter RD, Brinster RL. Expression and performance in transgenic pigs. J Reprod Fertil Suppl. 1990;40:235–245. [PubMed] [Google Scholar]
- 2.Kumar TR, Palapattu G, Wang P, Woodruff TK, Boime I, Byrne MC, Matzuk MM. Transgenic models to study gonadotropin function: the role of follicle-stimulating hormone in gonadal growth and tumorigenesis. Mol Endocrinol. 1999;13:851–865. doi: 10.1210/mend.13.6.0297. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Z, Sheng T, Lee CG, Homer RJ, Elias JA. Tetracycline-controlled transcriptional regulation systems: advances and application in transgenic animal modeling. Semin Cell Dev Bio. 2002;13:121–128. doi: 10.1016/s1084-9521(02)00018-6. [DOI] [PubMed] [Google Scholar]
- 4.Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 5.Miller CD, Miller WL. Transcriptional repression of the ovine follicle-stimulating hormone-beta gene by 17 beta-estradiol. Endocrinology. 1996;137:3437–3446. doi: 10.1210/endo.137.8.8754772. [DOI] [PubMed] [Google Scholar]
- 6.Wells KD, Foster JA, Moore K, Pursel VG, Wall RJ. Codon optimization, genetic insulation, and an rtTA reporter improve performance of the tetracycline switch. Transgenic Res. 1999;8:371–381. doi: 10.1023/a:1008952302539. [DOI] [PubMed] [Google Scholar]
- 7.Huang H-J, Sebastian J, Strahl BD, Wu JC, Miller WL. The promoter for the ovine follicle-stimulating hormone-β gene (FSHβ) confers FSHβ-like expression on luciferase in transgenic mice: regulatory studies in vivo and in vitro. Endocrinology. 2001;142:2260–2266. doi: 10.1210/endo.142.6.8202. [DOI] [PubMed] [Google Scholar]
- 8.Wu JC, Su P, Safwat NW, Sebastian J, Miller WL. Rapid, efficient isolation of murine gonadotropes and their use in revealing control of follicle-stimulating hormone by paracrine pituitary factors. Endocrinology. 2004;145:5832–5839. doi: 10.1210/en.2004-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzman K, Miller CD, Phillips CL, Miller WL. The gene encoding ovine follicle stimulating hormone β: isolation, characterization, and comparison to a related ovine genomic sequence. DNA Cell Biol. 1991;10:593–601. doi: 10.1089/dna.1991.10.593. [DOI] [PubMed] [Google Scholar]
- 10.Jorgensen JS, Quirk CC, Nilson JH. Multiple and overlapping combinatorial codes orchestrate hormone responsiveness and dictate cell-specific expression of the genes encoding luteinizing hormone. Endocr Rev. 2004;25:521–542. doi: 10.1210/er.2003-0029. [DOI] [PubMed] [Google Scholar]
- 11.Huang HJ, Wu JC, Su P, Zhirnov O, Miller WL. A novel role for bone morphogenetic proteins in the synthesis of follicle-stimulating hormone. Endocrinology. 2001;142:2275–2283. doi: 10.1210/endo.142.6.8159. [DOI] [PubMed] [Google Scholar]
- 12.Rugh R. The Mouse. Minneapolis, MN: Burgess Publishing Co; 1968. p. 31. [Google Scholar]
- 13.Graham KE, Nusser KD, Low MJ. LbetaT2 gonadotroph cells secrete follicle stimulating hormone (FSH) in response to activin A. J Endocrinol. 1999;162:R1–R5. doi: 10.1677/joe.0.162r001. [DOI] [PubMed] [Google Scholar]
- 14.Bilezikjian LM, Blount AL, Leal AM, Donaldson CJ, Fischer WH, Vale WW. Cell-specific transcriptional regulation of follicle-stimulating hormone-β by activin and gonadotropin-releasing hormone in the LβT2 pituitary gonadotrope cell model. Endocrinology. 2001;142:2284–2295. doi: 10.1210/endo.142.6.8185. [DOI] [PubMed] [Google Scholar]
- 15.Clutter AC, Kirby YL, Nielsen MK. Uterine capacity and ovulation rate in mice selected 21 generations on alternative criteria to increase litter size. J Anim Sci. 1994;72:577–583. doi: 10.2527/1994.723577x. [DOI] [PubMed] [Google Scholar]
- 16.Ford JJ, Zimmerman DR, Wise TH, Leymaster KA, Christenson RK. Increased plasma follicle-stimulating hormone concentrations in pre-pubertal gilts from lines selected for increased number of corpora lutea. J Anim Sci. 2001;79:1877–1882. doi: 10.2527/2001.7971877x. [DOI] [PubMed] [Google Scholar]
- 17.Bilezikjian LM, Blount AL, Leal AM, Donaldson CJ, Fischer WH, Vale WW. Autocrine/paracrine regulation of pituitary function by activin, inhibin and follistatin. Mol Cell Endocrinol. 2004;225:29–36. doi: 10.1016/j.mce.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Winters SJ, Moore JP. Intra-pituitary regulation of gonadotrophs in male rodents and primates. Reproduction. 2004;128:13–23. doi: 10.1530/rep.1.00195. [DOI] [PubMed] [Google Scholar]
- 19.D’Cruz OJ, Uckun FM. Lack of adverse effects on fertility of female CD-1 mice exposed to repetitive intravaginal gel-microemulsion formulation of a dual-function anti-HIV agent: aryl phosphate derivative of bromo-methoxy-zidovudine (compound WHI-07) J Appl Toxicol. 2001;21:317–322. doi: 10.1002/jat.762. [DOI] [PubMed] [Google Scholar]
- 20.McGee EA, Hsueh AJW. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 21.Dimaraki EV, Jaffe CA, Bowers CY, Marbach P, Barkan AL. Pulsatile and nocturnal growth hormone secretions in men do not require periodic declines of somatostatin. A J Physiol Endocrinol Metab. 2003;285:E163–E170. doi: 10.1152/ajpendo.00334.2002. [DOI] [PubMed] [Google Scholar]
- 22.Klerman EB, Adler GK, Jin M, Maliszewski AM, Brown EN. A statistical model of diurnal variation in human growth hormone. Am J Physiol Endocrinol Metab. 2003;285:E1118–1126. doi: 10.1152/ajpendo.00562.2002. [DOI] [PubMed] [Google Scholar]
- 23.Jin Y, Surabhi RM, Fresnoza A, Lytras A, Cattini PA. A role for A/ T-rich and Pit-1/GHF-1 in a distal enhancer located in the human growth hormone locus control region with preferential pituitary activity in culture and transgenic mice. Mol Endocrinol. 1999;13:1249–1266. doi: 10.1210/mend.13.8.0332. [DOI] [PubMed] [Google Scholar]
- 24.Greenwald R, Golub L. Biologic properties of non-antibiotic, chemically modified tetracyclines (CMTs): a structured, annotated bibliography. Curr Med Chem. 2001;8:237–242. doi: 10.2174/0929867013373624. [DOI] [PubMed] [Google Scholar]
- 25.Chrast-Balz J, Hooft Van Huijsduijnen R. Bi-directional switching with the tetracycline repressor and novel tetracycline antagonist. Nucleic Acids Res. 2004;24:2900–2904. doi: 10.1093/nar/24.15.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki T, Fujimoto K, Sakai K, Nemoto M, Nakai N, Tajima N. Gene and cell based therapy for diabetes mellitus: endocrine gene therapeutics. Endocr Pathol. 2003;14:141–144. doi: 10.1385/ep:14:2:141. [DOI] [PubMed] [Google Scholar]


