Abstract
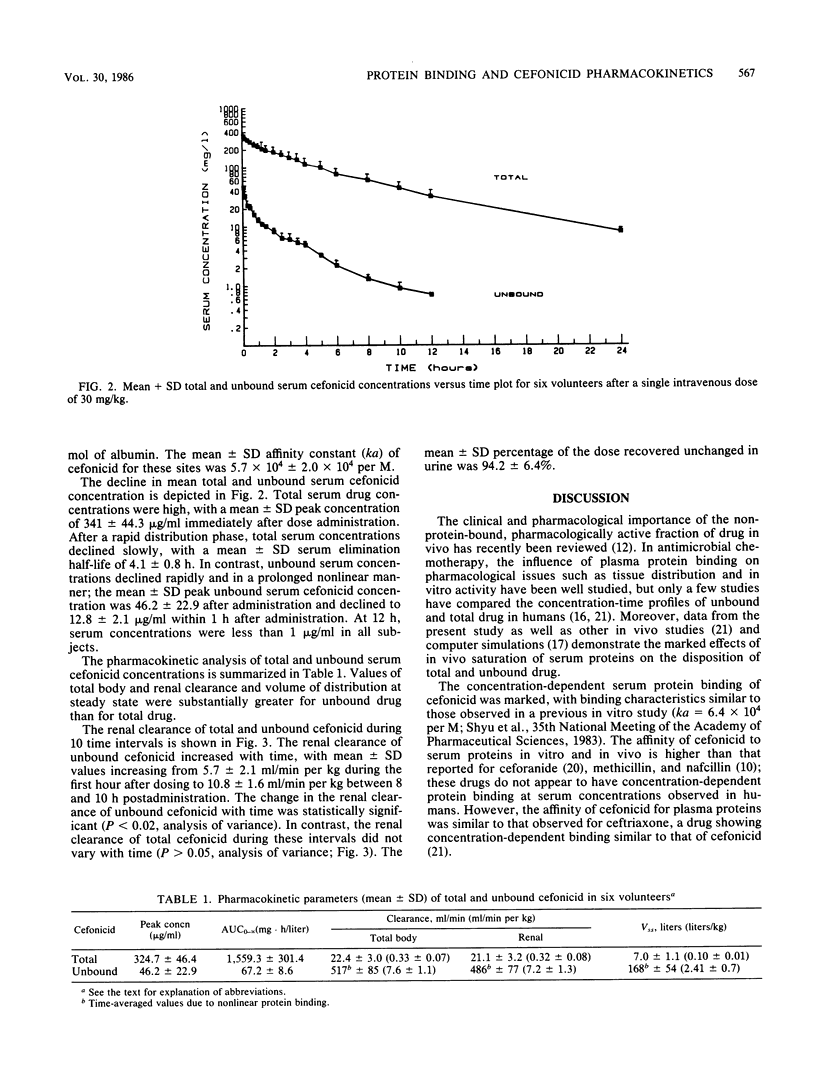
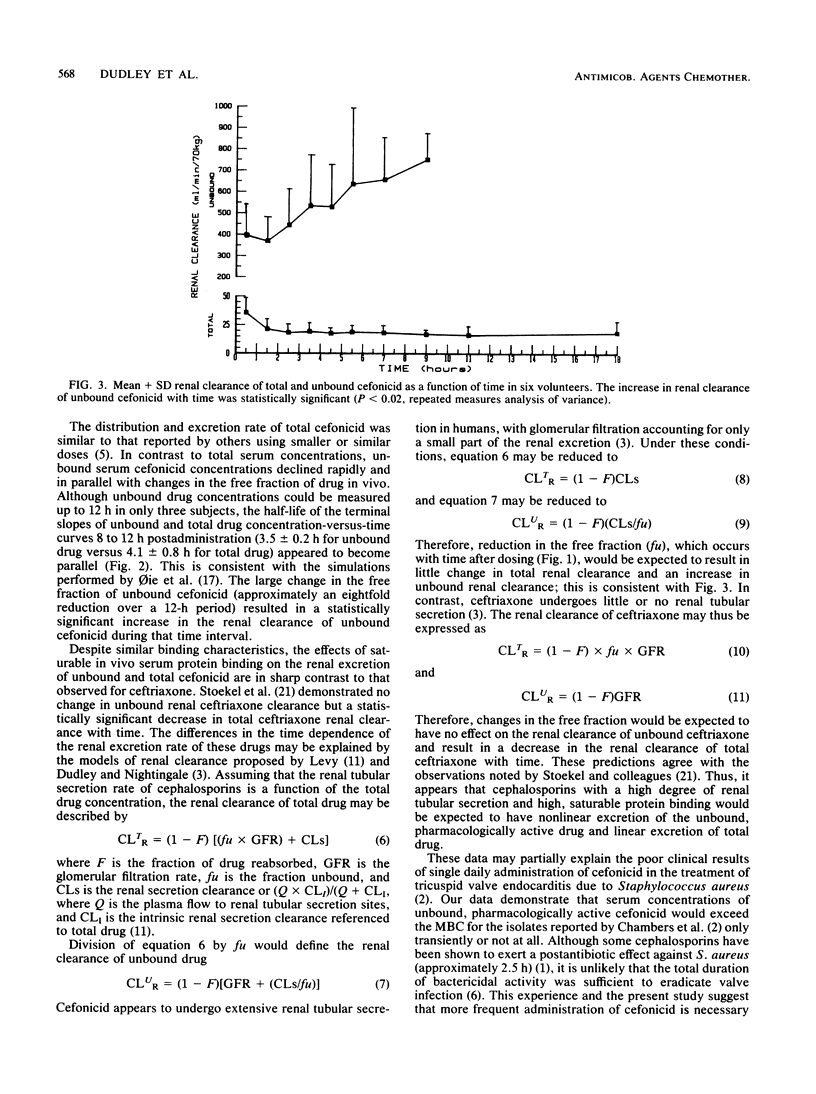
Previous studies have demonstrated high, concentration-dependent serum protein binding of cefonicid. To determine the in vivo pharmacokinetic significance of these observations, the pharmacokinetics of both total and unbound (non-protein-bound) cefonicid was studied in six volunteers after a single intravenous dose of 30 mg/kg. Saturable serum protein binding was observed in vivo; the mean +/- standard deviation free fraction of cefonicid was 17.6 +/- 6.1% immediately after administration and declined to a constant value of approximately 2% as total serum concentrations fell below 100 micrograms/ml. This nonlinear binding was associated with a pronounced decline in unbound serum cefonicid concentrations during the first 3 h after administration, with low or undetectable unbound drug concentrations by 12 h. Renal clearance of total cefonicid averaged 21.1 ml/min per kg and did not vary with time; in contrast, the mean +/- standard deviation unbound cefonicid renal clearance increased from 5.7 +/- 2.1 to 10.8 +/- 1.6 ml/min per kg with time (P less than 0.02). This study may partially explain the poor results obtained with single daily dosing of cefonicid in endocarditis. Dosage regimens of certain antimicrobial agents with high, saturable serum protein binding and extensive renal tubular secretion may be most appropriately designed based on unbound drug pharmacokinetics.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bundtzen R. W., Gerber A. U., Cohn D. L., Craig W. A. Postantibiotic suppression of bacterial growth. Rev Infect Dis. 1981 Jan-Feb;3(1):28–37. doi: 10.1093/clinids/3.1.28. [DOI] [PubMed] [Google Scholar]
- Chambers H. F., Mills J., Drake T. A., Sande M. A. Failure of a once-daily regimen of cefonicid for treatment of endocarditis due to Staphylococcus aureus. Rev Infect Dis. 1984 Nov-Dec;6 (Suppl 4):S870–S874. doi: 10.1093/clinids/6.supplement_4.s870. [DOI] [PubMed] [Google Scholar]
- Dudley M. N., Nightingale C. H., Quintiliani R., Tilton R. C. In vitro activity of cefonicid, ceforanide, and cefazolin against Staphylococcus aureus and Staphylococcus epidermidis and the effect of human serum. J Infect Dis. 1983 Jul;148(1):178–178. doi: 10.1093/infdis/148.1.178. [DOI] [PubMed] [Google Scholar]
- Dudley M. N., Quintiliani R., Nightingale C. H. Review of cefonicid, a long-acting cephalosporin. Clin Pharm. 1984 Jan-Feb;3(1):23–32. [PubMed] [Google Scholar]
- Gengo F. M., Mannion T. W., Nightingale C. H., Schentag J. J. Integration of pharmacokinetics and pharmacodynamics of methicillin in curative treatment of experimental endocarditis. J Antimicrob Chemother. 1984 Dec;14(6):619–631. doi: 10.1093/jac/14.6.619. [DOI] [PubMed] [Google Scholar]
- Gibaldi M., Koup J. R. Pharmacokinetic concepts - drug binding, apparent volume of distribution and clearance. Eur J Clin Pharmacol. 1981;20(4):299–305. doi: 10.1007/BF00618781. [DOI] [PubMed] [Google Scholar]
- Joos R. W., Hall W. H. Determination of binding constants of serum albumin for penicillin. J Pharmacol Exp Ther. 1969 Mar;166(1):113–118. [PubMed] [Google Scholar]
- Levy G. Effect of plasma protein binding on renal clearance of drugs. J Pharm Sci. 1980 Apr;69(4):482–483. doi: 10.1002/jps.2600690437. [DOI] [PubMed] [Google Scholar]
- Levy R. H., Moreland T. A. Rationale for monitoring free drug levels. Clin Pharmacokinet. 1984 Jan;9 (Suppl 1):1–9. doi: 10.2165/00003088-198400091-00001. [DOI] [PubMed] [Google Scholar]
- McNamara P. J., Gibaldi M., Stoeckel K. Volume of distribution terms for a drug (ceftriaxone) exhibiting concentration-dependent protein binding. I. Theoretical considerations. Eur J Clin Pharmacol. 1983;25(3):399–405. doi: 10.1007/BF01037955. [DOI] [PubMed] [Google Scholar]
- Nightingale C. H., Quintiliani R., Dudley M. N., Gough P., Hickingbotham M., Jordan N. S., Rose D., Toscani M. Tissue penetration and half-life of cefonicid. Rev Infect Dis. 1984 Nov-Dec;6 (Suppl 4):S821–S828. doi: 10.1093/clinids/6.supplement_4.s821. [DOI] [PubMed] [Google Scholar]
- Oie S., Gambertoglio J. G., Fleckenstein L. Comparison of the disposition of total and unbound sulfisoxazole after single and multiple dosing. J Pharmacokinet Biopharm. 1982 Apr;10(2):157–172. doi: 10.1007/BF01062333. [DOI] [PubMed] [Google Scholar]
- Oie S., Guentert T. W., Tozer T. N. Effect of saturable binding on the pharmacokinetics of drugs: a simulation. J Pharm Pharmacol. 1980 Jul;32(7):471–477. doi: 10.1111/j.2042-7158.1980.tb12971.x. [DOI] [PubMed] [Google Scholar]
- Peterson L. R., Gerding D. N. Influence of protein binding of antibiotics on serum pharmacokinetics and extravascular penetration: clinically useful concepts. Rev Infect Dis. 1980 May-Jun;2(3):340–348. doi: 10.1093/clinids/2.3.340. [DOI] [PubMed] [Google Scholar]
- Smyth R. D., Pfeffer M., Glick A., Van Harken D. R., Hottendorf G. H. Clinical pharmacokinetics and safety of high doses of ceforanide (BL-S786R) and cefazolin. Antimicrob Agents Chemother. 1979 Nov;16(5):615–621. doi: 10.1128/aac.16.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel K., McNamara P. J., Brandt R., Plozza-Nottebrock H., Ziegler W. H. Effects of concentration-dependent plasma protein binding on ceftriaxone kinetics. Clin Pharmacol Ther. 1981 May;29(5):650–657. doi: 10.1038/clpt.1981.90. [DOI] [PubMed] [Google Scholar]
- Stratton C. W., Reller L. B. Serum dilution test for bactericidal activity. I. Selection of a physiologic diluent. J Infect Dis. 1977 Aug;136(2):187–195. doi: 10.1093/infdis/136.2.187. [DOI] [PubMed] [Google Scholar]


