Abstract
Crystal structures of the lectin and epidermal growth factor (EGF)–like domains of P-selectin show ‘bent’ and ‘extended’ conformations. An extended conformation would be ‘favored’ by forces exerted on a selectin bound at one end to a ligand and at the other end to a cell experiencing hydrodynamic drag forces. To determine whether the extended conformation has higher affinity for ligand, we introduced an N-glycosylation site to ‘wedge open’ the interface between the lectin and EGF-like domains of P-selectin. This alteration increased the affinity of P-selectin for its ligand P-selectin glycoprotein 1 (PSGL-1) and thereby the strength of P-selectin-mediated rolling adhesion. Similarly, an asparagine-to-glycine substitution in the lectin-EGF-like domain interface of L-selectin enhanced rolling adhesion under shear flow. Our results demonstrate that force, by ‘favoring’ an extended selectin conformation, can strengthen selectin-ligand bonds.
Leukocyte migration to inflamed tissues and lymphocyte homing to peripheral lymphoid organs involves a multistep process1-6. In the first step, leukocytes tether and roll along vascular endothelia. Rolling exposes leukocytes to chemokines that activate integrins, which arrest leukocyte rolling and mediate leukocyte migration across the endothelia.
Rolling results from the hydrodynamic drag force acting on adherent cells. For rolling to be stable, the formation of new receptor-ligand bonds downstream must balance the dissociation of bonds upstream. Leukocyte tethering and rolling are mediated mainly by E-, L- and P-selectins. By initiating transient, rapidly reversible receptor-ligand interactions, these C-type lectin molecules allow a ‘zone of adhesion’ to move along a vessel wall7-9. In contrast, anti-body-antigen interactions are unable to support stable rolling over a wide range of shear stresses10.
Two features contribute to the ability of selectins to mediate stable leukocyte rolling. First, force increases the number of bonds that form between a rolling cell and its substrate, which effectively compensates for the shortening of receptor-ligand bond lifetimes that occurs as force on the bond increases11. Cell deformation and tether extension also promote leukocyte rolling11-13. Second, individual selectin-ligand bonds are more resistant to force than any other measured receptor-ligand bond8,11,14. As force on the receptor-ligand bond increases, the ‘off rate’ (koff) increases less for selectin–ligand bonds than for integrin–ligand or antibody–antigen bonds. Thus, selectins have enhanced mechanical stability. Furthermore, the application of a small force has been paradoxically reported to lengthen the lifetime of selectin–ligand bonds, whereas force beyond a threshold shortens the lifetime of selectin–ligand bonds15-17.
Selectins contain an N-terminal calcium-dependent lectin domain, an epidermal growth factor (EGF)-like domain, a variable number of short consensus repeats (SCRs), and transmembrane and cytoplasmic domains6,18-20. Selectin ligands consist mainly of mucin-like sialoglycoproteins such as P-selectin glycoprotein ligand 1 (PSGL-1), and all three selectin molecules bind the tetrasaccharide sialyl Lewis X (sLeX)21,22. The crystal structures of the lectin and EGF-like domains of human P-selectin, both bound and unbound to its high-affinity ligand PSGL-1 sulfoglycopeptide, SGP-3, have shown two distinct conformations of P-selectin. Ligand binding induces movements in ligand-binding loops of the lectin domain and a 52° change in orientation of the EGF-like domain23. The ligand-bound conformation is more extended. Throughout this report we use ‘extended’ and ‘bent’ to refer to the crystal structure conformations of P-selectin bound and unbound to ligand. The equilibrium between the bent and extended conformations would be shifted toward the extended conformation by an applied tensile force24. Some data suggest that optimal ligand binding requires extension of the P-selectin lectin domain a sufficient distance from the cell surface25. Removal of three SCR domains (approximately 90 Å) has no effect on rolling, but removal of four SCR domains (approximately 120 Å) results in decreased P-selectin–ligand bond strength under shear stress25. However, ligand-induced reorientation between the lectin and EGF-like domains of P-selectin does not result in a substantial change in the distance of the lectin domain above the cell surface. Comparing the structure of the extended and bent P-selectin conformers23, the difference in distance from the calcium ion that coordinates the fucose of sLeX to any disulfide in the EGF domain is approximately 5 Å.
Here we investigated altered binding properties that resulted from conformational changes in P-selectin, rather than extension of the P-selectin lectin domain above the cell surface. We tested the hypothesis that the extended selectin conformation has higher affinity for ligand. That is a reasonable hypothesis because the hydrodynamic force acting on a cell in shear flow conditions, which is applied as tensile force along the length of ligand-bound selectin molecules, would favor the more extended P-selectin conformation. We examined selectin molecules with two different types of alterations in the lectin–EGF-like domain interface: a glycan ‘wedge’ in P-selectin and an asparagine-to-glycine substitution in L-selectin. Each alteration increased the affinity of selectin for ligand and resulted in increased adhesiveness that gave rise to decreased rolling velocities. These results suggest that allostery at the selectin lectin–EGF-like domain interface regulates the affinity of selectin for ligand.
RESULTS
A glycan wedge in P-selectin
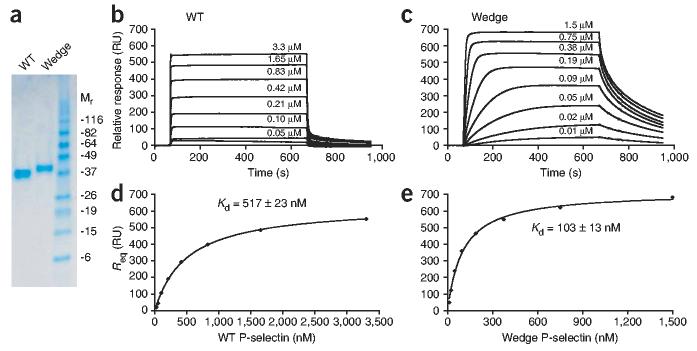
To introduce a wedge in the lectin–EGF-like domain interface of P-selectin, we focused on residues that were more exposed in the extended conformation than in the bent conformation (Fig. 1a,b). To mimic and stabilize the extended conformation, we introduced Q30N and K32T substitutions into the sequence of full-length P-selectin, which resulted in the generation of an Asn-X-Thr N-glycosylation consensus sequence (where ‘X’ is any amino acid ‘wedge mutant’). Gln30 protruded into a crevice in the lectin–EGF-like domain interface that was widened in the P-selectin conformation bound to ligand23 (Fig. 1a). Rolling a sphere over the protein surface with the radius of an N-acetylglucosamine residue (r = 2.8 Å) showed that Gln30 was eightfold more exposed in the ligand-bound extended conformation than in the non-ligand-bound bent conformation. Therefore, a glycan at this site was predicted to act as a wedge and thus favor the extended conformation. To confirm introduction of the N-linked glycosylation site, we produced fragments containing the lectin domain, EGF-like domain and the first SCR in 293S GnT1− HEK cells. This cell line produces glycoproteins with high-mannose, N-linked glycans sensitive to a recombinant fusion protein of endoglycosidase H and mannose-binding protein (endoglycosidase Hf). Wild-type and wedge-mutant P-selectin molecules migrated by reducing SDS-PAGE with apparent molecular weights of 36 kilodaltons (kDa) and 39 kDa, respectively (Fig. 1c). After endoglycosidase Hf digestion, each protein migrated with an apparent molecular weight of 33 kDa (Fig. 1c). Therefore, an additional N-linked glycan was present in the wedge mutant.
Figure 1.
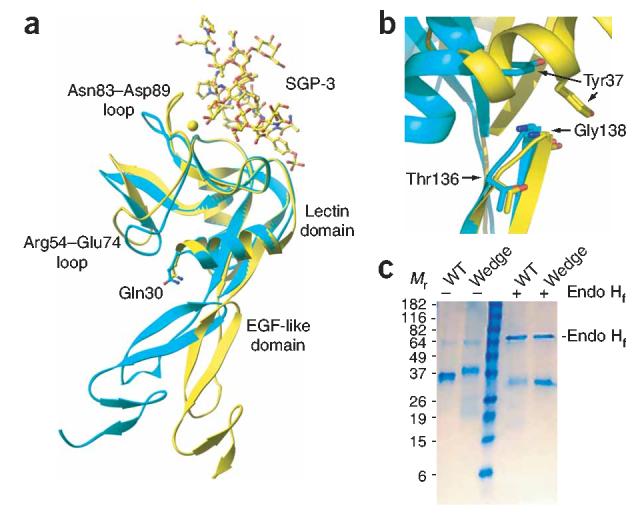
Selectin variants. (a) Superposition on the lectin domain of lectin and EGF-like domain fragments of P-selectin. Non-ligand-bound, cyan; bound to sulfoglycopeptide SGP-3, yellow23. Structure includes side chains of Gln30 in both conformations; the calcium ion of the lectin domain is a yellow sphere. (b) Structures of lectin and EGF-like domain fragments of P-selectin, presented as in a but superimposed on the EGF domain. Structure includes side chains of Thr136, Gly138 and Tyr37 for both P-selectin conformations. (c) Reducing SDS-PAGE of soluble wild-type (WT) and mutant (Wedge) P-selectin containing the lectin, EGF-like and the first SCR domains, produced in 293S GnT1− cells. Wild-type and mutant P-selectin migrate with an apparent Mr of 36 and 39 kDa, respectively, and deglycosylation with endoglycosidase Hf (Endo Hf) produces material with an apparent Mr of 33 kDa.
To study the effects of the wedge alteration on cell adhesion and rolling, we stably expressed full-length wild-type and wedge-mutant P-selectins in the Chinese hamster ovarian cell line CHO-K1. Staining with an SCR-specific monoclonal antibody (mAb) showed similar cell surface expression of wild-type and wedge-mutant P-selectin molecules (Fig. 2a). However, there was less staining of the mutant than wild-type P-selectin after incubation with multiple lectin-specific mAbs. These results suggest that introduction of the glycan wedge altered the conformation of P-selectin as intended. Although less likely, the possibility that all three lectin domain epitopes were partially ‘masked’ by the glycan introduced cannot be ruled out.
Figure 2.
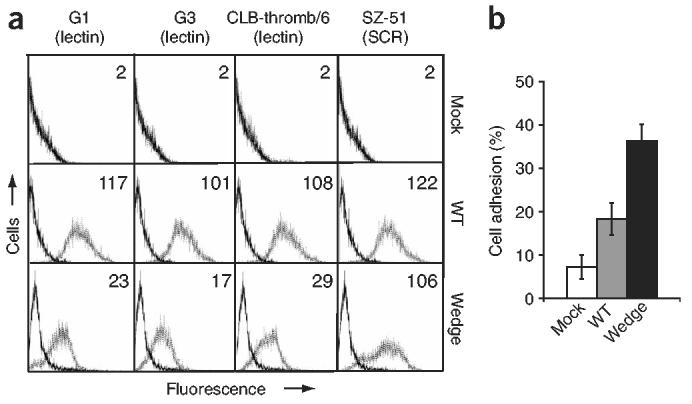
Wedge mutant P-selectin has increased adhesiveness to HL-60 cells. (a) Flow cytometry of CHO-K1 transfectants expressing wild-type or wedge mutant P-selectin, stained with control mAb X63 (solid lines) or various mAbs to lectin and SCR domains (dotted lines). Numbers in plots indicate mean channel fluorescence values. (b) Adhesion of CHO-K1 transfectants to HL-60 cells expressing PSGL-1. Adhesion, quantified as a percentage of input fluorescence. Each experiment included six replicates. Data represent the average of three experiments (error bars, mean ± s.e.m.). P = 0.025, wedge compared with wild-type. Mock, transfected with empty vector pcDNA3.1(+).
To measure cell-cell adhesion in the absence of flow, we assessed adhesion between P-selectin CHO-K1 transfectants and a human acute promyelocytic leukemia cell line, HL-60, that expresses PSGL-1. CHO-K1 cells expressing wedge-mutant P-selectin adhered significantly better than those expressing wild-type P-selectin (Fig. 2b), despite similar or slightly lower cell surface expression of mutant versus wild-type P-selectin molecules (Fig. 2a).
Next we examined the effects of the wedge mutation on P-selectin-mediated rolling under shear flow. We perfused CHO-K1 transfectants in a parallel-wall flow chamber in which three ligands (biotinylated sLeX, biotinylated PSGL-1.19ek peptide (the first 19 amino acids of mature PSGL-1 followed by an enterokinase cleavage site) and peripheral node addressin (PNAd)), each with different affinity for P-selectin, were adsorbed to the lower wall. We allowed cells to accumulate on the substrates at a wall shear stress of 0.3 dyn/cm2. We increased shear stress incrementally every 10 s and assessed rolling velocities and the percentage of cells remaining bound. The wedge mutation increased rolling adhesiveness on all three ligands, as demonstrated by slower rolling velocities (Fig. 3a-c). Over a wide range of wall shear stresses, wedge mutant expressing cells rolled approximately 90% more slowly than wild-type on sLeX (Fig. 3a) and 80% more slowly than wild-type on PNAd and PSGL-1.19ek (Fig. 3b,c). We found smaller differences in resistance to detachment induced by shear flow for mutant and wild-type P-selectin molecules (Fig. 3d-f). Wedge mutant expressing cells detached at two- to fourfold higher shear stress than wild-type on sLeX (Fig. 3a), at 1.5- to twofold higher shear stress than wild-type on PNAd (Fig. 3e) and at a shear stress similar to that of wild-type on PSGL-1.19ek (Fig. 3f).
Figure 3.
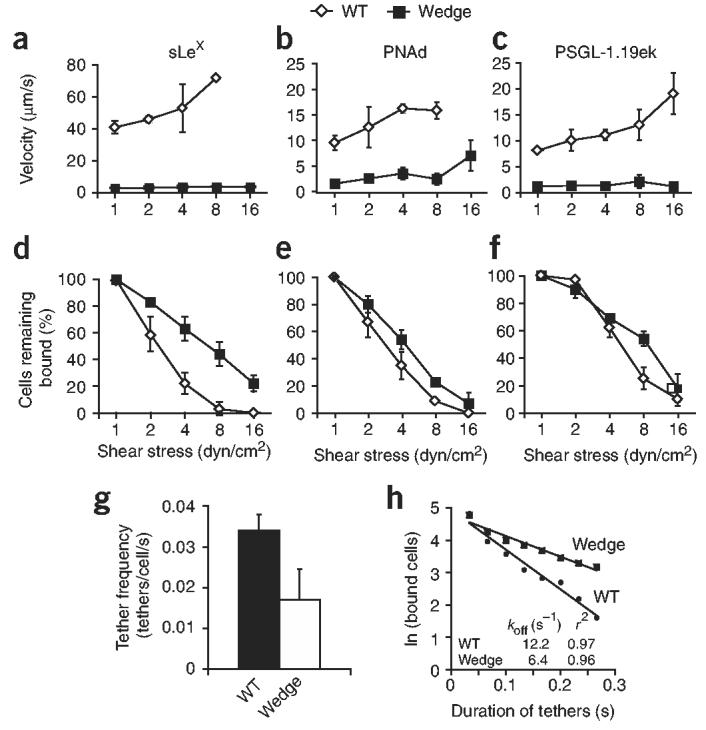
Rolling and transient tethering of wedge mutant P-selectin. (a–f) Mean velocity (a–c) and cells remaining bound (d–f), calculated at each shear stress, for CHO-K1 cells expressing WT or mutant P-selectin, perfused in a flow chamber over 5 μg/ml of biotinylated sLeX (a,d), a 1:25 dilution of PNAd (b,e) or 10 ng/ml of biotinylated PSGL-1.19ek (c,f). Cells were perfused at 0.3 dyn/cm2 for 30 s and shear stress was increased every 10 s. (g,h) Frequency of transient tether formation (g) and duration of transient tethers (h) for wild-type and mutant P-selectin, determined at a shear stress of 0.5 dyn/cm2 in conditions in which rolling was not observed (PSGL-1.19ek, 5 ng/ml for g and 3 ng/ml for h). Data (a–g) represent the average of three experiments (error bars, mean ± s.d.).
We also measured transient tethering to PGSL-1 at PSGL-1 concentrations below those required to support rolling (Fig. 3g,h). The frequency of tethering of the mutant was lower than that of wild-type P-selectin (Fig. 3g). Conversely, dissociation of wedge mutant transient tethers was slower than that of wild-type tethers (Fig. 3h).
We measured the PGSL-1 affinities of wild-type and mutant P-selectin molecules containing lectin, EGF-like and first SCR domains by surface plasmon resonance (SPR). We injected monomeric wild-type and mutant P-selectin molecules (Fig. 4a) over a flow cell containing immobilized PSGL-1–Fc (Fc region of human immunoglobulin G1) and an ethanolamine-blocked reference cell (Fig. 4b,c). The observed dissociation constants, which we obtained from nonlinear fits of the steady state equilibrium responses as a function of concentration, were 517 ± 23 nM and 103 ± 13 nM for wild-type and mutant P-selectin molecules, respectively (Fig. 4d,e). Qualitatively, the observed ‘on rate’ (kon) values were lower for the mutant, as more time was needed for the mutant than for wild-type to reach steady-state binding (Fig. 4b,c). This observation of slower kon for the mutant was consistent with the observation of fewer tethering events for the mutant (Fig. 3g). In addition, koff values were much lower for the mutant than for wild-type (Fig. 4b,c). That observation is consistent with lower koff values noted for wedge mutant transient tethers (Fig. 3h). These data did not fit well with a binding model of 1:1, as two koff values were needed to capture the dissociation phases. We tested a wide range of flow rates (2–100 μl/min), with minimal effect on the resulting data. The presence of EDTA completely abolished all interactions with the immobilized PSGL-1–Fc, consistent with the known calcium dependence of P-selectin–PGSL-1 interactions.
Figure 4.
Increased affinity of wedge mutant P-selectin. (a) Reducing SDS-PAGE of WT and wedge mutant P-selectin containing the lectin, EGF-like and first SCR domains, purified by size-exclusion chromatography. (b,c) SPR of WT monomeric material (0.026–3.3 μM; b) and wedge mutant monomeric material (0.012–1.5 μM, c), injected at twofold dilutions over immobilized PSGL-1–Fc and reference cell. Sensograms show the specific responses, as response units (RU; PSGL-1 Fc flow cell – ethanolamine-blocked reference cell). (c,d) Req for WT (c) and wedge mutant (d) plotted against their concentrations; curves are nonlinear fits to a 1:1 binding isotherm. Steady-state Kd values are the average ± s.d. of three experiments.
We also compared binding of various concentrations of soluble monomeric wild-type and wedge-mutant P-selectin molecules to CHO-K1 cells expressing PSGL-1 (Fig. 5a). Mutant P-selectin molecules bound strongly to PGSL-1 transfectants at 10 μg/ml and 100 μg/ml, whereas wild-type P-selectin did not bind (Fig. 5b). Notably, the binding of monomeric mutant P-selectin at 1 μg/ml was similar to that of wild-type P-selectin at 100 μg/ml (Fig. 5b). These results are consistent with the higher binding affinity and much lower koff values of mutant versus that of wild-type P-selectin, as measured by SPR.
Figure 5.
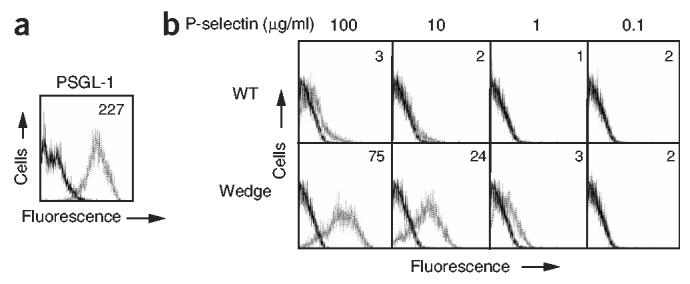
Increased binding of wedge mutant soluble P-selectin to PSGL-1 transfectants. (a) Flow cytometry of CHO-K1–PSGL-1–C2GnT1–FucT VII cells, stained with control mAb X63 (solid line) and PSGL-1-specific mAb PL2 (dotted line). (b) Flow cytometry of PSGL-1 transfectants incubated with 0.1, 1, 10 and 100 μg/ml of soluble WT or wedge P-selectin (dotted lines) or buffer alone (solid lines), then washed and stained with fluorescein isothiocyanate–conjugated P-selectin SCR–specific mAb AC1.2. Numbers in plots indicate mean channel fluorescence values.
Alterations in the L-selectin EGF-like domain
The importance of the lectin–EGF-like domain interactions in regulating rolling interactions in L-selectin was first demonstrated by the grafting of P-selectin residues 129 to 164 onto L-selectin26. That mutant, called the ‘LPL chimera’, retains the ligand specificity of L-selectin but demonstrates slower rolling under shear flow and a faster kon (ref. 27). The LPL chimera has 13 substitutions in the EGF domain and 4 substitutions in the first SCR domain26,28. Among all substituted residues, Thr136 and Gly138 are closest to the lectin domain (Fig. 1b). Crystal structures of E- and P-selectin23,29 show that only residue 138 (Gly in P-selectin, and Asn in L-selectin and E-selectin), participates in the interface. Residue 138 is at a position of considerable rearrangement in the lectin–EGF-like domain interface (Fig. 1b) and interacts mainly with residue 37 (Tyr in P-, L- and E-selectin) in the lectin domain (Fig. 1b).
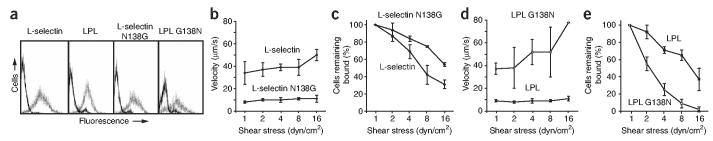
To test the importance of interface residue 138 in regulating rolling activity, we examined human chronic myelogenous leukemia K562 transfectants expressing L-selectin, the LPL chimera, L-selectin containing a N138G substiatution and an LPL chimera containing a G138N ‘back-substitution’. Staining with a lectin-specific mAb showed similar cell surface expression of all L-selectin molecules analyzed (Fig. 6a). We perfused transfectants into a parallel-wall flow chamber in which PNAd (diluted 1:25) was adsorbed to the lower wall. Over a range of wall shear stresses, L-selectin N138G transfectants rolled approximately 70% more slowly than wild-type transfectants (Fig. 6b). Furthermore, L-selectin N138G transfectants were more resistant than wild-type transfectants to detachment induced by increasing shear stress (Fig. 6c). Similarly, LPL transfectants rolled 70–90% more slowly than LPL G138N transfectants (Fig. 6d). Furthermore, higher shear stress was required for the detachment of LPL transfectants than for that of LPL G138N transfectants (Fig. 6e). These observations are consistent with earlier reports analyzing the LPL chimera27,28.
Figure 6.
Enhancement of rolling adhesiveness by the N138G substitution in L-selectin. (a) Flow cytometry showing similar cell surface expression of L-selectin, LPL, L-selectin N138G and LPL G138N. K562 transfectants were stained with control mAb X63 (solid lines) and lectin domain–specific mAb LAM1-3 (dotted lines). (b–e) Velocity (b,d) and cells remaining bound (c,e) of transfectants perfused into a flow chamber for 30 s at a shear stress of 0.3 dyn/cm2 over a 1:25 dilution of PNAd; shear stress was increased every 10 s. Mean velocity and percentage of cells remaining bound were calculated at each shear stress. Data represent the average of three experiments (error bars, mean ± s.d.).
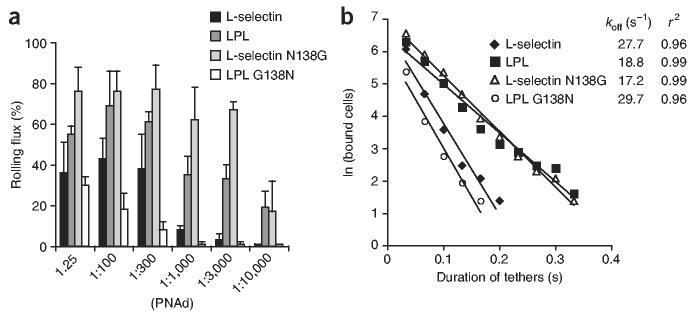
To examine the effects of L-selectin mutations on tethering and cell accumulation in shear flow, we perfused the four K562 transfectants at a constant wall shear stress of 0.6 dyn/cm2 for 1 min over varying concentrations of PNAd and determined the rolling flux (the percentage of cells in the focal plane near the substrate that were rolling). LPL and L-selectin N138G transfectants accumulated and rolled at PNAd concentrations less than 10% those required for rolling of either wild-type or LPL G138N transfectants (Fig. 7a). The ability of LPL and L-selectin N138G to support rolling at a much lower concentration and to increase rolling flux at all PNAd concentrations tested suggested that both mutants increase adhesiveness under flow by increasing the cellular kon value27. To determine cellular koff, we measured the durations of transient tethers at a shear stress of 0.6 dyn/cm2. In several experiments, the koff values of wild-type L-selectin and the LPL G138N ‘back mutant’ resembled each other and were higher than the koff values of LPL and L-selectin N138G mutants (Fig. 7b).
Figure 7.
The N138G substitution in L-selectin increases accumulation in shear flow and decreases transient tether koff. Transfectants expressing native L-selectin, LPL, L-selectin N138G or LPL G138N were perfused into a flow chamber at a shear stress of 0.6 dyn/cm2 over varying concentrations of PNAd. (a) After 1 min of perfusion, the percentage of cells in the field of view that were rolling was calculated. Data represent the average of three experiments (error bars, mean ± s.d.). (b) Durations of transient tethers determined at a shear stress of 0.6 dyn/cm2 in conditions in which rolling was not observed. Transient tethers were measured at the following PNAd dilutions: 1:300 for L-selectin; 1:10,000 for LPL and L-selectin N138G; and 1:150 for LPL G138N.
DISCUSSION
The adhesive activity of selectins in in vitro rolling assays closely mirrors their function in vivo7,30. The association of disease with polymorphic selectin amino acid substitution in vivo31,32 correlates with effects on rolling activity in vitro33-35. It has been shown that selectin-mediated rolling in vitro and in vivo requires a minimum hydrodynamic force or ‘shear threshold’36, and subsequent work on the shear threshold phenomenon has been done using in vitro flow chamber systems. Here we have used a mutational approach and in vitro flow chamber systems to show that changes in regions distant from selectin ligand-binding sites can substantially affect rolling activity. Such changes included alterations in the ability of the lectin and EGF-like domains to reorient relative to each other.
We introduced a glycan wedge between the lectin and EGF-like domains of P-selectin to stabilize the extended conformation noted before in the ligand-bound P-selectin crystal structure23. The site of that alteration is distant from the ligand-binding site. Cells expressing the wedge construct showed increased rolling adhesiveness over a wide range of shear stresses and a variety of ligands (sLeX, PNAd or PSGL-1). Those observations suggest that the orientation between the lectin and EGF-like domains is an important determinant of P-selectin function. To further understand the effect of the wedge mutation, we did SPR studies. The observed binding affinity of the mutant P-selectin to PSGL-1–Fc was fivefold higher than that of wild-type P-selectin. As stated earlier, the dissociation phases of the SPR profiles did not fit to a single exponential. However, they could be fit using two exponentials. Both of the observed dissociation rates for the mutant P-selectin (fast phase, 0.05 ± 0.01 s−1; slow phase, 0.005 ± 0.001 s−1) were substantially slower than the two rates for dissociation of wild-type P-selectin from PSGL-1–Fc (fast phase, 0.30 ± 0.03 s−1; slow phase, 0.021 ± 0.007 s−1). The observation of multiple kinetic phases could arise in different ways. First, there is heterogeneity in tyrosine sulfation of PSGL-1–Fc, and there are stepwise increases in affinity for glycopeptides that contain 0, 1, 2 and 3 sulfotyrosines over a total range of 40-fold (ref. 23). That could lead to a mixture of affinities and hence kinetic phases. However, models accounting for heterogeneous PSGL-1 still fail to capture the data (in which a single association and dissociation rate for each of the contributing interactions was assumed). Second, the phases may represent an inherent property of the P-selectin–PSGL-1 interaction. In a different experimental setup, two dissociation pathways for the P-selectin–PSGL-1 interaction have been noted37. Our SPR data were in agreement with transient tether measurements showing that the wedge mutant had a slower rate of tether formation and tether dissociation than wild-type P-selectin. The wedge mutation changed the affinity and kinetics of the interaction between P-selectin and PSGL-1, resulting in increases in both the binding of soluble P-selectin to cells expressing PSGL-1 and rolling adhesion. The results presented here have shown that P-selectin in an altered conformation has higher affinity for ligand and have demonstrated linkage between the physical properties of the molecular interaction and the resulting activity of rolling cells.
In addition, we demonstrated that a single amino acid substitution, N138G, in the interface between the lectin and EGF-like domains of L-selectin changed rolling activity considerably. As with the substitutions used to create the P-selectin wedge mutant, the N138G substitution in L-selectin is far from the ligand-binding site. This substitution increased the adhesiveness of rolling cells over a wide range of shear stress and increased resistance to detachment by shear stress. Most notably, the N138G substitution increased the efficiency with which cells in shear flow bound to the substrate and thus the tethering efficiency by more than tenfold. The reverse substitution, G138N, in the LPL chimera resulted in a return to wild-type L-selectin-like activity. Those observations are in agreement with findings obtained during previous analyses of the LPL chimera27 and show that the effects of substituting 17 different amino acids in the EGF-like and first SCR domains of L-selecten can be accounted for by a single amino acid substitution in the lectin–EGF-like domain interface.
Such data support the idea that the interactions that occur at residue 138, when force is applied to the selectin-ligand bond, are critical for modulating rolling. In the E-selectin structure, which adopts the same conformation as the bent form of P-selectin, Asn138 interacts with the lectin domain through van der Waals contact and water-mediated hydrogen bonds. In P-selectin, Gly138 interacts only with the lectin domain in the extended conformation. Among the interface residues in P-selectin, residue 138 experiences the most surface area ‘burial’ after extension of the two domains, which results in interaction mainly with Tyr37 of the lectin domain. Residue 138 is on the opposite side of the interface from the position of introduction of the glycan wedge.
Although conjectured, the importance of conformational changes in selectin-mediated rolling has not been demonstrated. Here we have shown that conformational changes, specifically those altering the lectin–EGF-like interface in selectin molecules, are a critical component of rolling activity. When the hydrodynamic force on a cell in shear flow is resisted by a selectin-substrate bond, a tensile force is applied over the length of the selectin molecule. Laws of physics and thermodynamics require that the applied force will lower the energy of the extended conformation relative to that of the bent conformation and will shift the equilibrium toward the extended conformation. At the molecular level, selectin-ligand bonds have high mechanical stability, meaning that as force increases, koff increases less than for other receptor-ligand bonds that have been studied8,10,14. Furthermore, a threshold force has been noted below which force decreases koff (‘catch-bond’ activity) and above which force increases koff (‘slip-bond’ activity)15,17,38. Both the high mechanical stability of selectins and their ‘catch-bond’ activity can be explained if force stabilizes a higher affinity conformation of selectins24,37.
One model accounts for the ‘catch-bond-like’ activity of selectins by considering two different bound states of selectins: the higher-affinity state is more populated when force is applied to the selectin-ligand bond37. Our data are consistent with that model and provide a biochemical explanation for the findings that selectin–ligand bonds are mechanically stronger than other receptor-ligand bonds tested and can show ‘catch-bond’ activity. Our results emphasize the importance of conformational change in the lectin–EGF-like domain interface in selectins and support the idea of a mechanism in which applied force enhances selectin–ligand binding by stabilizing a high-affinity, extended conformation.
METHODS
Cells and antibodies
CHO-K1, K562 and HL-60 cells were obtained from American Type Culture Collection. 293S GnT1− HEK cells39 were provided by P. Reeves (Massachusetts Institute of Technology, Cambridge, Massachusetts), and CHO-K1 cells expressing PSGL-1, core 2 β-(1,6)-N-acetylglucosaminyl-transferase I and α-(1,3)-fucosyltransferase VII (CHO-K1–PSGL-1–C2GnT1–FucT VII cells)40 were provided by M. Fukuda (Burnham Institute, La Jolla, California). CHO-K1 and 293S GnT1− cells were maintained in 10% FBS and DMEM supplemented with 2 mM l-glutamine, penicillin and streptomycin, and MEM nonessential amino acids. Before flow cytometry and flow chamber assays, CHO-K1 transfectants were collected with a solution of 0.5% trypsin plus 0.2 g EDTA·and 4 g Na per liter (Sigma) or 10 mM EDTA. K562 and HL-60 cells were maintained in RPMI medium containing 10% FBS supplemented with 2 mM l-glutamine plus penicillin and streptomycin. CHO-K1–PSGL-1–C2GnT1–FucT VII cells were maintained in DMEM supplemented with 10% FBS, 400 μg/ml of G418, 400 μg/ml hygromycin, 100 μg/ml zeocin, 2 mM l-glutamine, penicillin and streptomycin, and MEM nonessential amino acids. The mAbs G1, G3 (ref. 41), CLB-thromb/6 (ref. 42) and SZ-51 (ref. 43), specific for human P-selectin, and mAb PL2 (ref. 44), specific for human PGSL-1, were as described. Fluorescein isothiocyanate–conjugated mouse mAb AC1.2, specific for human P-selectin SCR (ref. 45), was purchased from Pharmingen, and fluorescein isothiocyanate–conjugated goat antibody to mouse immunoglobulin G was purchased from Zymed.
PCR-based mutagenesis
Plasmid pRc/RSV-P-selectin46 was provided by R. McEver (University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma), pSP65-LPL28 was provided by G. Kansas (Northwestern Medical School, Chicago, Illinois) and pcDNA 3.1 (+)-L-selectin was provided by M. Shimaoka (CBR Institute for Biomedical Research, Boston, Massachusetts). The LPL chimera sequence was subcloned into pcDNA 3.1 (−) using EcoRI and BamHI before PCR mutagenesis. Overlapping PCR was used to generate the Q30N-plus-K32T P-selectin mutant and the L-selectin N138G and LPL G138N mutants. Primers used for PCR-based mutagenesis are listed in Supplementary Table 1 online.
Generation of stable transfectants
CHO-K1 and K562 cells (20 × 106) were transfected by electroporation with 20 μg DNA at 250 mV and 500 μF and at 250 mV and 960 μF, respectively47. Transfectants, except for K562 L-selectin, were selected in medium containing 1 mg/ml of G418. K562 L-selectin transfectants, selected in medium containing 100 μg/ml hygromycin, were provided by A. Salas (CBR Institute for Biomedical Research, Boston, Massachusetts).
Generation and purification of soluble P-selectin
Wild-type and mutant P-selectin fragments containing the lectin, EGF-like and first SCR domains were generated by either subcloning into a pEF1-puror vector containing a tobacco etch virus cleavage site, six-histidine tag and streptavidin II tag48 or a pIRES2-EGFP vector containing a modified signal sequence from human κ light chain V region (Vx 014), a tobacco etch virus cleavage site, six-histidine tag and Szostak streptavidin-binding peptide49 (provided by C. Lu, CBR Institute for Biomedical Research, Boston, Massachusetts). Constructs were transfected into 293S GnT1− cells by calcium phosphate precipitation. Transfectants were cultured in DMEM containing 10% FBS, 1 mg/ml of G418, 2 mM l-glutamine, penicillin and streptomycin, and MEM nonessential amino acids. The medium was changed to Opti-MEM I (Gibco-BRL) supplemented with 5 mM l-glutamine for 4–6 d before supernatants were collected. Proteins were purified with Ni-NTA (Qiagen) and Strep-Tactin (IBA) resins. For deglycosylation, samples were digested overnight at 25 °C with endoglycosidase Hf (New England BioLabs). P-selectin was further purified by size-exclusion chromatography on a Superdex S75 HR10/30 column (Amersham Pharmacia Biotech) with 10 mM HEPES, 150 mM NaCl, 1 mM CaCl2 and 0.01% (volume/volume) Tween, pH 7.4.
Flow cytometry
Cells were collected, resuspended in L15 medium containing 2.5% FBS and incubated for 30 min at 4 °C with a 1:100 dilution of ascites fluid or a 1:10 dilution of the supernatant of control mAb X63. Cells were washed and incubated for 30 min at 4 °C with fluorescein isothiocyanate–conjugated goat antibody to mouse immunoglobulin G. Cells were washed, resuspended in PBS and analyzed on a FACScan (Becton Dickinson).
Adhesion assay
CHO-K1 transfectants were seeded overnight in a 96-well plate at a density of 5 × 104 cells/well. HL-60 cells were labeled for 15 min at 37 °C with 2 μg/ml of BCEF-AM (2′,7′-bis-(2-carboxyethyl)-5-(and 6)-carboxyfluorescein acetoxymethyl ester) in L15 medium. Cells were washed and resuspended in L15 medium containing 2.5% FCS, and 1 × 104 labeled cells were incubated for 15 min at 25 °C with CHO-K1 cells. Input fluorescence was measured at 535 nm with a fluorescence concentration analyzer (IDEXX). The wells were washed manually with L15 medium containing 2.5% FCS, and fluorescence was measured. This was repeated until fluorescence of cells transfected with pcDNA3.1(+) (mock) decreased to approximately 5% of the input fluorescence.
Flow chamber assay
PNAd at a dilution of 1:25 in PBS supplemented with 10 mM bicarbonate, pH 9, was adsorbed for 1 h at 37 °C to polystyrene plates, followed by a wash with PBS, pH 9, blocking for 1 h at 37 °C with PBS containing 2% human serum albumin and a wash with PBS, pH 9. For plates coated with PSGL-1.19ek, polystyrene plates were adsorbed for 16 h at 4 °C with 1 μg/ml of NeutrAvidin (Pierce Biotechnology), washed with PBS, pH 9, and blocked for 2 h at 4 °C with PBS containing 2% human serum albumin. Adsorption with 10 ng/ml of biotinylated PSGL-1.19ek (gift from R. Camphausen, Wyeth, Cambridge, Massachusetts) in Hanks' balanced-salt solution supplemented with 10 mM HEPES, pH 7.4, was done for 2 h at 4 °C. For sLeX, plates were adsorbed for 16 h at 4 °C with 5 μg/ml of NeutrAvidin, were washed with PBS, pH 9, blocked for 2 h at 4 °C with PBS containing 2% human serum albumin, and adsorbed with 5 μg/ml of biotinylated sLeX (Glycotech). Substrates assembled as the lower wall in a parallel-wall flow chamber and mounted on an inverted phase-contrast microscope. CHO-K1 or K562 transfectants were resuspended in Hanks' balanced-salt solution supplemented with 10 mM HEPES, pH 7.4, containing 2 mM Ca2+, and perfused through the flow chamber at a density of 0.5 × 106 cells/ml. For detachment assays, cells were perfused into the chamber and allowed to accumulate on the substrate for 30–60 s. Nonadherent cells were cleared by perfusion with Hanks' balanced-salt solution supplemented with 10 mM HEPES, pH 7.4, at a shear stress of 0.3 dyn/cm2 for 30 s, and wall shear stress was increased incrementally every 10 s. The number of cells remaining bound was calculated as a percentage of the number of cells rolling on the substrate at a shear stress of 1 dyn/cm2. Rolling velocity at each shear stress was calculated from the average displacement of cells during a 3-second interval. The number of rolling cells at 1 dyn/cm2 typically ranged between 30 and 60. For assessment of the rolling flux at varying dilutions of substrate, cells were perfused into the chamber at a constant wall shear stress of 0.6 dyn/cm2 for 1 min. Rolling flux was calculated as a percentage of the number of cells at the focal plane in the field of view. For transient tether assays, cells were perfused over low concentrations of substrate. Transient tethers were defined as pauses between cell movement at the hydrodynamic velocity. The duration of tethers was fit to first-order dissociation kinetics to determine koff values50. For determination of tether frequency, the number of tether events per second was divided by the number of cells in the field of view in the focal plane near the wall of the substrate50. Microscopic images of cells under flow were recorded on Hi-8 videotape with a Nikon plan 10× objective microscope. Analyses were made with a computerized imaging system consisting of a Pentium computer with a MVC 150/40-VL capture board (Imaging Technology).
SPR
A BIAcore 3000 instrument (Uppsala) was used for SPR, at 25 °C in 10 mM HEPES, 150 mM NaCl, 1 mM CaCl2 and 0.01% (volume/volume) Tween, pH 7.4. The PSGL-1–Fc chimera, provided by Thios Pharmaceuticals (Emeryville, California) was covalently coupled to a CM5 sensor chip (BIACore) using the amine-coupling kit (BIACore). PSGL-1 Fc at a concentration of 50 μg/ml in 10 mM sodium acetate, pH 3.8, was injected over the flow cell at a rate of 5 μl/min, giving 1,500 response units. ‘Unreacted’ sites and the reference flow cell were blocked with 1 M ethanolamine hydrochloride–NaOH, pH 8.5. Wild-type and mutant P-selectin were injected over the flow cells at a rate of 10 μl/min and bound analytes were removed by injection of 3 mM EDTA.
Soluble P-selectin binding assay
CHO-K1–PSGL-1–C2GnT1–FucT VII cells (5 × 104) in L15 medium were incubated for 30 min at 25 °C with 0.1, 1, 10 and 100 μg/ml of soluble wild-type and mutant P-selectin or with buffer alone (10 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM CaCl2 and 0.01% (volume/volume) Tween). Cells were washed with L15 medium and incubated for 30 min at 4 °C with fluorescein isothiocyanate–conjugated anti–P-selectin SCR (AC1.2). Cells were washed, resuspended in PBS and analyzed by flow cytometry.
Statistics
All nonlinear data fitting was done with the BIAevaluation (version 4.0.1) software package from BIACore (Sweden). Errors reported are the standard deviation of three independent measurements, unless otherwise noted.
Supplementary Material
ACKNOWLEDGMENTS
We thank R. Camphausen for suggestions and discussion. Supported by Kirschstein National Research Service Award (F32 AI052851 to U.T.P., F32 GM073529 to T.T.W. and HL-48675 to T.A.S).
Footnotes
Note: Supplementary information is available on the Nature Immunology website.
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
References
- 1.Rosen SD, Bertozzi CR. The selectins and their ligands. Curr. Opin. Cell Biol. 1994;6:663–673. doi: 10.1016/0955-0674(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 2.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multi-step paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 3.Kansas GS. Selectins and their ligands: Current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 4.Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol. Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 5.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 6.McEver RP. Selectins: lectins that initiate cell adhesion under flow. Curr. Opin. Cell Biol. 2002;14:581–586. doi: 10.1016/s0955-0674(02)00367-8. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 8.Alon R, Hammer DA, Springer TA. Lifetime of the P-selectin: carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 1995;374:539–542. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- 9.McEver RP. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb. Haemost. 2001;86:746–756. [PubMed] [Google Scholar]
- 10.Chen S, Alon R, Fuhlbrigge RC, Springer TA. Rolling and transient tethering of leukocytes on antibodies reveal specializations of selectins. Proc. Natl. Acad. Sci. USA. 1997;94:3172–3177. doi: 10.1073/pnas.94.7.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S, Springer TA. An automatic braking system that stabilizes leukocyte rolling by an increase in selectin bond number with shear. J. Cell Biol. 1999;144:185–200. doi: 10.1083/jcb.144.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramachandran V, Williams M, Yago T, Schmidtke DW, McEver RP. Dynamic alterations of membrane tethers stabilize leukocyte rolling on P-selectin. Proc. Natl. Acad. Sci. USA. 2004;101:13519–13524. doi: 10.1073/pnas.0403608101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yago T, et al. Distinct molecular and cellular contributions to stabilizing selectin-mediated rolling under flow. J. Cell Biol. 2002;158:787–799. doi: 10.1083/jcb.200204041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Chateau M, Chen S, Salas A, Springer TA. Kinetic and mechanical basis of rolling through an integrin and novel Ca2+-dependent rolling and Mg2+-dependent firm adhesion modalities for the α4β7-MAdCAM-1 interaction. Biochemistry. 2001;40:13972–13979. doi: 10.1021/bi011582f. [DOI] [PubMed] [Google Scholar]
- 15.Marshall BT, et al. Direct observation of catch bonds involving cell-adhesion molecules. Nature. 2003;423:190–193. doi: 10.1038/nature01605. [DOI] [PubMed] [Google Scholar]
- 16.Sarangapani KK, et al. Low force decelerates L-selectin dissociationfrom P-selectin glycoprotein ligand-1 and endoglycan. J. Biol. Chem. 2003;279:2291–2298. doi: 10.1074/jbc.M310396200. [DOI] [PubMed] [Google Scholar]
- 17.Yago T, et al. Catch bonds govern adhesion through L-selectin at threshold shear. J. Cell Biol. 2004;166:913–923. doi: 10.1083/jcb.200403144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 19.Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat. Rev. Immunol. 2004;4:325–336. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 20.Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 21.Foxall C, et al. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl LewisX oligosaccharide. J. Cell Biol. 1992;117:895–902. doi: 10.1083/jcb.117.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sako D, et al. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993;75:1179–1186. doi: 10.1016/0092-8674(93)90327-m. [DOI] [PubMed] [Google Scholar]
- 23.Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLeX and PSGL-1. Cell. 2000;103:467–479. doi: 10.1016/s0092-8674(00)00138-0. [DOI] [PubMed] [Google Scholar]
- 24.Konstantopoulos K, Hanley WD, Wirtz D. Receptor-ligand binding: ‘catch’ bonds finally caught. Curr. Biol. 2003;13:R611–R613. doi: 10.1016/s0960-9822(03)00529-3. [DOI] [PubMed] [Google Scholar]
- 25.Patel KD, Nollert MU, McEver RP. P-selectin must extend a sufficient length from the plasma membrane to mediate rolling of neutrophils. J. Cell Biol. 1995;131:1893–902. doi: 10.1083/jcb.131.6.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kansas GS, Spertini O, Stoolman LM, Tedder TF. Molecular mapping of functional domains of the leukocyte receptor for endothelium, LAM-1. J. Cell Biol. 1991;114:351–358. doi: 10.1083/jcb.114.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dwir O, Kansas GS, Alon R. An activated L-selectin mutant with conserved equilibrium binding properties but enhanced ligand recognition under shear flow. J. Biol. Chem. 2000;275:18682–18691. doi: 10.1074/jbc.M001103200. [DOI] [PubMed] [Google Scholar]
- 28.Kansas GS, et al. A role for the epidermal growth factor-like domain of P-selectin in ligand recognition and cell adhesion. J. Cell Biol. 1994;124:609–618. doi: 10.1083/jcb.124.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graves BJ, et al. Insight into E-selectin/ligand interaction from the crystal structure and mutagenesis of the lec/EGF domains. Nature. 1994;367:532–538. doi: 10.1038/367532a0. [DOI] [PubMed] [Google Scholar]
- 30.von Andrian UH, et al. Two-step model of leukocyte-endothelial cell interaction in inflammation: Distinct roles for LECAM-1 and the leukocyte β2 integrins in vivo. Proc. Natl. Acad. Sci. USA. 1991;88:7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wenzel K, Hanke R, Speer A. Polymorphism in the human E-selectin gene detected by PCR-SSCP. Hum. Genet. 1994;94:452–453. doi: 10.1007/BF00201614. [DOI] [PubMed] [Google Scholar]
- 32.Wenzel K, Ernst M, Rohde K, Baumann G, Speer A. DNA polymorphisms in adhesion molecule genes–a new risk factor for early atherosclerosis. Hum. Genet. 1996;97:15–20. doi: 10.1007/BF00218826. [DOI] [PubMed] [Google Scholar]
- 33.Revelle BM, Scott D, Beck PJ. Single amino acid residues in the E- and P-selectin epidermal growth factor domains can determine carbohydrate binding specificity. J. Biol. Chem. 1996;271:16160–16170. doi: 10.1074/jbc.271.27.16160. [DOI] [PubMed] [Google Scholar]
- 34.Rao RM, Haskard DO, Landis RC. Enhanced recruitment of Th2 and CLA-negative lymphocytes by the S128R polymorphism of E-selectin. J. Immunol. 2002;169:5860–5865. doi: 10.4049/jimmunol.169.10.5860. [DOI] [PubMed] [Google Scholar]
- 35.Rao RM, et al. The S128R polymorphism of E-selectin mediates neuraminidase-resistant tethering of myeloid cells under shear flow. Eur. J. Immunol. 2002;32:251–260. doi: 10.1002/1521-4141(200201)32:1<251::AID-IMMU251>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 36.Finger EB, et al. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature. 1996;379:266–269. doi: 10.1038/379266a0. [DOI] [PubMed] [Google Scholar]
- 37.Evans E, Leung A, Heinrich V, Zhu C. Mechanical switching and coupling between two dissociation pathways in a P-selectin adhesion bond. Proc. Natl. Acad. Sci. USA. 2004;101:11281–11286. doi: 10.1073/pnas.0401870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarangapani KK, et al. Low force decelerates L-selectin dissociation from P-selectin glycoprotein ligand-1 and endoglycan. J. Biol. Chem. 2004;279:2291–2298. doi: 10.1074/jbc.M310396200. [DOI] [PubMed] [Google Scholar]
- 39.Reeves PJ, Callewaert N, Contreras R, Khorana HG. Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc. Natl. Acad. Sci. USA. 2002;99:13419–13424. doi: 10.1073/pnas.212519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitoma J, et al. Extended core 1 and core 2 branched O-glycans differentially modulate Sialyl Lewis x-type L-selectin ligand activity. J. Biol. Chem. 2003;278:9953–9961. doi: 10.1074/jbc.M212756200. [DOI] [PubMed] [Google Scholar]
- 41.Geng J-G, et al. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990;343:757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- 42.Erbe DV, et al. P- and E- selectin use common sites for carbohydrate ligand recognition and cell adhesion. J. Cell Biol. 1993;120:1227–1235. doi: 10.1083/jcb.120.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu G, Xi X, Li P, Chu X, Ruan C. Preparation of a monoclonal antibody, SZ-51, that recognizes an α-granule membrane protein (GMP-140) on the surface of activated human platelets. Nouv. Rev. Fr. Hematol. 1990;32:231–235. [PubMed] [Google Scholar]
- 44.Moore KL, et al. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J. Cell Biol. 1995;128:661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larsen E, et al. PADGEM protein: A receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989;59:305–312. doi: 10.1016/0092-8674(89)90292-4. [DOI] [PubMed] [Google Scholar]
- 46.Setiadi H, Sedgewick G, Erlandsen SL, McEver RP. Interactions of the cytoplasmic domain of P-selectin with clathrin-coated pits enhance leukocyte adhesion under flow. J. Cell Biol. 1998;142:859–871. doi: 10.1083/jcb.142.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu C, Springer TA. The α subunit cytoplasmic domain regulates the assembly and adhesiveness of integrin lymphocyte function-associated antigen-1 (LFA-1) J. Immunol. 1997;159:268–278. [PubMed] [Google Scholar]
- 48.Takagi J, Erickson HP, Springer TA. C-terminal opening mimics “inside-out” activation of integrin α5β1. Nat. Struct. Biol. 2001;8:412–416. doi: 10.1038/87569. [DOI] [PubMed] [Google Scholar]
- 49.Keefe AD, Wilson DS, Seelig B, Szostak JW. One-step purification of recombinant proteins using a nanomolar-affinity streptavidin-binding peptide, the SBP-Tag. Protein Expr. Purif. 2001;23:440–446. doi: 10.1006/prep.2001.1515. [DOI] [PubMed] [Google Scholar]
- 50.Chen S, Springer TA. Selectin receptor-ligand bonds: Formation limited by shear rate and dissociation governed by the Bell model. Proc. Natl. Acad. Sci. USA. 2001;98:950–955. doi: 10.1073/pnas.98.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.