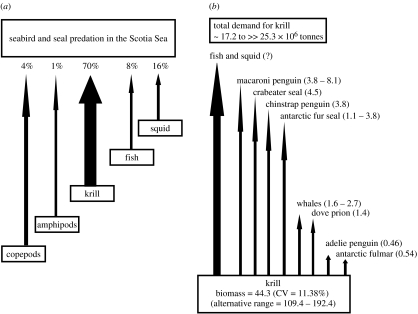
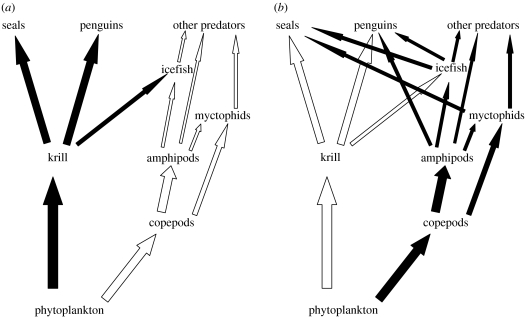
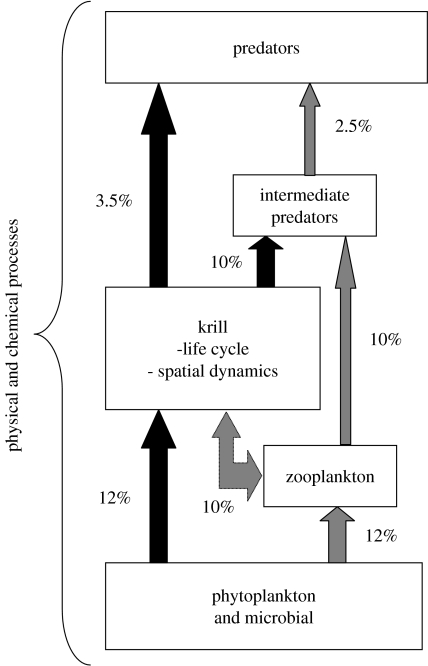
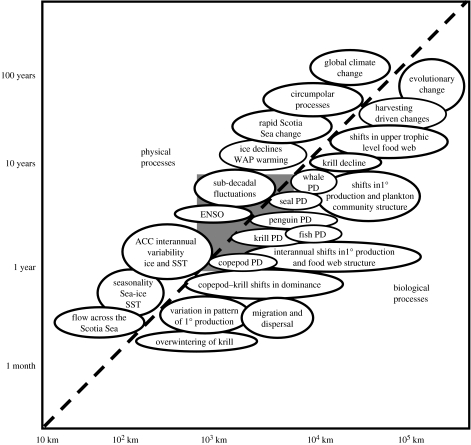
Abstract
The Scotia Sea ecosystem is a major component of the circumpolar Southern Ocean system, where productivity and predator demand for prey are high. The eastward-flowing Antarctic Circumpolar Current (ACC) and waters from the Weddell–Scotia Confluence dominate the physics of the Scotia Sea, leading to a strong advective flow, intense eddy activity and mixing. There is also strong seasonality, manifest by the changing irradiance and sea ice cover, which leads to shorter summers in the south. Summer phytoplankton blooms, which at times can cover an area of more than 0.5 million km2, probably result from the mixing of micronutrients into surface waters through the flow of the ACC over the Scotia Arc. This production is consumed by a range of species including Antarctic krill, which are the major prey item of large seabird and marine mammal populations. The flow of the ACC is steered north by the Scotia Arc, pushing polar water to lower latitudes, carrying with it krill during spring and summer, which subsidize food webs around South Georgia and the northern Scotia Arc. There is also marked interannual variability in winter sea ice distribution and sea surface temperatures that is linked to southern hemisphere-scale climate processes such as the El Niño–Southern Oscillation. This variation affects regional primary and secondary production and influences biogeochemical cycles. It also affects krill population dynamics and dispersal, which in turn impacts higher trophic level predator foraging, breeding performance and population dynamics. The ecosystem has also been highly perturbed as a result of harvesting over the last two centuries and significant ecological changes have also occurred in response to rapid regional warming during the second half of the twentieth century. This combination of historical perturbation and rapid regional change highlights that the Scotia Sea ecosystem is likely to show significant change over the next two to three decades, which may result in major ecological shifts.
Keywords: Scotia Sea, ecosystem, advection, Antarctic krill, heterogeneity, interannual variability
1. Introduction
Analysis of the operation of ocean ecosystems requires an understanding of how the structure of the ecosystem is determined by interactions between physical, chemical and biological processes. Such analysis needs to consider the interactions across a wide range of spatial (approx. 10 m–10 000 km) and temporal (minutes to centuries) scales, and across all trophic levels (primary producers to top predators; Murphy et al. 1988; Angel 1994; Werner et al. 2004). There are, however, few areas of the global ocean where there is sufficient knowledge to achieve such an integrated analysis (deYoung et al. 2004). Circulation patterns of the major ocean gyres, such as the North Atlantic and Pacific Oceans, involve movement of water masses through very different climatic regimes which favour distinctly different groups of organisms (Longhurst 1998). Generating comprehensive views of the operation of oceanic ecosystems is complicated as a result of such heterogeneity in species distribution and ecosystem structure (Murphy et al. 1988; Levin 1990; Longhurst 1998).
In contrast to other areas of the global ocean, the Southern Ocean has two major characteristics that make the development of large-scale integrated analyses a realistic possibility. The first is a circumpolar current with relatively constant environmental conditions along the streamlines, and the second is a simple food-web structure (Everson 1977; Hempel 1985a). A circumpolar eastward circulation that occurs within a restricted latitudinal belt dominates the flow (between approx. 50° and 65° S; Orsi et al. 1995). This current system, the Antarctic Circumpolar Current (ACC), transports around 130–140 Sv (million m3 s−1) eastward at Drake Passage (Cunningham et al. 2003), but shows significant atmospherically forced variability on time-scales from days to years (Hughes et al. 2003; Meredith et al. 2004b). This flow around the continent results in relatively consistent surface summer temperatures south of the Polar Front (PF) of approximately 4–5°C in the north and 0 to −1°C in areas just south of the Southern Boundary (SB) of the ACC (Sievers & Nowlin 1984; Whitworth & Nowlin 1987; Moore et al. 1997, 1999; Brandon et al. 2004).
Across the circumpolar current, there are therefore relatively consistent environmental conditions within which the ecosystem operates. Within this flow regime, the other major factor that determines the structure of the ecosystem is the marked seasonality of polar environments (Clarke 1988). Changes in solar irradiance and associated fluctuations in sea ice cover result in strong seasonal variation in upper ocean temperature and light levels (Okada & Yamanouchi 2002). This seasonal variation dominates the operation of Southern Ocean ecosystems in a number of ways. Temperature changes in surface waters as a result of fluctuations in irradiance have direct impacts on the physiological processes of many marine species, and temperature tolerances are a major determinant of the geographical boundaries of species distributions (Mackintosh 1936, 1960; Hempel 1985b; Longhurst 1998; Peck et al. 2004; Peck 2005). However, for most species, it is marked seasonal fluctuations in the availability of food that drives key biological processes (Laws 1983; Clarke 1985a; Peck et al. 2005). During summer, there is a short period of only two to three months (or less in the highest latitudes) when conditions are favourable for primary production. The resulting phytoplankton blooms are often dominated by species of large diatoms (Laws 1983; Clarke 1985a; Hempel 1985a,b; Clarke & Leakey 1996; Smetacek et al. 2004). As with the rest of the world ocean, microbial systems are a key feature of Southern Ocean ecosystems and can dominate the processes of production in many regions and through the winter months (Smetacek et al. 2004). The seasonality propagates through the food web, so consumers must be able to make full use of the short summer periods to breed and survive during the low production periods of winter (Laws 1983; Clarke 1985a). Such physical and biological conditions favour the two extremes of smaller species that can develop quickly in response to favourable conditions and large-bodied predators that are often highly mobile. The smaller microbial and meso-planktonic species opportunistically use available resources and have strategies in place to survive periods of low production. The large, mid- and higher trophic level species, such as penguins and seals, have relatively long lifespans (often greater than 10 years) and are highly mobile (foraging over hundreds to thousands of kilometres), and many move away from the area during the periods of low production (Clarke 1985b; Croxall 1992). The extreme seasonality in production also means that there is little capacity to build-up long food chains involving many steps to the highest trophic levels (Everson 1977; Clarke 1985b). Southern Ocean ecosystems therefore have an apparently simple structure, dominated by short food chains that also make them tractable for analysing large-scale system operation (Everson 1977; Laws 1983; Hempel 1985a; Clarke 1985b). The dominant food-web pathway from diatoms–zooplankton–predators also provides an important focus for studying end-to-end ecosystem processes linking primary production and highest trophic level predators.
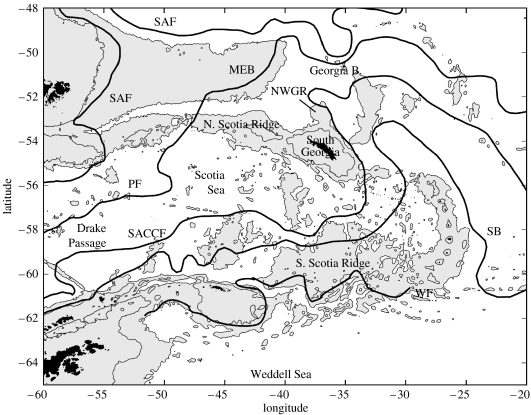
Although there is a consistency in the structure and composition of the Southern Ocean ecosystem, it is not operationally homogeneous (Hempel 1985a). South of the ACC exist large subpolar gyres in the Ross and Weddell Seas, and there are often complex current systems at the shelf break and on the shelf of Antarctica, such as the Antarctic coastal current (Hempel 1985a; Orsi et al. 1995). The flow of the ACC is strongly topographically constrained in many places around its circulation, with the greatest restriction occurring at Drake Passage. Here, the ACC flows through a ‘choke point’ between South America and the Antarctic Peninsula and emerges into the Scotia Sea. The Drake Passage and Scotia Sea region is therefore an important area in the connection of the global ocean (Cunningham et al. 2003). To the east of Drake Passage, the ACC encounters one of the biggest topographic barriers in the Southern Ocean, the Scotia Arc, which forms the northern, southern and eastern boundaries of the Scotia Sea (figure 1). The southern section of the Scotia Sea also receives input of waters from the shelf of the Antarctic Peninsula and the Weddell Sea (Whitworth et al. 1994).
Figure 1.
The Scotia Sea and surrounding areas showing the general position of the major frontal systems in relation to bottom topography. SAF, sub-Antarctic Front; PF, Polar Front; SACCF, Southern Antarctic Circumpolar Front; SB, Southern ACC Boundary; WF, Weddell Front; MEB, Maurice Ewing Bank; NWGR, North West Georgia Rise (see text for references; depth contours shown for 1000 and 2000 m).
The combination of strong flow and mixing in an area of rugged bathymetry makes the Scotia Sea one of the most physically energetic regions of the Southern Ocean. As a result, the Scotia Sea ecosystem has different operational characteristics to those in other regions of the ACC. Over much of the oceanic Southern Ocean, the concentration of chlorophyll is low even though macro-nutrient concentrations are high (termed high-nutrient, low-chlorophyll or HNLC regions). In contrast, extensive blooms of large diatoms occur across the Scotia Sea during spring. The result is a high-nutrient, high-chlorophyll region, although at times nutrient concentrations can become sufficiently depleted to become locally limiting (Holm-Hansen et al. 2004b; Korb et al. 2005). The enhanced production supports some of the largest and most diverse concentrations of seabirds and marine mammals anywhere on Earth (Everson 1977, 1984). Antarctic krill (Euphausia superba Dana; hereafter krill) are the major link between primary production and vertebrate predators in the Southern Ocean food webs. This is particularly marked in the Scotia Sea, where about half of the overall krill population occurs (Atkinson et al. 2004). Historically, the Scotia Sea is also where the majority of harvesting of seals, whales and fishes in the Southern Ocean occurred (Everson 1977, 2001; Laws 1984). Although the krill fishery has declined during the last decade, a significant fishery still exits, operating almost exclusively in the Scotia Sea and the Antarctic Peninsula regions (Everson 1977, 2001; Everson et al. 2000a).
The Scotia Sea ecosystem is therefore a key part of the Southern Ocean ecosystem and understanding its operation has become more urgent as evidence has emerged that rapid environmental change is occurring in the western Scotia Sea and west Antarctic Peninsula (WAP) region (Vaughan et al. 2003; Meredith & King 2005). Recent analyses have also suggested that krill abundance has reduced by over 50% in the Scotia Sea during the last 30 years and there are indications that some of the krill-dependent predator populations are in decline (Reid & Croxall 2001; Atkinson et al. 2004). Suggestions that these ecological changes are linked to the climate-related variations have been provided support by evidence that changes in the Scotia Sea ecosystem are linked to Southern Ocean and southern hemisphere scale variations (Forcada et al. 2005; Murphy et al. submitted; Trathan et al. in press).
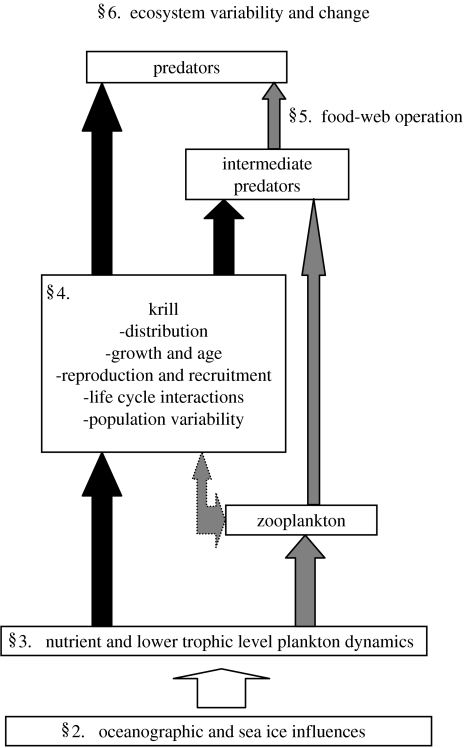
Predicting how the Scotia Sea ecosystem will respond to the climate-related changes presents a major challenge. Traditional views of food webs have tended to consider the network of biological interactions in isolation from the environment. Such an approach is not realistic because it does not take account of process interactions of different organisms at different scales, or the ontogenetic and seasonal changes in trophic interactions. Including all such complexity is impossible, so a pragmatic scale-based approach that focuses on key species within the system is more realistic (Murphy et al. 1988; deYoung et al. 2004). Such an approach is tractable for the Scotia Sea ecosystem owing to the importance of krill. To analyse the operation of the Scotia Sea ecosystem, therefore, requires detailed analyses of the krill population dynamics as well as knowledge of the trophic interactions (figure 2). An analysis of the Scotia Sea ecosystem also requires consideration of the wider links to surrounding regions owing to the open nature of the ecosystem. Here, we concentrate on the Southern Ocean region of the Scotia Sea south of the PF, but consider the wider links of the ecosystem to surrounding areas of the Antarctic Peninsula, the northern Weddell Sea and the regions east and north of the Scotia Arc. Earlier Southern Ocean whole system reviews were produced by Everson (1977) and Miller & Hampton (1989). Priddle et al. (1998a) also considered carbon flows through the food web to highest trophic levels. Lower trophic level dynamics in Southern Ocean ecosystems have been reviewed recently by Smetacek et al. (2004) and Smith & Lancelot (2004). Specific reviews of aspects of the dynamics of krill populations have been discussed by Siegel (2005), of predators by Ainley et al. (2005) and Trathan et al. in press and the response of the wider ecosystem to change by Smetacek & Nicol (2005). In this paper, we review the operation of the ecosystem in the Scotia Sea and surrounding areas, focusing on the dominant krill centred food web (figure 2 illustrates the structure of this paper in relation to the food web).
Figure 2.
Schematic of the Scotia Sea food web as considered in this review. Developing the approach of deYoung et al. (2004), the major focus is on krill, their life history and interactions, with reduced detail on other groups and trophic levels. Numbered headings refer to the major sections and organization of this paper.
2. Oceanography and sea ice
Flow through Drake Passage commenced when an initial shallow gateway opened around 50 Myr ago, but deep throughflow started only around 34–30 Myr ago, immediately after the onset of spreading of the west Scotia Ridge (Livermore et al. in press). The western side of the Scotia Sea is bounded by Drake Passage, while the other sides are formed by the Scotia Arc (figure 1). It extends over approximately 750 km north–south and approximately 2000 km to the east from Drake Passage, encompassing an area of approximately 1.5×106 km2. The waters of the ACC enter the Scotia Sea through Drake Passage, deflect northwards and then cross the Scotia Arc that rises from depths of around 3000–5000 m as a chain of islands from the Antarctic Peninsula to the tip of South America. Along this arc are a series of island groups and seamounts. Much of the central abyssal plain of the Scotia Sea is 3000–4000 m deep with a gradual shallowing from west to east. Across the region, there are submarine structures and seamounts such as the Shackleton Fracture Zone, the Pirie, Bruce, Discovery and Herdman Banks and the North West Georgia Rise (NWGR) to the north of South Georgia.
(a) Upper-ocean circulation and characteristics in the Scotia Sea
The ACC is split into several fronts, which are at their narrowest meridional constriction within Drake Passage and which then diverge as the ACC spreads into the Scotia Sea (Orsi et al. 1995; Brandon et al. 2004). Following Orsi et al. (1995), the fronts are termed (from north to south) the sub-Antarctic Front (SAF), the PF, the Southern ACC Front (SACCF) and the SB (figure 1). The SAF and PF veer northward upon entering the Scotia Sea and cross the complex bathymetry of the North Scotia Ridge (Zenk 1981). North of the North Scotia Ridge, the PF separates into two branches over the Falkland Plateau, with one branch topographically tied to the southern flank of the Maurice Ewing Bank and the other branch continuing northward over the plateau. The transport is approximately equally split between these branches, with the classical signature of the PF being found in the former (Trathan et al. 1997, 2000; Arhan et al. 2002; Naveira Garabato et al. 2002). The SACCF has a more eastward course, but loops around South Georgia anticyclonically from the south before retroflecting eastward (Thorpe et al. 2002; Meredith et al. 2003c). The SB also maintains a mostly eastward course through the Scotia Sea, but has a northward topographically induced loop in the vicinity of the South Sandwich Island arc (figure 1).
South of the ACC in the Scotia Sea lies the waters of the Weddell–Scotia Confluence (WSC), formed from waters spilling off the shelf at the tip of the Antarctic Peninsula that are injected into oceanic waters flowing eastward (Whitworth et al. 1994). It should be noted that the flux of shelf waters into the WSC is not the only route for such waters to enter the deep ocean from close to the tip of the Peninsula: Meredith et al. (2003a) showed that downslope convection occurs to the north of Elephant Island, with waters dense enough to contribute to the deep waters of the Scotia Sea, including Antarctic Bottom Water.
This downslope convection is strongly seasonal and concentrated in the austral winter. It is speculated that the flux of shelf waters into the WSC will be similarly time dependent. The WSC is bounded to the north by the SB and to the south by the Weddell Front (WF) (figure 1). It has been suggested recently that the WF originates from a branching of the Antarctic Slope Front close to the northwestern limit of the Weddell Sea (Heywood et al. 2004). Historical observations have often depicted the WSC to be characterized by abundant eddies and meanders, but it is now thought that at least some of this complexity is caused by the fronts being strongly steered by the convoluted bathymetry of the South Scotia Ridge.
Close to South Georgia, the flow regime is dominated by the SACCF. The extent of the SACCF retroflection has been revised since Orsi et al. (1995) first represented it schematically reaching to 43° W; Thorpe et al. (2002) compiled historical hydrographic measurements and found that the retroflection only extended as far as 36° W. Subsequently, Meredith et al. (2003c) showed that the SACCF is steered away from the shelf of South Georgia by the NWGR, which rises 2000 m above the seabed. It has also been shown that the course of the SACCF in this region is traceable using sea surface temperature (SST) imagery from satellite-borne radiometers (Meredith et al. 2003c). Such imagery revealed a complex eddy field north of South Georgia, and this probably accounts for the debate on the westward extent of the SACCF retroflection. Waters on the shelf of South Georgia can differ in potential temperature and salinity characteristics from those off-shelf, due to retention processes coupled with freshwater inputs from land and warming through insolation (Brandon et al. 1999, 2000; Meredith et al. 2005). The transition between the shelf and off-shelf waters can be abrupt or gradual, with implications for baroclinic advection around the shelf break (Brandon et al. 1999, 2000; Meredith et al. 2005).
Although the circulation in the Scotia Sea broadly follows the pathways of the ACC fronts, it is important to recognize the role of bathymetry. Not only does this steer the ACC fronts themselves, but it also controls the circulation in the zones between the fronts. For example, Meredith et al. (2003a) presented trajectories of passive drogued drifters in the Georgia Basin and demonstrated a general anticyclonic circulation around the island shelf from the south. However, some of the drifters did not move to the east in the vicinity of the SACCF retroflection, but continued to circulate anticyclonically around the periphery of the Georgia Basin before joining the PF to the west and north of the basin. Clearly, the advective pathways can be more strongly influenced by direct topographic steering than by the ACC frontal pathways in such circumstances. Also of note is the presence of a variable, but often intense, warm-core anticyclonic circulation above the NWGR, with velocities as large as 50 cm s−1. Meredith et al. (2003a) presented dimensional analysis which showed that the features of this circulation were consistent with those of a stratified Taylor column and demonstrated the strong impact that it can have on primary production and biogeochemistry.
(b) Sea ice dynamics
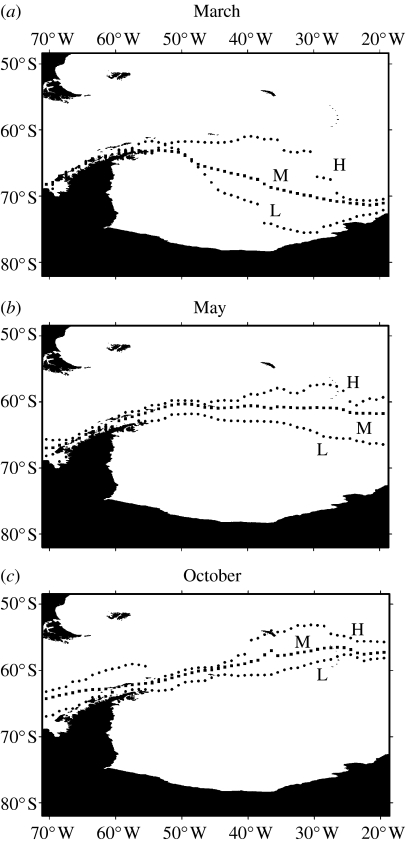
During winter in the Scotia Sea, sea ice extends out over the southern areas of the ACC (figures 1 and 3). The ice is generated mainly in the Weddell Sea, drifting northwards driven by ocean currents and surface winds (Murphy et al. 1995; Harms et al. 2001; Parkinson 2002, 2004). The minimum ice extent in summer occurs across the Weddell Sea between February and March, with sea ice advancing across the southern Scotia Arc around May (figure 3). Although the maximum northward extent of sea ice across the Scotia Sea during winter usually occurs during September or October, it can occur anytime between July and November. The timing of both advance and retreat shows significant interannual variation (figure 3) and is related to changes in air temperatures and wind speed and direction reflecting regional atmospheric dynamics (Allison 1997). The mean position of the maximum winter sea ice extent generally occurs in the area of the mean summer position of the SB of the ACC (figures 1 and 3). However, in extreme years, it can occur much further north in the region of the SACCF or indeed much further south around the position of the WF. It should be noted however that there is little information on the positions of the fronts when sea ice covers the region. The average concentration of sea ice across the area during winter is between 50 and 15%, and at this time it will be approximately 0.3–0.5 m thick (Allison 1997) and drifts north and eastward at speeds ranging from 1 to 15 cm s−1. These characteristics result in an eastward drifting marginal ice zone (MIZ) comprising variable sized ice floes separated by leads and more extensive areas of open water (Allison 1997). In spring, there is an asymmetric southward retreat of sea ice, with sea ice in the east retreating earlier than that in the west (October and November). The MIZ in the west is also more limited in north–south extent by the Antarctic Peninsula than areas in the eastern Scotia Sea. Some areas in the western Scotia Sea remain ice covered until late in the spring (November and December). In areas where the sea ice retreats slowly, the upper water column can be stabilized by melt water input, generating shallow surface mixed layers (10–30 m; Bianchi et al. 1992; Lancelot et al. 1993; Figueiras et al. 1994; Parkinson 1994; Park et al. 1999). However, the retreat of sea ice across the Scotia Sea during spring is often rapid and probably mainly wind driven (Sullivan et al. 1988; Comiso et al. 1993; Parkinson 1994).
Figure 3.
Seasonal and interannual changes in extent of sea ice across the Scotia Sea and Weddell Sea. Mean positions of the 15% ice edge are shown for three months in the year along with the position of the ice edge in a year of extreme north and south extent. (a) Mean sea ice extent in March (M) and extent in March 1986 (L) and 2004 (H). (b) Mean sea ice extent in October (M) and extent in 1989 (L) and 1987 (H). (c) Mean sea ice extent in May (M) and extent in May 1999 (L) and 1992 (H). Sea ice data from 1979 to 2005 from DMSP–SSMI passive microwave data produced by NOAA/NCEP.
(c) Physical variability and long-term change
With the development of satellite-derived data series of over 25 years duration, we can now consider variability and change across the Scotia Sea system. On interannual time-scales, connections between remotely sensed SST close to South Georgia and the El Niño–Southern Oscillation (ENSO) have been demonstrated (Trathan & Murphy 2002). These studies show a 2–3 year lag between ENSO variability in the equatorial Pacific and response around South Georgia, implying a significant component of oceanic advection in the signal propagation (Trathan & Murphy 2002; Murphy et al. submitted). More recently, Meredith et al. (2005) examined 5 years of hydrographic data from close to South Georgia and noted particularly cold waters in early 1998. These were shown to be linked directly to the very strong 1997/1998 El Niño event (Meredith et al. 2005). Murphy et al. (submitted) have further shown that the propagating oceanic signal dominates the interannual variation from the Central and west Pacific sector through to the Scotia Sea, but further support the view that short-term (less than six months) direct impacts from atmospheric effects did occur during the major El Niño event. In contrast, variation in the WAP region appears to be dominated by the direct ENSO-related atmospheric effects rather than the signal that is propagated in the ACC (Fraser & Hofmann 2003; Quetin & Ross 2003; Meredith et al. 2004a).
The interannual changes in SST associated with these large-scale processes are also closely correlated with sea ice variation across the region (Fedulov et al. 1996). Warm periods coincide with winters of reduced ice extent and duration, while in the coldest years the ice extends further north generating longer winters in the southern Scotia Sea (Trathan et al. 2006; Murphy et al. submitted). These changes are linked with the passage of warm and cold anomalies in ocean SST through the region from the South Pacific sector of the Southern Ocean (White & Peterson 1996; Murphy et al. submitted). Further work is needed to fully determine the climatic forcings of interannual and longer period variability in the Scotia Sea.
There is also marked decadal and longer term change occurring in physical environments around the Scotia Sea. There is clear evidence that the region around the Antarctic Peninsula is one of the most rapidly warming on the planet, with increases in air and SSTs, and decreases in winter sea ice cover (Smith & Stammerjohn 2001; Stammerjohn et al. 2003; Vaughan et al. 2003; Meredith & King 2005). The longest record of sea ice dynamics for anywhere in the Southern Ocean also comes from the southern Scotia Sea (Murphy et al. 1995). Records of the duration of fast ice in the South Orkney Islands have shown a significant decline in the mean duration of fast ice between the first and the second half of the twentieth century (Murphy et al. 1995; de la Mare 1997). There are also some indications that upper water column temperatures around South Georgia increased between the first and the second half of the century and that this was related to changes in sea ice extent (Whitehouse et al. 1996a). This warming was followed by a period of glacier retreat at South Georgia (Gordon & Timmis 1992). Taken together there is evidence that there was an abrupt and rapid change in the physical environment of the Scotia Sea in the middle of the last century. There are also clear indications that further changes have been occurring over the last three decades. Meredith & King (2005) recently showed a warming of the upper ocean on the west of the Peninsula over the second half of the last century. There has also been a reduction in the mean duration of winter sea ice around the WAP and across the Scotia Sea during the last 25 years (Parkinson 2002). It is likely that this more recent regional change reflects a downstream influence of the regional warming that is occurring around the Antarctic Peninsula in areas where sea ice formation occurs.
3. Nutrient and plankton dynamics
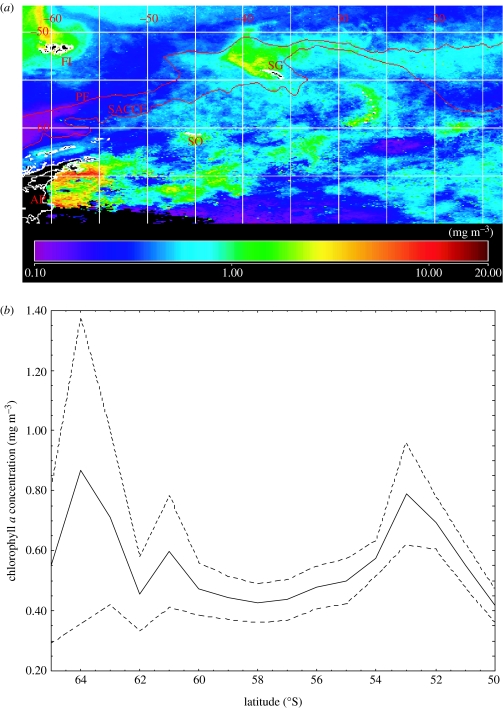
In contrast to much of the Southern Ocean, which is characterized by HNLC conditions, the Scotia Sea is an area of both high nutrient concentration and high productivity (Holm-Hansen et al. 2004a,b). However, the production regimes are highly variable and reflect the large-scale variation in physical and chemical conditions across the region. Pre-bloom surface macro-nutrient concentrations (nitrate, silicate and phosphate) are generally high (surface values of nitrate (NO3)>30 mol m−3; silicic acid (Si(OH)4)>60 mmol m−3; phosphate (PO4)>2 mmol m−3; Whitehouse et al. 1996a, 2000; Atkinson et al. 2001), with a gradient from south to north of reducing nutrient concentration. Across the central Scotia Sea, summer surface chlorophyll a concentrations are moderate, between 0.4 and 1.0 mg m−3, with some areas of higher concentration (>1.0 mg m−3; figure 4; Holm-Hansen et al. 2004a,b; Korb et al. 2005). Most areas of enhanced mean surface chlorophyll a concentrations (>1.0 mg m−3) occur around and downstream of islands, across shelf areas, within frontal jets and in areas recently covered by sea ice (figures 3 and 4; Mitchell et al. 1991; Bianchi et al. 1992; Treguer & Jacques 1992; Comiso et al. 1993; Perez et al. 1994; de Baar et al. 1995; Clarke & Leakey 1996; Korb & Whitehouse 2004; Korb et al. 2004, 2005; Holm-Hansen et al. 2004a,b). Productivity is also variable and Korb et al. (2005) estimated primary production rates of approximately 0.31 g C m−2 d−1 in central oceanic regions compared to rates between 0.72 and 2.04 g C m−2 d−1 across the shelf areas, around the Scotia Arc and in the region of the retreating ice edge in the southern Scotia Sea. These rates are similar to the empirically derived estimates of Holm-Hansen et al. (2004b) of between 0.60 and 0.99 g C m−2 d−1 for the entire Scotia Sea during January and February. In the northern areas of enhanced production, where the summer season can extend over about five months, annual productivity may be very high and has been estimated to be approximately 30–40 g C m−2 around South Georgia (Whitehouse et al. 1996a). Further west, near to Drake Passage, where waters of the ACC have recently emerged from the South Pacific sector, concentrations of chlorophyll a are much lower (approx. 0.1 mg m−3; figure 4; Holm-Hansen et al. 2004a). These waters are similar to much of the Pacific and Indian Ocean sectors of the Southern Ocean with high concentrations of nutrients (silicate, phosphate and nitrate, i.e. HNLC; Korb et al. 2005).
Figure 4.
(a) Mean concentration of chlorophyll a (mg m−3) derived from the summer (December–February) SeaWiFS data for the period from 1998 to 2005. The position of the PF and SACCF are also shown. (b) Mean and 95% confidence intervals of the December–February concentration of chlorophyll a (mg m−3) calculated in 1° latitude bands across the Scotia Sea from 55 to 30° W. Data are from the SeaWiFS Project and the NASA Giovanni Ocean Color Project.
The development of blooms in the southern Scotia Sea is affected by the timing and pattern of sea ice retreat during spring (Sullivan et al. 1988; Comiso et al. 1993; Korb et al. 2004, 2005). In the areas of the northern Scotia Arc, blooms are more regular and predictable. Over much of the summer, blooms develop in the shelf areas around South Georgia (figure 4; Atkinson et al. 2001; Korb & Whitehouse 2004; Korb et al. 2004, 2005). These blooms extend to the north in the retroflective area of the SACCF. A large spatially extended bloom is often established downstream of the island (Korb & Whitehouse 2004). This bloom can extend downstream from the island more than 2750 km to the east and at times enhanced chlorophyll a concentrations are observed beyond the prime meridian (Korb et al. 2004). These megablooms, which can occur over an area of between approximately 0.07 and 0.5×106 km2 and can last for over five months, are potentially globally important in export of carbon from the surface to the seabed (Schlitzer 2002). In areas where intense phytoplankton blooms form, such as north of South Georgia, macronutrients can be reduced to or near to limiting concentrations (approx. 10 mmol m−3 NO3, approx. 1 mmol m−3 Si(OH)4 and approx. 0.3 mmol m−3 PO4; Whitehouse et al. 1996a,b; Korb & Whitehouse 2004; Korb et al. 2005).
These views of large-scale chlorophyll a distribution across the Scotia Sea are based on satellite data (Comiso et al. 1993; Korb et al. 2004; Holm-Hansen et al. 2004a,b), which are known to underestimate high chlorophyll a concentrations in large blooms (>5 mg m−3; Korb et al. 2004). A further problem is that satellites cannot detect sub-surface chlorophyll maxima which are known to occur in the Scotia Sea (Korb et al. 2004; Holm-Hansen et al. 2005; Whitehouse et al. submitted). Such sub-surface production is likely to be regionally and temporally important, but presently remains an uncertain aspect of the operation of the food web.
It is likely that the waters of the Scotia Sea are naturally iron enriched and this promotes high productivity of large diatoms throughout the region. There is now good evidence, from artificial iron enrichment experiments, that a lack of iron in surface waters is a major factor limiting phytoplankton growth (de Baar et al. 1995; Boyd 2002c). The natural iron enrichment in the Scotia Sea is likely to come from a range of sources, including shelf water inputs from the Antarctic Peninsula region associated with the WSC, upwelling and interaction of the ACC with the shelf sediments of the Scotia Arc introducing dissolved iron into surface waters (de Baar et al. 1995; Korb et al. 2004, 2005; Holm-Hansen et al. 2004b). This enhanced concentration of iron, which is a crucial micronutrient in the growth process of large diatoms, is considered to be the major factor that allows phytoplankton to bloom across the Scotia Sea (Hart 1942; Korb & Whitehouse 2004; Korb et al. 2004, 2005; Holm-Hansen et al. 2004a). A recent study of phytoplankton growth across the northern Scotia Arc region gave further support to this view. Holeton et al. (2005) obtained direct iron measurements which showed enhanced iron concentrations around South Georgia that arose from a benthic source. A range of indirect evidence gives further support for the view that iron concentrations are high and a major factor generating the large phytoplankton blooms across the Scotia Sea; these include the dominance of large diatoms, large depletions of NO3 concentration, observed nutrient deficit ratios and high phytosynthetic efficiency (Korb & Whitehouse 2004; Korb et al. 2004, 2005; Holm-Hansen et al. 2004a; Holeton et al. 2005; BAS 2006, unpublished data).
In the Scotia Sea, we therefore see a region of transition with waters of low iron concentration in the west that emerge from Drake Passage (Korb et al. 2004; Holm-Hansen et al. 2004a) and then mix with waters of high iron concentration that have recently flowed around and across the Antarctic Peninsula shelf and southern Scotia Arc. The iron levels of these waters are likely to be further enhanced as the currents flow over the northern Scotia Arc, allowing blooms to develop around the shelf areas. Over time, these blooms develop downstream away from the shelf areas; therefore, they are a function of both the flow and the iron enhancement (Korb et al. 2005). The strong gradient north–south in irradiance and ice cover and duration will affect the timing and development of the planktonic system.
Although iron is important in phytoplankton growth, a range of studies have shown that realized population growth rates are the result of multiple controls (Lancelot et al. 2000; Holm-Hansen et al. 2004b; Korb et al. 2005). The interactive effects of light, nutrients (micro and macro), temperature and grazing will all be important in determining the concentrations of phytoplankton (Smith & Lancelot 2004; Holm-Hansen et al. 2004b). Silicic acid levels decrease further north across the Scotia Sea and, as previously noted, are more likely to become limiting late in the season in northern areas where summer lasts longer. The long-lasting blooms observed in these areas are also therefore likely to show shifts in species composition from diatoms to non-siliceous species. It is also likely that the dynamics and fate of iron from the Scotia Sea will also be important in determining the food-web structure downstream. Indeed, studies of the food-web operation along the region north of the Scotia Arc may reveal the time-scales for iron recycling and its fate in the food web (Smetacek et al. 2004). Of the grazing controls on production, the impacts of meso- and macro-zooplankton on phytoplankton production can often be low, particularly during summer when blooms have already developed (Atkinson et al. 2001). However, krill and copepods also exploit microbial and heterotrophic production, so grazing impacts on new production will be determined by food-web structure and interactions (Atkinson et al. 1996; Atkinson & Snyder 1997; Pakhomov et al. 1997a,b; Giesenhagen et al. 1999; Lancelot et al. 2000).
Like much of the global ocean, microbial populations are undoubtedly an important component of Scotia Sea nutrient and production systems, but relatively little is known about connections between the microbial components and higher trophic levels and much of the relevant work comes from adjacent areas of the Weddell Sea and Antarctic Peninsula and elsewhere in the Southern Ocean (Lancelot et al. 1991; Mordy et al. 1995; Wright & van den Enden 2000; Walsh et al. 2001). During summer, the dominant pathway for energy flow in the Scotia Sea will be through new production by the larger diatoms, but ammonium is likely to be an important nitrogen source over the Scotia Sea (Priddle et al. 2003). Outside of the summer period, the recycling pathways are much more important. The seasonal changes in relative importance of new versus recycled production however is unknown (Cota et al. 1992; Mordy et al. 1995). In winter, the microbial communities associated with the sea ice are important in the food web (Becquevort et al. 1992; Garrison & Close 1993; Mordy et al. 1995). Bacteria have an important role in transferring energy through the consumption of dissolved organic matter and are in turn consumed by protozoa which are fed upon by smaller zooplankton (Bak et al. 1992; Kuparinen & Bjornsen 1992; Grossmann 1994; Tupas et al. 1994; Mordy et al. 1995; Moran et al. 2001). These microbial systems introduce important temporal delays into the food web, making key compounds, organic substrates and energy available at times during the season when little new production is available. This will be particularly important in maintaining energy flows in the food web during autumn and winter in the Scotia Sea, where extensive meso- and macro-zooplankton populations require food (Walsh et al. 2001; Smith & Lancelot 2004). The recycling pathways are likely to be the major components of coastal food webs around the Scotia Sea. In more pelagic waters during winter, there will be significant sea ice-associated microbial production in the drifting ice habitat of the MIZ of the southern Scotia Sea that will maintain higher trophic level production (Garrison & Close 1993; Ackley & Sullivan 1994; Murphy et al. 1998a). Temporal delays in the food web introduced by recycling will also result in a spatial disconnect between regions of production and consumption as the material is advected in the ocean (Garrison & Buck 1991; Becquevort et al. 1992; Garrison & Close 1993; Grossmann 1994; Grossmann & Dieckmann 1994).
The high productivity of the Scotia Sea ecosystem makes it an important region for examining the effects of natural iron fertilization on the development of planktonic systems. The impacts on the wider operation and structure of the ecosystem provide a valuable natural contrast with much of the rest of the oceanic Southern Ocean.
4. Krill in the Scotia Sea food web
(a) Krill distribution in the Scotia Sea
The physical environment sets the context within which any species must operate. For Antarctic krill, the biggest influence may have been the opening of Drake Passage and the development of the ACC to generate the relatively isolated circumpolar Southern Ocean (Patarnello et al. 1996; Jarman et al. 2000; Zane & Patarnello 2000; Livermore et al. 2005, in press). This has generated an oceanic environment in the Scotia Sea which is the most advective in the world (Cunningham et al. 2003). The life cycle of Antarctic krill, which appears to have originated at about the time the ACC became established (Patarnello et al. 1996; Jarman et al. 2000), will have developed in this dispersive system, which had characteristics similar to the general pattern of oceanic circulation and seasonality that is observed today (Spiridonov 1996).
Understanding the factors controlling the large-scale distribution of krill has become a major focus of research during the last 5–10 years. These studies have been advanced by the development of complementary large-scale modelling, field studies and data syntheses (Murphy et al. 1998b; Murphy & Reid 2001; Atkinson et al. 2004; Hofmann & Murphy 2004; Murphy et al. 2004a,b; Siegel 2005; Fach & Klinck 2006; Fach et al. 2006; Nicol et al. 2000; Nicol 2006). The large-scale distribution of krill is a function of production (recruitment and growth), mortality, retention and dispersal. The resultant circumpolar distribution is highly asymmetric (Marr 1962; Mackintosh 1973), with at least half of the entire krill population occurring in the southwest Atlantic sector of the Southern Ocean (Atkinson et al. 2004). The distribution of krill in the Scotia Sea also extends further north than in any other region of the Southern Ocean, with high densities occurring north of 53° S (figure 5a). Elsewhere in the Southern Ocean, krill tend to occur mainly near the continent (between approx. 75 and 65° S). Marr (1962) suggested that the large-scale distribution was dominated by the surface currents generally, and that the ACC, WSC and outflows from the Weddell Sea were the major determinants of the horizontal distribution in the Scotia Sea. A recent modelling study has further indicated that the mixing of surface waters in the Scotia Sea is a key determinant of the large-scale distribution of krill and brings together plankton from around the Southern Ocean (Thorpe et al. submitted).
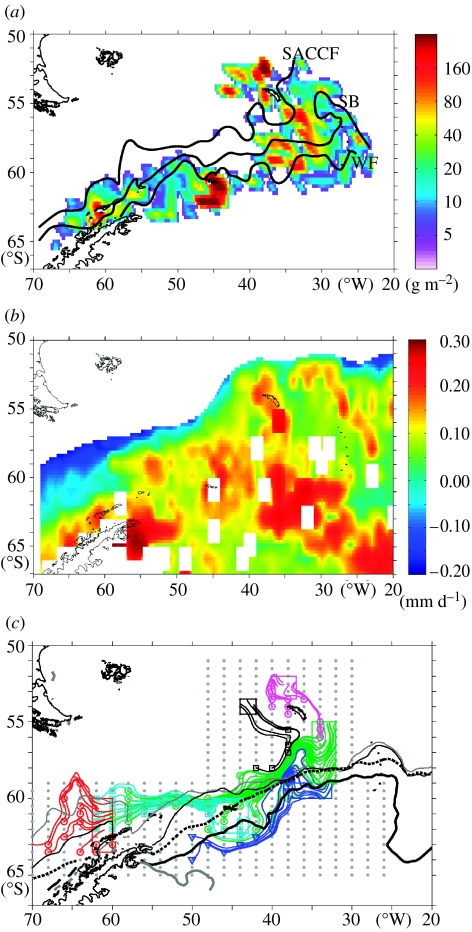
Figure 5.
(a) Krill biomass across the Scotia Sea based on CCAMLR Synoptic Survey during January and February 2000. The positions of the major fronts as determined during the survey are also shown (Murphy et al. 2004a). (b) Estimated growth rates (mm d−1) of krill across the Scotia Sea during January and February 2000. Values based on empirical relationships derived by Atkinson et al. (2006; Calculations use Model 3, Table 5, for all krill sampled) and Tarling et al. (2006) using satellite-derived mean SST field and chlorophyll a (SeaWiFS) concentrations for January and February and assuming a mean length of 40 mm. Blank cells are where no data were available or where the SST was less than −1 or greater than 5°C. (c) Lagrangian particle tracks passing through major biomass regions (a) based on tracks from previous three months using output from the OCCAM circulation model (Murphy et al. 2004a). (a,c) Reproduced from Murphy et al. 2004a with permission from Elsevier.
Within the Scotia Sea, high and relatively predictable concentrations of krill occur in waters less than 1000 m deep (Miller & Hampton 1989; Murphy et al. 1997). Detailed analyses of fishery and acoustic survey data from around South Georgia in the northern Scotia Sea have shown that maximal values occur in the shelf-break region (Murphy et al. 1997; Trathan et al. 1998a; Trathan et al. 2003). However, significant amounts of krill also occur in oceanic waters across the Scotia Sea (Siegel 2005). The Discovery Investigations (1925–1951) found large concentrations of krill in off-shelf regions (Marr 1962). The most recent comprehensive acoustic survey to date also showed a large biomass of krill in the central southern Scotia Sea during summer 2000 (figure 5a; Hewitt et al. 2004). Further support to this view is given in an analysis of historical net data, which shows large amounts of krill in off-shelf areas during summer (Atkinson et al. 2004). These central Scotia Sea regions were also areas of commercial whaling and are strongly influenced by oceanic frontal systems (Hofmann et al. 1998; Tynan 1998; Hofmann & Murphy 2004; Murphy et al. 2004a,b). During autumn and winter, a combination of dispersal and mortality leads to a decline in the abundance of krill across northern oceanic regions. Higher abundances are maintained across shelf and off-shelf areas further south through retention, recruitment and seasonal dispersal (see discussions in Marr 1962).
(b) Krill growth and age in the Scotia Sea
Growth of krill is highly variable, and a function of animal size and maturity, food availability and temperature (Ross et al. 2000; Fach et al. 2002; Reid et al. 2002; Daly 2004; Atkinson et al. 2006; Candy & Kawaguchi 2006; Kawaguchi et al. 2006). The Scotia Sea during summer appears to be a generally favourable habitat for krill growth and development. Atkinson et al. (2006) and Tarling et al. (2006) recently measured post-larval growth rates based on samples from across the entire Scotia Sea in mid-summer. They derived empirical relationships between krill growth rates, size and development state with local temperature and chlorophyll a concentration. Here, we use these relationships to estimate growth rates across the whole Scotia Sea during summer (figure 5b). Highest growth rates, predicted for the 2000 season, are across the southeast Scotia Arc, down to the eastern Weddell Sea, across the southern Scotia Sea and in the east Antarctic Peninsula region. Predicted growth rates are consistently above zero over most of the area, except in the more northern and warmer regions nearer the PF. Calculations of carbon flux indicate that rates of growth of krill of approximately 3–6% of animal body mass per day would have occurred in the high growth regions where the animals were 20–30 mm long. This was mainly in the central and the eastern Scotia Sea. Further west and north 40–60 mm animals would have shown lower rates of 0.5 to 2%. The relationships indicate that higher temperature regions, to the north of the Scotia Sea, are poor areas for krill growth and especially for larger animals. However, it should be noted that significant growth rates can be maintained by larger animals in relatively warmer more northern regions if the chlorophyll a concentrations are sufficiently high. Areas to the north and northeast of South Georgia show consistent blooms during summer in areas where temperatures can be more than 3°C, and these could be areas where positive growth rates could be maintained.
As there are currently no reliable methods to age individual krill, the variability in growth rate makes it difficult to examine the development of individual year classes (Miller & Hampton 1989). There is a fragmented picture of year class development at South Georgia compared to that from the WAP. Analyses of predator diet data have suggested that growth rates across the Scotia Sea over extended summer periods are sufficient for animals to reach a size of between 35 and 40 mm in 1 year having overwintered only once (1+ age class; Reid et al. 2002). This view of rapid growth in the north compared to southern regions of the Scotia Sea is also supported by analyses of year class fluctuation across the Scotia Sea (Brierley et al. 1999; Reid 2002; Reid et al. 2002). However, analyses of length frequency distributions from net samples have suggested that the same year classes dominate around South Georgia and the Elephant Island and WAP regions (Quetin & Ross 2003; Siegel et al. 2003). In this interpretation, which is based on a view that the size of age classes is the same across the Scotia Sea, the animals would have overwintered twice (2+ age class) before they appear in the population at South Georgia (Siegel 2005).
This lack of agreement arises from the absence of a definitive ageing method, the capacity of krill to shrink in conditions of low food availability, and the short duration of the available time-series of recruitment strength data in which the mean size of cohorts are highly variable and consecutive year classes tend to occur together. Uncertainty in identifying exactly which year the animals were spawned affects our ability to interpret interannual changes in abundance. The predator and net series also relate to different parts of the krill population (Reid et al. 1996b; Murphy et al. 1998b; Watkins et al. 1999; Murphy & Reid 2001). The net sampling has occurred around the whole island on and off the shelf, which combined with the small mesh size of the nets can sample size groups in the year before they dominate the predator diet (Watkins et al. 1999). Most of the sampling of the length frequency of krill in the diet of predators is based on animals that forage in the west of the region, often mainly over the shelf (Reid et al. 1996b). Comparison of net and predator data has shown that the sampling needs to be local and contemporaneous to be comparable (Reid et al. 1996b). Thus, discrepancies can arise through mismatches in the scale of sampling, thereby generating difficulties in interpretation of population processes across the region.
(c) Krill reproduction and recruitment in the Scotia Sea
Spawning followed by successful recruitment probably occurs to some extent right across the southern Scotia Arc and the Scotia Sea between about November and February (Marr 1962; Hofmann & Husrevoglu 2003; Tarling et al. in press). Depending on food availability, krill can probably spawn several times in a year (Ross & Quetin 1986; Siegel 2005). Mature krill have been found throughout the Scotia Sea in both on- and off-shelf areas, and a recent study off South Georgia has shown that krill complete spawning and produce viable eggs in the region (Tarling et al. 2006b). Around the Antarctic Peninsula region mature krill appear to migrate to the shelf-break regions to spawn (Siegel 2005). Eggs sink rapidly to depths of greater than 500 m, so spawning in shallow shelf areas is unlikely to be viable as it would result in physical damage when the eggs come into contact with the substrate and predation from the benthos (Marr 1962; Miller & Hampton 1989; Hofmann & Husrevoglu 2003). Larvae develop as they return to the surface where they begin to feed, in a process that takes about two to three weeks to complete. Model simulations of egg development and larval hatching have shown that there are restricted regions of the shelf–slope, where the sinking eggs come into contact with upwelling, relatively warm Upper Circumpolar Deep Water, where egg development and larval hatching and ascent can be successfully completed (Hofmann & Husrevoglu 2003). In the Scotia Sea sector, these areas are restricted to the WAP and a few places around the east Antarctic Peninsula. Although shelf–slope regions around the Scotia Arc do not favour egg development and larval retention, the model simulations indicate that oceanic waters right across the Scotia Sea are suitable for spawning and larval development (Hofmann & Husrevoglu 2003; Tarling et al. 2006b). This suggestion is supported by large-scale surveys of larval distribution that have shown that the Scotia Sea is an area where high densities of larval krill occur during the summer months (Marr 1962; Brinton 1985; Ward et al. 2004). These larvae are generally considered to have come mainly from the major spawning regions further south along the southern Scotia Arc and around the Antarctic Peninsula (Marr 1962; Fach et al. 2006). Through a combination of further spawning and drift, the distribution of larvae then develops across the southern regions of the Scotia Sea and north towards South Georgia in the east. However, recent findings have focused on the fate of larvae spawned and released over oceanic waters across the Scotia Sea (Murphy et al. 2004a; Tarling et al. 2006). Analyses of spawning status have shown that krill at South Georgia probably complete their maturation process and spawn over slope and off-shelf areas, where eggs and larvae will be rapidly transported away from the island (Murphy et al. 2004a; Tarling et al. 2006b).
Winter survival and growth of the larval krill produced during summer require access to alternative food sources. Sea ice is considered to be a key overwintering habitat for krill generally (Quetin & Ross 1991; Spiridonov 1995). Ice algae, which develop on the undersurface and within sea ice, are an important source of energy that help sustain krill during the periods of low water column productivity (Daly & Macaulay 1991; Quetin & Ross 1991, 2001; Melnikov & Spiridonov 1996; Quetin et al. 1996; Ross & Quetin 1999; Fraser & Hofmann 2003; Meyer et al. 2002; Pakhomov et al. 2004). The sea ice also acts as a potential refuge from predators, reducing mortality rates (Daly & Macaulay 1991). A relationship has been found between sea ice conditions during winter and krill recruitment around the Antarctic Peninsula (Siegel & Loeb 1995; Loeb et al. 1997; Quetin & Ross 2003; Siegel et al. 2003; Siegel 2005). It also appears that consecutive years of extensive sea ice are required to generate large year classes around the WAP (Loeb et al. 1997; Fraser & Hofmann 2003; Quetin & Ross 2003). However, the extension of this concept that greater winter sea ice extents lead to better food and refuge conditions as a linear function in every region is likely to be too simplistic to explain changes across the whole region. Sea ice conditions vary across the region, with an area in the west around the Peninsula where the MIZ is small compared to areas further east, where low-concentration sea ice cover can extend over much of the Scotia Sea. Sea ice conditions around the WAP are dependent on factors to the west, with much of the ice brought into the region on ocean currents and driven by wind. Areas around the tip of the Antarctic Peninsula into the Scotia Sea will be affected by conditions in the Weddell Sea as well as further west. It is therefore surprising that a simple relationship of krill recruitment with ice extent appears to dominate, given the complexity of the processes generating the distribution of sea ice. Recent studies have suggested that the relationships between krill recruitment and sea ice are more complex. In the Palmer and Marguerite Bay region, years of enhanced recruitment were found to be associated with winters of average ice conditions (Quetin & Ross 2001, 2003). Algal concentration and abundance in sea ice will not depend on sea ice extent, but will be a function of the degree of open water, the floe size and thickness, and may also be dependent on when the ice formed and under what conditions. The complexity of the habitat for krill has been highlighted by Daly (2004), who showed that larval grazing on sea ice algae in southern areas of the WAP is low in winter, but becomes more important in spring as the ice melts and the light levels increase. The successful survival of krill through various critical stages of the life cycle will therefore be a complex function of interaction between sea ice habitats in winter and open ocean regions in summer (Quetin & Ross 2001, 2003; Siegel 2005). Final recruitment success, when animals are at least 1 year old, will reflect conditions over at least the previous 2 years, which would have affected maturation and spawning of mature animals and larval survival in summer and winter.
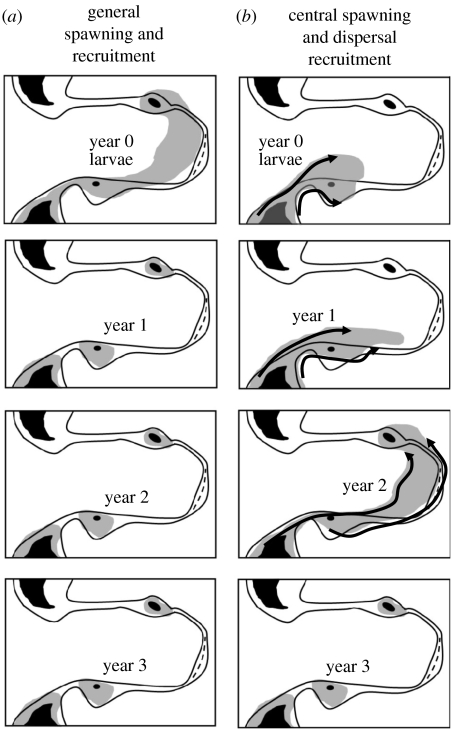
An apparent consistency of year class recruitment at the Antarctic Peninsula and across the Scotia Sea has been noted and a number of explanations have been proposed (Priddle et al. 1988; Murphy et al. 1998b; Brierley et al. 1999; Quetin & Ross 2001, 2003; Siegel et al. 2003; Atkinson et al. 2004). Spawning may be occurring right across the region, under similar large-scale physical and growth conditions, generating successful regional recruitment (figure 6). Alternatively, spawning and larval survival may be occurring mainly in a central area in the south with drift taking the older individuals into a larger habitat over the next 1–2 years (figures 5c and 6; Priddle et al. 1988; Hofmann et al. 1998; Murphy et al. 1998b; Quetin & Ross 2003; Hofmann & Murphy 2004; Fach et al. 2006). Analyses of sea ice data indicate that conditions show marked variation across the region, suggesting that recruitment success will also vary. We also know that large animals occur in off-shelf areas in regions of rapid current flow, indicating that there is significant transport of both larvae and adult krill, and a general oceanic mixing of year classes across the region (Hofmann & Murphy 2004; Murphy et al. 2004a,b). These observations tend to support the view of a central spawning region with dispersal into the larger habitat (figure 6), but this is based on limited information. At local scales, around islands, how the krill get onto the shelf from off-shelf areas is unknown, although vertical migration strategies may be important in areas where exchanges of water occur at depth (Murphy et al. 2004a). In some areas close to the Scotia Arc, larvae may be entrained back onto the shelf by cross-shelf transfers of water associated with upwelling or surface water mass exchange (Dinniman & Klinck 2004; Klinck et al. 2004). These exchange mechanisms are likely to be important in larval retention along the Antarctic Peninsula and around the South Orkneys. However, there is no evidence that there is a significant larval recruitment onto the shelf in more northern regions such as South Georgia (Ward et al. 1990; Watkins et al. 1999; Atkinson et al. 2001).
Figure 6.
Schematic of two alternative spawning and recruitment scenarios that can both generate concordance in recruitment across the region. (a) Spawning occurs generally across the region and then recruitment is maintained in all shelf regions. (b) Spawning and successful survival during the first year occur mainly in central and southern areas of the Scotia Sea, and the year class is dispersed through interactions with the ocean and sea ice over the next 1–2 years. Intermediate scenarios between these extremes can also be envisaged.
The implication of this view of krill dynamics is that a large proportion of the young produced will be immediately lost from the regional system (Murphy et al. 2004a,b). This can be a viable strategy for a species as long as some animals are retained in the major spawning zones or there is some reverse mixing against the flow towards the south and west. However, this view of a broadcast spawner, in which the majority of the larvae drift away from a central favoured habitat and are lost from the population, may not be appropriate for this species. The chances of successful development of larvae in the pelagic areas of the Scotia Sea will depend on food availability (Meyer et al. 2002, 2003; Ross et al. 2000; Meyer & Oettl 2005). As previously mentioned, the southern Scotia Sea and Arc show variable but moderate chlorophyll a concentrations that are likely to be adequate for krill growth (Atkinson et al. 2004).
(d) Krill–habitat interactions in the Scotia Sea
This large-scale view of krill dynamics indicates that the more northerly regions of the Scotia Arc will be unfavourable areas for krill with an apparent lack of larval recruitment and low growth rates as a result of high temperatures (Atkinson et al. 2006). A longer growing season at low latitudes may offset this situation, but it raises the question of whether these northern areas are part of a linked system where animals are returned south to the major spawning areas or whether they are effectively a dead end, where krill are consumed, starve or are transported out of the system. A direct active migration (Siegel 1988; Nicol 2006), towards favoured spawning areas in the south, would be successful even in the rapid flow of the ACC with a sustained swimming speed of 15 cm s−1. Such a sustained swimming speed may be possible for krill (Marr 1962; Kils 1982; Miller & Hampton 1989), although there is no evidence of a large-scale migratory strategy (Marr 1962). The proposed evidence of active directed horizontal migration of krill over extended distances could also be largely explained by small-scale interaction effects and interactions with larger scale environmental structure. One such small-scale strategy would be a vertical migration to exploit changes in flow speed and direction with depth (Hardy 1967). Simulation studies have shown that diurnal vertical migration in surface waters (less than 200 m) can modify the direction that krill are transported within the main current flow (Murphy et al. 2004a). Krill do not however appear to undertake a deep (greater than 300 m) migration during winter, although there are suggestions that vertical migration during winter in shelf and slope regions may be more important than first recognized (Siegel 2005; Taki et al. 2005). Even if such a seasonal vertical migration does occur, there is little southward flow at any depth in the Scotia Sea sector as intermediate water masses enter through Drake Passage and not from the Atlantic Sector, so a deep winter migration will not move krill south in the Scotia Sea.
A change in vertical distribution in the water column through the year does however occur owing to the winter association with the sea ice, which is a crucial part of the life history. The association with the sea ice has so far been assumed to be a strategy for accessing alternative sources of food and for the avoidance of predators (Loeb et al. 1997). However, the association may also be a strategy for retention and life cycle closure. The direction of the drift of ice is different from the underlying ocean circulation because the motion of ice is mainly wind driven (Thorndike & Colony 1982; Steele et al. 1997). A recent modelling study (Thorpe et al. submitted) has suggested that drifting with the sea ice over winter can generate retention of krill in southern regions where conditions for larval growth over the whole year are most favourable. A strong physical association of the krill with the sea ice could lead to a rapid southward redistribution as the retreat of sea ice in spring is often wind driven. This process may be particularly important in the Antarctic Peninsula region where the sea ice tends to move towards the continent from the Bellingshausen Sea region rather than offshore and northwards (Stammerjohn et al. 2003).
The link with the sea ice will also be important in generating the large-scale distribution of krill. Simulations of the growth and development of larval krill (Fach et al. 2002) showed that krill drifting east from the Antarctic Peninsula region would encounter sea ice advancing north across the region (Murphy et al. 1998b). Thus, larvae would be entrained in the west and central Scotia Sea during autumn. Modelling studies (Thorpe et al. submitted) indicate that krill entrained with the sea ice in the southern Scotia Sea in autumn would drift east and north with it during winter. During the spring ice retreat, the krill would either be entrained into the water column in the eastern or southern Scotia Sea or remain with the ice as it retreats and become entrained in the eastern Weddell. Further drift and entrainment in sea ice in the following season may release the krill into the favourable growth conditions of the Scotia Sea or in the Antarctic Coastal Current in the following year (figure 5c). The sea ice interaction is therefore potentially important in generating the distribution of krill in the Scotia Sea (figure 5; Murphy et al. 2004a).
During winter, the sea ice zone across the Scotia Sea system will also provide a very different habitat to that of the WAP region and probably favours ice algae growth even during mid-winter (Garrison & Close 1993). Day length in mid-winter across the southern Scotia Sea is more than 5 h, whereas there is no daylight in areas further south in the Weddell Sea and along the WAP. The sea ice zone will be an area of ice divergence where leads and floes are consistently changing, generating a MIZ system right across the southern Scotia Sea. During winter, the mean concentration of sea ice is approximately 42%. Even for the peak months of July, August and September, the concentration averages below 50%. An area of approximately 0.5×106 km2 is covered by sea ice at this time and over 0.17×106 km2 will be covered by sea ice less than 30% in concentration. These characteristics of low-concentration sea ice and relatively high irradiance are likely to favour the growth of sea ice algae and other components of the microbial community across the southern Scotia Sea during winter (Garrison & Close 1993). These are areas where krill are known to occur during spring and are likely to be an important habitat for krill and the whole food web during winter (Marr 1962; Hopkins et al. 1993b; Brierley et al. 2002a).
Although the South Georgia population depends on inputs from areas further south, there does appear to be some local retention of krill over a number of years (Reid et al. 1999b; Murphy & Reid 2001). There are consistent changes over weeks to months and between summer and winter and also between years in krill length in the diet of Antarctic fur seals (Reid et al. 1999a). This raises key questions about how krill overwinter in these more northern regions. At South Georgia, krill overwinter on shelf where they are the target of a fishery that operates over a series of banks off the north coast (Murphy et al. 1997). However, we do not know what these krill feed on during winter, nor do we have much information on the winter dynamics of local plankton populations. We do know from studies around the Antarctic Peninsula that krill can feed on benthic material, so a benthic food source may be available (Ligowski 2000; Daly 2004). We also know that krill occur near the sea bed in other regions. Activity recorder studies on penguins foraging from Signy Island have shown that at times they are feeding close to the bottom around 200 m and consuming krill (Takahashi et al. 2003). Krill can also feed on a range of planktonic species and groups other than large diatoms, including microbial species and meso-zooplankton (Marr 1962; Quetin & Ross 1991; Hopkins et al. 1993b; Huntley et al. 1994; Pakhomov et al. 1997a, 2004; Atkinson et al. 2002; Meyer et al. 2002, 2003; Daly 2004). These alternative food sources will be important in allowing the krill to survive in winter away from the sea ice, and further information on krill diet in winter in areas outside the sea ice is required.
There have also been suggestions that the Scotia Sea krill stock is maintained by two separate inputs of krill from populations in the Weddell Sea and the WAP regions (Siegel 2005). There are some indications of an east–west split in krill dynamics with different-sized krill dominating in the east or west in some years. However, there is no physical or planktonic community distinction between these areas, indicating that there is no simple ecological distinction (Marin 1987; Ward et al. 2004, 2006). It is also likely that krill are produced right across the region in areas of the WAP, the southern Scotia Arc, Weddell Sea and possibly right across the Scotia Sea (Hofmann & Husrevoglu 2003; Tarling et al. in press). However, we do not observe large numbers of larval krill regularly around South Georgia, and those that are present do not appear to recruit successfully to the local population (Ward et al. 1990). It is possible that larval development may be constrained by high temperatures in these more northerly regions. The view of a mixed Scotia Sea population is also supported by the observed consistency of recruitment success across the region and indicates that a discrete two-source view is inappropriate (Fach et al. 2006). Modelling studies have also suggested that krill will recruit into the Scotia Sea from right across the southern region (around the Antarctic Peninsula and northern Weddell Sea) and that successful recruitment will be a complex function of krill life cycle and feeding interactions (Fach et al. 2006). The apparent east–west split might therefore be the result of a combination of oceanic and sea ice interactions (Siegel et al. 2004; Siegel 2005). Krill larvae generated around the Antarctic Peninsula (east or west) will be moved eastwards over winter and will emerge in areas in the eastern Scotia Sea in spring (Fach et al. 2002, 2006; figure 5c). This could result in a separation in the distribution of different year classes, with annual waves of recruitment moving east, associated with local year class retention in shelf areas (figures 5c and 6).
Larger scale closure of the life cycle of krill from eggs to mature adult and spawning may involve connections between krill in areas that occur outside the Scotia Sea. Simulation studies indicate that after the ice retreats, a lot of the krill in the Scotia Sea would be transported out of the region to the east around the South Sandwich Islands (Murphy et al. 2004a). Such eastward movement may facilitate transport to areas further south in the eastern Weddell Gyre and Lazerev Sea (Thorpe et al. submitted). The link with sea ice areas in the southern Scotia Sea requires more specific study, focusing on larvae in ice edge regions encompassing both oceanic and neritic waters particularly during spring and autumn. Understanding the links and potential sources in this highly distributed system requires large-scale coupled simulations of the life cycle in association with oceanic and sea ice dynamics.
These analyses of population dynamics indicate that the central southern Scotia Sea and Arc may be a much more important habitat for maintenance of the krill population across the whole area than previously considered. The habitat of the central southern Scotia Sea appears particularly crucial in both winter and summer and will be a valuable focus for studies to determine larger scale controls on the distribution of krill.
(e) Krill population variability and change in the Scotia Sea
A number of studies have developed integrated analyses of krill population dynamics across the Scotia Sea. These built on earlier studies of variability of the ecosystem such as those of Maslennikov & Solyankin (1988) and Priddle et al. (1988). Together these have shown that fluctuations in the numbers of larval krill produced and their subsequent survival is the major driver of variation in the abundance of krill across the Scotia Sea (Murphy et al. 1998b; Murphy & Reid 2001; Reid et al. 2002). The importance of year class strength in driving changes in abundance in krill populations in the WAP and Elephant Island regions has been known for some time (Quetin & Ross 1991, 2003; Siegel & Loeb 1995; Ross & Quetin 1999; Quetin & Ross 2001). However, despite the evidence of a large-scale relationship between krill density and sea ice extent (Atkinson et al. 2004), the situation at South Georgia and across the northern Scotia Sea is more complicated. Smaller size/age (less than 30 mm and 1-year-old) classes of krill are generally not observed at South Georgia (Watkins et al. 1999). Size classes of older age groups merge together as the animals increase in size owing to the asymptotic nature of krill growth (Priddle et al. 1988). As noted earlier, this has made it difficult to determine whether abundance changes are driven by individual year class variations or bulk changes across all year classes. Initial studies suggested that bulk shifts in distribution of all age groups, linked to large-scale atmospherically driven changes in ocean currents, were generating the observed variation (Priddle et al. 1988). Subsequently, Murphy et al. (1995) and Fedulov et al. (1996) showed that these changes were also linked to sea ice changes further south and that they affected the availability of krill to the fishery. Model studies showed how the observed rapid reductions and recoveries in abundance could be the result of year class fluctuations in a system where older age groups dominated (Murphy et al. 1998b). Further analyses of krill size in the diet of Antarctic fur seals at South Georgia showed consistent changes in length frequency between years (Reid et al. 1999a), indicating that year class fluctuations were generating the observed abundance and biomass changes at South Georgia (Murphy & Reid 2001; Reid et al. 2002). The abundance changes are therefore driven by the influx of a large cohort of young krill which dominate the population and maintain regional biomass for 1–2 years. The biomass then declines until the next influx event. For the northern Scotia Sea, there is therefore a second-stage distributional effect on top of the original recruitment variation occurring elsewhere (Murphy et al. 1998b). The two effects are however linked, i.e. cold periods favour recruitment success and disperse krill further north, so it is unlikely to be possible to simply separate physical and biological effects.
These events of influx of young krill into the northern Scotia Sea are strongly related to the physical conditions across the Scotia Sea. At South Georgia, it is krill that have overwintered at least once under the ice that are transported to the island during the early summer (Murphy et al. 1998b). The further north the sea ice extends across the region, the colder the conditions in the north (Fedulov et al. 1996; Whitehouse et al. 1996a). Analyses of recruitment of krill into the population at South Georgia (Murphy et al. submitted) and particle-tracking studies, including interactions with sea ice (Thorpe et al. submitted), indicate that more extensive winter sea ice leads to enhanced dispersal and transport of young krill into northern regions. The effect is that during cold periods, influx recruitment is enhanced, while there is little or no influx during warm periods. In years of little or no flux, mortality rates will also increase as the predators attempt to maintain food supply, reducing abundance more quickly (Murphy & Reid 2001; Constable et al. 2003). These interactive effects of varying krill abundance and predator demand mean that mortality will be a key process in determining the interannual variability and may enhance the amplitude of the observed variation. However, although we can estimate the mortality rates of older krill, rates for larval and juvenile krill are unknown (Murphy & Reid 2001; Siegel 2005). Higher temperatures are likely to exacerbate the decline in krill biomass through reduced rates of growth (Atkinson et al. 2006), and may also affect survival. As the duration of the warm period extends over 2–3 years, biomass declines further so that the lowest biomasses occur at the end of the warm period (Reid et al. 1999a; Murphy & Reid 2001). A recent study of population changes at South Georgia (Murphy et al. submitted) indicates that influx events are most clearly detected after the warmest, lowest biomass years. During the colder periods, influx events are less obvious as the biomass is generally higher due to consecutive years of reasonable or high recruitment.
The influx of krill to the South Georgia area depends on transport from the southern Scotia Sea in spring (Murphy et al. 2004a,b). Some of the transport is associated with the SACCF which has been shown to be important in advecting krill (Hofmann et al. 1998; Fach et al. 2002, 2006; Murphy et al. 2004b). Further analyses of fluctuations in the position of the SACCF have also indicated that this may affect the large-scale transport of krill across the Scotia Sea (Thorpe et al. 2002; Trathan et al. 2003; see also Priddle et al. 1988).
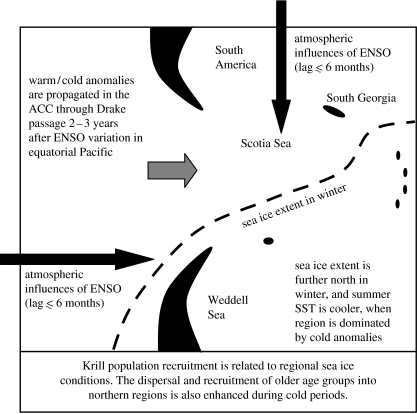
The sea ice and SST variation in the Scotia Sea is related to larger scale atmospherically driven changes (Murphy et al. 1995; Turner 2004; Murphy et al. submitted). ENSO variation influences the region oceanically through a signal that propagates across the southern Pacific sector and through Drake Passage into the South Atlantic region, 2–3 years after the variation in the ENSO region (figure 7; Murphy et al. 1995; Trathan & Murphy 2002; Murphy et al. submitted). During the most intense ENSO periods, the signal can be modified by direct, short-term, atmospherically driven changes (Meredith et al. 2005; Murphy et al. submitted). Low SST in the South Atlantic is also associated with greater sea ice in winter (Trathan et al. 2006; Murphy et al. submitted). As previously noted, these changes in sea and SST affect the recruitment in southern ice-covered regions and dispersal of older age groups to the north. This will introduce biological lags that affect the northern Scotia Sea through dispersal of krill 2–3 years after the recruitment in the south. The coherent nature of the physical variability provides the potential for prediction of physical and biological changes in the Scotia Sea.
Figure 7.
Schematic of the main physical processes generating variation in the Scotia Sea ecosystem. These factors also affect krill recruitment and dispersal across the region, generating observed correlations of changes in krill density and biomass and higher trophic level predator foraging and breeding performance with sea ice and larger scale indices of oceanic and climatic variation.
A lack of information on seasonal changes in krill abundance limits our understanding of these interannual fluctuations. Seasonal variation in krill abundance has been recorded in the Antarctic Peninsula region and may be a key aspect of the interannual fluctuations (Siegel 2005). Data from krill predators at South Georgia also indicate that there are marked seasonal changes in krill population structure in northern regions (Reid et al. 1999b) and biomass peaks during the summer (Brierley et al. 2002b; BAS unpublished data). In the northern regions, changes in timing of influx, growth and mortality during the season will all affect local krill abundance and hence their availability to predators. There is little knowledge of these processes and it is important that further information on seasonal changes in krill abundance across the Scotia Sea is obtained.
On longer time-scales, analyses of historical net data have shown a decline (50–80%) in the abundance of krill across the Scotia Sea over the last 30 years (Atkinson et al. 2004). Across the region, annual krill density is related positively to the previous winter sea ice cover in the Scotia Sea (Atkinson et al. 2004). Although it is tempting to infer a causal relationship from these observations, sea ice changes over the last three decades have been complex and show marked interannual, sub-decadal and decadal changes (Murphy et al. 1995; Murphy et al. submitted). The long-term decline in overall abundance could be interpreted as a stock that has become more dependent on fewer years of successful recruitment and which are consequently subject to high mortality rates (Murphy et al. 1998b; Reid et al. 1999b; Murphy et al. submitted). The effect on population dynamics will therefore be expressed more clearly than in higher biomass periods that dominated two to three decades ago. When the population size was larger, single year class fluctuations would have had less effect on biomass (Murphy et al. 1998b). With this view, we would expect that across the Scotia Sea, correlations between environmental variation and krill abundance fluctuations will be stronger now than in previous periods when biomass was higher. However, as both recruitment and dispersal of krill are related to sea ice, it is difficult to determine whether the changes in density in the Scotia Sea are due to reductions in overall abundance or changes in distribution (Murphy et al. 1998b; Constable et al. 2003).
(f) Krill in the Scotia Sea food web
The analysis of the life cycle of krill highlights the spatial operation of the krill population in the Scotia Sea. Krill are therefore a variable and dynamic component of the food web across the Scotia Sea (figure 8). As we have noted, krill are omnivorous and consume other zooplankton or microbial groups. In sea ice-covered regions, they can consume sea ice algae and, in shelf areas, have been found to be feeding on benthic algae (Ligowski 2000). However, a major source of energy for krill during spring and summer is diatoms (Cadee et al. 1992; Pond et al. 2005). Each year, as the ice retreats, large blooms of diatoms occur across the Scotia Sea, particularly in the regions of the Scotia Arc and downstream (Holm-Hansen et al. 2004b), and these are exploited by krill (figure 8). Later in the spring, as the ice retreats southward, large blooms of diatoms in southern regions will fuel the growth of post-larval krill in areas where they are retained over the shelf or as they drift north across the Scotia Sea (figure 8). In the more northern regions, krill will benefit from the occurrence of large blooms associated with the Scotia Arc that are maintained for extended periods during summer (Atkinson et al. 2001; Korb et al. 2005).
Figure 8.
Schematic of seasonal development (winter, spring and summer) of the Scotia Sea ecosystem, highlighting major spatial connections and the development of the spatial distribution of krill.
The resultant krill distribution is highly heterogeneous with the highest densities occurring in areas of shelf around the Scotia Arc, but with a significant biomass in areas off-shelf, particularly at the ice edge during spring (Marr 1962; Atkinson et al. 2004; Hewitt et al. 2004; Siegel 2005). Large numbers of predators require land-based breeding sites during summer, from where they operate over a restricted local area as centrally placed foragers (Croxall et al. 1988). These areas occur on islands around the Scotia Arc where enhanced concentrations of krill are found on the shelf. These are also the regions where most of the commercial fishing for krill occurs (Murphy et al. 1997). There is therefore a spatially heterogeneous demand for krill, with intense hotspots, where krill concentrations are high and predator demand is greatest (figure 8; Murphy & Reid 2001). In areas of low production or retention of recruits, the demand for prey is maintained by the advective influx of krill. Rates of mortality of krill across the Scotia Sea will therefore be highly heterogeneous (Murphy & Reid 2001).
Within these regions of high concentration, krill show further spatial structure associated with meso-scale (tens to hundreds of kilometres) physical features, such as frontal regions, plumes and the edges of submarine canyons (Brinton 1985; Watkins et al. 1986; Murphy et al. 1988; Witek et al. 1988; Miller & Hampton 1989; Watkins & Murray 1998). Biological and physical process interactions within these aggregations generate swarms in response to predatory and feeding stimuli (Murphy et al. 1988). This patchiness across a range of scales allows a variety of predator species with very different foraging strategies to exploit krill (Murphy et al. 1988). The longevity of krill is also an important factor in their role in the food web. The long lifespan of krill (5–7 years) means that at a population level, they are able to cope with the strong seasonal and interannual variability (Murphy et al. 1998b; Fraser & Hofmann 2003). By exploiting a wide range of food sources, they can survive periods of starvation. Long lifespan allows for the potential of spawning several times during their life and means they can cope with unfavourable years (Fraser & Hofmann 2003). This combination of high abundance, longevity, dispersal and heterogeneity is why krill have such a central role in the food web of the Scotia Sea (figure 9).
Figure 9.
Predator links in the Scotia Sea food web. (a) Proportional consumption of different groups of prey by the major predators. (b) Estimates of annual consumption of krill (106 tonnes yr−1) by the main krill predators. Where available a range of estimates are given to illustrate the uncertainty. Information on predator diet and consumption is from Croxall et al. (1984, 1985), Boyd (2002a) and Reilly et al. (2004). Estimates of krill standing stock are from Hewitt et al. (2004) and values in parentheses are from Demer & Conti (2005). Estimates are based mainly on summer studies and are likely to overestimate the importance of krill in the diet (Croxall et al. 1985). Values for fish and squid consumption have not been included, but may be very large (Kock 1985; Pusch et al. 2004).
5. Food-web operation
The Scotia Sea ecosystem encompasses high production regions around areas of shelf and sea ice retreat during spring, and low production regions in the west near Drake Passage. The advective nature of the system also makes a narrow geographical view inappropriate. Some locally detailed studies, mainly based on summer data, have been undertaken on various aspects of the ecosystem (Croxall et al. 1985; Gowing & Garrison 1992; Hopkins et al. 1993a,b; Bathmann et al. 2000; Atkinson et al. 2001), but there have been few attempts at a broader synthesis (Everson 1977; Hempel 1985a,b). The focus for carbon flow analyses has been on primary production and lower trophic level interactions where the flows are greatest (Bathmann et al. 2000). In contrast, analyses of variability have focused mainly on higher predator interactions with their prey, which has generated datasets extending back almost 30 years (Croxall et al. 1988; Reid & Croxall 2001). Here, we highlight some of the key features of the food web, considering trophic and spatial links.
(a) Trophic links
Within the lower trophic levels, a particular focus has been on the role of copepods and their interactions with krill. Copepods in the northern Scotia Sea, around South Georgia, can at times be the dominant grazers (Ward et al. 1995; Atkinson & Snyder 1997; Pakhomov et al. 1997b; Atkinson et al. 1996, 1999). Shreeve et al. (2005) found that daily gross krill production was 0.022 g C m−2 d−1 compared with 0.026 g C m−2 d−1 of the older stages (CIV and CV) of the copepod Calanoides acutus (which represented approx. 25% of the total copepod biomass at South Georgia). These analyses give a valuable view of the summer situation and indicate that copepods may be the dominant zooplankton secondary producers across the Southern Ocean (Shreeve et al. 2005). Developing more detailed analyses of the relative impact of krill and copepods on production requires a seasonal view. These are highly dynamic systems where the timing of interactions will generate multiple and varying controls that are not easily resolved by short-term sampling programmes. The situation is complicated by associated spatial changes in the operation of the food web. Krill biomass around South Georgia is greatest on the shelf where local temperatures and chlorophyll a concentrations are generally low. In contrast, copepods tend to dominate in warmer waters, off-shelf to the west, where chlorophyll a concentrations are higher. Both competitive interactions and ‘bottom-up’ processes (physical and chemical) in the food web have been invoked to explain these relationships between copepods and krill (Atkinson & Snyder 1997; Priddle et al. 1997, 2003; Atkinson et al. 1999; Shreeve et al. 2005; Ward et al. 2005).
The extended lifespan of the larger zooplankton species may be an adaptation to survive in highly seasonal and variable systems. However, they may also be an important factor in determining the influence of zooplankton on lower trophic levels (Priddle et al. 2003; Shreeve et al. 2005). Overwintering zooplankton will affect the development of phytoplankton populations during spring. Large numbers of zooplankton rising from depth in spring (copepods; Atkinson et al. 1997; Ward et al. 1997; Tarling et al. 2004), or already present in surface waters (krill), will have an instantaneously high grazing impact, affecting the net growth rates of phytoplankton (Lancelot et al. 1991, 1993). Krill grazing impacts may therefore affect bloom development even though their impact on overall productivity during the summer is low. This may be particularly important in the Scotia Sea where copepods and krill are a dominant part of the food web.
The relative importance of grazing through copepods and krill will impact the fate of carbon (Cadee et al. 1992; Gonzalez 1992; Ross et al. 1998; Schnack-Schiel & Isla 2005). Krill generate large faecal pellets that sink quickly (rapidly removing carbon to depth), whereas copepod waste material is smaller and likely to sink less rapidly. However, copepods spend a large part of the year in diapause at depths of greater than 1000 m where they may die, generating a direct carbon flux at depth, whereas krill remain in surface waters. The complexities of such vertical interaction effects in the food web, including links to microbial systems, are largely unknown but are likely to be important in determining vertical fluxes of carbon (Tarling & Johnson 2006). These life cycle and behavioural effects demonstrate that analyses of carbon fluxes in the Scotia Sea, and indeed biogeochemical cycles generally, will require detailed knowledge of the life cycles of key planktonic species, particularly krill and copepods (Giesenhagen et al. 1999).
Of the other zooplankton groups, two in particular deserve further study in the Scotia Sea. Firstly, salps are distributed across the Scotia Sea and have an important role in regional biogeochemical cycles and food webs (Foxton 1966; Fortier et al. 1994; Pakhomov et al. 2002). However, it is unclear whether they are as important as in other regions around the Southern Ocean, particularly in the WAP region and in warmer areas to the north (Marchant & Murphy 1994; Loeb et al. 1997; Atkinson et al. 2004; Kawaguchi et al. 2004; Smetacek et al. 2004). This may be the result of high concentrations of diatoms that adversely affect salp feeding, or the result of competitive or predatory interactions with other species (Smetacek et al. 2004). Recent suggestions that their abundance is increasing in high-latitude Southern Ocean ecosystems (Pakhomov et al. 2002; Atkinson et al. 2004) make the need to improve understanding more urgent.
The second group for which information is very limited are the predatory amphipods, particularly Themisto gaudichaudi (Pakhomov & Perissinotto 1996). These amphipods can become a major component (more than 50%) in the diet of mackerel icefish around South Georgia in years of low krill abundance and are also consumed by a range of other pelagic and seabird predators (Rodhouse et al. 1992; Kock et al. 1994; Pakhomov & Perissinotto 1996; Reid et al. 1997b; Bocher et al. 2001). It is likely that they are a significant predator on a wide range of species, including krill and copepods (Pakhomov & Perissinotto 1996) and may exert predatory control on the dynamics and interactions of lower level species (Bocher et al. 2001).
Top-down controls are important in Scotia Sea planktonic ecosystems (Atkinson & Snyder 1997; Priddle et al. 2003; Shreeve et al. 2005), but much more specific studies of plankton interaction effects are required to elucidate mechanisms and generate dynamic models. Feedbacks within the food web will also affect the dynamics of phytoplankton productivity (Priddle et al. 2003; Shreeve et al. 2005). For example, large aggregations of krill grazing on phytoplankton can generate locally high concentrations of ammonium through excretion (Priddle et al. 1997, 2003; Atkinson & Whitehouse 2000). As ammonium is a preferred nitrogen source for many phytoplankton species, this will lead to enhanced phytoplankton growth rates (Priddle et al. 1998b). Ammonium levels can also be enhanced around local predator colonies, again increasing potential growth rates of phytoplankton (Whitehouse et al. 1999). Such interaction and feedback effects are likely to be particularly significant in the Scotia Sea, which has such high concentrations of copepods, krill and higher predators.
Across the Scotia Sea, there is no simple relationship between the structure of plankton communities and any major physical features, such as frontal boundaries between water masses. In recent analyses of zooplankton community structure, Ward et al. (2004, 2006) found that the major Scotia Sea frontal systems did not act as significant boundaries for species or between communities. Instead, the variation of community structure in spring was dominated by north–south differences in the state of development of the different zooplankton species. Species in communities further north were generally in a more advanced state of development than areas to the south during mid-summer. This development-related variation has been generally linked to timing changes in water temperature and sea ice cover related to latitude and seasonal variation in production and planktonic system development (Hempel 1985a; Marin 1987; Atkinson & Sinclair 2000; Ward et al. 2004, 2006). This difference was further shown in analysis and modelling of the development status of key copepod species (Tarling et al. 2004). Overwintering stages reach the surface waters earlier in the season in the more northern regions (Voronina 1970). The short and later season also impacts the overall life cycle, with animals in the north completing their life cycle in a single year, whereas further south, more of the population take 2 years (Tarling et al. 2004). These variations in timing, growth and development will affect interactions with krill and modify the dynamics of the food web and will be a valuable focus for the next generation of model studies.
Even though krill dominate the energy flows to higher trophic levels (figure 9), the pathways of energy transfer through the food web are complex (Croxall et al. 1984, 1985; Hopkins et al. 1993a,b). Most of the studies of krill consumption are from analyses of the diet of land-based predators (figure 9). However, krill are also consumed by a wide range of pelagic species, especially squid and fish, although consumption estimates are extremely uncertain (Kock 1985; Rodhouse & Nigmatullin 1996; Pakhomov et al. 1996; Pusch et al. 2004). The importance of copepods as prey items in the food web is even less well quantified than for krill. Shreeve et al. (2005) recently discussed the potential importance of the copepods in the food web and noted that C. acutus is an important part of the diet of the mesopelagic fish, Electrona antarctica. Another copepod species, Drepanopus forcipatus, is consumed by the larvae of the commercially exploited icefish (Champsocephalus gunnari). Copepods are also important to the flying seabirds such as Antarctic prions (Pachyptila desolata) and diving petrels (Pelecanoides sp.; Reid et al. 1997a,b). Groups and species other than copepods and krill can also be locally or seasonally important in the diet of a range of pelagic and land-based predators. For example, the diet of icefish varies markedly around South Georgia; euphausids other than krill (e.g. Thysanoessea spp.), the amphipod T. gaudichaudi or mysids (Siegel & Muhlenhardtsiegel 1988) are a significant component of the diet in different areas. These groups and species are also an important, but variable, component of the diet of other fish, seabird and squid predators (Kock et al. 1994; Croxall et al. 1999; Everson et al. 1999; Bocher et al. 2001; Dickson et al. 2004). Variations in trophic links as zooplankton and fish species grow and develop are largely unknown. The impact of chaetognaths, jellyfish and other pelagic species on young stages of fish and krill is also unknown, but may be an important component of variability of recruitment of fish and krill.
Around South Georgia and across the Scotia Arc, demersal and pelagic predators such as fish and squid provide alternatives to krill as prey for higher predators during the summer (figure 9). For example, the mackerel icefish, C. gunnari, which is semi-demersal on the shelf, is an important prey item for Antarctic fur seals at South Georgia (Reid & Arnould 1996; Everson et al. 1999). Equally, squid are an important prey for several groups of higher predators including seabirds, seals and toothed whales (Rodhouse et al. 2001; Collins & Rodhouse 2006). Mesopelagic species of myctophid fish are also important in the diet of various predators, including Antarctic fur seals (Reid & Arnould 1996), squid (Rodhouse et al. 1992) and king penguins (Olsson & North 1997). A number of these predator species forage in the vicinity of the PF and Rodhouse et al. (1992, 1994) considered explicitly some of the trophic links in this region. Further south in winter in MIZs, E. antarctica can be a more important prey item in the diet of flying seabirds (Ainley et al. 1991; Hopkins et al. 1993b). Salps and jellyfish also appear to be a potentially significant dietary component in a range of pelagic and land-based predators but their importance is unknown (Catry et al. 2004). As well as being key components of the diet of many of the predators, squid and myctophid fish species will also be important in links between pelagic and mesopelagic communities (Collins & Rodhouse 2006). There will also be important pelagic–benthic links, and a number of species develop in shallow waters and migrate deeper to shelf–slope regions as they grow. For example, in coastal ecosystems along the northern Scotia Arc, the commercially exploited Patagonian toothfish (Dissostichus eleginoides) will be a significant predator with dynamic trophic interactions that vary between pelagic and benthic systems as it grows. Short-term variations in trophic interactions involving diurnal and seasonal changes in depth are also known to be important. However, although descriptive analyses are available, quantitative studies of abundance and fluxes associated with most of these interactions are not available.
These less well-known alternative pathways to the traditionally studied krill links will be important in maintaining the ecosystem structure and determining the dynamics of individual species. These alternative pathways cannot however support the same level of predator demand as the krill–predator pathway, because more complex pathways involve more trophic transfers and associated energy losses at each step (figures 10 and 11).
Figure 10.
Schematic illustration of alternative pathways in part of the Scotia Sea food web, showing shifts between (a) years when krill are abundant across the Scotia Sea and (b) years when krill are scarce. Major pathways shown as black arrows.
Figure 11.
Estimated transfer efficiencies in the Scotia Sea krill-based food web with transfer efficiencies for alternative routes through other zooplankton species and intermediate predators. Based on Priddle et al. (1998a).
The upper trophic level structure of the food web is dominated by different predator species across the Scotia Sea. In the north, there are extensive land areas that are not covered by snow during the summer which allow access to suitable breeding sites for macaroni penguins and Antarctic fur seals, which are the major krill predators (Croxall et al. 1984, 1985; Boyd 2002a). Further south, in regions covered by sea ice for much of the year, it is chinstrap or adelie penguins and Weddell or crabeater seals that are the major krill consumers (Croxall et al. 1985; Trathan et al. 1996; Priddle et al. 1998a; Boyd 2002b; Takahashi et al. 2003; Lynnes et al. 2004). The dietary differences and specific habitat requirements that generate niche separation of the many predator species across the Scotia Sea have been described in detail. Krill predators often dominate local Scotia Sea food webs, but large numbers of other seabird species, such as petrels and albatrosses, are dependent on groups other than krill, particularly copepods, amphipods, fishes and squid (Croxall et al. 1984, 1985; Reid et al. 1996a; Lynnes & Rodhouse 2002; Xavier et al. 2003a,b, 2004). We have some knowledge of the diet and foraging of many of these species, particularly in one or two localities such as South Georgia, Signy Island and around the Antarctic Peninsula, but little information for much of the area (Croxall et al. 1985; Reid et al. 1996a, 2004).
There is also very little data on the overall abundance and distribution of most of these predator species across the Scotia Sea. Much more detailed information is required on trophic links at local and regional scales combined with data on geographical distribution and abundance. There are major gaps in our knowledge about the operation of the mesopelagic systems across the Scotia Sea and the links between pelagic and benthic systems. These systems will be crucial, both in terms of the fate of upper ocean production (and hence carbon) and in terms of the effects on the long-term dynamics of pelagic food webs. It is not possible to examine all pathways in the food web, so we need to focus effort. Specific studies of the role of key predator groups for which we have very little information, such as the whales and crabeater seals, will be important. With a changing climate, copepod and mesopelagic fish interactions, that are crucial in more sub-Antarctic ecosystems, are likely to become more important across the northern Scotia Sea. Focusing effort on these pathways as alternatives to energy flow through krill will be a valuable basis for future research on trophic links.
(b) Spatial operation of the food web
In a highly advective physical environment, the food-web structure can only be maintained by a combination of local and externally generated production. That both local (see also Atkinson et al. 2001; Gilpin et al. 2002) and external production are important in krill population processes and in maintaining food webs was recognized as far back as the Discovery Investigations (Marr 1962; Hardy 1967; Mackintosh 1972, 1973). This view has been supported by field analyses that have shown that large amounts of krill do occur in high-flow areas off South Georgia (Ward et al. 2003; Murphy et al. 2004b). These flows will be important in taking krill around the island and onto the shelf, although the exact pathways and connections will be complex and variable (Thorpe et al. 2002; Meredith et al. 2003b,c). Field measurements have shown that the flows will be variable, but at times their influence will dominate the local food-web structure (Murphy et al. 2004a).
Even conservative estimates suggest that local krill production around islands such as South Georgia is rapidly consumed through predation. Calculations of predator demand are however complex, and it is especially difficult to account for all the potential demand (Boyd 2002a; Murphy et al. 2004b; Shreeve et al. 2005). The relatively low growth rate of krill in warm regions of high predator demand around South Georgia during summer further indicates that production will not supply local demand in key regions of the Scotia Sea (Atkinson et al. 2006). Such a large impact should generate rapid changes in numbers that would need to be replenished to maintain local krill populations (Murphy et al. 1998b). The lack of very young krill on the shelf at South Georgia, combined with the strong flow of the ACC past the island, further indicates that the population in the northern regions is maintained by recruitment from further south (Ward et al. 1990; Thorpe et al. 2002; Meredith et al. 2003c; Murphy et al. 2004b). Variations in these inputs will have profound effects on the krill population dynamics and the local system operation. Input of krill into more northern regions probably peaks during spring and early summer following the retreat of the sea ice in the southern Scotia Sea, and is likely to be lowest in winter (Murphy & Reid 2001; Murphy et al. 2004a,b). On top of these input effects, further variation will be introduced through fluctuations in rates of growth and mortality. Growth rates may be highest in early spring and summer, while mortality rates are likely to peak in mid to late summer. Together these factors will generate the apparent mid-season peak in the biomass of krill, the timing of which is likely to vary.
Although it is difficult to estimate the total predator demand for krill, it is clear that top-down control on plankton communities will be important in areas around the major predator colonies in the Scotia Sea (Reid et al. 2004; Murphy et al. 2004b). At South Georgia, in areas of shelf to the east of Cumberland Bay, the concentration of krill is variable, but usually exceeds that observed in areas further west around Bird Island, where the variation is lower (Brierley et al. 1997). The majority of seabird and seal demand for krill around South Georgia is concentrated in the west around Bird Island (Croxall et al. 1985; Trathan et al. 1996). Long-term data on predator breeding performance has demonstrated that there are periods where krill availability in the western regions has been sufficiently low that they result in catastrophic mortalities of predator offspring (Croxall et al. 1988; Reid & Croxall 2001; Reid et al. 2005; Trathan et al. 2006). During these periods of reduced krill biomass, foraging trips become extended as the predators forage further offshore in the search for prey (Boyd & Murray 2001). These factors indicate that predator demand is at times sufficient to deplete local krill biomass. This top-down influence will in turn modify the dynamics of the planktonic communities on the shelf, although the actual effects are unknown. The processes of replenishment of plankton on the shelf through cross-shelf exchange are unknown as we have little real understanding of the detailed circulation on the shelf (Brandon et al. 1999; Meredith et al. 2003b; Meredith et al. 2005). This is also true in terms of understanding the links along the shelf around South Georgia between areas that are fished in winter, which are mainly in the east, and areas further west where the predators forage during spring and summer (Murphy et al. 1997). Development of high-resolution models of on-shelf circulation will be an important component in generating the required understanding.
The role of fronts in transferring material across the Scotia Sea is likely to be important, as these are areas of high flow rate (Hofmann et al. 1998; Nicol 2006; Murphy et al. 2004b). However, analyses of the large-scale distribution of krill during summer indicate that a simple view of a ‘conveyor belt’ of krill across the Scotia Sea connecting the Antarctic Peninsula region to South Georgia is not appropriate (Hewitt et al. 2004; Murphy et al. 2004a). The distribution of krill and other plankton in spring will be a function of the timing and pattern of sea ice retreat, affecting local production, krill emergence from the MIZ and copepod migration from depth. Thus, the summer distribution of plankton across the Scotia Sea will be strongly dependent on the system development during spring (Murphy et al. 2004a). The importance of advective transfers and dispersal of species other than krill, many of which are more planktonic, such as copepods, has not been the focus of as much study, but is likely to be important. The importance of advection in generating the observed distribution of phytoplankton was demonstrated in a detailed analysis of the planktonic system on the northern side of South Georgia (Ward et al. 2002). The analysis showed that the planktonic system around the island was strongly influenced by inflows associated with the SACCF. Enhanced chlorophyll a concentrations coincided with the centre of the high-flow region of the SACCF. It was estimated that the chlorophyll a and depleted nutrient concentrations in the region would have taken two to three months to develop. In this same area, copepods were found to be more advanced in development state compared to surrounding communities and krill abundance was also high. Model simulations of transport pathways indicated that the system would have been associated with areas of the southern Scotia Sea during early spring. At that time, the planktonic communities would have been within the MIZ, indicating spatial connections between the planktonic systems of the ice edge during spring and South Georgia during summer.
Predator foraging is also important in the spatial transfer of energy in the ecosystem and the operation of the food web (Croxall et al. 1984; Reid et al. 2004). During summer, many of the predator species forage over large distances with foraging bouts taking generally a few days to a week (Croxall et al. 1984, 1985). Of the dominant krill foragers, the macaroni penguins forage directly across the shelf areas and the major direction of the flow, potentially maximizing the volume of water sampled. Fur seals forage further out over the shelf, spending more time in the off-shelf regions, where krill are advected in the current flow (Boyd et al. 1994, 2002 Boyd 1996, 1999; Staniland & Boyd 2002, 2003; Staniland et al. 2004). The flying seabirds also travel large distances (more than 1000 km) to obtain prey (Croxall & Briggs 1991; Croxall et al. 1997, 2005; Xavier et al. 2003c; Catry et al. 2004; Phillips et al. 2005). Black-browed albatrosses, which consume large amounts of krill, forage on the shelf and in areas of the southern Scotia Sea as far as the South Orkney Islands. Species such as the grey-headed albatross and king penguins, which consume more squid and fish, operate more widely across the Scotia Sea or in areas further north around the PF (Collins & Rodhouse 2006).
The predators in the South Georgia ecosystem, and at other islands across the Scotia Sea, are therefore not only dependent on local production, but also forage out across the Scotia Sea and surrounding areas, bringing energy back to feed their young in the large and spatially restricted colonies (Croxall et al. 1984, 1985). This concept was developed in predator–prey modelling by Murphy (1995) and it generates a distance–demand relationship away from the centre of foraging. The result is intense heterogeneity in the demand for prey, which is centred around the islands with most of the demand within approximately 200–250 km of the islands. This concept has been further developed by Trathan et al. (1998b) and Reid et al. (2004), who used data from large-scale at-sea observations to derive more specific species-based relationships of foraging in relation to distance from land in the Scotia Sea. Such analyses provide the basis for generating spatially distributed demand maps, and hence prey mortality distributions, across the Scotia Sea.
Food webs in the Scotia Sea also show marked seasonal changes with major shifts in structure between summer and winter. Thus, for example, around South Georgia the diet of fur seals changes during late summer and autumn, from being dominated by krill to one where myctophid and other fish species are proportionately more important (Reid & Arnould 1996). Many of the higher trophic level species also show shifts in foraging areas or disperse across the region during winter (Boyd et al. 1998). This includes many of the krill-eating species of penguins, such as the adelie and chinstrap penguins in the southern regions, which disperse as the sea ice advances north from about May. In the north, fur seals disperse south towards the advancing sea ice, but also north across the PF to areas of the Patagonian Shelf (Boyd et al. 1998, 2002). However, a significant number of animals remain in areas around the islands such as South Georgia during winter (Boyd et al. 1998, 2002). Macaroni penguins also disperse away from South Georgia during the winter months, but there is little available information on winter distribution. Many of the seabirds also leave the region, as illustrated by the wandering albatross which forages right around the Southern Ocean. Other species and groups leave the Southern Ocean completely and move north across the southern hemisphere. This large-scale dispersal is most well known for the whales, which migrate north along the east coast of South America and the west coast of Africa. There is therefore a movement out of the region of a potentially significant, but unknown, proportion of the upper trophic level predators. This dispersal across the Scotia Sea, the Southern Ocean and southern hemisphere is crucial in understanding the operation of the food web. The effect is to reduce the upper trophic level demand for energy during the low production winter season, so that a significant proportion of the potential demand leaves the ecosystem during the winter (figure 8). More broadly, it also means that these predators connect the dynamic operation of the Scotia Sea ecosystem with ocean ecosystems across the South Atlantic.
Improving our understanding of the spatial and temporal operation of Scotia Sea food webs requires a multi-scale approach. In particular, we need to understand how localized food webs, such as around the island areas of the Scotia Arc, interact as part of the larger scale Scotia Sea and Southern Ocean ecosystems. Understanding how physical and biological processes and interactions operating over different scales impact the regional food web will be crucial in analyses of the long-term dynamics of the ecosystem (figure 12).
Figure 12.
Schematic of the temporal and spatial scales of the main physical and biological processes important in determining the dynamics of the Scotia Sea ecosystem (based on Murphy et al. 1988). The 1 : 1 relationship is based on the scales of physical mixing in the oceans. Note the physical and biological processes are illustrated offset above and below this line respectively for clarity. The shaded grey block illustrates the natural spatial and temporal scale of Scotia Sea processes. We include processes above and below this scale to approximately 105 km and a few hundred years and down to approximately 200 km and approximately two to three months. PD, population dynamics.
6. Ecosystem variability and long-term change
As we have noted, the major focus of studies aimed at understanding the factors generating interannual variability in Scotia Sea ecosystems has been on changes in the distribution and population dynamics of krill. However, the development of that focus has been generated in part through studies of the impact of variability on other trophic levels in the food web (Croxall et al. 1988; Priddle et al. 1988; Constable et al. 2003). Unique long-term monitoring datasets of the breeding biology and population size of upper trophic level predators across the Scotia Sea highlighted that there were years in which availability of krill was very low and predator breeding performance was significantly reduced (Boyd & Murray 2001; Fraser & Hofmann 2003). These impacts have been shown across a range of predators for which krill are a significant component of their diet, including the land-based breeding predators, such as macaroni penguins, gentoo penguins, Antarctic fur seals, black-browed and grey-headed albatrosses and Antarctic prions at South Georgia, and chinstrap and adelie penguins at Signy Island and on the Antarctic Peninsula (Priddle et al. 1988; Boyd & Murray 2001; Reid et al. 1997a, 2005). Pelagic predators are also affected as shown in the earliest observations of interannual variability, which were revealed by changes in the distribution and feeding of fin and blue whale species between years (Priddle et al. 1988 and references therein). Growth and condition indices of the mackerel icefish also show marked interannual changes (Everson et al. 1997, 2000b; Everson & Kock 2001). These changes have shown that predator performance can be used to monitor changes in krill availability (Boyd & Murray 2001; Reid & Croxall 2001). However, although indices of predator performance do identify periods of low krill abundance, they cannot resolve changes in abundance above approximately 25–30 g m−2 (Boyd & Murray 2001). Above this level, increases in density do not generate improved performance of predators (Reid et al. 2005). It is likely that above this concentration foraging constraints, competitive effects and other density-dependent factors dominate the dynamics. More recently, relationships have been revealed between the variation in predator performance and local and large-scale indices of physical variation (Forcada et al. 2006; Trathan et al. 2006; Trathan et al. in press). At South Georgia, warm conditions in the previous season precede low reproductive success in penguins and seals (Forcada et al. 2006; Trathan et al. 2006; Trathan et al. in press). Detailed studies of krill population dynamics have shown that these relationships reflect changes in krill availability which are linked to changes in the ocean and sea ice regimes (Murphy et al. 1998b; Murphy & Reid 2001; Constable et al. 2003; Murphy et al. submitted).
The variability reveals shifts in food-web structure between years of high and low krill availability (figure 10). At South Georgia, when krill are scarce, the diet of the large numbers of seals, penguins and fish shifts. Fur seals consume mackeral icefish and myctophids, penguins consume fishes and amphipods, and icefish consume more amphipods (Croxall et al. 1999). Energy flows through these alternative pathways are insufficient to support the demand required to generate a large number of offspring, so we see failures in reproductive performance (figure 11; Croxall et al. 1988). However, the switching does allow the survival of adults and hence maintains the populations during years of low krill abundance. The switching therefore reveals a property of the food web that buffers the system response to variability. These weak pathways can appear unimportant in terms of energy flow compared to the main krill-related flows, but they are crucial in maintaining the system in the longer term. These alternative, weaker interaction pathways linking production to highest trophic level predators are likely to be crucial in determining the dynamics of the food web and its stability properties (Rooney et al. 2006).
There are also direct physical effects that modify the food-web operation when the region is dominated by warm or cold conditions. During colder, longer duration winters, the sea ice extends further north and more ice obligate species will occur across the Scotia Sea. Leopard seals (Hydrurga leptonyx) usually occur in ice-associated environments, but they are present around South Georgia in winter although their abundance varies between years. Jessopp et al. (2004) analysed a time-series of leopard seal occurrence from South Georgia. They found that in winters which are cooler and of longer duration, when winter sea ice extends further north, leopard seals occur in greater numbers at South Georgia, arriving earlier and leaving later. It is also likely that at these times, the general influence of ice-associated species will extend further north across the region. Of the predator species, the role of crabeater seals is likely to be particularly significant across the region at these times.
The physical and biological process interactions underpinning the structure of the food web make it sensitive to regional and hemisphere scale changes in climate (Trathan & Murphy 2002; Murphy et al. submitted). As we have noted, changes in this region not only reflect local processes, but are also linked to global scale processes. This has given us a short-term predictive capacity that will be tested over the coming years.
The rapid regional reductions in sea ice concentration and SST associated with increases in air temperature are generating major changes in the Scotia Sea ecosystem (Murphy et al. 1995; Vaughan et al. 2003; Meredith & King 2005). Along the Antarctic Peninsula, shifts in the breeding distributions of penguins have been related to reduced ice extents (Fraser et al. 1992; Smith et al. 1999). Across the Scotia Sea, the abundance of krill has declined by between 50 and 80% over the last 30 years (Atkinson et al. 2004). These declines have been linked to changes in sea ice distribution and, as we have discussed, probably reflect regional variations in recruitment and dispersal (Murphy et al. 1998b). At South Georgia, there have also been significant changes in penguin and albatross populations (Reid & Croxall 2001; Barlow et al. 2002). However, although the changes have been linked to a general reduction in krill abundance over the last 20 years (Reid & Croxall 2001), other effects are also likely to be important. Although there have been clear regional changes in sea ice and ocean temperatures, the effects on the ecological system are complicated by the long-term dynamics of the food web (May 1979; May et al. 1979). The regional food web has been strongly perturbed over the last two centuries, as a result of harvesting of seals, whales, fishes and krill (Everson 1977; Murphy 1995). It has been suggested that the reductions in whales and seals may have generated a ‘krill surplus’ and that this will have been used by other groups of predators (Laws 1985). Although the extent to which this occurred has been debated, the exploitation will have had long-term consequences (Everson 1977; Croxall 1992; Murphy 1995). Populations of many of the exploited whale species have not recovered to pre-exploitation levels. In contrast, Antarctic fur seal populations have recovered rapidly from very low numbers (less than hundreds) in the first half of the twentieth century to around 2–3 million just around South Georgia, while their range has expanded across the Scotia Sea. These large-scale and rapid ecological changes are likely to be generating intense competition for krill (Barlow et al. 2002) and be a major factor influencing the dynamics of the food web. It is also worth noting that two species whose populations are expanding in the northern Scotia Sea, the Antarctic fur seal and king penguin, both exploit the myctophid P. choriodon as part of their diet. This myctophid is the major component of the diet of both predators in sub-Antarctic areas, outside the main krill zones. The success of these predator species may reflect a shifting competitive balance favouring species that can exploit prey other than krill.
Over the last 30 years, the Scotia Sea and surrounding regions have also been the major area of exploitation of living resources in the Southern Ocean (Everson 2001). It is currently the main area where krill are harvested and is the focus for the development of ecosystem-based approaches to the management of exploited fish and krill stocks (Constable et al. 2000). The long-term effects of the disturbance are unclear as we have little knowledge of the population size of the major predator species across the Scotia Sea (Everson 1977; Everson 1984). If we are to predict the long-term dynamics of key species in the ecosystem, we need much better information on the size of populations, their distribution and their dynamics across the Scotia Sea. We also need to consider the larger scale physical processes affecting the region (such as ACC, ENSO and Southern Annular Mode variability), as well as the biological processes (such as krill transport processes and predator migration) and their interactions (figure 12; Murphy et al. 1988, 1998b, submitted; Constable et al. 2003; Fraser & Hofmann 2003; Ainley et al. 2005; see also Clarke et al. 2007a,b). This will be crucial in developing models of the response of the system to change and in developing long-term sustainable approaches to management of exploitation. This is a key region in global fisheries and an area where the potential for expansion of the demand of fisheries in the coming years is generating real concern, particularly among scientists involved in developing management procedures within the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR). It is urgent that a comprehensive understanding of the operation of this regional ecosystem is developed. The Scotia Sea system is changing so quickly that in a short time regions in the north may not be influenced by polar waters and could undergo rapid changes in ecosystem structure.
7. Concluding comments
The food web of the Scotia Sea is highly heterogeneous, widely distributed but dynamically connected. The fundamental determinant of the operation of the Scotia Sea ecosystem is the ocean circulation and its interaction with the Scotia Arc. The interaction of the flow with the regional topography generates a highly energetic environment of intense vertical and horizontal mixing. The ecosystem is therefore dominated by the flows of the major current systems (the ACC and the WSC), but it is also strongly influenced by the seasonality manifest most clearly by the advance of sea ice across the region during winter. This combination of mixing and seasonality generates a unique environment in the ACC which is high in both nutrients and chlorophyll a. The generation of megablooms of large diatoms is the result of the flow of the ACC across the Scotia Arc, affecting macro- and micro-nutrient concentrations. These blooms are a particularly consistent feature across the northern Scotia Sea towards the PF. This production fuels the food web, and krill are particularly reliant on the development of these large diatom blooms during spring. Krill are the major link between lower trophic level production and consumption by higher trophic level predators across the Scotia Sea. The regional food web, which has developed in this dispersive and seasonal context, is therefore highly distributed with production at higher trophic levels maintained by advective flows. The advective regime that disperses krill is therefore a fundamental factor in determining the structure of the whole ecosystem. Energy is generated in restricted regions in spring, particularly in areas of the retreating ice edge in the south. This energy is dispersed across the region and over the summer to be used in more northern regions in areas where large numbers of predators are concentrated during breeding.
The role of krill in the ecosystem is crucial, not only owing to their high abundance, but also because of a number of biological characteristics that make them a major prey item for many of the predators. Krill are heterogeneously distributed over a wide range of scales from tens of metres to hundreds of kilometres. This makes them available as prey over very different scales, so that predators of very different size and foraging strategies can all exploit krill as food. This heterogeneity generates a highly spatially structured and variable ecosystem in which food-web connections involve complex spatial as well as temporal and trophic interactions. Relative to other species of zooplankton, krill attain a large maximum size (approx. 60 mm) and are long lived (5–7 years). This makes them available to large-bodied predators over extended periods, especially during periods of reduced primary production. This longevity is therefore important in allowing them to be dispersed across the Scotia Sea, surviving through winter and across low production regions, connecting regions of production with remote areas of consumption. Krill production and development are not limited to the shelf areas of the Scotia Sea and the central Scotia Sea area is a key region for overwintering of post-larval and larval krill. The interaction with the sea ice during winter and spring will be crucial in determining survival and dispersal during summer. The retreating ice edge across the Scotia Sea generates high productivity which drives the regional production of krill. The MIZ across the southern Scotia Sea in winter and spring is therefore a key habitat for krill, where production of both sea ice algae and phytoplankton will occur during winter and spring. The sea ice is therefore a link between the southern and northern Scotia Sea and between winter and summer.
The importance of krill to the higher trophic level predators means that any examination of Scotia Sea food webs requires not only descriptions of distribution, abundance and production, but also detailed knowledge of the life cycles of key species. The debate about whether advection is important in transporting krill has developed to focus on quantifying the relative roles of these different processes and the factors controlling their operation. This requires a specific focus on key stages of the life history of krill, particularly the larval and juvenile phases in oceanic waters during spring. Developing the models of krill requires detailed analyses of the operation of the life cycle. This will require coupled physical–biological models that involve not only the oceanic system but also the sea ice. Taking account of the interactions of krill at different scales will be important and will require a multi-scale modelling strategy that links behavioural and population processes with physical models which can resolve appropriate physical processes. In particular, this will require high-resolution shelf models embedded in lower resolution oceanic models.
To develop a wider understanding of the dynamics of the food web, the detailed life cycle operation of krill and other key species will need to be analysed in the context of the regional food-web interactions. Improving knowledge of winter processes across the Scotia Sea is crucial, as it is gaining more information on poorly studied species or groups such as the fishes (especially mesopelagics), squid, crabeater seals and whales. Developing studies of the importance of alternative pathways involving groups such as copepods and myctophid fish will also be valuable. The importance of large-scale physical and biological interactions in determining the dynamics of the Scotia Sea ecosystem has also highlighted that any analyses and modelling must take account of the wider circumpolar and southern hemisphere atmospheric, oceanic and biospheric connections (figure 12). The Scotia Sea ecosystem is highly spatially and temporally variable, so future research effort aimed at understanding the effects of a changing environment must be focused on aspects of the food web that determine long-term behaviour of the system. To deal with the complexity, we need to focus effort on analyses of food-web structure that centre on the dominant energy flow pathway (through krill) and some of the alternative weaker pathways to higher predators. These links will be crucial in determining the operation of the food web and its long-term dynamics (Rooney et al. 2006).
In analysing the Scotia Sea ecosystem, the biggest challenge we face is to determine what has happened in the last two centuries of major ecological change. This requires detailed analyses of long-term datasets and the development of models that account for the spatial and temporal complexity of the ecosystem operation. This will allow the development of models to predict the responses of Southern Ocean ecosystems to change and procedures for the sustainable exploitation of resources. Developing such models is an urgent requirement because we know that there is rapid regional change occurring in the ocean and sea ice. There is clear evidence that there was a stepwise change in the physical regime of the Scotia Sea during the last century. This change has occurred simultaneously with the ecological changes driven by harvesting. Ecological systems are dynamic and particular states represent dynamic equilibria or transient effects, so they are always varying and changing. However, the rapid changes over the last 20–30 years on top of the changes that have occurred in the last two centuries may already be driving the Scotia Sea ecosystem into a very different operational state. It is likely that we will see major changes over the next 50 years, with the potential for extremely rapid and locally catastrophic changes in species distribution and abundance across the northern Scotia Sea.
8. Summary
The ecosystem of the Scotia Sea and the Antarctic Peninsula region is undergoing some of the most rapid regional environmental and ecological change in any area of the ocean.
Advection and the interaction of the circulation with the regional bathymetry generate intense mixing and are major factors determining the structure of the Scotia Sea ecosystem.
Interactions of the circulation with the regional topography probably generate elevated concentrations of iron in regions of high macronutrients. This fuels regular blooms of extended duration (more than one month) across areas around the northern Scotia Sea. In the south, blooms are more irregular and associated with areas of melt water stabilization, following the spring retreat of the sea ice, or with shallow areas of the Scotia Arc. The variability of the sea ice system and its spring retreat results in highly variable productivity.
Krill are a long-lived and key species in the food web, maintaining the majority of higher trophic level production. The dynamics of their population operates across the Scotia Sea and is linked to adjacent regions of the Weddell Sea and Antarctic Peninsula. Analyses of the operation of the Scotia Sea food web require a detailed understanding of the spatial and temporal dynamics of krill populations.
Krill occur in predictable densities across the Scotia Arc. High concentrations of krill also occur across the central oceanic regions of the Scotia Sea in areas seasonally covered by sea ice. The drifting MIZ of the central Scotia Sea is likely to favour ice-associated production, and its relatively low latitude for a polar region means that the light cycle will fuel production even during winter. These central sea ice-covered regions will be a key overwintering and spring habitat for krill, connecting areas further south with regions to the north.
There is marked variability in the spatial structure of the food web across the Scotia Sea. There is also marked temporal variation in the connections within the food web. During winter many of the higher predators disperse or leave the region, reducing the energetic requirements at higher trophic levels.
The food-web structure is maintained by horizontal advection of energy, for which krill are the key vector. Biological dispersals and active movements further maintain the regional food-web structure.
There is clear evidence of top-down control effects of grazers on the primary production systems. Grazing and predatory impacts on the plankton affect the dynamics of the plankton community, and shifts between krill and copepods affect the regional production and phytoplankton community development.
There is also evidence that top-down control is exerted by higher predators on macro-plankton in shelf regions. High local demand for krill reduces density and variance in the distribution of krill, which in turn affects plankton dynamics.
There is marked interannual variability in the operation of the Scotia Sea ecosystem that is driven by changes in regional sea ice and SST conditions that are linked to hemispheric-scale variations (linked to ENSO).
These variations affect the population dynamics and dispersal of krill across the Scotia Sea during spring and summer. This generates a reduction in the recruitment of krill into northern regions during warm periods.
The removal of the large seal and whale predators over the past two centuries has undoubtedly generated long-term top-down cascade effects and modified the local plankton populations. These effects are probably continuing today and will affect the interpretation of the ecosystem responses to change.
There has been marked regional climate and oceanic change over the last 20 years. A rapid change occurred in the duration of winter sea ice across the Scotia Sea between the first and the second half of the last century.
A decline in krill abundance has been linked to changes in sea ice, but these changes are confounded by ecological shifts in the predators.
The Scotia Sea ecosystem has many key features that make it ideal for examining the effects of harvesting and climate change on processes in large-scale oceanic ecosystems, from primary production through to the highest level predators.
Acknowledgments
We are grateful to our many colleagues and national and international collaborators who have worked with us on many of these topics. We thank the BAS logistical and technical support teams, the officers and crew of the RRS James Clark Ross and all those involved with Bird Island, South Georgia. This paper is a contribution to the BAS DYNAMOE and DISCOVERY 2010 Programmes. We are also grateful for the comments of two anonymous referees, which greatly improved the manuscript.
Footnotes
One contribution of 8 to a Theme Issue ‘Antarctic ecology: from genes to ecosystems. I.’
References
- Ackley S.F, Sullivan C.W. Physical controls on the development and characteristics of Antarctic sea ice biological communities—a review and synthesis. Deep Sea Res. Part I Oceanogr. Res. Pap. 1994;41:1583–1604. doi:10.1016/0967-0637(94)90062-0 [Google Scholar]
- Ainley D.G, Fraser W.R, W O, Smith J, Hopkins T.L, Torres J.J. The structure of upper level pelagic food webs in the Antarctic: effect of phytoplankton distribution. J. Mar. Syst. 1991;2:111–122. doi:10.1016/0924-7963(91)90017-O [Google Scholar]
- Ainley D.G, Clarke E.D, Arrigo K, Fraser W.R, Kato A, Barton K.J, Wilson P.R. Decadal-scale changes in the climate and biota of the Pacific sector of the Southern Ocean, 1950s to the 1990s. Antarct. Sci. 2005;17:171–182. doi:10.1017/S0954102005002567 [Google Scholar]
- Allison I. Physical processes determining the Antarctic sea ice environment. Aust. J. Phys. 1997;50:759–771. doi:10.1071/P96113 [Google Scholar]
- Angel, M. V. 1994 Spatial distribution of marine organisms: patterns and processes. In Large-scale ecology and conservation biology (ed. P. J. Edwards, R. M. May & N. R. Webb), pp. 59–109. Oxford, UK: Blackwell Scientific Publications.
- Arhan M, Garabato A.C.N, Heywood K.J, Stevens D.P. The Antarctic circumpolar current between the Falkland Islands and South Georgia. J. Phys. Oceanogr. 2002;32:1914–1931. doi:10.1175/1520-0485(2002)032<1914:TACCBT>2.0.CO;2 [Google Scholar]
- Atkinson A, Sinclair J.D. Zonal distribution and seasonal vertical migration of copepod assemblages in the Scotia Sea. Polar Biol. 2000;23:46–58. doi:10.1007/s003000050007 [Google Scholar]
- Atkinson A, Snyder R. Krill–copepod interactions at South Georgia, Antarctica, I. Omnivory by Euphausia superba. Mar. Ecol. Prog. Ser. 1997;160:63–76. [Google Scholar]
- Atkinson A, Whitehouse M.J. Ammonium excretion by Antarctic krill Euphausia superba at South Georgia. Limnol. Oceanogr. 2000;45:55–63. [Google Scholar]
- Atkinson A, Shreeve R.S, Pakhomov E.A, Priddle J, Blight S.P, Ward P. Zooplankton response to a phytoplankton bloom near South Georgia, Antarctica. Mar. Ecol. Prog. Ser. 1996;144:195–210. [Google Scholar]
- Atkinson A, SchnackSchiel S.B, Ward P, Marin V. Regional differences in the life cycle of Calanoides acutus (Copepoda: Calanoida) within the Atlantic sector of the Southern Ocean. Mar. Ecol. Prog. Ser. 1997;150:99–111. [Google Scholar]
- Atkinson A, Ward P, Hill A, Brierley A.S, Cripps G.C. Krill–copepod interactions at South Georgia, Antarctica, II. Euphausia superba as a major control on copepod abundance. Mar. Ecol. Prog. Ser. 1999;176:63–79. [Google Scholar]
- Atkinson A, Whitehouse M.J, Priddle J, Cripps G.C, Ward P, Brandon M.A. South Georgia, Antarctica: a productive, cold water, pelagic ecosystem. Mar. Ecol. Prog. Ser. 2001;216:279–308. [Google Scholar]
- Atkinson A, Meyer B, Stubing D, Hagen W, Schmidt K, Bathmann U.V. Feeding and energy budgets of Antarctic krill Euphausia superba at the onset of winter: II. Juveniles and adults. Limnol. Oceanogr. 2002;47:953–966. [Google Scholar]
- Atkinson A, Siegel V, Pakhomov E, Rothery P. Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature. 2004;432:100–103. doi: 10.1038/nature02996. doi:10.1038/nature02996 [DOI] [PubMed] [Google Scholar]
- Atkinson A, Shreeve R.S, Hirst A.G, Rothery P, Tarling G.A, Pond D.W, Korb R.E, Murphy E.J, Watkins J.L. Natural growth rates in Antarctic krill (Euphausia superba): II. Predictive models based on food, temperature, body length, sex, and maturity stage. Limnol. Oceanogr. 2006;51:973–987. [Google Scholar]
- Bak R.P.M, Boldrin A, Nieuwland G, Rabitti S. Biogenic particles and nano picoplankton in water masses over the Scotia-Weddell Sea Confluence, Antarctica. Polar Biol. 1992;12:219–224. doi:10.1007/BF00238263 [Google Scholar]
- Barlow K.E, Boyd I.L, Croxall J.P, Reid K, Staniland I.J, Brierley A.S. Are penguins and seals in competition for Antarctic krill at South Georgia? Mar. Biol. 2002;140:205–213. doi:10.1007/s00227-001-0691-7 [Google Scholar]
- Bathmann, U., Priddle, J., Treguer, P., Lucas, M., Hall, J. & Parslow, J. 2000 Plankton ecology and biogeochemistry in the Southern Ocean: a review of the Southern Ocean JGOFS. In The changing ocean carbon cycle: a midterm synthesis of the joint global ocean flux study, vol. 5 (ed. R. Hanson, H. Ducklow & J. G. Field), pp. 300–337. Cambridge, UK: Cambridge University Press.
- Becquevort S, Mathot S, Lancelot C. Interactions in the microbial community of the marginal ice zone of the northwestern Weddell Sea through size distribution analysis. Polar Biol. 1992;12:211–218. doi:10.1007/BF00238262 [Google Scholar]
- Bianchi F, et al. Phytoplankton distribution in relation to sea ice, hydrography and nutrients in the northwestern Weddell Sea in early spring 1988 during EPOS. Polar Biol. 1992;12:225–235. doi:10.1007/BF00238264 [Google Scholar]
- Bocher P, Cherel Y, Labat J.P, Mayzaud P, Razouls S, Jouventin P. Amphipod-based food web: Themisto gaudichaudii caught in nets and by seabirds in Kerguelen waters, southern Indian Ocean. Mar. Ecol. Prog. Ser. 2001;223:261–276. [Google Scholar]
- Boyd I.L. Temporal scales of foraging in a marine predator. Ecology. 1996;77:426–434. doi:10.2307/2265619 [Google Scholar]
- Boyd I.L. Foraging and provisioning in Antarctic fur seals: interannual variability in time-energy budgets. Behav. Ecol. 1999;10:198–208. doi:10.1093/beheco/10.2.198 [Google Scholar]
- Boyd I.L. Estimating food consumption of marine predators: Antarctic fur seals and macaroni penguins. J. Appl. Ecol. 2002a;39:103–119. doi:10.1046/j.1365-2664.2002.00697.x [Google Scholar]
- Boyd I.L. The krill eating crabeater seal. In: Macdonald D.W, editor. Encyclopedia of mammals. Academic Press; New York, NY: 2002b. [Google Scholar]
- Boyd P.W. The role of iron in the biogeochemistry of the Southern Ocean and equatorial Pacific: a comparison of in situ iron enrichments. Deep Sea Res. Part II: Topical Stud. Oceanogr. 2002c;49:1803–1821. doi:10.1016/S0967-0645(02)00013-9 [Google Scholar]
- Boyd I.L, Murray A.W.A. Monitoring a marine ecosystem using responses of upper trophic level predators. J. Anim. Ecol. 2001;70:747–760. doi:10.1046/j.0021-8790.2001.00534.x [Google Scholar]
- Boyd I.L, Arnould J.P.Y, Barton T, Croxall J.P. Foraging behavior of Antarctic fur seals during periods of contrasting prey abundance. J. Anim. Ecol. 1994;63:703–713. doi:10.2307/5235 [Google Scholar]
- Boyd I.L, McCafferty D.J, Reid K, Taylor R, Walker T.R. Dispersal of male and female Antarctic fur seals (Arctocephalus gazella) Can. J. Fish. Aquat. Sci. 1998;55:845–852. doi:10.1139/cjfas-55-4-845 [Google Scholar]
- Boyd I.L, Staniland I.J, Martin A.R. Spatial distribution of foraging by female Antarctic fur seals. Ecology. 2002;242:285–294. [Google Scholar]
- Brandon M.A, Murphy E.J, Whitehouse M.J, Trathan P.N, Murray A.W.A, Bone D.G, Priddle J. The shelf break front to the east of the sub-Antarctic island of South Georgia. Cont. Shelf Res. 1999;19:799–819. doi:10.1016/S0278-4343(98)00112-5 [Google Scholar]
- Brandon M.A, Murphy E.J, Trathan P.N, Bone D.G. Physical oceanographic conditions to the northwest of the sub-Antarctic Island of South Georgia. J. Geophys. Res. Oceans. 2000;105:23 983–23 996. doi:10.1029/2000JC900098 [Google Scholar]
- Brandon M.A, et al. Physical oceanography in the Scotia Sea during the CCAMLR 2000 survey, austral summer 2000. Deep Sea Res. Part II Topical Stud. Oceanogr. 2004;51:1301–1321. doi:10.1016/j.dsr2.2004.06.006 [Google Scholar]
- Brierley A.S, Watkins J.L, Murray A.W.A. Interannual variability in krill abundance at South Georgia. Mar. Ecol. Prog. Ser. 1997;150:87–98. [Google Scholar]
- Brierley A.S, Demer D.A, Watkins J.L, Hewitt R.P. Concordance of interannual fluctuations in acoustically estimated densities of Antarctic krill around South Georgia and Elephant Island: biological evidence of same-year teleconnections across the Scotia Sea. Mar. Biol. 1999;134:675–681. doi:10.1007/s002270050583 [Google Scholar]
- Brierley A.S, et al. Antarctic krill under sea ice: elevated abundance in a narrow band just south of ice edge. Science. 2002a;295:1890–1892. doi: 10.1126/science.1068574. doi:10.1126/science.1068574 [DOI] [PubMed] [Google Scholar]
- Brierley A.S, et al. Significant intra-annual variability in krill distribution and abundance at South Georgia revealed by multiple acoustic surveys during 2000/01. CCAMLR Sci. 2002b;9:71–82. [Google Scholar]
- Brinton E. The oceanographic structure of the eastern Scotia Sea—III. Distributions of euphausiid species and their developmental stages in 1981 in relation to hydrography. Deep Sea Res. 1985;32:1153–1180. doi:10.1016/0198-0149(85)90001-9 [Google Scholar]
- Cadee G.C, Gonzalez H, Schnackschiel S.B. Krill diet affects fecal string settling. Polar Biol. 1992;12:75–80. [Google Scholar]
- Candy S.G, Kawaguchi S. Modelling growth of Antarctic krill. II. Novel approach to describing the growth trajectory. Mar. Ecol. Prog. Ser. 2006;306:17–30. [Google Scholar]
- Catry P, Phillips R.A, Phalan B, Silk J.R.D, Croxall J.P. Foraging strategies of grey-headed albatrosses Thalassarche chrysostoma: integration of movements, activity and feeding events. Mar. Ecol. Prog. Ser. 2004;280:261–273. [Google Scholar]
- Clarke A. Energy flow in the Southern Ocean food web. In: Siegfried W.R, Condy P.R, Laws R.M, editors. Fourth SCAR symposium on Antarctic biology, Antarctic nutrient cycles and food webs. Springer; Wilderness, South Africa: 1985a. pp. 573–581. [Google Scholar]
- Clarke A. Food webs and interactions: and overview of the Antarctic system. In: Bonner W.N, Walton D.W.H, editors. Antarctica. Permagon Press Ltd; Oxford, UK: 1985b. p. 381. [Google Scholar]
- Clarke A. Seasonality in the Antarctic marine environment. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 1988;90:461–473. doi:10.1016/0305-0491(88)90285-4 [Google Scholar]
- Clarke A, Leakey R.J.G. The seasonal cycle of phytoplankton, macronutrients, and the microbial community in a nearshore Antarctic marine ecosystem. Limnol. Oceanogr. 1996;41:1281–1294. [Google Scholar]
- Clarke A, Johnston N.M, Murphy E.J, Rogers A.D. Antarctic ecology from genes to ecosystems: the impact of climate change and the importance of scale. Phil. Trans. R. Soc. B. 2007a;362:5–9. doi: 10.1098/rstb.2006.1943. doi:10.1098/rstb.2006.1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A, Murphy E.J, Meredith M.P, King J.C, Peck L.S, Barnes D.K.A, Smith R.C. Climate change and the marine ecosystem of the western Antarctic Peninsula. Phil. Trans. R. Soc. B. 2007b;362:149–166. doi: 10.1098/rstb.2006.1958. doi:10.1098/rstb.2006.1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M.A, Rodhouse P.G. Southern Ocean cephalopods. Adv. Mar. Biol. 2006;50:193–265. doi: 10.1016/S0065-2881(05)50003-8. [DOI] [PubMed] [Google Scholar]
- Comiso J.C, McClain C.R, Sullivan C.W, Ryan J.P, Leonard C.L. Coastal zone color scanner pigment concentrations in the Southern-Ocean and relationships to geophysical surface-features. J. Geophys. Res. Oceans. 1993;98:2419–2451. [Google Scholar]
- Constable A.J, de la Mare W.K, Agnew D.J, Everson I, Miller D. Managing fisheries to conserve the Antarctic marine ecosystem: practical implementation of the Convention on the Conservation of Antarctic Marine Living Resources (CCAMLR) Ices J. Mar. Sci. 2000;57:778–791. doi:10.1006/jmsc.2000.0725 [Google Scholar]
- Constable A.J, Nicol S, Strutton P.G. Southern Ocean productivity in relation to spatial and temporal variation in the physical environment. J. Geophys. Res. Oceans. 2003;108 art. no.-8079. [Google Scholar]
- Cota G.F, Smith W.O, Nelson D.M, Muench R.D, Gordon L.I. Nutrient and biogenic particulate distributions, primary productivity and nitrogen uptake in the Weddell–Scotia Sea marginal ice-zone during winter. J. Mar. Res. 1992;50:155–181. [Google Scholar]
- Croxall J.P. Southern-Ocean environmental-changes—effects on Seabird, seal and whale populations. Phil. Trans. R. Soc. B. 1992;338:319–328. [Google Scholar]
- Croxall J.P, Briggs D.R. Foraging economics and performance of polar and subpolar Atlantic seabirds. Polar Res. 1991;10:561–578. [Google Scholar]
- Croxall J.P, Ricketts C, Prince P.A. The impacts of seabirds on marine resources, especially krill, at South Georgia. In: Whittow G.C, Rahn H, editors. Seabird energetics. Plenum; New York, NY: 1984. pp. 285–318. [Google Scholar]
- Croxall J.P, Prince P.A, Ricketts C. Relationship between prey life cycles and the extent, nature and timing of seal and seabird predation in the Scotia Sea. In: Siegfried W.R, Condy P.R, Laws R.M, editors. Antarctic nutrient cycles and food webs. Springer; Berlin, Germany: 1985. pp. 137–146. [Google Scholar]
- Croxall J.P, McCann T.S, Prince P.A, Rothery P. Reproductive performance of seabirds and seals at South Georgia and Signy Island, South Orkney Islands, 1976–1987: implicatyions for Southerrn Ocean monitoring studies. In: Sahrhage D, editor. Antarctic Ocean resources variability. Springer; Berlin, Germany: 1988. pp. 261–285. [Google Scholar]
- Croxall J.P, Prince P.A, Reid K. Dietary segregation of krill-eating South Georgia seabirds. J. Zool. 1997;242:531–556. [Google Scholar]
- Croxall J.P, Reid K, Prince P.A. Diet, provisioning and productivity responses of marine predators to differences in availability of Antarctic krill. Mar. Ecol. Prog. Ser. 1999;177:115–131. [Google Scholar]
- Croxall J.P, Silk J.R.D, Phillips R.A, Afanasyev V, Briggs D.R. Global circumnavigations: tracking year-round ranges of nonbreeding albatrosses. Science. 2005;307:249–250. doi: 10.1126/science.1106042. doi:10.1126/science.1106042 [DOI] [PubMed] [Google Scholar]
- Cunningham S.A, Alderson S.G, King B.A, Brandon M.A. Transport and variability of the Antarctic circumpolar current in Drake Passage. J. Geophys. Res. Oceans. 2003:108. [Google Scholar]
- Daly K.L. Overwintering growth and development of larval Euphausia superba: an interannual comparison under varying environmental conditions west of the Antarctic Peninsula. Deep Sea Res. Part II Topical Stud. Oceanogr. 2004;51:2139–2168. doi:10.1016/j.dsr2.2004.07.010 [Google Scholar]
- Daly K.L, Macaulay M.C. Influence of physical and biological mesoscale dynamics on the seasonal distribution and behavior of Euphausia superba in the Antarctic marginal ice zone. Mar. Ecol. Prog. Ser. 1991;79:37–66. [Google Scholar]
- de Baar H.J.W, de Jong J.T.M, Bakker D.C.E, Loscher B.M, Veth C, Bathmann U, Smetacek V. Importance of iron for plankton blooms and carbon-dioxide drawdown in the Southern-Ocean. Nature. 1995;373:412–415. doi:10.1038/373412a0 [Google Scholar]
- Demer D.A, Conti S.G. New target-strength model indicates more krill in the Southern Ocean. Ices J. Mar. Sci. 2005;62:25–32. doi:10.1016/j.icesjms.2004.07.027 [Google Scholar]
- de la Mare W.K. Abrupt mid-twentieth-century decline in Antarctic sea-ice extent from whaling records. Nature. 1997;389:57–60. doi:10.1038/37956 [Google Scholar]
- deYoung B, Heath M, Werner F, Chai F, Megrey B, Monfray P. Challenges of modeling ocean basin ecosystems. Science. 2004;304:1463–1466. doi: 10.1126/science.1094858. doi:10.1126/science.1094858 [DOI] [PubMed] [Google Scholar]
- Dickson J, Morley S.A, Mulvey T. New data on Martialia hyadesi feeding in the Scotia Sea during winter; with emphasis on seasonal and annual variability. J. Mar. Biol. Assoc. UK. 2004;84:785–788. doi:10.1017/S0025315404009944h [Google Scholar]
- Dinniman M.S, Klinck J.M. A model study of circulation and cross-shelf exchange on the west Antarctic Peninsula continental shelf. Deep Sea Res. Part II Topical Stud. Oceanogr. 2004;51:2003–2022. doi:10.1016/j.dsr2.2004.07.030 [Google Scholar]
- Everson I. FAO; Rome, Italy: 1977. The living resources of the Southern Ocean. [Google Scholar]
- Everson I. In: Marine interactions. Antarctic ecology. Laws R.M, editor. vol. 1. Academic Press; London, UK: 1984. pp. 783–820. [Google Scholar]
- Everson I. Southern Ocean fisheries. In: Steele J, Turekian K, Thorpe S.A, editors. Encyclopedia of ocean sciences. Academic Press; San Diego, CA: 2001. pp. 2858–2865. [Google Scholar]
- Everson I, Kock K.H. Variations in condition indices of mackerel icefish at South Georgia from 1972 to 1997. CCAMLR Sci. 2001;8:119–132. [Google Scholar]
- Everson I, Kock K.H, Parkes G. Interannual variation in condition of the mackerel icefish. J. Fish Biol. 1997;51:146–154. doi: 10.1111/j.1095-8649.1997.tb02520.x. doi:10.1111/j.1095-8649.1997.tb02520.x [DOI] [PubMed] [Google Scholar]
- Everson I, Parkes G, Kock K.H, Boyd I.L. Variation in standing stock of the mackerel icefish Champsocephalus gunnari at South Georgia. J. Appl. Ecol. 1999;36:591–603. doi:10.1046/j.1365-2664.1999.00425.x [Google Scholar]
- Everson I, Agnew D.J, Miller D.G.M. Krill fisheries and the future. In: Everson I, editor. Krill: biology, ecology and fisheries. Blackwell Science; Oxford, UK: 2000a. pp. 345–348. [Google Scholar]
- Everson I, Kock K.H, Ellison J. Inter-annual variation in the gonad cycle of the mackerel icefish. J. Fish Biol. 2000b;57:103–111. doi:10.1111/j.1095-8649.2000.tb02247.x [Google Scholar]
- Fach B.A, Klinck J.M. Transport of Antarctic krill (Euphausia superba) across the Scotia Sea. Part I: circulation and particle tracking simulations. Deep Sea Res. 2006;53:987–1010. doi:10.1016/j.dsr.2006.03.006 [Google Scholar]
- Fach B.A, Hofmann E.E, Murphy E.J. Modeling studies of antarctic krill Euphausia superba survival during transport across the Scotia Sea. Mar. Ecol. Prog. Ser. 2002;231:187–203. [Google Scholar]
- Fach B.A, Hofmann E.E, Murphy E.J. Transport of Antarctic krill (Euphausia superba) across the Scotia Sea. Part II: krill growth and survival. Deep Sea Res. 2006;53:1011–1043. doi:10.1016/j.dsr.2006.03.007 [Google Scholar]
- Fedulov P.P, Murphy E.J, Shulgovsky K.E. Environment–krill relations in the South Georgia marine ecosystem. CCAMLR Sci. 1996;3:13–30. [Google Scholar]
- Figueiras F.G, Perez F.F, Pazos Y, Rios A.F. Light and productivity of Antarctic phytoplankton during austral summer in an ice-edge region in the Weddell–Scotia Sea. J. Plankton Res. 1994;16:1459–1459. [Google Scholar]
- Forcada J, Trathan P.N, Reid K, Murphy E.J. The effects of global climate variability in pup production of Antarctic fur seals. Ecology. 2005;86:2408–2417. [Google Scholar]
- Forcada J, Trathan P.N, Reid K, Murphy E.J, Croxall J.P. Contrasting population changes in sympatric penguin species in association with climate warming. Global Change Biol. 2006;12:411–423. doi:10.1111/j.1365-2486.2006.01108.x [Google Scholar]
- Fortier L, Lefevre J, Legendre L. Export of biogenic carbon to fish and to the deep-ocean—the role of large planktonic microphages. J. Plankton Res. 1994;16:809–839. [Google Scholar]
- Foxton P. The distribution and life-history of Salpa thompsoni Foxton species, Salpa gerlachei Foxton. Discov. Rep. 1966;34:1–116. [Google Scholar]
- Fraser W.R, Hofmann E.E. A predator's perspective on causal links between climate change, physical forcing and ecosystem response. Mar. Ecol. Prog. Ser. 2003;265:1–15. [Google Scholar]
- Fraser W.R, Trivelpiece W.Z, Ainley D.G, Trivelpiece S.G. Increases in Antarctic penguin populations—reduced competition with whales or a loss of sea ice due to environmental warming. Polar Biol. 1992;11:525–531. doi:10.1007/BF00237945 [Google Scholar]
- Garrison D.L, Buck K.R. Surface-layer sea ice assemblages in Antarctic pack ice during the austral spring—environmental conditions, primary production and community structure. Mar. Ecol. Prog. Ser. 1991;75:161–172. [Google Scholar]
- Garrison D.L, Close A.R. Winter ecology of the sea-ice biota in Weddell sea pack ice. Mar. Ecol. Prog. Ser. 1993;96:17–31. [Google Scholar]
- Giesenhagen H.C, Detmer A.E, de Wall J, Weber A, Gradinger R.R, Jochem F.J. How are Antarctic planktonic microbial food webs and algal blooms affected by melting of sea ice? Primary production and community structure. Aquat. Microb. Ecol. 1999;20:183–201. [Google Scholar]
- Gilpin L.C, Priddle J, Whitehouse M.J, Savidge G, Atkinson A. Primary production and carbon uptake dynamics in the vicinity of South Georgia—balancing carbon fixation and removal. Mar. Ecol. Prog. Ser. 2002;242:51–62. [Google Scholar]
- Gonzalez H.E. The distribution and abundance of krill fecal material and oval pellets in the Scotia and Weddell Seas (Antarctica) and their role in particle-flux. Polar Biol. 1992;12:81–91. doi:10.1007/BF00239968 [Google Scholar]
- Gordon J.E, Timmis R.J. Glacier fluctuations on South-Georgia during the 1970s and early 1980s. Antarct. Sci. 1992;4:215–226. [Google Scholar]
- Gowing M.M, Garrison D.L. Abundance and feeding ecology of larger protozooplankton in the ice edge zone of the Weddell and Scotia Seas during the austral winter. Deep Sea Res. Part a Oceanogr. Res. Pap. 1992;39:893–919. doi:10.1016/0198-0149(92)90128-G [Google Scholar]
- Grossmann S. Bacterial activity in sea ice and open water of the Weddell Sea, Antarctica—a microautoradiographic study. Microb. Ecol. 1994;28:1–18. doi: 10.1007/BF00170244. doi:10.1007/BF00170244 [DOI] [PubMed] [Google Scholar]
- Grossmann S, Dieckmann G.S. Bacterial standing stock, activity, and carbon production during formation and growth of sea-ice in the Weddell Sea, Antarctica. Appl. Environ. Microbiol. 1994;60:2746–2753. doi: 10.1128/aem.60.8.2746-2753.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy A. Great waters. A voyage of natural history to study whales, plankton and the waters of the Southern Ocean in the old Royal Research Ship Discovery with the results brought up to date by the findings of the R.R.S. Discovery II. Collins; London, UK: 1967. [Google Scholar]
- Harms S, Fahrbach E, Strass V.H. Sea ice transports in the Weddell Sea. J. Geophys. Res. Oceans. 2001;106:9057–9073. doi:10.1029/1999JC000027 [Google Scholar]
- Hart T.J. In Discovery reports. vol. 21. Cambridge University Press; Cambridge, UK: 1942. Phytoplankton periodicity in Antarctic surface waters; pp. 263–348. [Google Scholar]
- Hempel G. Antarctic marine food webs. In: Siegfried W.R, Condy P.R, Laws R.M, editors. Fourth SCAR Symp. on Antarctic biology, Antarctic nutrient cycles and food webs. Springer; Wilderness, South Africa: 1985a. pp. 266–270. [Google Scholar]
- Hempel G. On the biology of polar seas, particularly the Southern Ocean. In: Gray J.S, Christiansen M.E, editors. Marine biology of polar regions and effects of stress on marine organisms. Wiley; Chichester, UK: 1985b. pp. 3–33. [Google Scholar]
- Hewitt R.P, et al. Biomass of Antarctic krill in the Scotia Sea in January/February 2000 and its use in revising an estimate of precautionary yield. Deep Sea Res. Part II Topical Stud. Oceanogr. 2004;51:1215–1236. doi:10.1016/j.dsr2.2004.06.011 [Google Scholar]
- Heywood K.J, Garabato A.C.N, Stevens D.P, Muench R.D. On the fate of the Antarctic Slope Front and the origin of the Weddell front. J. Geophys. Res. Oceans. 2004;109 art. no.-C06021. [Google Scholar]
- Hofmann E.E, Husrevoglu Y.S. A circumpolar modeling study of habitat control of Antarctic krill (Euphausia superba) reproductive success. Deep Sea Res. Part II Topical Stud. Oceanogr. 2003;50:3121–3142. doi:10.1016/j.dsr2.2003.07.012 [Google Scholar]
- Hofmann E.E, Murphy E.J. Advection, krill, and Antarctic marine ecosystems. Antarct. Sci. 2004;16:487–499. doi:10.1017/S0954102004002275 [Google Scholar]
- Hofmann E.E, Klinck J.M, Locarnini R.A, Fach B, Murphy E. Krill transport in the Scotia Sea and environs. Antarct. Sci. 1998;10:406–415. [Google Scholar]
- Holeton C.L, Nedelec F, Sanders R, Brown L, Moore C.M, Stevens D.P, Heywood K.J, Statham P.J, Lucas C.H. Physiological state of phytoplankton communities in the Southwest Atlantic sector of the Southern Ocean, as measured by fast repetition rate fluorometry. Polar Biol. 2005;29:44–52. doi:10.1007/s00300-005-0028-y [Google Scholar]
- Holm-Hansen O, et al. Temporal and spatial distribution of chlorophyll-a in surface waters of the Scotia Sea as determined by both shipboard measurements and satellite data. Deep Sea Res. Part II Topical Stud. Oceanogr. 2004a;51:1323–1331. doi:10.1016/j.dsr2.2004.06.004 [Google Scholar]
- Holm-Hansen O, et al. Factors influencing the distribution, biomass, and productivity of phytoplankton in the Scotia Sea and adjoining waters. Deep Sea Res. Part II Topical Stud. Oceanogr. 2004b;51:1333–1350. doi:10.1016/j.dsr2.2004.06.015 [Google Scholar]
- Holm-Hansen O, Kahru M, Hewes C.D. Deep chlorophyll a maxima (DCMs) in pelagic Antarctic waters. II. Relation to bathymetric features and dissolved iron concentrations. Mar. Ecol. Prog. Ser. 2005;297:71–81. [Google Scholar]
- Hopkins T.L, Ainley D.G, Torres J.J, Lancraft T.M. Trophic structure in open waters of the marginal ice-zone in the Scotia-Weddell Confluence region during spring (1983) Polar Biol. 1993a;13:389–397. doi:10.1007/BF01681980 [Google Scholar]
- Hopkins T.L, Lancraft T.M, Torres J.J, Donnelly J. Community structure and trophic ecology of zooplankton in the Scotia Sea marginal ice-zone in winter (1988) Deep Sea Res. Part I Oceanogr. Res. Pap. 1993b;40:81–105. doi:10.1016/0967-0637(93)90054-7 [Google Scholar]
- Hughes C.W, Woodworth P.L, Meredith M.P, Stepanov V, Whitworth T, III, Pyne A. Coherence of Antarctic sea levels, southern hemisphere annular mode, and flow through Drake Passage. Geophys. Res. Lett. 2003;30 doi:10.1029/2003GL017240 [Google Scholar]
- Huntley M.E, Nordhausen W, Lopez M.D.G. Elemental composition, metabolic activity and growth of Antarctic krill Euphausia superba during winter. Mar. Ecol. Prog. Ser. 1994;107:23–40. [Google Scholar]
- Jarman S.N, Elliott N.G, Nicol S, McMinn A. Molecular phylogenetics of circumglobal Euphausia species (Euphausiacea: Crustacea) Can. J. Fish. Aquat. Sci. 2000;57:51–58. doi:10.1139/cjfas-57-S3-51 [Google Scholar]
- Jessopp M.J, Forcada J, Reid K, Trathan P.N, Murphy E.J. Winter dispersal of leopard seals (Hydrurga leptonyx): environmental factors influencing demographics and seasonal abundance. J. Zool. 2004;263:251–258. doi:10.1017/S0952836904005102 [Google Scholar]
- Kawaguchi S, Siegel V, Litvinov F, Loeb V, Watkins J. Salp distribution and size composition in the Atlantic sector of the Southern Ocean. Deep Sea Res. Part II Topical Stud. Oceanogr. 2004;51:1369–1381. doi:10.1016/j.dsr2.2004.06.017 [Google Scholar]
- Kawaguchi S, Candy S.G, King R, Naganobu M, Nicol S. Modelling growth of Antarctic krill. I. Growth trends with sex, length, season, and region. Mar. Ecol. Prog. Ser. 2006;306:1–15. [Google Scholar]
- Kils U. In BIOMASS Scientific Series. vol. 3. SCAR; College Station, TX: 1982. Swimming behavior, swimming performance and energy balance of Antarctic krill Euphausia superba. pp. 1–122. [Google Scholar]
- Klinck J.M, Hofmann E.E, Beardsley R.C, Salihoglu B, Howard S. Water-mass properties and circulation on the west Antarctic Peninsula Continental Shelf in Austral Fall and Winter 2001. Deep Sea Res. Part II Topical Stud. Oceanogr. 2004;51:1925–1946. doi:10.1016/j.dsr2.2004.08.001 [Google Scholar]
- Kock K.-H. Krill consumption by Antarctic Nototheniod fish. In: Siegfried W.R, Condy P.R, Laws R.M, editors. Antarctic nutrient cycles and food webs. Springer; Berlin, Germany: 1985. pp. 437–451. [Google Scholar]
- Kock K.H, Wilhelms S, Everson I, Groger J. Variations in the diet composition and feeding intensity of Mackerel Icefish Champsocephalus gunnari at South Georgia (Antarctic) Mar. Ecol. Prog. Ser. 1994;108:43–57. [Google Scholar]
- Korb R.E, Whitehouse M. Contrasting primary production regimes around South Georgia, Southern Ocean: large blooms versus high nutrient, low chlorophyll waters. Deep Sea Res. Part I Oceanogr. Res. Pap. 2004;51:721–738. doi:10.1016/j.dsr.2004.02.006 [Google Scholar]
- Korb R.E, Whitehouse M.J, Ward P. SeaWiFS in the southern ocean: spatial and temporal variability in phytoplankton biomass around South Georgia. Deep Sea Res. Part II Topical Stud. Oceanogr. 2004;51:99–116. doi:10.1016/j.dsr2.2003.04.002 [Google Scholar]
- Korb R.E, Whitehouse M.J, Thorpe S.E, Gordon M. Primary production across the Scotia Sea in relation to the physico-chemical environment. J. Mar. Syst. 2005;57:231–249. doi:10.1016/j.jmarsys.2005.04.009 [Google Scholar]
- Kuparinen J, Bjornsen P.K. Spatial-distribution of bacterioplankton production across the Weddell–Scotia confluence during early austral summer 1988–1989. Polar Biol. 1992;12:197–204. [Google Scholar]
- Lancelot C, Billen G, Veth C, Becquevort S, Mathot S. Modeling carbon cycling through phytoplankton and microbes in the Scotia–Weddell Sea area during sea ice retreat. Mar. Chem. 1991;35:305–324. [Google Scholar]
- Lancelot C, Mathot S, Veth C, Debaar H. Factors controlling phytoplankton ice-edge blooms in the marginal ice-zone of the Northwestern Weddell Sea during sea-ice retreat 1988—field observations and mathematical modeling. Polar Biol. 1993;13:377–387. doi:10.1007/BF01681979 [Google Scholar]
- Lancelot C, Hannon E, Becquevort S, Veth C, De Baar H.J.W. Modeling phytoplankton blooms and carbon export production in the Southern Ocean: dominant controls by light and iron in the Atlantic sector in Austral spring 1992. Deep Sea Res. Part I Oceanogr. Res. Pap. 2000;47:1621–1662. doi:10.1016/S0967-0637(00)00005-4 [Google Scholar]
- Laws R.M. The ecology of the Southern Ocean. Am. Sci. 1985;73:26–40. [Google Scholar]
- Laws R.M. Antarctica: a convergence of life. New Sci. 1983;99:608–616. [Google Scholar]
- Laws R.M, editor. Antarctic ecology. Academic Press; London, UK: 1984. [Google Scholar]
- Levin S.A. Physical and biological scales and the modelling of predator–prey interactions in large marine ecosystems. In: Sherman K, Alexander L.M, Gold B.D, editors. Large marine ecosystems: patterns, processes and yields. American Association for the Advancement of Science; Washington DC: 1990. pp. 179–187. [Google Scholar]
- Ligowski R. Benthic feeding by krill, Euphausia superba Dana, in coastal waters off West Antarctica and in Admiralty Bay, South Shetland Islands. Polar Biol. 2000;23:619–625. doi:10.1007/s003000000131 [Google Scholar]
- Livermore R, Nankivell A, Eagles G, Morris P. Paleogene opening of Drake Passage. Earth Planet. Sci. Lett. 2005;236:459–470. doi:10.1016/j.epsl.2005.03.027 [Google Scholar]
- Livermore, R. A., Hillenbrand, C.-D., Meredith, M. P. & Eagles, G. In press. Drake Passage and Cenozoic climate: an open and shut case? Geochem. Geophys. Geosyst
- Loeb V, Siegel V, HolmHansen O, Hewitt R, Fraser W, Trivelpiece W, Trivelpiece S. Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature. 1997;387:897–900. doi:10.1038/43174 [Google Scholar]
- Longhurst A.R. Academic Press; San Diego, CA: 1998. Ecological geography of the sea. [Google Scholar]
- Lynnes A.S, Rodhouse P.G. A big mouthful for predators: the largest recorded specimen of Kondakovia longimana (Cephalopoda: Onychoteuthidae) Bull. Mar. Sci. 2002;71:1087–1090. [Google Scholar]
- Lynnes A.S, Reid K, Croxall J.P. Diet and reproductive success of Adelie and chinstrap penguins: linking response of predators to prey population dynamics. Polar Biol. 2004;27:544–554. doi:10.1007/s00300-004-0617-1 [Google Scholar]
- Mackintosh N.A. Distribution of the macroplankton in the Atlantic sector of the Antarctic. Discov. Rep. 1936;9:65–160. [Google Scholar]
- Mackintosh N.A. The pattern of distribution of the Antarctic fauna. Proc. R. Soc. B. 1960;153:624–631. [Google Scholar]
- Mackintosh N. Life cycle of Antarctic krill in relation to ice and water conditions. Discov. Rep. 1972;XXXVI:1–94. [Google Scholar]
- Mackintosh N. Distribution of post-larval krill in the Antarctic. Discov. Rep. 1973;36:95–156. [Google Scholar]
- Marchant H.J, Murphy E.J. Interactions at the base of the marine food chain. In: El-Sayed S.Z, editor. Southern Ocean ecology: the BIOMASS perspective. Cambridge University Press; Cambridge, UK: 1994. pp. 267–286. [Google Scholar]
- Marin V. The oceanographic structure of the Eastern Scotia Sea. 4. Distribution of copepod species in relation to hydrography in 1981. Deep Sea Res. Part a Oceanogr. Res. Pap. 1987;34:105–121. doi:10.1016/0198-0149(87)90125-7 [Google Scholar]
- Marr J. The natural history and geography of the Antarctic krill (Euphausia superba Dana) Discov. Rep. 1962;32:33–464. [Google Scholar]
- Maslennikov V.V, Solyankin E.V. Patterns of fluctuations in the hydrological conditions of the Antarctic and their effect on the distribution of Antarctic krill. In: Sahrhage D, editor. Antarctic ocean and resources variability. Springer; Berlin, Heidelberg, Germany; New York, NY: 1988. pp. 209–213. [Google Scholar]
- May R.M. Ecological interactions in the Southern Ocean. Nature. 1979;277:86–89. doi:10.1038/277086a0 [Google Scholar]
- May R.M, Beddington J.R, Clark C.W, Holt S.J, Laws R.M. Management of multispecies fisheries. Science. 1979;205:267–277. doi: 10.1126/science.205.4403.267. doi:10.1126/science.205.4403.267 [DOI] [PubMed] [Google Scholar]
- Melnikov I.A, Spiridonov V.A. Antarctic krill under perennial ice in the western Weddell Sea. Antarct. Sci. 1996;8:323–329. [Google Scholar]
- Meredith M.P, King J.C. Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys. Res. Lett. 2005;32:L19604. doi:10.1029/2005GL024042 [Google Scholar]
- Meredith M.P, Hughes C.W, Foden P.R. Downslope convection north of Elephant Island, Antarctic Peninsula: influence on deep waters and dependence on ENSO. Geophys. Res. Lett. 2003a;30:1462. doi:10.1029/2003GL017074 [Google Scholar]
- Meredith M.P, Watkins J.L, Murphy E.J, Cunningham N.J, Wood A.G, Korb R, Whitehouse M.J, Thorpe S.E, Vivier F. An anticyclonic circulation above the northwest Georgia rise, Southern Ocean. Geophys. Res. Lett. 2003b;30:2061. doi:10.1029/2003GL018039 [Google Scholar]
- Meredith M.P, Watkins J.L, Murphy E.J, Ward P, Bone D.G, Thorpe S.E, Grant S.A, Ladkin R.S. Southern ACC front to the northeast of South Georgia: pathways, characteristics, and fluxes. J. Geophys. Res. Oceans. 2003c;108 art. no.-3162. [Google Scholar]
- Meredith M.P, Renfrew I.A, Clarke A, King J.C, Brandon M.A. Impact of the 1997/98 ENSO on the upper waters of Marguerite Bay, western Antarctic Peninsula. J. Geophys. Res. 2004a;109:C09013. doi:10.1029/2003JC001784 [Google Scholar]
- Meredith M.P, Woodworth P.L, Hughes C.W, Stepanov V. Changes in the ocean transport through Drake Passage during the 1980s and 1990s, forced by changes in the Southern Annular Mode. Geophys. Res. Lett. 2004b;31:L21305. doi:10.1029/2004GL021169 [Google Scholar]
- Meredith M.P, Brandon M.A, Murphy E.J, Trathan P.N, Thorpe S.E, Bone D.G, Chernyshkov P.P, Sushin V.A. Variability in hydrographic conditions to the east and northwest of South Georgia, 1996–2001. J. Mar. Syst. 2005;53:143–167. doi:10.1016/j.jmarsys.2004.05.005 [Google Scholar]
- Meyer B, Oettl B. Effects of short-term starvation on composition and metabolism of larval Antarctic krill Euphausia superba. Mar. Ecol. Prog. Ser. 2005;292:263–270. [Google Scholar]
- Meyer B, Atkinson A, Stubing D, Oettl B, Hagen W, Bathmann U.V. Feeding and energy budgets of Antarctic krill Euphausia superba at the onset of winter—I. Furcilia III larvae. Limnol. Oceanogr. 2002;47:943–952. [Google Scholar]
- Meyer B, Atkinson A, Blume B, Bathmann U.V. Feeding and energy budgets of larval Antarctic krill Euphausia superba in summer. Mar. Ecol. Prog. Ser. 2003;257:167–177. [Google Scholar]
- Miller D.G.M, Hampton I. In Biological investigations of marine Antarctic systems and stocks (BIOMASS) vol. 9. SCAR and SCOR; Cambridge, UK: 1989. Biology and ecology of the Antarctic krill (Euphausia superba Dana): a review. [Google Scholar]
- Mitchell B.G, Brody E.A, Holmhansen O, McClain C, Bishop J. Light limitation of phytoplankton biomass and macronutrient utilization in the Southern-Ocean. Limnol. Oceanogr. 1991;36:1662–1677. [Google Scholar]
- Moore J.K, Abbott M.R, Richman J.G. Variability in the location of the Antarctic Polar Front (90 degrees–20 degrees W) from satellite sea surface temperature data. J. Geophys. Res. Oceans. 1997;102:27 825–27 833. doi:10.1029/97JC01705 [Google Scholar]
- Moore J.K, Abbott M.R, Richman J.G. Location and dynamics of the Antarctic polar front from satellite sea surface temperature data. J. Geophys. Res. Oceans. 1999;104:3059–3073. [Google Scholar]
- Moran X.A.G, Gasol J.M, Pedros-Alio C, Estrada M. Dissolved and particulate primary production and bacterial production in offshore Antarctic waters during austral summer: coupled or uncoupled? Mar. Ecol. Prog. Ser. 2001;222:25–39. [Google Scholar]
- Mordy C.W, Penny D.M, Sullivan C.W. Spatial-distribution of bacterioplankton biomass and production in the marginal ice-edge zone of the Weddell–Scotia Sea during austral winter. Mar. Ecol. Prog. Ser. 1995;122:9–19. [Google Scholar]
- Murphy E.J. Spatial structure of the Southern-Ocean ecosystem—predator–prey linkages in Southern-Ocean food webs. J. Anim. Ecol. 1995;64:333–347. doi:10.2307/5895 [Google Scholar]
- Murphy E.J, Reid K. Modelling Southern Ocean krill population dynamics: biological processes generating fluctuations in the South Georgia ecosystem. Mar. Ecol. Prog. Ser. 2001;217:175–189. [Google Scholar]
- Murphy E, Morris D.J, Watkins J.L, Priddle J. Scales of interaction between Antarctic krill and the environment. In: Sahrhage D, editor. Antarctic Ocean and resources variability. Springer; Berlin, Gemany: 1988. pp. 120–130. [Google Scholar]
- Murphy E.J, Clarke A, Symon C, Priddle J. Temporal variation in Antarctic sea-ice—analysis of a long- term fast-ice record from the South-Orkney Islands. Deep Sea Res. Part I Oceanogr. Res. Pap. 1995;42:1045–1062. doi:10.1016/0967-0637(95)00057-D [Google Scholar]
- Murphy E, Trathan P, Everson I, Parkes G, Daunt F. Krill fishing in the Scotia Sea in relation to bathymetry, including the detailed distribution around South Georgia. CCAMLR Sci. 1997;4:1–17. [Google Scholar]
- Murphy E.J, et al. Carbon flux in ice–ocean–plankton systems of the Bellingshausen Sea during a period of ice retreat. J. Mar. Syst. 1998a;17:207–227. doi:10.1016/S0924-7963(98)00039-6 [Google Scholar]
- Murphy E.J, et al. Interannual variability of the South Georgia marine ecosystem: biological and physical sources of variation in the abundance of krill. Fish. Oceanogr. 1998b;7:381–390. doi:10.1046/j.1365-2419.1998.00081.x [Google Scholar]
- Murphy E.J, Thorpe S.E, Watkins J.L, Hewitt R. Modeling the krill transport pathways in the Scotia Sea: spatial and environmental connections generating the seasonal distribution of krill. Deep Sea Res. Part II Topical Stud. Oceanogr. 2004a;51:1435–1456. doi:10.1016/j.dsr2.2004.06.019 [Google Scholar]
- Murphy E.J, Watkins J.L, Meredith M.P, Ward P, Trathan P.N, Thorpe S.E. Southern Antarctic circumpolar current front to the northeast of South Georgia: horizontal advection of krill and its role in the ecosystem. J. Geophys. Res. Oceans. 2004b;109 art. no.-C01029. [Google Scholar]
- Murphy, E. J., Trathan, P. N., Watkins, J. L., Reid, K., Meredith, M. P., Forcada, J. & Johnston, N. M. Submitted. Climate-driven fluctuations in remote ocean ecosystems revealed in prey and predator responses to ENSO. Proc. R. Soc. B
- Naveira Garabato A.C, Heywood K.J, Stevens D.P. Modification and pathways of Southern Ocean deep water masses in the Scotia Sea. Deep Sea Res. II. 2002;49:681–705. doi:10.1016/S0967-0645(02)00156-X [Google Scholar]
- Nicol S. Krill, currents, and sea ice: Euphausia superba and its changing environment. Bioscience. 2006;56:111–120. doi:10.1641/0006-3568(2006)056[0111:KCASIE]2.0.CO;2 [Google Scholar]
- Nicol S, Kitchener J, King R, Hosie G, de la Mare W.K. Population structure and condition of Antarctic krill (Euphausia superba) off East Antarctica (80–150 degrees E) during the Austral summer of 1995/1996. Deep Sea Res. Part II Topical Stud. Oceanogr. 2000;47:2489–2517. doi:10.1016/S0967-0645(00)00033-3 [Google Scholar]
- Okada I, Yamanouchi T. Seasonal change of the atmospheric heat budget over the Southern Ocean from ECMWF and ERBE data. J. Clim. 2002;15:2527–2536. doi:10.1175/1520-0442(2002)015<2527:SCOTAH>2.0.CO;2 [Google Scholar]
- Olsson O, North A.W. Diet of the king penguin Aptenodytes patagonicus during three summers at South Georgia. Ibis. 1997;139:504–512. [Google Scholar]
- Orsi A.H, Whitworth T, Nowlin W.D. On the meridional extent and fronts of the Antarctic circumpolar current. Deep Sea Res. Part I Oceanogr. Res. Pap. 1995;42:641–673. doi:10.1016/0967-0637(95)00021-W [Google Scholar]
- Pakhomov E.A, Perissinotto R. Trophodynamics of the hyperiid amphipod Themisto gaudichaudi in the South Georgia region during late austral summer. Mar. Ecol. Prog. Ser. 1996;134:91–100. [Google Scholar]
- Pakhomov E.A, Perissinotto R, McQuaid C.D. Prey composition and daily rations of myctophid fishes in the Southern Ocean. Mar. Ecol. Prog. Ser. 1996;134:1–14. [Google Scholar]
- Pakhomov E.A, Perissinotto R, Froneman P.W, Miller D.G.M. Energetics and feeding dynamics of Euphausia superba in the South Georgia region during the summer of 1994. J. Plankton Res. 1997a;19:399–423. [Google Scholar]
- Pakhomov E.A, Verheye H.M, Atkinson A, Laubscher R.K, TauntonClark J. Structure and grazing impact of the mesozooplankton community during late summer 1994 near South Georgia, Antarctica. Polar Biol. 1997b;18:180–192. doi:10.1007/s003000050175 [Google Scholar]
- Pakhomov E.A, Froneman P.W, Perissinotto R. Salp/krill interactions in the Southern Ocean: spatial segregation and implications for the carbon flux. Deep Sea Res. Part II Topical Stud. Oceanogr. 2002;49:1881–1907. doi:10.1016/S0967-0645(02)00017-6 [Google Scholar]
- Pakhomov E.A, Atkinson A, Meyer B, Oettl B, Bathmann U. Daily rations and growth of larval krill Euphausia superba in the Eastern Bellingshausen Sea during austral autumn. Deep Sea Res. Part II Topical Stud. Oceanogr. 2004;51:2185–2198. doi:10.1016/j.dsr2.2004.08.003 [Google Scholar]
- Park M.G, Yang S.R, Kang S.H, Chung K.H, Shim J.H. Phytoplankton biomass and primary production in the marginal ice zone of the northwestern Weddell Sea during austral summer. Polar Biol. 1999;21:251–261. doi:10.1007/s003000050360 [Google Scholar]
- Parkinson C.L. Spatial patterns in the length of the sea-ice season in the Southern Ocean, 1979–1986. J. Geophys. Res. Oceans. 1994;99:16 327–16 339. doi:10.1029/94JC01146 [Google Scholar]
- Parkinson C.L. Trends in the length of the Southern Ocean sea-ice season, 1979–99. Ann. Glaciol. 2002;34:435–440. [Google Scholar]
- Parkinson C.L. Southern Ocean sea ice and its wider linkages: insights revealed from models and observations. Antarct. Sci. 2004;16:387–400. doi:10.1017/S0954102004002214 [Google Scholar]
- Patarnello T, Bargelloni L, Varotto V, Battaglia B. Krill evolution and the Antarctic ocean currents: evidence of vicariant speciation as inferred by molecular data. Mar. Biol. 1996;126:603–608. doi:10.1007/BF00351327 [Google Scholar]
- Peck L.S. Prospects for survival in the Southern Ocean: vulnerability of benthic species to temperature change. Antarct. Sci. 2005;17:497–507. doi:10.1017/S0954102005002920 [Google Scholar]
- Peck L.S, Webb K.E, Bailey D.M. Extreme sensitivity of biological function to temperature in Antarctic marine species. Funct. Ecol. 2004;18:625–630. doi:10.1111/j.0269-8463.2004.00903.x [Google Scholar]
- Peck L.S, Barnes D.K.A, Willmott J. Responses to extreme seasonality in food supply: diet plasticity in Antarctic brachiopods. Mar. Biol. 2005;147:453–463. doi:10.1007/s00227-005-1591-z [Google Scholar]
- Perez F.F, Tokarczyk R, Figueiras F.G, Rios A.F. Water masses and phytoplankton biomass distribution during summer in the Weddell Sea marginal ice-zone. Oceanol. Acta. 1994;17:191–199. [Google Scholar]
- Phillips R.A, Silk J.R.D, Croxall J.P. Foraging and provisioning strategies of the light-mantled sooty albatross at South Georgia: competition and co-existence with sympatric pelagic predators. Mar. Ecol. Prog. Ser. 2005;285:259–270. [Google Scholar]
- Pond D.W, Atkinson A, Shreeve R.S, Tarling G, Ward P. Diatom fatty acid biomarkers indicate recent growth rates in Antarctic krill. Limnol. Oceanogr. 2005;50:732–736. [Google Scholar]
- Priddle J, Croxall J.P, Everson I, Heywood R.B, Murphy E.J, Prince P.A, Sear C.B. Large-scale fluctuations in distribution and abundance of krill—a discussion of possible causes. In: Sahrhage D, editor. Antarctic ocean and resources variability. Springer; Berlin, Germany: 1988. pp. 169–182. [Google Scholar]
- Priddle J, Whitehouse M.J, Atkinson A, Brierley A.S, Murphy E.J. Diurnal changes in near-surface ammonium concentration—interplay between zooplankton and phytoplankton. J. Plankton Res. 1997;19:1305–1330. [Google Scholar]
- Priddle J, Boyd I.L, Whitehouse M.J, Murphy E.J, Croxall J.P. Estimates of Southern Ocean primary production—constraints from predator carbon demand and nutrient drawdown. J. Mar. Syst. 1998a;17:275–288. doi:10.1016/S0924-7963(98)00043-8 [Google Scholar]
- Priddle J, Nedwell D.B, Whitehouse M.J, Reay D.S, Savidge G, Gilpin L.C, Murphy E.J, Ellis-Evans J.C. In Annals of Glaciology. vol. 27. International Glaciological Society; Cambridge, UK: 1998b. Re-examining the Antarctic Paradox: speculation on the Southern Ocean as a nutrient-limited system. pp. 661–668. [Google Scholar]
- Priddle J, Whitehouse M.J, Ward P, Shreeve R.S, Brierley A.S, Atkinson A, Watkins J.L, Brandon M.A, Cripps G.C. Biogeochemistry of a Southern Ocean plankton ecosystem: using natural variability in community composition to study the role of metazooplankton in carbon and nitrogen cycles. J. Geophys. Res. Oceans. 2003;108 art. no.-8082. [Google Scholar]
- Pusch C, Hulley P.A, Kock K.H. Community structure and feeding ecology of mesopelagic fishes in the slope waters of King George Island (South Shetland Islands, Antarctica) Deep Sea Res. Part I Oceanogr. Res. Pap. 2004;51:1685–1708. doi:10.1016/j.dsr.2004.06.008 [Google Scholar]
- Quetin L.B, Ross R.M. Behavioural and physiological characteristics of the Antarctic krill, Euphausia superba. Am. Zool. 1991;31:49–63. [Google Scholar]
- Quetin L.B, Ross R.M. Environmental variability and its impact on the reproductive cycle of Antarctic krill. Am. Zool. 2001;41:74–89. doi:10.1668/0003-1569(2001)041[0074:EVAIIO]2.0.CO;2 [Google Scholar]
- Quetin L.B, Ross R.M. Episodic recruitment in Antarctic krill Euphausia superba in the Palmer LTER study region. Mar. Ecol. Prog. Ser. 2003;259:185–200. [Google Scholar]
- Quetin L.B, Ross M.M, Frazer T.K, Haberman K.L. Factors affecting distribution and abundance of zooplankton, with an emphasis on Antarctic krill, Euphausia superba. Antarct. Res. Ser. 1996;70:357–371. [Google Scholar]
- Reid K. Growth rates of Antarctic fur seals as indices of environmental conditions. Mar. Mammal Sci. 2002;18:469–482. doi:10.1111/j.1748-7692.2002.tb01049.x [Google Scholar]
- Reid K, Arnould J.P.Y. The diet of Antarctic fur seals Arctocephalus gazella during the breeding season at South Georgia. Polar Biol. 1996;16:105–114. [Google Scholar]
- Reid K, Croxall J.P. Environmental response of upper trophic-level predators reveals a system change in an Antarctic marine ecosystem. Proc. R. Soc. B. 2001;268:377–384. doi: 10.1098/rspb.2000.1371. doi:10.1098/rspb.2000.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K, Croxall J.P, Prince P.A. The fish diet of black-browed albatross Diomedea melanophris and grey-headed albatross D. chrysostoma at South Georgia. Polar Biol. 1996a;16:469–477. [Google Scholar]
- Reid K, Trathan P.N, Croxall J.P, Hill H.J. Krill caught by predators and nets: differences between species and techniques. Mar. Ecol. Prog. Ser. 1996b;140:13–20. [Google Scholar]
- Reid K, Croxall J.P, Edwards T.M. Interannual variation in the diet of the Antarctic Prion Pachyptila desolata at South Georgia. Emu. 1997a;97:126–132. doi:10.1071/MU97016 [Google Scholar]
- Reid K, Croxall J.P, Edwards T.M, Hill H.J, Prince P.A. Diet and feeding ecology of the diving petrels Pelecanoides georgicus and P. urinatrix at South Georgia. Polar Biol. 1997b;17:17–24. doi:10.1007/s003000050100 [Google Scholar]
- Reid K, Barlow K.E, Croxall J.P, Taylor R.I. Predicting changes in the Antarctic krill, Euphausia superba, population at South Georgia. Mar. Biol. 1999a;135:647–652. doi:10.1007/s002270050665 [Google Scholar]
- Reid K, Watkins J.L, Croxall J.P, Murphy E.J. Krill population dynamics at South Georgia 1991–1997, based on data from predators and nets. Mar. Ecol. Prog. Ser. 1999b;177:103–114. [Google Scholar]
- Reid K, Murphy E.J, Loeb V, Hewitt R.P. Krill population dynamics in the Scotia Sea: variability in growth and mortality within a single population. J. Mar. Syst. 2002;36:1–10. doi:10.1016/S0924-7963(02)00131-8 [Google Scholar]
- Reid K, Sims M, White R.W, Gillon K.W. Spatial distribution of predator/prey interactions in the Scotia Sea: implications for measuring predator/fisheries overlap. Deep Sea Res. Part II Topical Stud. Oceanogr. 2004;51:1383–1396. doi:10.1016/j.dsr2.2004.06.007 [Google Scholar]
- Reid K, Croxall J.P, Briggs D.R, Murphy E.J. Antarctic ecosystem monitoring: quantifying the response of ecosystem indicators to variability in Antarctic krill. Ices J. Mar. Sci. 2005;62:366–373. doi:10.1016/j.icesjms.2004.11.003 [Google Scholar]
- Reilly S, Hedley S, Borberg J, Hewitt R, Thiele D, Watkins J, Naganobu M. Biomass and energy transfer to baleen whales in the South Atlantic sector of the Southern Ocean. Deep Sea Res. Part II Topical Stud. Oceanogr. 2004;51:1397–1409. doi:10.1016/j.dsr2.2004.06.008 [Google Scholar]
- Rodhouse P.G, Nigmatullin C.M. Role as consumers. Phil. Trans. R. Soc. B. 1996;351:1003–1022. [Google Scholar]
- Rodhouse P.G, White M.G, Jones M.R.R. Trophic relations of the Cephalopod Martialia hyadesi (Teuthoidea, Ommastrephidae) at the Antarctic Polar Front, Scotia Sea. Mar. Biol. 1992;114:415–421. doi:10.1007/BF00350032 [Google Scholar]
- Rodhouse P.G, Piatkowski U, Murphy E.J, White M.G, Bone D.G. Utility and Limits of biomass spectra—the Nekton community sampled with the Rmt-25 in the Scotia Sea during austral summer. Mar. Ecol. Prog. Ser. 1994;112:29–39. [Google Scholar]
- Rodhouse P.G, Elvidge C.D, Trathan P.N. Remote sensing of the global light fishing fleet: an analysis of interactions with oceanography, other fisheries and predators. Adv. Mar. Biol. 2001;39:261–303. [Google Scholar]
- Rooney N, McCann K, Gellner G, Moore J.C. Structural asymmetry and the stability of diverse food webs. Nature. 2006;442:265–269. doi: 10.1038/nature04887. doi:10.1038/nature04887 [DOI] [PubMed] [Google Scholar]
- Ross R.M, Quetin L.B. How productive are Antarctic krill? BioScience. 1986;36:264. doi:10.2307/1310217 [Google Scholar]
- Ross R.M, Quetin L.B. Environmental variability and its impact on the reproductive cycle of antarctic krill, Euphausia superba. Am. Zool. 1999;39:3A–3A. [Google Scholar]
- Ross R.M, Quetin L.B, Haberman K.L. Interannual and seasonal variability in short-term grazing impact of Euphausia superba in nearshore and offshore waters west of the Antarctic Peninsula. J. Mar. Syst. 1998;17:261–273. doi:10.1016/S0924-7963(98)00042-6 [Google Scholar]
- Ross R.M, Quetin L.B, Baker K.S, Vernet M, Smith R.C. Growth limitation in young Euphausia superba under field conditions. Limnol. Oceanogr. 2000;45:31–43. [Google Scholar]
- Schlitzer R. Carbon export fluxes in the Southern Ocean: results from inverse modeling and comparison with satellite-based estimates. Deep Sea Res. Part II Topical Stud. Oceanogr. 2002;49:1623–1644. doi:10.1016/S0967-0645(02)00004-8 [Google Scholar]
- Schnack-Schiel S.B, Isla E. The role of zooplankton in the pelagic–benthic coupling of the Southern Ocean. Sci. Mar. 2005;69:39–55. [Google Scholar]
- Shreeve R.S, Tarling G.A, Atkinson A, Ward P, Goss C, Watkins J. Relative production of Calanoides acutus (Copepoda: Calanoida) and Euphausia superba (Antarctic krill) at South Georgia, and its implications at wider scales. Mar. Ecol. Prog. Ser. 2005;298:229–239. [Google Scholar]
- Siegel V.A. A concept of seasonal variation of krill (Euphausia superba) distribution and abundance west of the Antarctic Peninsula. In: Sahrhage D, editor. Antarctic Ocean and resources variability. Springer; Berlin, Heidelberg, Germany; New York, NY: 1988. pp. 219–230. [Google Scholar]
- Siegel V. Distribution and population dynamics of Euphausia superba: summary of recent findings. Polar Biol. 2005;29:1–22. doi:10.1007/s00300-005-0058-5 [Google Scholar]
- Siegel V, Loeb V. Recruitment of Antarctic krill Euphausia superba and possible causes for its variability. Mar. Ecol. Prog. Ser. 1995;123:45–56. [Google Scholar]
- Siegel V, Muhlenhardtsiegel U. On the occurrence and biology of some Antarctic Mysidacea (Crustacea) Polar Biol. 1988;8:181–190. doi:10.1007/BF00443451 [Google Scholar]
- Siegel V, Ross R.M, Quetin L.B. Krill (Euphausia superba) recruitment indices from the western Antarctic Peninsula: are they representative of larger regions? Polar Biol. 2003;26:672–679. doi:10.1007/s00300-003-0537-5 [Google Scholar]
- Siegel V, Kawaguchi S, Ward P, Litvinov F, Sushin V, Loeb V, Watkins J. Krill demography and large-scale distribution in the southwest Atlantic during January/February 2000. Deep Sea Res. Part II Topical Stud. Oceanogr. 2004;51:1253–1273. doi:10.1016/j.dsr2.2004.06.013 [Google Scholar]
- Sievers H.A, Nowlin W.D. The stratification and water masses at Drake Passage. J. Geophys. Res. Oceans. 1984;89:489–514. [Google Scholar]
- Smetacek V, Nicol S. Polar ocean ecosystems in a changing world. Nature. 2005;437:362–368. doi: 10.1038/nature04161. doi:10.1038/nature04161 [DOI] [PubMed] [Google Scholar]
- Smetacek V, Assmy P, Henjes J. The role of grazing in structuring Southern Ocean pelagic ecosystems and biogeochemical cycles. Antarct. Sci. 2004;16:541–558. doi:10.1017/S0954102004002317 [Google Scholar]
- Smith W.O, Lancelot C. Bottom-up versus top-down control in phytoplankton of the Southern Ocean. Antarct. Sci. 2004;16:531–539. doi:10.1017/S0954102004002305 [Google Scholar]
- Smith R.C, Stammerjohn S.E. In Annals of Glaciology. vol. 33. International Glaciological Society; Cambridge, UK: 2001. Variations of surface air temperature and sea-ice extent in the western Antarctic Peninsula region. pp. 493–500. [Google Scholar]
- Smith R.C, et al. Marine ecosystem sensitivity to climate change. Bioscience. 1999;49:393–404. doi:10.2307/1313632 [Google Scholar]
- Spiridonov V.A. Spatial and temporal variability in reproductive timing of Antarctic krill (Euphausia superba Dana) Polar Biol. 1995;15:161–174. doi:10.1007/BF00239056 [Google Scholar]
- Spiridonov V.A. A scenario of the late-Pleistocene–Holocene changes in the distributional range of Antarctic krill (Euphausia superba) Pubbl. Stazione Zool. Napoli I: Mar. Ecol. 1996;17(1-3):519–541. [Google Scholar]
- Stammerjohn S.E, Drinkwater M.R, Smith R.C, Liu X. Ice–atmosphere interactions during sea-ice advance and retreat in the western Antarctic Peninsula region. J. Geophys. Res. Oceans. 2003;108:3329. [Google Scholar]
- Staniland I.J, Boyd I.L. Why travel further to get the same food? Antarctic fur Seal Arctocephalus gazella as foragers on patchy prey. Anim. Behav. 2002 [Google Scholar]
- Staniland I.J, Boyd I.L. Variation in the foraging location of Antarctic fur seals (Arctocephalus gazella) and the effects on diving behaviour. Mar. Mammal Sci. 2003;19:331–343. doi:10.1111/j.1748-7692.2003.tb01112.x [Google Scholar]
- Staniland I.J, Reid K, Boyd I.L. Comparing individual and spatial influences on foraging behaviour in Antarctic fur seals Arctocephalus gazella. Mar. Ecol. Prog. Ser. 2004;275:263–274. [Google Scholar]
- Steele M, Zhang J, Rothrock D, Stern H. The force balance of sea ice in a numerical model of the Arctic Ocean. J. Geophys. Res. 1997;102:21 061–21 079. doi:10.1029/97JC01454 [Google Scholar]
- Sullivan C.W, McClain C.R, Comiso J.C, Smith W.O. Phytoplankton standing crops within an Antarctic Ice Edge assessed by satellite remote-sensing. J. Geophys. Res. Oceans. 1988;93:12 487–12 498. [Google Scholar]
- Takahashi A, Dunn M.J, Trathan P.N, Sato K, Naito Y, Croxall J.P. Foraging strategies of chinstrap penguins at Signy Island, Antarctica: importance of benthic feeding on Antarctic krill. Mar. Ecol. Prog. Ser. 2003;250:279–289. [Google Scholar]
- Taki K, Hayashi T, Naganobu M. Characteristics of seasonal variation in diurnal vertical migration and aggregation of Antarctic krill (Euphausia superba) in the Scotia Sea, using Japanese fishery data. CCAMLR Sci. 2005;12:163–172. [Google Scholar]
- Tarling G.A, Johnson M.L. Satiation gives krill that sinking feeling. Curr. Biol. 2006;16:R83–R84. doi: 10.1016/j.cub.2006.01.044. doi:10.1016/j.cub.2006.01.044 [DOI] [PubMed] [Google Scholar]
- Tarling G.A, Shreeve R.S, Ward P, Atkinson A, Hirst A.G. Life-cycle phenotypic composition and mortality of Calanoides acutus (Copepoda: Calanoida) in the Scotia Sea: a modelling approach. Mar. Ecol. Prog. Ser. 2004;272:165–181. [Google Scholar]
- Tarling G.A, Shreeve R.S, Hirst A.G, Atkinson A, Pond D.W, Murphy E.J, Watkins J.L. Natural growth rates in Antarctic krill (Euphausia superba): I. Improving methodology and predicting intermolt period. Limnol. Oceanogr. 2006;51:959–972. [Google Scholar]
- Tarling, G. A., Cuzin-Roudy, J., Thorpe, S. E. Shreeve, R. S., Ward, P. W. & Murphy E. J. In press. Recruitment of Antarctic krill (Euphausia superba Dana) in the South Georgia region: adult fecundity and the fate of early developmental stages. Mar. Ecol. Prog. Ser
- Thorndike A.S, Colony R. Sea ice motion in response to geostrophic winds. J. Geophys. Res. 1982;87:5845–5852. [Google Scholar]
- Thorpe S.E, Heywood K.J, Brandon M.A, Stevens D.P. Variability of the southern Antarctic circumpolar current front north of South Georgia. J. Mar. Syst. 2002;37:87–105. doi:10.1016/S0924-7963(02)00197-5 [Google Scholar]
- Thorpe, S. E., Murphy, E. J. & Watkins, J. L. Submitted. Circumpolar connections between Antarctic krill (Euphausia superba Dana) populations: investigating the roles of ocean and sea ice transport. Deep Sea Res. Part II: Topical Stud. Oceanogr
- Trathan, P. N., Forcada, J. & Murphy, E. J. In press. Environmental forcing and Southern Ocean marine predator populations: effects of climate change and variability. Phil. Trans. R. Soc. B362 (doi:10.1098/rstb.2006.1953) [DOI] [PMC free article] [PubMed]
- Trathan P.N, Murphy E.J. Sea surface temperature anomalies near South Georgia: relationships with the Pacific El Nino regions. J. Geophys. Res. Oceans. 2002;108 art. no.-8075. [Google Scholar]
- Trathan P.N, Croxall J.P, Murphy E.J. Dynamics of Antarctic penguin populations in relation to interannual variability in sea ice distribution. Polar Biol. 1996;16:321–330. [Google Scholar]
- Trathan P.N, Brandon M.A, Murphy E.J. Characterization of the Antarctic Polar Frontal Zone to the north of South Georgia in summer 1994. J. Geophys. Res. Oceans. 1997;102:10 483–10 497. doi:10.1029/97JC00381 [Google Scholar]
- Trathan P.N, Everson I, Murphy E.J, Parkes G.B. Analysis of haul data from the South Georgia krill fishery. CCAMLR Sci. 1998a;5:9–30. [Google Scholar]
- Trathan P.N, Murphy E.J, Croxall J.P, Everson I. Use of at-sea distribution data to derive potential foraging ranges of macaroni penguins during the breeding season. Mar. Ecol. Prog. Ser. 1998b;169:263–275. [Google Scholar]
- Trathan P.N, Brandon M.A, Murphy E.J, Thorpe S.E. Transport and structure within the Antarctic circumpolar current to the north of South Georgia. Geophys. Res. Lett. 2000;27:1727–1730. doi:10.1029/1999GL011131 [Google Scholar]
- Trathan P.N, Brierley A.S, Brandon M.A, Bone D.G, Goss C, Grant S.A, Murphy E.J, Watkins J.L. Oceanographic variability and changes in Antarctic krill (Euphausia superba) abundance at South Georgia. Fish. Oceanogr. 2003;12:569–583. doi:10.1046/j.1365-2419.2003.00268.x [Google Scholar]
- Trathan, P. N., Murphy, E. J., Forcada, J., Croxall, J. P., Reid, K. & Thorpe, S. E. 2006 Physical forcing in the southwest Atlantic: ecosystem control. In Top Predators in marine ecosystems: their role in monitoring and management, vol. 12 (ed. I. L. Boyd, S. Wanless & C. J. Camphuysen), pp. 28–45. Cambridge, UK: Cambridge University Press.
- Treguer P, Jacques G. Dynamics of nutrients and phytoplankton, and fluxes of carbon, nitrogen and silicon in the Antarctic Ocean. Polar Biol. 1992;12:149–162. doi:10.1007/BF00238255 [Google Scholar]
- Tupas L.M, Koike I, Karl D.M, Holmhansen O. Nitrogen-metabolism by heterotrophic bacterial assemblages in Antarctic Coastal waters. Polar Biol. 1994;14:195–204. doi:10.1007/BF00240524 [Google Scholar]
- Turner J. The El Nino–southern oscillation and Antarctica. Int. J. Climatol. 2004;24:1–31. doi:10.1002/joc.965 [Google Scholar]
- Tynan C.T. Ecological importance of the Southern boundary of the Antarctic circumpolar current. Nature. 1998;392:708–710. doi:10.1038/33675 [Google Scholar]
- Vaughan D.G, Marshall G.J, Connolley W.M, Parkinson C, Mulvaney R, Hodgson D.A, King J.C, Pudsey C.J, Turner J. Recent rapid regional climate warming on the Antarctic Peninsula. Climat. Change. 2003;60:243–274. doi:10.1023/A:1026021217991 [Google Scholar]
- Voronina, N. M. 1970 Seasonal cycles of some common Antarctic copepod species. In Antarctic Ecology, vol. 1 (ed. M. Holdgate), pp. 160–172. London, UK; New York, NY: Academic Press.
- Walsh J.J, Dieterle D.A, Lenes J. A numerical analysis of carbon dynamics of the Southern Ocean phytoplankton community: the roles of light and grazing in effecting both sequestration of atmospheric CO2 and food availability to larval krill. Deep Sea Res. Part I Oceanogr. Res. Pap. 2001;48:1–48. doi:10.1016/S0967-0637(00)00032-7 [Google Scholar]
- Ward P, Atkinson A, Peck J.M, Wood A.G. Euphausiid life-cycles and distribution around South Georgia. Antarct. Sci. 1990;2:43–52. [Google Scholar]
- Ward P, Atkinson A, Murray A.W.A, Wood A.G, Williams R, Poulet S.A. The summer zooplankton community at South Georgia—biomass, vertical migration and grazing. Polar Biol. 1995;15:195–208. doi:10.1007/BF00239059 [Google Scholar]
- Ward P, Atkinson A, SchnackSchiel S.B, Murray A.W.A. Regional variation in the life cycle of Rhincalanus gigas (Copepoda: Calanoida) in the Atlantic sector of the Southern Ocean—re-examination of existing data (1928 to 1993) Mar. Ecol. Prog. Ser. 1997;157:261–275. [Google Scholar]
- Ward P, et al. The southern antarctic circumpolar current front: physical and biological coupling at South Georgia. Deep Sea Res. Part I Oceanogr. Res. Pap. 2002;49:2183–2202. doi:10.1016/S0967-0637(02)00119-X [Google Scholar]
- Ward P, Whitehouse M, Brandon M, Shreeve R, Woodd-Walker R. Mesozooplankton community structure across the antarctic circumpolar current to the north of South Georgia: Southern Ocean. Mar. Biol. 2003;143:121–130. doi:10.1007/s00227-003-1019-6 [Google Scholar]
- Ward P, Grant S, Brandon M, Siegel V, Sushin V, Loeb V, Griffiths H. Mesozooplankton community structure in the Scotia Sea during the CCAMLR 2000 Survey: January–February 2000. Deep Sea Res. Part II Topical Stud. Oceanogr. 2004;51:1351–1367. doi:10.1016/j.dsr2.2004.06.016 [Google Scholar]
- Ward P, et al. Phyto- and zooplankton community structure and production around South Georgia (Southern Ocean) during Summer 2001/02. Deep Sea Res. Part I Oceanogr. Res. Pap. 2005;52:421–441. doi:10.1016/j.dsr.2004.10.003 [Google Scholar]
- Ward P, Shreeve R, Atkinson A, Korb B, Whitehouse M, Thorpe S, Pond D, Cunningham N. Plankton community structure and variability in the Scotia Sea: austral summer 2003. Mar. Ecol. Prog. Ser. 2006;309:75–91. [Google Scholar]
- Watkins J.L, Murray A.W.A. Layers of Antarctic krill, Euphausia superba: are they just long krill swarms? Mar. Biol. 1998;131:237–247. doi:10.1007/s002270050316 [Google Scholar]
- Watkins J.L, Morris D.J, Ricketts C, Priddle J. Differences between swarms of Antarctic krill and some implications for sampling krill populations. Mar. Biol. 1986;93:137–146. doi:10.1007/BF00428662 [Google Scholar]
- Watkins J.L, Murray A.W.A, Daly H.I. Variation in the distribution of Antarctic krill Euphausia superba around South Georgia. Mar. Ecol. Prog. Ser. 1999;188:149–160. [Google Scholar]
- Werner F.E, Aretxabaleta A, Edwards K. Modelling marine ecosystems and their environmental forcing. In: Stenseth N, Ottersen G, Hurrell J.W, Belgrano A, editors. Marine ecosystems and climate variation: the North Atlantic. A comparative perspective. Oxford University Press; Oxford, UK: 2003. pp. 33–48. [Google Scholar]
- White W.B, Peterson R.G. An Antarctic circumpolar wave in surface pressure, wind, temperature and sea-ice extent. Nature. 1996;380:699–702. doi:10.1038/380699a0 [Google Scholar]
- Whitehouse M.J, Priddle J, Symon C. Seasonal and annual change in seawater temperature, salinity, nutrient and chlorophyll a distributions around South Georgia, South Atlantic. Deep Sea Res. Part I Oceanogr. Res. Pap. 1996a;43:425–443. doi:10.1016/0967-0637(96)00020-9 [Google Scholar]
- Whitehouse M.J, Priddle J, Trathan P.N, Brandon M.A. Substantial open-ocean phytoplankton blooms to the north of South Georgia, South Atlantic, during summer 1994. Mar. Ecol. Prog. Ser. 1996b;140:187–197. [Google Scholar]
- Whitehouse M.J, Priddle J, Brandon M.A, Swanson C. A comparison of chlorophyll/nutrient dynamics at two survey sites near South Georgia, and the potential role of planktonic nitrogen recycled by land-based predators. Limnol. Oceanogr. 1999;44:1498–1508. [Google Scholar]
- Whitehouse M.J, Priddle J, Brandon M.A. Chlorophyll/nutrient characteristics in the water masses to the north of South Georgia, Southern Ocean. Polar Biol. 2000;23:373–382. doi:10.1007/s003000050458 [Google Scholar]
- Whitehouse, M. J., Korb, R. E., Atkinson, A., Thorpe, S. E. & Gordon, M. Submitted. Formation, transport and decay of an intense phytoplankton bloom within the High Nutrient Low Chlorophyll belt of the Southern Ocean. J. Mar. Syst.
- Whitworth T, Nowlin W.D. Water masses and currents of the Southern-Ocean at the Greenwich Meridian. J. Geophys. Res. Oceans. 1987;92:6462–6476. [Google Scholar]
- Whitworth T, Nowlin W.D, Orsi A.H, Locarnini R.A, Smith S.G. Weddell Sea Shelf Water in the Bransfield Strait and Weddell–Scotia confluence. Deep Sea Res. Part I Oceanogr. Res. Pap. 1994;41:629–641. doi:10.1016/0967-0637(94)90046-9 [Google Scholar]
- Witek Z, Kalinowski J, Grelowski A. Formation of Antarctic krill concentrations in relation to hydrodynamic processes and social behaviour. In: Sahrhage D, editor. Antarctic Ocean and resources variability. Springer; Berlin, Germany: 1988. pp. 237–244. [Google Scholar]
- Wright S.W, van den Enden R.L. Phytoplankton community structure and stocks in the East Antarctic marginal ice zone (BROKE survey, January–March 1996) determined by CHEMTAX analysis of HPLC pigment signatures. Deep Sea Res. Part II Topical Stud. Oceanogr. 2000;47:2363–2400. doi:10.1016/S0967-0645(00)00029-1 [Google Scholar]
- Xavier J.C, Croxall J.P, Reid K. Interannual variation in the diets of two albatross species breeding at South Georgia: implications for breeding performance. Ibis. 2003a;145:593–610. doi:10.1046/j.1474-919X.2003.00196.x [Google Scholar]
- Xavier J.C, Croxall J.P, Trathan P.N, Rodhouse P.G. Inter-annual variation in the cephalopod component of the diet of the wandering albatross, Diomedea exulans breeding at Bird Island, South Georgia. Mar. Biol. 2003b;142:611–622. [Google Scholar]
- Xavier J.C, Croxall J.P, Trathan P.N, Wood A.G. Feeding strategies and diets of breeding grey-headed and wandering albatrosses at South Georgia. Mar. Biol. 2003c;143:221–232. doi:10.1007/s00227-003-1049-0 [Google Scholar]
- Xavier J.C, Trathan P.N, Croxall J.P, Wood A.G, Podesta G, Rodhouse P.G. Foraging ecology and interactions with fisheries of wandering albatrosses (Diomedea exulans) breeding at South Georgia. Fish. Oceanogr. 2004;13:324–344. doi:10.1111/j.1365-2419.2004.00298.x [Google Scholar]
- Zane L, Patarnello T. Krill: a possible model for investigating the effects of ocean currents on the genetic structure of a pelagic invertebrate. Can. J. Fish. Aquat. Sci. 2000;57:16–23. doi:10.1139/cjfas-57-S3-16 [Google Scholar]
- Zenk W. Detection of overflow events in the Shag Rocks passage, Scotia Ridge. Science. 1981;213:1113–1114. doi: 10.1126/science.213.4512.1113. doi:10.1126/science.213.4512.1113 [DOI] [PubMed] [Google Scholar]