Abstract
The Antarctic Peninsula is experiencing one of the fastest rates of regional climate change on Earth, resulting in the collapse of ice shelves, the retreat of glaciers and the exposure of new terrestrial habitat. In the nearby oceanic system, winter sea ice in the Bellingshausen and Amundsen seas has decreased in extent by 10% per decade, and shortened in seasonal duration. Surface waters have warmed by more than 1 K since the 1950s, and the Circumpolar Deep Water (CDW) of the Antarctic Circumpolar Current has also warmed. Of the changes observed in the marine ecosystem of the western Antarctic Peninsula (WAP) region to date, alterations in winter sea ice dynamics are the most likely to have had a direct impact on the marine fauna, principally through shifts in the extent and timing of habitat for ice-associated biota. Warming of seawater at depths below ca 100 m has yet to reach the levels that are biologically significant. Continued warming, or a change in the frequency of the flooding of CDW onto the WAP continental shelf may, however, induce sublethal effects that influence ecological interactions and hence food-web operation. The best evidence for recent changes in the ecosystem may come from organisms which record aspects of their population dynamics in their skeleton (such as molluscs or brachiopods) or where ecological interactions are preserved (such as in encrusting biota of hard substrata). In addition, a southwards shift of marine isotherms may induce a parallel migration of some taxa similar to that observed on land. The complexity of the Southern Ocean food web and the nonlinear nature of many interactions mean that predictions based on short-term studies of a small number of species are likely to be misleading.
Keywords: food web, ice, krill, oceanography, physiology, temperature
1. Introduction
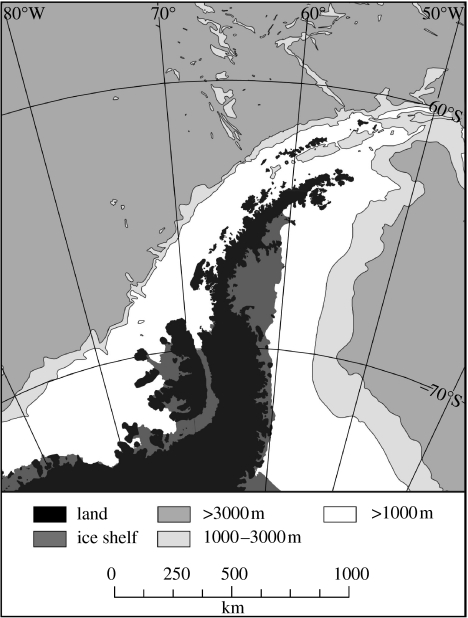
The Antarctic Peninsula (figure 1) is one of the three areas of the globe presently experiencing rapid climate change (King 1994; Smith et al. 1996; King & Harangozo 1998; Vaughan et al. 2003). The Second Assessment Report of the Intergovernmental Panel on Climate Change (IPCC; Nicholls et al. 1995) demonstrated two areas of rapid atmospheric warming at northern high latitudes, namely northwestern North America, and an area centred on the Siberian Plateau. A subsequent analysis by Hansen et al. (1999) revealed a third region of warming centred on the Antarctic Peninsula and Bellingshausen Sea. In each of these areas, mean annual temperatures have warmed by more than 1.5 K since 1950, compared with a global mean increase of ca 0.6 K. The third assessment report of the IPCC (Folland et al. 2001) confirmed that these three areas have warmed rapidly in the period 1976–2000.
Figure 1.
The Antarctic Peninsula. The 1000 m isobath marks the conventional transition from the continental shelf to the continental slope round Antarctica, and the 3000 m isobath marks an arbitrary transition from the slope to the abyssal plain.
Although relatively few meteorological records from Antarctic stations extend further than 50 years, those that do are a particularly important indicator of climate change (Vaughan et al. 2003). An unweighted mean of the trends determined for the continental stations is 0.6±1.5 K (century)−1. Although almost identical to the global mean warming during the twentieth century (0.6±0.2 K; Houghton et al. 2001), this mean figure masks considerable variability between sites. There is no significant trend, either warming or cooling, along much of the coast of East Antarctica and for some sites the record suggests a cooling trend (e.g. Halley and Amundsen–Scott at South Pole; Turner et al. 2005). In contrast, the mean warming for the Antarctic Peninsula stations is 3.7±1.6 K (century)−1 unweighted, and 3.4 K (century)−1 when weighted by length of record (Vaughan et al. 2003). The causes of this warming are not completely understood, and coupled climate models (including those forced with greenhouse gases) currently fail to reproduce it (King et al. 2003). However, recent data have shown a strong correlation between regional atmospheric circulation and air temperature in Antarctic Peninsula, and it seems likely that the observed trend towards higher temperatures has been accompanied by a shift towards more cyclonic atmospheric circulation (Turner et al. 2005). Turner et al. (2006) have also reported a significant warming of the troposphere over Antarctica in winter.
The warming over the Antarctic Peninsula has had a profound influence on the terrestrial ice sheet. The annual duration of melting conditions has increased markedly on the Antarctic Peninsula (Vaughan 2006) and the majority of glaciers in this region have retreated during the past 50 years, with the average rate of retreat accelerating (Cook et al. 2005). There have also been spectacular collapses of several ice sheets, including the Wordie Ice Shelf in the 1980s (Doake & Vaughan 1991), the northern part of the Larsen Ice Shelf (Larsen A) and the small ice shelf in Prince Gustav Channel in 1995, and the middle section of the Larsen Ice Shelf (Larsen B) in 2002. Overall, the rapid regional warming of the Antarctic Peninsula has resulted in the loss of seven ice shelves in the past 50 years (Vaughan & Doake 1996).
Significant changes have also been observed in sea ice adjacent to the western Antarctic Peninsula (WAP). Comparison of satellite data with the sparse ship observations taken during the middle of the twentieth century has suggested a reduction in the spatial extent of winter sea ice in the Bellingshausen Sea (King & Harangozo 1998). Satellite data have indicated a shortening of the sea ice season in the Bellingshausen and Amundsen seas during the period 1979–1999, and a significant decrease in cover of 9.7±1.5% per decade (Jacobs & Comiso 1993; Parkinson 2002; Zwally et al. 2002). This is the only sector of the Antarctic to show a significant decrease. The data for the WAP area itself have revealed a 40% reduction in annual mean sea ice extent over a 26-year period, caused principally by a reduction in the duration of winter sea ice (Smith & Stammerjohn 2001).
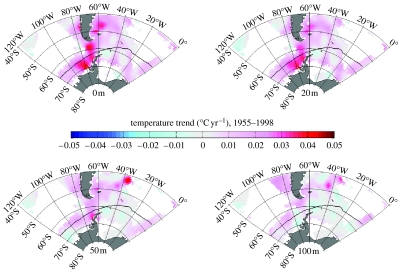
The precise role of the ocean in the regional climate change of the Antarctic Peninsula remains unclear, although there is increasingly strong evidence for linkage between oceanic processes, sea ice and atmospheric cyclonic activity (Yuan & Martinson 2001; Marshall et al. 2004; Lachlan-Cope 2005; Harangozo 2006) with teleconnection to the Pacific (Yuan & Martinson 2001; Liu et al. 2002; Yuan 2004; Ducklow et al. 2007). Unfortunately, there are relatively few direct observations of temperature change in the Southern Ocean, and those that do exist are often based on isolated observations. This makes the detection of a trend against the background of natural variability very difficult. Gille (2002) used data from Autonomous Lagrangian Circulation Explorer floats to suggest a warming of ca 0.17 K between the shipboard vertical temperature profiles taken in the 1950s and those recorded in the 1980s. These data are for depths of 700–1000 m, and they suggest that the deeper waters of the Antarctic Circumpolar Current (ACC) are warming faster than the mean rate calculated for the global ocean (which is ca 0.1 K between 1955 and 1995 in the top 1000 m; Levitus et al. 2000, 2005; Barnett et al. 2005). Robertson et al. (2002) reported a warming of ca 0.012 K yr−1 in Weddell Deep Water (WDW) from the 1970s to the 1990s, and more recently Smedsrud (2005) has reported a warming rate of ca 0.032 K per decade in the WDW along the Greenwich meridian over the period 1977–2001. In addition, Meredith & King (2005) have detected a profound warming of the summer ocean surface in the Bellingshausen Sea during the second half of the twentieth century (more than 1 K since the 1950s; figure 2). This marked surface warming has been accompanied by a significant increase in the salinity of the summer ocean surface (more than 0.25 since the 1950s), as a result of mixed layer processes driven by reduced sea ice formation (Meredith & King 2005).
Figure 2.
Trends in ocean summer surface temperature over the period 1955–1998, for four different depth levels (surface 20, 50 and 100 m). Grid cells with no data are left white. Note that the marked warming trend observed close to the Antarctic Peninsula is strongly intensified towards the surface, and decays to almost 0 by 100 m depth. Reproduced, with permission, from Meredith & King (2005).
In this paper, we review the effects of this rapid regional climate change on the marine ecosystem, developing themes discussed previously (Clarke & Harris 2003), and providing a more specific context for the general review of Smetacek & Nicol (2005). We confine ourselves to the waters overlying the continental shelf to the west of the Antarctic Peninsula, from Marguerite Bay (approx. 68 °S) to the Scotia Sea (approx. 60 °S), and in doing so refer extensively to the work of the Palmer Long-Term Ecological Research (Pal-LTER) programme (Smith et al. 2003; Ducklow et al. 2006, 2007). We also draw on examples from the Antarctic terrestrial environment; this is because the signal of regional climate change is far stronger on land than in the sea, and the responses of the biota are more clearly established. While not all of these will be relevant to the marine system, they do provide a powerful pointer as to what types of response we should be looking for.
(a) The oceanographic setting
The properties of the surface waters of the Southern Ocean are dominated by exchange with the atmosphere, sea ice dynamics and interaction with the deeper water (Klinck 1998; Smith & Klinck 2002). The oceanic source water for coastal waters around Antarctica is Circumpolar Deep Water (CDW), the voluminous warm saline water mass that occupies the mid-levels of the ACC. This is usually divided into upper (shallower) and lower components (UCDW and LCDW, respectively). The UCDW is characterized by a maximum in potential temperature, relatively high nutrient contents and a low oxygen content (Hofmann et al. 1996; Meredith et al. 2004). The uppermost waters over the continental shelf interact with the atmosphere and cryosphere, and are typically very much colder than the UCDW. They are traditionally referred to as Antarctic Surface Water (AASW); they typically cool and gain salt from sea ice formation as summer moves into winter, then warm and freshen from winter to summer (Klinck 1998).
Thus, the AASW is thermally highly variable and solar heating can lead to strong stratification with very warm surface layers (e.g. sometimes exceeding 2°C, and occasionally reaching 5°C in Marguerite Bay; Meredith et al. 2004; British Antarctic Survey 1997–2006, unpublished observations). It is these surface waters that have recently been shown to be experiencing a rapid warming in the WAP area (Meredith & King 2005). Beneath the surface layers, typically below, ca 20 m, AASW shows a strong seasonality in temperature, but rarely exceeds +2°C. This seasonality is seen down to depths ca 100 m, the approximate maximum depth of the winter mixed layer (Meredith et al. 2004). As winter progresses into summer, this cold deep layer is capped by warmer, fresher waters, resulting in a subsurface temperature minimum that is commonly known as winter water (WW; Mosby 1936). Winter water temperatures are usually very close to the freezing point, but over the continental shelf of the WAP, vertical mixing with the warmer waters above leads to higher temperatures (Ducklow et al. 2007). The coastal oceanography around Antarctica is thus dominated by the AASW at depths down to ca 100 m, including WW at the lower levels.
The area of the WAP is unusual in that the southern boundary of the ACC abuts the continental shelf and thus is not separated spatially from the continental shelf by the presence of a subpolar gyre (as typifies the Weddell and Ross seas). This close proximity of the ACC to the continental shelf allows UCDW to flood onto the shelf, principally through glacially carved canyons (Hofmann & Klinck 1998; Klinck 1998; Dinniman & Klinck 2004). These incursions of UCDW are important in bringing heat and nutrients onto the continental shelf, and may influence primary production and the operation of the food web (Smith et al. 1998; Prezelin et al. 2000). They may also be important in influencing the production of meltwater from beneath floating ice shelves, and hence in the dynamics of the shelves themselves (Jacobs et al. 1996). Over the period 1993–2002, UCDW flooded large areas of the WAP continental shelf in most years, and this appears to be a reasonably consistent feature of shelf oceanography in this region (Ducklow et al. 2007).
The continental shelf around Antarctica is unusually deep (Clarke & Johnston 2003). As a result, most of the shelf is well below the AASW. This is a different situation from that on most continental shelves elsewhere, where the seabed is frequently within, or close to, the depth of the seasonal mixed layer. For the continental shelf benthos around Antarctica, there are thus two depth ranges which differ significantly in their thermal characteristics and hence their likely response to rapid regional warming. The first is the depth range of the seabed that is bathed in AASW (from the surface to approx. 100 m). Here, organisms will be subject to thermal variability over a range of temporal scales (Clarke 2001) with a strong seasonal signal at many sites. At depths below this, the seabed will be thermally more stable, though subject to changes associated with intermittent incursions of relatively warm UCDW onto the shelf.
A second important factor in the oceanography of the WAP area is the influence of glacial meltwater. Seasonal variability in the volume and spatial extent of glacial meltwater play a critical role in oceanic ecosystem processes, and particularly primary production (Dierssen et al. 2002). Water column stability and a shallow mixed layer are essential to phytoplankton bloom development (Mitchell & Holm-Hansen 1991); adding a thin lens of freshwater to the ocean surface will greatly increase its stability, hence enabling phytoplankton to remain within a favourable light environment by preventing mixing to depths where light is limiting. In the WAP region, lower salinities are typically associated with a transition from a diatom-dominated system to one dominated by smaller cryptophytes (Moline et al. 2004).
(b) The historical context
It is important to set the recent rapid regional warming of the Antarctic Peninsula in a historical context. The coastal marine ecosystem of Antarctica is subject to variability on a range of time-scales, from tidal to geological (Clarke 2001). In order to understand the significance of current regional climate change, we need to distinguish the relative importance of the various processes operating over the different time-scales. On the geological time-scale, the nearshore marine environment of Antarctic has cooled from the warmth of the late Cretaceous to the present polar conditions (Lear et al. 2000; Zachos et al. 2001). Although the overall trajectory of the climate has been one of cooling, this has been interrupted by episodic warming, such as the Late Palaeocene thermal maximum which appears to be related to a massive release of methane from marine sediment clathrates (Dickens 2000; Zachos et al. 2003), and in the Pliocene.
At the shorter time-scales, ecologists have tended to concentrate on variability in the range from seasonal to decadal (Clarke 1988; Murphy et al. 1995; Ainley et al. 2005), whereas evolutionary biologists are concerned more with Milankovitch climate cycles or global climate change over millions of years (Clarke & Crame 1989, 1992, 2003; Clarke et al. 2004a,b). Only recently has attention been directed at time-scales intermediate between these two, primarily using evidence from the climate record in ice cores and marine sediments. Recent ice cores have provided insights into variability over the last eight glacial cycles (Wolff et al. 2006), and sediment cores have revealed variability in the WAP marine system on time-scales ranging from less than a hundred to tens of thousands of years. Thus, the record of magnetic susceptibility from sediments in the Palmer Deep reveals variability in the silt to clay ratio and microfossil composition at frequencies of 1800, 400, 200, 100 and 50 years (Leventer et al. 1996). This variability has been interpreted as indicative of changes in production, linked to the extent of glaciation, long-term variability in the dynamics of the ACC, principally flux of UCDW onto the continental shelf and solar variability (Warner & Domack 2002).
These important climate records also reveal the mid-Holocene warm period (ca 9000–6700 years BP) when subpolar diatoms appear in the sediment record (Leventer et al. 2002). At this time, neither the Prince Gustav Channel nor the King George VI ice shelves existed (Pudsey & Evans 2001; Bentley et al. 2005), and the Ross Sea region of Antarctic was also experiencing a warm period (Lyons et al. 1997). These records are also important in indicating clearly that despite a long history of variability, the current rate of atmospheric warming is unprecedented in the recent geological record (Domack et al. 2005a).
2. Predicted environmental changes along the western Antarctic Peninsula
Although the air temperature of the northern Antarctic Peninsula has been increasing over the past 50 years, in the absence of a firm understanding of the mechanism, we cannot predict future climate with any degree of certainty. There are two opposing hypotheses as to the cause of the recent warming, namely that the observed warming is a response to changed climate forcing (principally greenhouse gases), or that it reflects natural internal variability of the climate system. On a global scale, there is very strong evidence from models and observations that we can reject the internal variability hypothesis with some confidence (Folland et al. 2001). However, on a regional scale, this becomes increasingly difficult and until we can reproduce the observed warming in models, we cannot entirely rule out the possibility that the recent climate warming of the Antarctic Peninsula region is a function of natural internal variability. The present generation of general circulation models (King et al. 2003) do not reproduce this regional warming and hence we are constrained in our ability to predict future climate in the region.
Warming trends for the northern Antarctic Peninsula, as described by linear trends fitted to data for 1950–2000, are 0.109±0.085 K yr−1 in winter and 0.027±0.016 K yr−1 in summer. If these rates of warming continue, then a simplistic forward projection would suggest that mean winter air temperatures in the northern Antarctic Peninsula will be above 0°C by 2100. This is, however, a very unrealistic prediction because it would suggest that winters will then be warmer than summers. We know that the present rate of winter warming cannot continue indefinitely, because winter temperatures in the WAP region are controlled strongly by sea ice extent in the Bellingshausen Sea, and once this ice has gone, further winter warming cannot be sustained (Lachlan-Cope 2005). Simple unconstrained forward projection of current seasonal warming trends is thus not a meaningful way of predicting future climate.
If we cannot reject natural internal variability as the cause of regional warming, then a plausible scenario for any time in the future would be that conditions will lie somewhere within the range of variability observed to date (although this assumes that the historical record has sampled all possible states of variability in the system). However, it seems unlikely that the future climate of the Antarctic Peninsula will be determined by natural variability alone. Increasing concentrations of greenhouse gases are expected to lead to varying degrees of surface warming over most of the globe, and while the Antarctic Peninsula is unlikely to be immune from such warming, predicting future climate for this region is far from straightforward.
More reliable predictions can be made on the basis of IPCC scenarios for global climate change. IPCC Scenario A2 (a heterogeneous world with continuously increasing population) would suggest annual mean air temperatures in the northern Antarctic Peninsula of +0.2°C in 2100, an increase of 3–4 K over the Faraday mean for the period 1961–1990. IPCC Scenario B2 (slower population increase and technological development than Scenario A2) would suggest an annual mean air temperature of −0.8°C in 2100, an increase of 3 K. If the Antarctic Peninsula follows the IPCC globally averaged surface temperature increase, then the mean annual air temperature for the northern Antarctic Peninsula would increase by between 1.4 and 5.8 K by 2100. These would seem to be the most soundly based predictions, but a necessary caveat is that they are based on models which cannot yet reproduce the observed regional warming of the mid-to-late twentieth century. Although these models work very well at global or larger regional scales, they fail to reproduce the details of the finer regional scales and we cannot have a great deal of confidence in their ability to forecast future climate at these scales.
The variability of these estimates indicates the degree of uncertainty in our ability to predict future climate scenarios for the Antarctic Peninsula. Nevertheless, on the basis of current evidence, it seems likely that warming will continue, and that by the end of the present century atmospheric climate will be considerably warmer than it is today. This warming will, however, continue to be spatially and seasonally heterogeneous. At present, warming is greatest in winter in the middle and lower Antarctic Peninsula (as demonstrated by the Faraday/Vernadsky and Rothera data series), whereas further north to the South Shetland Islands the summertime warming trend increases and the wintertime trend decreases. On the northeastern side of the Antarctic Peninsula, summertime warming exceeds wintertime warming, and is greater than the summertime warming anywhere else on the Antarctic Peninsula. It is this rapid summer warming that caused the collapse of the Prince Gustav and Larsen ice shelves, and it appears to be associated with a strengthening of the circumpolar westerlies (i.e. the Southern Annular Mode (SAM) becoming more positive; Marshall et al. 2006). Some of this strengthening may be attributed to increased greenhouse forcing (Marshall et al. 2004).
While considerable attention has been directed at the regional increase in air temperature, more relevant for the marine ecosystem are changes in seawater temperature. Unfortunately, a great deal of uncertainty surrounds any prediction of oceanic temperature changes in water west of the Antarctic Peninsula, and the current generation of global circulation models predict only very small increases in upper level temperatures close to the Antarctic Peninsula (e.g. a comparison of the greenhouse gas Hadley Centre HadCM3 model with present conditions suggests a warming of less than 0.25 K at 5 m by 2100; T. Lachlan-Cope 2006, personal communication). Measurements by the Pal-LTER programme suggest that the flux of heat from the CDW to the upper waters over the WAP continental shelf has increased in recent years (Ducklow et al. 2007; D. G. Martinson 2006, personal communication). This could be the result of small increases in the core temperature of the CDW (reported by Gille (2002) for lower latitudes), and/or increases in the flux of CDW onto the WAP continental shelf. It has been argued that this latter process might be associated with an increase in isothermal tilt across the ACC in response to increases in the strength of westerly winds over the Southern Ocean (Thompson & Solomon 2002), but at present, this remains conjecture. Nevertheless, it is generally accepted that the CDW is unlikely to be immune from the large-scale warming of the global ocean (Levitus et al. 2000).
There is also considerable uncertainty concerning future upper-layer ocean warming over the WAP continental shelf. The warming demonstrated by Meredith & King (2005) is clearly dependent on associated changes in the atmosphere and sea ice fields, and it is a logical presumption that continued atmospheric warming and sea ice retreat will be linked with further warming of the summertime surface ocean. The magnitude of such warming is a matter for conjecture, but based on the trend observed in the second half of the twentieth century (a summer ocean warming of more than 1 K associated with an increase in annual mean air temperature of nearly 3 K), then a further increase in mean annual air temperature of between 1.4 and 5.8 K (see above) could be associated with a further increase of 1–2 K in summer surface oceanic temperature. This prediction is, however, surrounded by so many uncertainties that it is little more than an educated guess.
It is also likely that the Bellingshausen and Amundsen seas will continue to experience reductions in ice cover, enhancing those changes already apparent (Smith et al. 1996, 2003; Stammerjohn & Smith 1997; Smith & Stammerjohn 2001). The timing of the changes in sea ice dynamics will be important, for these will influence when sunlight reaches the underlying water column to drive primary production. Such changes will also have powerful consequences for those organisms that depend on sea ice as habitat.
The widespread retreat of glaciers and the collapse of ice shelves will expose increasingly large areas of coastal water to sunlight and hence will increase the total volume of seawater supporting primary production and thereby driving the oceanic food web (both pelagic and benthic). Although it has long been recognized that benthic and demersal organisms exist under ice shelves, sometimes at substantial distances from the open water (Littlepage & Pearse 1962; Heywood & Light 1975; Lipps et al. 1977, 1979; Hain & Melles 1994; Domack et al. 2005b), these populations must be sustained by particulate organic material advected from open water by currents. The collapse of ice shelves does, however, expose new areas of coastal ocean to sunlight for primary production, either in the water column immediately above or, in shallower areas, on the seabed itself. A final consideration is that an increase in the flux of meltwater from the land will influence water column stability, and likely also the availability of essential micronutrients such as iron, both of which will promote primary production. However, it should be noted that while limitation of phytoplankton production by micronutrient limitation has been noted for Antarctic shelf waters in the Ross Sea (Sedwick et al. 2000), at present we lack measurements of micronutrients for the WAP region.
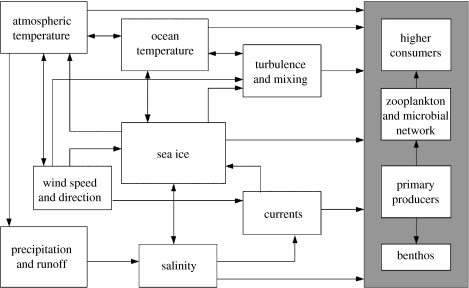
The complexity of the interactions between the physical environment and biological processes (figure 3) makes it almost impossible to predict the net outcome of these many and varied changes. Thus, we should be wary of simplistic predictions that regional warming will lead to particular ecosystem or organismal responses. However, there is some general agreement that enhanced stability of the water column from increased freshwater input is likely to increase primary productivity; however, where decreased sea ice cover allows a greater deepening of the summer wind-mixed layer, production may decrease with a shift in balance from diatoms to smaller flagellates (Walsh et al. 2001).
Figure 3.
Conceptual diagram illustrating the variety of physical environmental factors forcing biological processes in the Southern Ocean, and emphasizing the central importance of sea ice to the western Antarctic Peninsula oceanic ecosystem. Only the key forcing factors are shown. Modified from Barange (2002).
The regional warming being experienced by the Antarctic Peninsula can be envisaged as a southward progression of isotherms. The coupling between air temperature, sea ice dynamics and both terrestrial and oceanic ecology leads to a general prediction that there will be an associated southward migration of those ecosystem features which exhibit a meridional variation. Examples might include plant diversity on land (Peat et al. in press) and those aspects of the nearshore marine system tied intimately to temperature or ice (Ducklow et al. 2007).
3. Environmental variability and ecological response
Having established as best we can how the marine environment of the WAP may change over the next century or so, the obvious question is how will the marine ecosystem respond? We will examine this question at three different scales of ecological organization, namely individuals, populations and the ecosystem as a whole; but first we make some general points to set the scene.
There are two aspects of environmental variability that are relevant to any discussion of the impact of the present regional warming on the marine ecosystem of Antarctica. This first is that the fauna we observe today is the result of a long period of climatic cooling from a warm, ice-free, marine environment in the Late Mesozoic (Lear et al. 2000; Zachos et al. 2001). Associated with this cooling was the onset of continental glaciation. The growth of the continental ice sheet resulted in the loss of many nearshore habitats, and a marked drop in diversity of some taxa (e.g. teleost fish and decapods) coupled with a radiation of others (e.g. amphipods, isopods and echinoids; Clarke & Crame 1989, 1992; Poulin et al. 2002; Clarke et al. 2004a,b). However, the cooling has been interrupted by warmer periods; for example, a variety of evidence has indicated that the Antarctic experienced significant warming during the Holocene (Lyons et al. 1997; Jones et al. 2000; Leventer et al. 2002; Warner & Domack 2002; Emslie et al. 2003; Emslie & Woehler 2005). This period was characterized by the absence of some ice shelves, the presence of subpolar diatoms in sediment cores, and a more southerly distribution of penguin colonies (Emslie & McDaniel 2002). The latter indicates a quite different pattern of ice and open water in the relatively recent past. Conversely, molecular evidence indicates that at the last glacial maximum Ade´lie penguins, Pygoscelis adeliae, were restricted to a small number of locations, from which they have subsequently recolonized Antarctica as the ice receded (Ritchie et al. 2004). Clearly, the Antarctic Peninsula has experienced widespread and significant climate change in the relatively recent past, and at least some parts of the flora and fauna have responded with changes in distribution, both geographically and bathymetrically (Brey et al. 1996).
The second aspect is that the population dynamics of the current fauna has been selected to cope with the strong interannual variability characteristic of many aspects of the marine environment. Particularly important in the Southern Ocean is variability in sea ice and ocean associated with the El Niño/Southern Oscillation (ENSO; Murphy et al. 1995; Marshall & King 1998; Harangozo 2000; Yuan & Martinson 2001; Kwok & Comiso 2002; Meredith et al. 2004), and the SAM (Hall & Visbeck 2002; Marshall et al. 2004). These large-scale processes induce a strong interannual variability in the recruitment and growth of both pelagic and benthic organisms (Ross et al. 2000; Quetin & Ross 2003; Grange et al. 2004). While organisms are adapted to cope with historical levels of such variability, it is not clear that they will necessarily be able to withstand a significant increase in the frequency of years with low recruitment or slow growth, such as might be induced by an increase in the frequency of ENSO events.
(a) Biotic responses to climate change: some general points
If the extent of environmental variability is small, then many organisms can cope with a variety of physiological adjustments (Hochachka & Somero 2002; Peck 2005a,b). When a population of organisms experiences an environmental challenge outside the normal range of phenotypic variability, they may respond in one of the three ways (Clarke 1996):
Migration: the species shifts to a more favourable area.
Adaptation: the species evolves to shift the phenotypic reaction norm to match the new environment.
Extinction: the species fails to adapt or migrate, and becomes extinct.
The recent fossil history of marine organisms provides examples of all the three. Thus, the Pleistocene has been characterized by climate variability on Milankovitch time-scales, with major changes in global sea-level driven by variation in the size of the polar icecaps (Dynesius & Jansson 2000; Jansson & Dynesius 2002). Along the linear Pacific coast of the United States, the principal response of the shallow-water fauna has been to migrate (Valentine & Jablonski 1991; Roy et al. 1995, 1996). This was possible because the organisms had somewhere to migrate to, and the current fauna appears to be responding in the same way to current climate change (Barry et al. 1995; Sagarin et al. 1999). Similar changes are also underway in the intertidal faunas of Northwest Europe (Southward et al. 1995, 2005; Lima et al. 2006; Mieszkowska et al. 2006). By contrast, in the scattered archipelago of the Indo-West Pacific changes in global sea-level have had major consequences for the extent and distribution of shallow-water habitat, and here a widespread response to Milankovitch-driven climate cycles has been diversification (Palumbi 1996, 1997; Briggs 2003). Thus, present evidence suggests that the response of a particular marine fauna to climatic change is dependent critically on its geographical environment: linear coasts offer the possibility of migration, scattered archipelagos tend to fragment ranges and thereby drive speciation and extinction. In the Southern Ocean only along the coasts of Victoria Land and the Antarctic Peninsula do organisms have linear coasts along which to migrate.
The continental shelf fauna of Antarctica is isolated from the rest of the world by temperature, strong frontal systems associated with the ACC, and a wide expanse of deep ocean. This isolation, although strong in comparison with that of continental faunas elsewhere, is not complete (Clarke et al. 2005; Barnes et al. 2006a; Lewis et al. 2006). While oceanographic mechanisms for transporting zooplankton and larval benthic invertebrates into and out from Antarctica clearly exist, the critical factor in determining changes in biodiversity in response to climate change is whether propagules transported to new habitats can survive there. This is analogous to the Antarctic terrestrial environment, where a major limitation to change in diversity is not the arrival of new propagules, but suitable conditions for the establishment of viable populations (Clarke 2003a; Convey 2003). For marine organisms, a major factor is the temperature sensitivity; can new arrivals to Antarctica survive the low temperatures, and can the existing fauna cope with increases in water temperature?
4. Responses of individual marine species to climate change
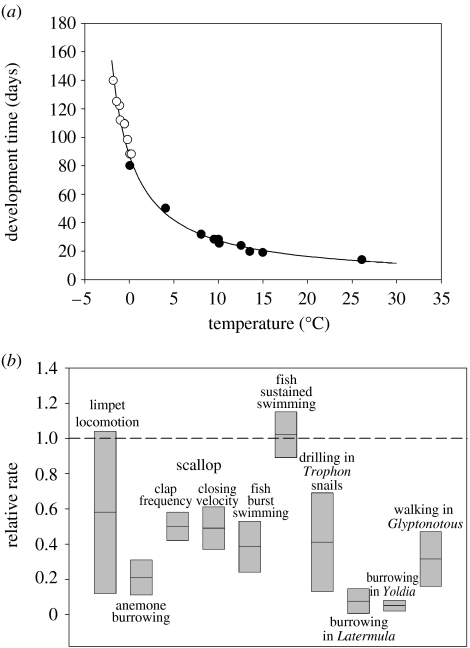
It is now well established that marine organisms adapted to low temperatures typically exhibit physiological performances below those of related species from warmer water (Clarke 1983, 2003b; Peck 2005a,b). Examples of this temperature limitation of performance include growth (e.g. Clarke et al. 2004a,b; Heilmayer et al. 2004; Barnes et al. under review), development rate (Bosch et al. 1987; Ross et al. 1988; Stanwell-Smith & Peck 1998; Peck et al. 2006) and locomotor activity (Peck et al. 2004a,b; figure 4). In part, this reduced performance is caused by limitations in the system itself, and in part by limitations in metabolic energy supply (Clarke 2003b). This might imply that a warming of sea temperature could be beneficial by increasing the ability of many organisms to perform physiologically.
Figure 4.
Temperature limitation of physiological performance in Antarctic marine ectotherms. (a) Development rate in echinoderm embryos from polar, temperate and tropical locations (redrawn from Bosch et al. 1987 and Stanwell-Smith & Peck 1998). (b) Comparative rate of various locomotor activities in polar marine ectotherms compared with a range of temperate water relatives. Data are expressed as a ratio, such that a value of 1 indicates equality of rates in polar and temperate species, and a value less than 1 indicates a slower rate in the polar species. The size of the box indicates the range of values observed, and the midline indicates the mean (redrawn from Peck et al. 2004b).
The reason this is not necessarily so is that in many species a concomitant of adaptation to low temperature is a significantly reduced ability to tolerate increased temperature (i.e. they are stenothermal; Somero & DeVries 1967). It is not clear whether a stenothermal physiology is an inevitable consequence of adaptation to a low temperature, or selection for a low-energy lifestyle (Clarke 2003b), but it does render at least some members of the marine fauna vulnerable to rising environmental temperatures. Although the early studies involved relatively few species and typically brief acclimation periods (Somero & DeVries 1967), recent work has confirmed that in a wide range of taxa whole-organism physiological performance is significantly impaired at temperatures only slightly above those experienced in the field (figure 5). There is increasing evidence that at least part of this impaired performance at elevated temperatures is caused by a reduction in the ability of the organism to supply oxygen for the generation of ATP by mitochondria (Pörtner et al. 1999; Pörtner 2001; Peck et al. 2002, 2004a,b; Peck 2005a,b).
Figure 5.
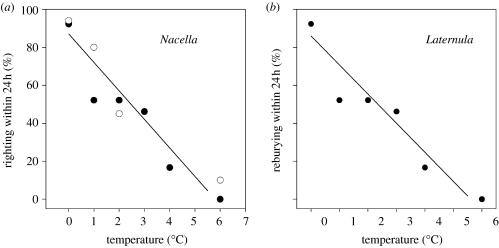
The effect of temperature on performance in two Antarctic marine invertebrates. (a) The limpet Nacella concinna; data show the proportion of limpets capable of righting within 24 h when turned over. The different symbols indicate experiments undertaken in 2001 (open circles) and 2002 (filled circles). (b) The infaunal bivalve Laternula elliptica; data show the proportion of individuals capable of successfully reburying within 24 h when removed from the sediment. In both cases, the line shows the least-squares regression fitted following arcsine transformation of the data (redrawn from Peck et al. 2004a,b).
These results are important in that they emphasize ecologically significant sublethal effects at temperatures below the physiological limits established by traditional thermal tolerance experiments (e.g. determination of LD50 values). Recent work has also suggested that Antarctic species may lack the otherwise universal heat-shock response, whereby organisms respond to a thermal challenge (either cooling or warming) by the expression of a class of stress or chaperone proteins (Hofmann et al. 2005). It is possible that this is because adaptation to the very low temperatures of the Southern Ocean requires continuous expression of some types of chaperone protein; this would thus constitute an additional cost to low-temperature adaptation, and also potentially limit the ability of Antarctic organisms to respond to additional thermal challenges. This intriguing area needs further research.
Although work to date has been restricted to a fairly limited range of taxa and short-term acclimation periods, these results clearly indicate that in the absence of any acclimation or evolutionary adjustment in physiological performance, many subtidal Antarctic organisms will be severely impacted by even very small changes in temperature. The Antarctic intertidal environment fauna is proving to be more diverse than expected (Waller et al. 2006), and these organisms are regularly subject to considerably wider daily, tidal and seasonal variability in temperature than subtidal organisms. This poses the question of the extent to which we might expect Antarctic marine species to adapt evolutionarily to the projected environmental change.
(a) Acclimation and evolutionary responses to environmental change in Antarctic marine organisms
Although it is now clear from short-term experiments that many marine Antarctic ectotherms suffer an impaired physiological performance at temperatures only slightly above those experienced in the environment, we know very little about their ability to acclimate in the longer term. It has frequently been assumed that Antarctic marine organisms have a relatively poor ability to acclimate to warmer temperatures, and there are a few studies that provide support for this. Thus, Bailey (2001) was unable to acclimate the Antarctic scallop Adamussium colbecki to 4°C, at which temperature 50% mortality had occurred within 19 days. However, a recent study has suggested that for some Antarctic species, the ability to acclimate to higher temperatures may have been underestimated (Seebacher et al. 2005). Furthermore, the marine fauna in the shallows around the island of South Georgia includes many typical Antarctic species (Barnes et al. 2006d). Nearshore shallow seawater temperature around South Georgia typically varies between −1 and ca +4°C (Barnes et al. 2006c); endemic Antarctic organisms at South Georgia thus live at temperatures typically 2 K above those along much of the WAP. At present, however, we have insufficient empirical data to decide whether or not Antarctic marine organisms in general are likely to be able to acclimate to warmer temperatures under the rates of change they are likely to experience in the future.
Neither do we know the likelihood that they will be able to adapt evolutionarily. Lynch & Lande (1993) examined the possibility of evolutionary change in the face of environmental temperature change using a population genetics model and a number of simplifying assumptions. They concluded that populations would be able to adapt to track moderate rates of environmental change, given sufficient genetically determined phenotypic variance in the population. The thermal capacity of water means that aquatic habitats tend to change temperature far more slowly than the terrestrial realm, and this might suggest that environmental change in the marine ecosystem of the Antarctic Peninsula would probably be sufficiently slow not to be problematical for most species; however, in the absence of firm data on population structure in respect of phenotypic physiological performance, this remains a speculative conclusion. A further unknown is the extent to which high rates of geneflow through larval dispersal may limit the ability of local populations to adapt to changing circumstances, although local oceanographic conditions will also influence the spatial patterns of such geneflow.
In many intermediate latitudes, where environmental temperature typically varies strongly seasonally, one widely observed consequence of global climate change has been a change in the timing of key life cycle events (phenology). The observation of changes in phenology in many terrestrial species (Fitter & Fitter 2002; Parmesan & Yohe 2003; Root et al. 2003; Visser et al. 2004) has led to concerns that ecosystems may become disrupted by the development of mismatches between previously synchronized events (Thomas et al. 2001; Edwards & Richardson 2004). The likelihood of significant life cycle changes in an Antarctic diving beetle has already been indicated (Arnold & Convey 1998; Convey 2000, 2003), and a possible long-term change in the physiological condition of a terrestrial arthropod also reported (Block & Convey 2001). However, in the Antarctic marine system, seasonal temperature changes are typically very small, and it currently seems unlikely that ecosystem mismatches caused by differential changes in phenology will be a major problem in the WAP system, except for those species where key life cycle events are tied intimately to sea ice. Here, changes in dynamics, and in particular changes in the timing of sea ice formation and melt, have the potential to affect the phenology and population dynamics of ice-dependent organisms significantly.
In general, we must conclude that current evidence suggests that the projected rates of environmental change for the oceanic system of the WAP may cause physiological problems for at least some species. However, species do not live in isolation, and competitive effects frequently limit the distribution of species to regions more restricted than those predicted purely on the basis of thermal tolerance (for a discussion of this in relation to climate change see Clarke 1996; Chown & Clarke 2000). Therefore, we also need to consider responses to environmental change at the assemblage or community level.
5. Community level responses to climate change
While a physiological understanding of individual responses to environmental variability is essential (Helmuth et al. 2005), a simple bioclimate envelope approach to predicting the consequences of environmental change is incomplete (Pearson & Dawson 2003). Experimental studies have demonstrated clearly that the response of an individual species to a changed environment is modified by the presence of competitive species (Davis et al. 1998). Therefore, we cannot consider species in isolation; we must examine the impact of environmental change on the community as a whole. Unfortunately, it is this level, between populations and ecosystems, for which we have least information in Antarctica.
In the terrestrial environment, warming of local areas by a few degrees has produced dramatically different community composition and biomass (Convey et al. 2002). Atmospheric warming along the Antarctic Peninsula is likely to increase available habitat for terrestrial and intertidal communities, and increases in the populations of some plants have already been reported (Fowbert & Lewis Smith 1994; Lewis Smith 1994, 2001). Continued warming will also increase the possibility of colonization, and changes have been observed in aspects of population dynamics (Lewis Smith & Convey 2001).
In both the terrestrial realm and the intertidal and shallows of the marine environment, community development and performance will also be influenced by the increased levels of springtime UV-B radiation resulting from stratospheric ozone depletion. The complexity of the interactions between temperature, ice cover, light and UV radiation makes prediction of the underlying community dynamics exceptionally difficult. It does, however, appear likely that the identity of the dominant species in benthic communities, and hence overall assemblage structure, may change in response to variations in temperature and food availability.
It is also likely that changes in disturbance by ice will affect the assemblage structure of benthic communities. Increased ice scour following further ice shelf collapse will be likely to increase patchiness of shallower shelf areas, and the intensity of ice scour has been shown to influence the cover, diversity and taxonomic composition of subtidal encrusting communities in Marguerite Bay (Brown et al. 2004). Studies of the intertidal and shallow subtidal encrusting communities in the Arctic have shown that while pioneer and dominant species are similar across large scales, the precise details of succession vary markedly between sites (Barnes & Kuklinski 2004). These results indicate that changes in the frequency and intensity of ice scour are likely to have significant effects on the diversity and assemblage composition of the benthic fauna, with associated changes in ecosystem function.
6. Ecosystem level responses to climate change
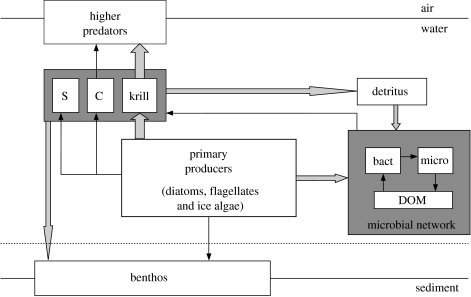
The basic structure of the Southern Ocean food web is similar to that of oceanic food webs elsewhere (figure 6). The fundamental topology is branched, with significant pathways from primary producers to zooplankton herbivores, to the microbial loop (Pomeroy 1974, 2000; Azam et al. 1983), and to the benthos. The zooplankton herbivores include copepods and salps in addition to Antarctic krill, Euphausia superba, and each of these may dominate production and hence energy flow in different places at different times. The suite of higher predators includes pelagic groups, such as fish, squid and whales, together with numerous seabirds and seals that are land based for breeding and hence act as central-place foragers. The food web thus varies both spatially and temporally, and in many places is driven by advective processes associated with the powerful ACC (Hofmann & Murphy 2004). This is quite different from the traditional picture for the Southern Ocean of a short, linear, food chain incorporating just two steps linking phytoplankton through krill to a suite of higher predators that includes the largest organisms ever to exist on Earth. The dominant pathways for the continental shelf food web of the WAP area in summer have been estimated from inverse modelling (Ducklow et al. 2006), and are shown here in a simplified form (figure 6). These indicate that despite the branched nature of the food web overall, in summer, energy flow tends to be dominated by the flux from phytoplankton through krill to a range of higher predators.
Figure 6.
The basic structure of the Southern Ocean food web. Note that the fundamental topology is branched, with carbon fixed by primary producers being used by three principal and competing pathways (to zooplankton consumers, the microbial network and the benthos). The arrows show only the major routes for energy flow, with the size indicating the dominant pathways for the western Antarctic Peninsula (WAP) oceanic food web over the continental shelf in summer, based on the inverse modelling results of Ducklow et al. (2006). The major route for energy flow is from phytoplankton to zooplankton consumers (and predominantly Antarctic krill) and thereby to higher predators, with secondary pathways to the microbial network both directly from the primary producers and also from zooplankton via the detrital pathway. In the WAP area, flux to copepods and salps in the zooplankton, or directly to the benthos, are important but secondary. Note that in other areas of the Antarctic, and possibly at other times in the WAP area, the relative importance of the major pathways will be different. Modified from Clarke & Harris (2003).
To date, most discussions of the likely impact of regional warming on the Antarctic oceanic ecosystem have tended to concentrate on the consequences for Antarctic krill, or their dependent predators. While these are undoubtedly important, there may well be more subtle effects on the food web as a whole. Patterns of energy flow through food webs can be dominated by a small number of species (as in the WAP in summer; figure 6), and perturbation of these strong interactions has the potential to cause dramatic changes in communities. However, there is increasing evidence that the network of many weak interactions play an important role in stabilizing communities (Berlow 1999) and also that the degree of omnivory (defined as feeding at more than one trophic level) exerts a strong effect on ecosystem functioning and stability (Bruno & O'Connor 2005). Perturbation to these weak interactions, or extinction of some of the species participating in them, thus has the potential to cause significant disturbance to food-web structure and dynamics.
Critical to the outcome of any environmental perturbation will be what particular species do in the food web; disturbances to primary producers for example have the potential to impair ecosystem processes and reduce the ability of the system as a whole to respond to extreme events (Lawton 1994). Loss of species elsewhere in the food web may trigger a cascade of secondary extinctions, the nature and extent of which depend on the level of connectance within the web (Eklöf & Ebenman 2006). Critical to these effects is the level of functional diversity in the food web, although there is no real consensus over how best to measure this. One extreme view is that every species is functionally unique and hence any extinction event represents a loss of functional diversity within the system. A more generally accepted view is that some species are sufficiently similar in functional terms that the overall suite of species can be divided into functional groups, such as feeding guilds (Loreau et al. 2001) or biogeochemical groups (Le Quéré et al. 2005; Hood et al. 2006). Petchey & Gaston (2002) have shown that functional group structure exerts a strong influence on the role of extinction in driving loss of ecosystem function. The Antarctic marine system, although generally richer in species than comparable Arctic systems, has a low species richness in comparison with tropical reef systems (Clarke & Johnston 2003). While the overall species abundance structure of Antarctic marine assemblages appears to be similar to that of tropical systems (Clarke in press), the Antarctic lacks the large number of rare species that characterize some tropical systems (Bouchet et al. 2002). At present, we have no idea whether this difference is important in terms of how these different systems operate or their relative resilience to environmental perturbation. There is also some evidence that the Antarctic marine food web, at least in the benthic subsystem, may be characterized by increased levels of carnivory (Taylor et al. 1980) and fewer trophic specialists than elsewhere (Dauby et al. 2001a,b).
While the absolute changes in oceanic temperature recorded to date are, in physiological terms, small, it is possible that continued warming will induce subtle sublethal effects on physiological performance which have the potential to disrupt ecological relationships. Although theoretical studies of food webs are starting to reveal important insights into stability and resilience to environmental perturbation (Martinez 1994; Johnson 2000; Fulton et al. 2003; Dunne et al. 2004), at present, we have insufficient knowledge of the structure of the Southern Ocean food web to make a judgement as to whether it is more or less likely to be able to resist climate warming. In the context of the marine food web of the WAP, we do not as yet know to what extent the flat trophic structure of benthic community in many parts of Antarctica (Jarre-Teichmann et al. 1997), the prevalence of tropic generalists, or the dominance of the pelagic food web by Antarctic krill and its predators, renders the overall system more or less resilient to perturbation.
7. What biological changes have been observed to date?
There are two problems in assessing whether the regional warming evident in the WAP area has lead to biological changes in the marine ecosystem. The first is the need to distinguish a climate-related change from the background of marked variability over a wide range of spatial and temporal scales (Dayton 1989; Murphy et al. 1995, 1998). The second is that we have relatively few ecological data series of sufficient length to detect change. Two groups of organisms, however, for which we do have some data are zooplankton (and especially Antarctic krill, E. superba), and penguins.
Research on Antarctic krill extends back over a century, and recently Atkinson et al. (2004) have demonstrated a long-term decline in the biomass of krill in the Scotia Sea sector. The reasons for this apparent decline are far from clear, though it is tempting to point to a long-term regional warming, with a consequent shift in the balance between zooplankton assemblages dominated by krill and those dominated by salps. It has long been known that there are strong interannual differences in the balance between salps and Antarctic krill as dominant herbivores in the WAP area (Loeb et al. 1997). The difficulty is distinguishing a secular change, or a switch between alternative system states, against the background of strong interannual variability. A more detailed, but shorter term, data series for the WAP area does suggest a switch in the dominance of krill and salps since 1999 (Ross et al. in press). If such a change is indeed underway, then the very different roles played in the Southern Ocean food web by krill and salps mean that significant wide-scale changes may result. Salps appear to have fewer predators at higher levels in the food web than do krill, and the potential thus exists for major shifts in the structure of the upper levels of the WAP food web.
In the WAP area, penguins are important higher predators, because of their high abundance and biomass. As mobile, long-lived predators, penguins integrate the effects of variability in the physical and biological environment over large spatial and temporal scales (Fraser & Trivelpiece 1996). Penguin populations have been monitored at various locations in the WAP area since the 1970s. During this period, there has been a long-term decline in the population of the more southerly, ice-associated, Ade´lie penguin, Pygoscelis adeliae, in the vicinity of Anvers Island, accompanied by an increase in the more northerly chinstrap (P. antarctica) and gentoo (P. papua) penguins (Fraser et al. 1992; Fraser & Hofmann 2003; Ducklow et al. 2007). Indeed, it is clear that the latter two species are relatively recent arrivals in the Anvers Island region, with founder populations being established only in 1976 and 1994, respectively (Ducklow et al. 2007) and no fossil evidence of their presence for at least the past 700 years (Emslie et al. 1998). The reasons for these population changes are not clear, but appear to be related to both regional and local influences (Fraser & Trivelpiece 1996), and similar population changes have been observed further north at Signy Island (Forcada et al. 2006). At the regional scale, population decrease in Adélie penguins appears to be linked to the decline in winter sea ice extent, whereas at the local scale, the driving factor appears to be the interaction between snowfall, landscape and reproductive success (Fraser & Patterson 1997; Patterson et al. 2003), although there are many other complicating factors (Ducklow et al. 2007). Although at a broad level, the change in penguin populations in the Anvers Island region appears to be related directly to regional climate change mediated through changes in sea ice dynamics, conversant with the hypothesis that a southward migration of isotherms is matched by a southward movement of ecosystem features, detailed studies have revealed the intricacies of the relationship at the local level.
There are very few long-term studies of benthic marine invertebrates in the WAP area, and none of sufficient length to detect putative effects of regional climate change. The only viable approach is thus to use taxa that record their individual growth history in a skeleton. Antarctic molluscs and brachiopods are often very long-lived (Brey et al. 1995) and their skeletons thus have the potential to provide a record of growth performance over the past half century, although one problem is that the variability of the Antarctic marine ecosystem can lead to the growth checks in shells being intermittent rather than annual (Peck & Brey 1996).
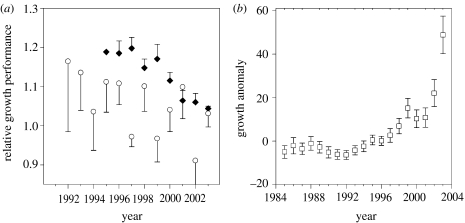
The most useful organisms for investigating past variability in growth in Southern Ocean benthos have proved to be bryozoans. Bryozoans are clonal organisms, and some erect forms record annual growth in carbonate skeletons. Since colonies may live in excess of 20 years, these skeletons can provide a valuable record of variability in growth which may then be related to environmental factor (Barnes 1995). To date, no bryozoans from the WAP area have been analysed, but Barnes et al. (2006b, under review) have studied long-term variability in the growth of three species of Cellarinella from the Weddell Sea. One species exhibited a significant increase in growth performance, whereas the other two showed a long-term decline (figure 7). Although all three species exhibited significant interannual variability in growth performance, there was no significant correlation in performance among the three taxa. Thus, although no clear climate change signal emerges from this study of three closely related taxa sampled from one region, the data would suggest that studies of long-lived organisms that leave a record of aspects of their population dynamics in their skeletons will be an important means of documenting the response of benthic organisms to past climate change.
Figure 7.
Long-term variability in growth in three species of the erect bryozoan Cellarinella from the Weddell Sea. Growth determined from number of zooids produced annually, measured only in living colonies so growth can be ascribed to both colony age and calendar year, and relative growth rate and anomaly calculated using a standard exponential growth rate model for bryozoans. (a) Cellarinella watersi (black diamonds) and C. rogickae (open circles); data presented as relative growth rate anomaly, plotted as mean and s.e. In both species, the decline in growth rate since the 1990s is significant (model 1 least-squares regression, p<0.05), but there is no significant correlation between the two species in growth performance (p>0.05). Redrawn from Barnes et al. (under review). (b) Cellarinella nutti, with data presented as absolute growth anomaly. The overall increase in growth rate since the 1980s is significant (model 1 least-squares regression, p<0.05); note the strikingly strong growth performance in 2003. Redrawn from Barnes et al. (2006b).
8. Concluding remarks
It is now clear that the regional atmospheric warming of the Antarctic Peninsula is linked to oceanographic changes. The extent of winter sea ice in the Bellingshausen and Amundsen seas has decreased at a rate of almost 10% per decade, the sea ice season has shortened, surface temperatures have increased by more than 1 K since the 1950s, and the deeper waters of the ACC have also warmed. It is inevitable that some of these changes will have had an impact on the Southern Ocean food web, though the complexity of the linkages involved means that simple conclusions based on studies of a few species over a short period of time may be misleading. It is likely, however, that organisms whose life history is tied intimately to sea ice will already have been affected. Current rates of oceanic warming are, however, slow and it is likely that far more significant pressure in the short term will come from fishing and other human activity (Clarke & Harris 2003).
Acknowledgments
This paper emanated from the work of the Antarctic Peninsula 2101 (AP2101) project at the British Antarctic Survey, which was convened to review the potential state of the Antarctic Peninsula marine and terrestrial environments at the turn of the twenty-first century. The group comprised Andrew Clarke, John Turner, John King, Eugene Murphy, Peter Convey, David Vaughan and Martin Jarvis. However, the paper also makes extensive use of the results of the Palmer Long-Term Ecological Research (Pal-LTER) programme (PIs Ray Smith and latterly Hugh Ducklow) of the US National Science Foundation, and the Rothera Oceanographic and Biological Time-series (RaTS) project of the British Antarctic Survey. Pete Convey provided extensive advice on the responses of terrestrial organisms, and Dominic Hodgson was extremely helpful with advice on Holocene climate change in Antarctica. We thank Hugh Ducklow and three referees for their constructive comments, which greatly improved the paper.
Footnotes
One contribution of 8 to a Theme Issue ‘Antarctic ecology: from genes to ecosystems. I’.
References
- Ainley D.G, Clarke E.D, Arrigo K, Fraser W.R, Kato A, Barton K.J, Wilson P.R. Decadal-scale changes in the climate and biota of the Pacific sector of the Southern Ocean, 1950s to the 1990s. Antarctic Sci. 2005;17:171–182. doi:10.1017/S0954102005002567 [Google Scholar]
- Arnold R, Convey P. The life history of the diving beetle, Lancetes angusticollis (Curtis) (Coleoptera: Dytiscidae), on sub-Antarctic South Georgia. Polar Biol. 1998;20:153–160. doi:10.1007/s003000050291 [Google Scholar]
- Atkinson A, Siegel V, Pakhomov E.A, Rothery P. Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature. 2004;432:100–103. doi: 10.1038/nature02996. doi:10.1038/nature02996 [DOI] [PubMed] [Google Scholar]
- Azam F, Fenchel T, Field J.G, Gray J.S, Meyerreil L.A, Thingstad F. The ecological role of water column microbes in the sea. Mar. Ecol. Prog. Ser. 1983;10:257–263. [Google Scholar]
- Bailey D.M. University of St Andrews; St Andrews, UK: 2001. The thermal dependence of swimming and muscle physiology in temperate and Antarctic scallops. [Google Scholar]
- Barange M. Influence of climate variability and change on the structure, dynamics and exploitation of marine ecosystems. In: Hester H.E, Harrison R.M, editors. Global environmental change. Issues in environmental sciences and technology. vol. 17. Royal Society of Chemistry; Cambridge, UK: 2002. pp. 57–82. [Google Scholar]
- Barnes D.K.A. Seasonal and annual growth in erect species of Antarctic bryozoans. J. Exp. Mar. Biol. Ecol. 1995;188:181–198. doi:10.1016/0022-0981(95)00003-A [Google Scholar]
- Barnes D.K.A, Kuklinski P. Variability of competition at scales of 101, 103, 105, and 106 m: encrusting Arctic community patterns. Mar. Biol. 2004;145:361–372. doi:10.1007/s00227-004-1320-z [Google Scholar]
- Barnes D.K.A, Hodgson D.A, Convey P, Allen C.S, Clarke A. Incursion and excursion of Antarctic biota: past, present and future. Global Ecol. Biogeogr. 2006a;15:121–142. doi:10.1111/j.1466-822X.2006.00216.x [Google Scholar]
- Barnes D.K.A, Webb K, Linse K. Slow growth of Antarctic bryozoans increases over 20 years and is anomalously high in 2003. Mar. Ecol. Prog. Ser. 2006b;314:187–195. [Google Scholar]
- Barnes, D. K. A., Fuentes, V., Clarke, A., Schloss, I. R. & Wallace, M. I. 2006c Spatial and temporal variation in shallow seawater temperatures around Antarctica. Deep-Sea Res. II53, 853–865.
- Barnes, D. K. A., Linse, K., Waller, C., Morley, S. A., Enderlein, P., Fraser, K. P. P. & Brown, M. 2006d Shallow benthic fauna communities of South Georgia Island. Polar Biol.29, 223–228.
- Barnes, D. K. A., Webb, K. E. & Linse, K. Under review. Inter-specific, -generic and morphologic comparison of bryozoan growth shows polar species are slowest, and slowing. Oecologia
- Barnett T.P, Pierce D.W, AchutaRao K.M, Glecker P.J, Santer B.D, Gregory J.M, Washington W.M. Penetration of human-induced warming into the world's oceans. Science. 2005;309:284–287. doi: 10.1126/science.1112418. doi:10.1126/science.1112418 [DOI] [PubMed] [Google Scholar]
- Barry J.P, Baxter C.H, Sagarin R.D, Gilman S.E. Climate-related, long-term faunal changes in a California rocky intertidal community. Science. 1995;267:672–675. doi: 10.1126/science.267.5198.672. doi:10.1126/science.267.5198.672 [DOI] [PubMed] [Google Scholar]
- Bentley M.J, Hodgson D.A, Sugden D.E, Roberts S.J, Smith J.A, Leng M.J, Bryant C. Early Holocene retreat of the George VI Ice Shelf, Antarctic Peninsula. Geology. 2005;33:173–176. doi:10.1130/G21203.1 [Google Scholar]
- Berlow E.L. Strong effects of weak interactions in ecological communities. Nature. 1999;398:330–334. doi:10.1038/18672 [Google Scholar]
- Block W, Convey P. Seasonal and long-term variation in body water content of an Antarctic springtail—a response to climate change? Polar Biol. 2001;24:764–770. doi:10.1007/s003000100282 [Google Scholar]
- Bosch I, Beauchamp K.A, Steele M.E, Pearse J.S. Development, metamorphosis and seasonal abundance of embryos and larvae of the Antarctic sea urchin Sterechinus neumayeri. Biol. Bull. 1987;173:126–135. doi: 10.2307/1541867. [DOI] [PubMed] [Google Scholar]
- Bouchet P, Lozouet P, Maestrati P, Heros V. Assessing the magnitude of species richness in tropical marine environments: exceptionally high numbers of molluscs at a New Caledonia site. Biol. J. Linn. Soc. 2002;75:421–436. doi:10.1046/j.1095-8312.2002.00052.x [Google Scholar]
- Brey T, Peck L.S, Gutt J, Hain S, Arntz W.E. Population dynamics of Magellania fragilis, a brachiopod dominating a mixed-bottom macrobenthic assemblage on the Antarctic shelf. J. Mar. Biol. Assoc. UK. 1995;75:857–869. [Google Scholar]
- Brey T, Dahm C, Gorny M, Klages M, Stiller M, Arntz W.E. Do Antarctic benthic invertebrates show an extended level of eurybathy? Antarctic Sci. 1996;8:3–6. [Google Scholar]
- Briggs J.C. Marine centres of origin as evolutionary engines. J. Biogeogr. 2003;30:1–18. doi:10.1046/j.1365-2699.2003.00810.x [Google Scholar]
- Brown K.M, Fraser K.P.P, Barnes D.K.A, Peck L.S. Links between the structure of an Antarctic shallow-water community and ice-scour frequency. Oecologia. 2004;141:121–129. doi: 10.1007/s00442-004-1648-6. doi:10.1007/s00442-004-1648-6 [DOI] [PubMed] [Google Scholar]
- Bruno J.F, O'Connor M.I. Cascading effects of predator diversity and omnivory in a marine food web. Ecol. Lett. 2005;8:1048–1056. doi:10.1111/j.1461-0248.2005.00808.x [Google Scholar]
- Chown S.L, Clarke A. Stress and the geographic distribution of marine and terrestrial animals. In: Storey K.B, Storey J.M, editors. Environmental stressors and gene responses. Elsevier; Amsterdam, The Netherlands: 2000. pp. 41–54. [Google Scholar]
- Clarke A. Life in cold water: the physiological ecology of polar marine ectotherms. Oceanogr. Mar. Biol. Annu. Rev. 1983;21:341–453. [Google Scholar]
- Clarke A. Seasonality in the Antarctic marine ecosystem. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1988;90:461–473. doi:10.1016/0305-0491(88)90285-4 [Google Scholar]
- Clarke A. The influence of climate change on the distribution and evolution of organisms. In: Johnston I.A, Bennett A.F, editors. Animals and temperature: phenotypic and evolutionary adaptation. Society for Experimental Biology seminar series. vol. 59. Cambridge University Press; Cambridge, UK: 1996. pp. 375–407. [Google Scholar]
- Clarke A. Benthic organisms and environmental variability in Antarctica: responses to seasonal, decadal and long-term change. Ocean Polar Res. 2001;23:433–440. [Google Scholar]
- Clarke A. Evolution, adaptation and diversity: global ecology in an Antarctic context. In: Huiskes A.H.L, Gieskes W.W.C, Rozema J, Schorno R.M.L, van der Vries S.M, Wolff W.J, editors. Antarctic biology in a global context; proceedings of the VIII SCAR biology symposium. Backhuys Publishers; Leiden, The Netherlands: 2003. pp. 3–17. [Google Scholar]
- Clarke A. Costs and consequences of evolutionary temperature adaptation. Trends Ecol. Evol. 2003b;18:573–581. doi:10.1016/j.tree.2003.08.007 [Google Scholar]
- Clarke, A. In press. Temperature and marine macroecology. In Marine macroecology (ed. K. Roy & J. D. Witman). Chicago, IL: University of Chicago Press.
- Clarke A, Crame J.A. The origin of the Southern Ocean marine fauna. In: Crame J.A, editor. Origins and evolution of the Antarctic biota. The Geological Society; London, UK: 1989. pp. 253–268. [Google Scholar]
- Clarke A, Crame J.A. The Southern Ocean benthic fauna and climate change: a historical perspective. Phil. Trans. R. Soc. B. 1992;338:299–309. [Google Scholar]
- Clarke A, Crame J.A. The importance of historical processes in global patterns of diversity. In: Blackburn T.M, Gaston K.J, editors. Macroecology: concepts and consequences. vol. 43. Blackwell; Oxford, UK: 2003. pp. 130–151. [Google Scholar]
- Clarke A, Harris C.M. Polar marine ecosystems: major threats and future change. Environ. Conserv. 2003;30:1–25. doi:10.1017/S0376892903000018 [Google Scholar]
- Clarke A, Johnston N.M. Antarctic marine benthic diversity. Oceanogr. Mar. Biol. Annu. Rev. 2003;41:47–114. [Google Scholar]
- Clarke A, Aronson R.B, Crame J.A, Gili J.-M, Blake D.B. Evolution and diversity of the benthic fauna of the Southern Ocean continental shelf. Antarctic Sci. 2004a;16:559–568. doi:10.1017/S0954102004002329 [Google Scholar]
- Clarke A, Prothero-Thomas E, Beaumont J.C, Chapman A.L, Brey T. Growth in the limpet Nacella concinna from contrasting sites in Antarctica. Polar Biol. 2004b;28:62–71. [Google Scholar]
- Clarke A, Barnes D.K.A, Hodgson D. How isolated is Antarctica? Trends Ecol. Evol. 2005;20:1–3. doi: 10.1016/j.tree.2004.10.004. doi:10.1016/j.tree.2004.10.004 [DOI] [PubMed] [Google Scholar]
- Convey P. Environmental change and Antarctic terrestrial life histories: fact and prediction. In: Davison W, Howard-Williams C, Broady P, editors. Antarctic ecosystems: models for wider ecological understanding. New Zealand Natural Sciences, Canterbury University; Christchurch, New Zealand: 2000. pp. 243–251. [Google Scholar]
- Convey, P. 2003 Antarctic Peninsula climate change: signals from terrestrial biology. In Antarctic Peninsula climate variability: a historical and paleonenvironmental perspective, vol. 79 (ed. E. Domack, A. Burneet, A. Leventer, P. Convey, M. Kirby & R. Bindschadler), Antarctic research series, pp. 45–158. Washington, DC: American Geophysical Union.
- Convey P, Pugh P.J.A, Jackson C, Murray A.W, Ruhland C.T, Xiong F.S, Day T.A. Response of Antarctic terrestrial microarthropods to long-term climate manipulations. Ecology. 2002;83:3130–3140. [Google Scholar]
- Cook A.J, Fox A.J, Vaughan D.G, Ferrigno J.G. Retreating glacier fronts on the Antarctic Peninsula over the past half-century. Science. 2005;308:541–544. doi: 10.1126/science.1104235. doi:10.1126/science.1104235 [DOI] [PubMed] [Google Scholar]
- Dauby P, Scailteur Y, Chapelle G, de Broyer C. Potential impact of the main benthic amphipods on the eastern Weddell Sea shelf ecosystem. Polar Biol. 2001a;24:657–662. doi:10.1007/s003000100265 [Google Scholar]
- Dauby P, Scailteur Y, de Broyer C. Trophic diversity within the eastern Weddell Sea amphipod community. Hydrobiologia. 2001b;443:69–86. doi:10.1023/A:1017596120422 [Google Scholar]
- Davis A.J, Lawton J.H, Shorrocks B, Jenkinson L.S. Individualistic species responses invalidate simple physiological models of community dynamics under global environmental change. J. Anim. Ecol. 1998;67:600–612. doi:10.1046/j.1365-2656.1998.00223.x [Google Scholar]
- Dayton P.K. Interdecadal variation in an Antarctic sponge and its predators resulting from oceanographic shifts. Science. 1989;245:1484–1486. doi: 10.1126/science.245.4925.1484. doi:10.1126/science.245.4925.1484 [DOI] [PubMed] [Google Scholar]
- Dickens G.R. Methane oxidation during the Late Palaeocene Thermal Maximum. Bulletin de la Société Geologique de France. 2000;171:37–49. [Google Scholar]
- Dierssen H.M, Smith R.C, Vernet M. Glacial meltwater dynamics in coastal waters west of the Antarctic Peninsula. Proc. Natl Acad. Sci. USA. 2002;99:1790–1795. doi: 10.1073/pnas.032206999. doi:10.1073/pnas.032206999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinniman M.S, Klinck J.M. A model study of circulation and cross-shelf exchange on the west Antarctic Peninsula continental shelf. Deep-Sea Res. II. 2004;51:2003–2022. doi:10.1016/j.dsr2.2004.07.030 [Google Scholar]
- Doake C.S.M, Vaughan D.G. Rapid disintegration of the Wordie Ice Shelf in response to atmospheric warming. Nature. 1991;350:328–330. doi:10.1038/350328a0 [Google Scholar]
- Domack E, et al. Stability of the Larsen B ice shelf on the Antarctic Peninsula during the Holocene epoch. Nature. 2005a;436:681–685. doi: 10.1038/nature03908. doi:10.1038/nature03908 [DOI] [PubMed] [Google Scholar]
- Domack E, Ishman S, Leventer A, Sylva S, Willmott V, Huber B. A chemotrophic ecosystem found beneath Antarctic ice shelf. EOS Trans. Am. Geophys. Union. 2005b;86:269–276. [Google Scholar]
- Ducklow, H. W., Fraser, W. R., Quetin, L. B., Ross, R. M., Smith, R. C., Stammerjohn, S. E., Vernet, M. & Daniels, R. M. 2006 Water column processes in the West Antarctic Peninsula and the Ross Sea: interannual variations and foodweb structure. Deep-Sea Res. II53, 834–852.
- Ducklow, H. W., Baker, K., Fraser, W. R., Martinson, D. G., Quetin, L. B., Ross, R. M., Smith, R. C., Stammerjohn, S. & Vernet, M. 2007 Marine ecosystems: the West Antarctic Peninsula. Phil. Trans. R. Soc. B362, 67–94. (doi:10.1098/rstb.2006.1955) [DOI] [PMC free article] [PubMed]
- Dunne J.A, Williams R.J, Martinez N.D. Network structure and robustness of marine food webs. Mar. Ecol. Prog. Ser. 2004;273:291–302. [Google Scholar]
- Dynesius M, Jansson R. Evolutionary consequences of changes in species' geographical distributions driven by Milankovitch climate oscillations. Proc. Natl Acad. Sci. USA. 2000;97:9115–9120. doi: 10.1073/pnas.97.16.9115. doi:10.1073/pnas.97.16.9115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M, Richardson A.J. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature. 2004;430:881–884. doi: 10.1038/nature02808. doi:10.1038/nature02808 [DOI] [PubMed] [Google Scholar]
- Eklöf A, Ebenman B. Species loss and secondary extinctions in simple and complex model communities. J. Anim. Ecol. 2006;75:239–246. doi: 10.1111/j.1365-2656.2006.01041.x. doi:10.1111/j.1365-2656.2006.01041.x [DOI] [PubMed] [Google Scholar]
- Emslie S.D, McDaniel J.D. Adelie penguin diet and climate change during the middle to late Holocene in northern Marguerite Bay, Antarctic Peninsula. Polar Biol. 2002;25:222–229. [Google Scholar]
- Emslie S.D, Woehler E.J. A 9000-year record of Adélie penguin occupation and diet in the Windmill Islands, East Antarctica. Antarctic Sci. 2005;17:57–66. doi:10.1017/S0954102005002427 [Google Scholar]
- Emslie S.D, Fraser W.R, Smith R.C, Walker W. Abandoned penguin colonies and environmental change in the Palmer Station area, Anvers Island, Antarctic Peninsula. Antarctic Sci. 1998;10:257–268. [Google Scholar]
- Emslie S.D, Berkman P.A, Ainley D.G, Coats L, Polito M. Late-Holocene initiation of ice-free ecosystems in the southern Ross Sea, Antarctica. Mar. Ecol. Prog. Ser. 2003;262:19–25. [Google Scholar]
- Fitter A.H, Fitter R.S.R. Rapid changes in flowering time in British plants. Science. 2002;296:1689–1691. doi: 10.1126/science.1071617. doi:10.1126/science.1071617 [DOI] [PubMed] [Google Scholar]
- Folland C, et al. Observed climate variability and change. In: Houghton J.T, Ding Y, Griggs D.J, Noguer M, van den Linden P.J, Dai X, Maskell K, Johnson C.A, editors. Climate change 2001: the scientific basis. Cambridge University Press; Cambridge, UK: 2001. pp. 99–182. [Google Scholar]
- Forcada J, Trathan P.N, Reid K, Murphy E.J, Croxall J.P. Contrasting population changes in sympatric penguin species in association with climate warming. Global Change Biol. 2006;12:1–13. doi:10.1111/j.1365-2486.2006.01108.x [Google Scholar]
- Fowbert J.A, Lewis Smith R.I. Rapid population increase in native vascular plants in the Argentine Islands, Antarctic Peninsula. Arctic Alpine Res. 1994;26:290–296. doi:10.2307/1551941 [Google Scholar]
- Fraser W.R, Hofmann E.E. A predator's perspective on causal links between climate change, physical forcing and ecosystem response. Mar. Ecol. Prog. Ser. 2003;265:1–15. [Google Scholar]
- Fraser W.R, Patterson D.L. Human disturbance and long-term changes in Adelie penguin populations: a natural experiment at Palmer Station, Antarctic Peninsula. In: Battaglia B, Valencia J, Walton D.W.H, editors. Antarctic communities: species, structure and survival. Cambridge University Press; Cambridge, UK: 1997. pp. 445–452. [Google Scholar]
- Fraser W.R, Trivelpiece W.Z. Factors controlling the distribution of seabirds: winter-summer heterogeneity in the distribution of Adélie penguin populations. In: Ross R.M, Hofmann E.E, Quetin L.B, editors. Foundations for ecological research west of the Antarctic Peninsula. Antarctic research series. vol. 70. American Geophysical Union; Washington, DC: 1996. pp. 257–272. [Google Scholar]
- Fraser W.R, Trivelpiece W.Z, Ainley D.G, Trivelpiece S.G. Increases in Antarctic penguin populations: reduced competition with whales or loss of sea ice due to environmental warming? Polar Biol. 1992;11:525–531. doi:10.1007/BF00237945 [Google Scholar]
- Fulton E.A, Smith A.D.M, Johnson C.R. Effect of complexity on marine ecosystem models. Mar. Ecol. Prog. Ser. 2003;253:1–16. [Google Scholar]
- Gille S.T. Warming of the Southern Ocean since the 1950s. Science. 2002;295:1275–1277. doi: 10.1126/science.1065863. doi:10.1126/science.1065863 [DOI] [PubMed] [Google Scholar]
- Grange L.J, Tyler P.A, Peck L.S, Cornelius N. Long-term interannual cycles of the gametogenic ecology of the Antarctic brittle star Ophionotus victoriae. Mar. Ecol. Prog. Ser. 2004;278:141–155. [Google Scholar]
- Hain S, Melles M. Evidence for a marine molluscan fauna beneath ice shelves in the Lazarev and Weddell Seas, Antarctica, from shells of Adamussium colbecki and Nacella (Patinigera) cf concinna. Antarctic Sci. 1994;6:29–36. doi:10.1017/S0954102094000040 [Google Scholar]
- Hall A, Visbeck M. Synchronous vaiability in the southern hemisphere atmosphere, sea ice, and ocean resulting from the annular mode. J. Climate. 2002;15:3043–3057. doi:10.1175/1520-0442(2002)015<3043:SVITSH>2.0.CO;2 [Google Scholar]
- Hansen J.E, Ruedy R, Glascoe J, Sato M. GISS analysis of surface temperature change. J. Geophys. Res. 1999;104:30 997–31 022. doi:10.1029/1999JD900835 [Google Scholar]
- Harangozo S. A search for ENSO teleconnections in the west Antarctic Peninsula climate in austral winter. Int. J. Climatol. 2000;20:663–679. doi:10.1002/(SICI)1097-0088(200005)20:6<663::AID-JOC493>3.0.CO;2-I [Google Scholar]
- Harangozo S. Atmospheric circulation impacts on winter maximum sea ice extent in the west Antarctic Peninsula region (1979–2001) Geophys. Res. Lett. 2006;33:L02502. doi:10.1029/2005GL024978 [Google Scholar]
- Heilmayer O, Brey T, Pörtner H.O. Growth efficiency and temperature in scallops: a comparative analysis of species adapted to different temperatures. Funct. Ecol. 2004;18:641–647. doi:10.1111/j.0269-8463.2004.00905.x [Google Scholar]
- Helmuth B, Kingsolver J.G, Carrington E. Biophysics, physiological ecology, and climate change: does mechanism matter? Annu. Rev. Physiol. 2005;67:177–201. doi: 10.1146/annurev.physiol.67.040403.105027. doi:10.1146/annurev.physiol.67.040403.105027 [DOI] [PubMed] [Google Scholar]
- Heywood R.B, Light J.J. First direct evidence of life under Antarctic shelf ice. Nature. 1975;254:591–592. doi:10.1038/254591a0 [Google Scholar]
- Hofmann E.E, Klinck J.M. Thermohaline variability of the waters overlying the west Antarctic Peninsula continental shelf. In: Jacobs S.S, Weiss R.F, editors. Ocean, ice and atmosphere: interactions at the Antarctic continental margin. Antarctic research series. vol. 75. American Geophysical Union; Washington, DC: 1998. pp. 67–81. [Google Scholar]
- Hofmann E.E, Murphy E.J. Advection, krill, and Antarctic marine ecosystems. Antarctic Sci. 2004;16:487–499. doi:10.1017/S0954102004002275 [Google Scholar]
- Hochachka P.W, Somero G.N. Oxford University Press; Oxford, UK: 2002. Biochemical adaptation: mechanism and process in physiological evolution. [Google Scholar]
- Hofmann E.E, Klinck J.M, Lascara C.M, Smith D.A. Water mass distribution and circulation west of the Antarctic Peninsula and including Bransfield Strait. In: Ross R.M, Hofmann E.E, Quetin L.B, editors. Foundations for ecological research west of the Antarctic Peninsula. Antarctic research series. vol. 70. American Geophysical Union; Washington, DC: 1996. pp. 61–80. [Google Scholar]
- Hofmann G.E, Lund S.G, Place S.P, Whitmer A.C. Some like it hot, some like it cold: the heat shock response is found in New Zealand but not Antarctic notothenioid fishes. J. Exp. Mar. Biol. Ecol. 2005;316:79–89. doi:10.1016/j.jembe.2004.10.007 [Google Scholar]
- Hood R.R, et al. Pelagic functional group modeling: progress, challenges and prospects. Deep-Sea Res. II. 2006;53:459–512. doi:10.1016/j.dsr2.2006.01.025 [Google Scholar]
- Houghton J.T, Ding Y, Griggs D.J, Noguer M, van der Linden P.J, Xiaosu D. Cambridge University Press for the Intergovernmental Panel on Climate Change; Cambridge, UK: 2001. Climate change 2001: the scientific basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change (IPCC) [Google Scholar]
- Jacobs S.S, Comiso J.C. A recent sea-ice retreat west of the Antarctic Peninsula. Geophys. Res. Lett. 1993;20:1171–1174. [Google Scholar]
- Jacobs S.S, Hellmer H.H, Jenkins A. Antarctic ice sheet melting in the southeast Pacific. Geophys. Res. Lett. 1996;23:957–960. doi:10.1029/96GL00723 [Google Scholar]
- Jansson R, Dynesius M. The fate of clades in a world of recurrent climatic change: Milankovitch oscillations and evolution. Annu. Rev. Ecol. Syst. 2002;33:741–777. doi:10.1146/annurev.ecolsys.33.010802.150520 [Google Scholar]
- Jarre-Teichmann A, et al. Trophic flows in the benthic shelf community of the eastern Weddell Sea, Antarctica. In: Battaglia B, Valencia J, Walton D.W.H, editors. Antarctic communities: species, structure and survival. Cambridge University Press; Cambridge, UK: 1997. pp. 118–134. [Google Scholar]
- Johnson K.H. Trophic-dynamic considerations in relating species diversity to ecosystem resilience. Biol. Rev. 2000;75:347–376. doi: 10.1017/s0006323100005508. doi:10.1017/S0006323100005508 [DOI] [PubMed] [Google Scholar]
- Jones V.J, Hodgson D.A, Chepstow-Lusty A. Palaeolimnological evidence for marked Holocene environmental changes on Signy Island, Antarctica. The Holocene. 2000;10:43–60. doi:10.1191/095968300673046662 [Google Scholar]
- King J.C. Recent climate variability in the vicinity of the Antarctic Peninsula. Int. J. Climatol. 1994;14:357–369. [Google Scholar]
- King J.C, Harangozo S.A. Climate change in the western Antarctic Peninsula since 1945: observations and possible causes. Ann. Glaciol. 1998;27:571–575. [Google Scholar]
- King J.C, Turner J, Marshall G.J, Connolley W.M, Lachlan-Cope T.A. Antarctic Peninsula climate variability and its causes as revealed by analysis of instrumental records. In: Domack E, Leventer A, Burnett A, Bindschadler R, Convey P, Kirby M, editors. Antarctic Peninsula climate variability: historical and paleoenvironmental perspectives. Antarctic research series. vol. 79. American Geophysical Union; Washington, DC: 2003. pp. 17–30. [Google Scholar]
- Klinck J.M. Heat and salt changes on the continental shelf west of the Antarctic Peninsula between January 1993 and January 1994. J. Geophys. Res. Oceans. 1998;103:7617–7636. doi:10.1029/98JC00369 [Google Scholar]
- Kwok R, Comiso J.C. Southern Ocean climate and sea ice anomalies associated with the Southern Oscillation. J. Climate. 2002;15:487–501. doi:10.1175/1520-0442(2002)015<0487:SOCASI>2.0.CO;2 [Google Scholar]
- Lachlan-Cope T. Role of sea ice in forcing the winter climate of Antarctica in a global climate model. J. Geophys. Res. 2005;110:D03110. doi:10.1029/2004JD004935 [Google Scholar]
- Lawton J.H. What do species do in ecosystems? Oikos. 1994;71:367–374. [Google Scholar]
- Le Quéré C, et al. Ecosystem dynamics based on plankton functional types for global ocean biogeochemistry models. Global Change Biol. 2005;11:2016–2040. [Google Scholar]
- Lear C.H, Elderfield H, Wilson P.H. Cenozoic deep-sea temperatures and global ice volumes from Mg/Ca in benthic foraminiferal calcite. Science. 2000;287:269–272. doi: 10.1126/science.287.5451.269. doi:10.1126/science.287.5451.269 [DOI] [PubMed] [Google Scholar]
- Leventer A, Domack E.W, Ishman S.E, Brachfield S, McClennen C.E, Manley P. Productivity cycles of 200–300 years in the Antarctic Peninsula area: understanding linkages among the sun, atmosphere, oceans, sea ice, and biota. Geol. Soc. Am. Bull. 1996;108:1626–1644. doi:10.1130/0016-7606(1996)108<1626:PCOYIT>2.3.CO;2 [Google Scholar]
- Leventer A, Domack E, Barkoukis A, McAndrews B, Murray J. Laminations from the Palmer Deep: a diatom-based interpretation. Paleoceanography. 2002;17:1–15. doi:10.1029/2001PA000624 Art. No. 1027. [Google Scholar]
- Levitus S, Antonov J.L, Boyer T.P, Stephens C. Warming of the world ocean. Science. 2000;287:2225–2229. doi:10.1126/science.287.5461.2225 [Google Scholar]
- Levitus S, Antonov J, Boyer T. Warming of the world ocean, 1955–2003. Geophys. Res. Lett. 2005;32:L02604. doi:10.1029/2004GL021592 [Google Scholar]
- Lewis, P. N., Bergstrom, D. M. & Whinam, J. 2006. Barging in: a temperate marine community travels to the subantarctic. Biol. Invasions, 8, 787–795.
- Lewis Smith R.I. Vascular plants as bioindicators of regional warming in Antarctica. Oecologia. 1994;99:322–328. doi: 10.1007/BF00627745. doi:10.1007/BF00627745 [DOI] [PubMed] [Google Scholar]
- Lewis Smith R.I. Plant colonisation response to climate change in the Antarctic. Folia Facultatis Scientiarium Naturalium Universitatis Masarykianae Brunensis, Geographia. 2001;25:19–33. [Google Scholar]
- Lewis Smith R.I, Convey P. Enhanced sexual reproduction in bryophytes at high latitudes in the maritime Antarctic. J. Bryol. 2001;24:107–117. doi:10.1179/037366802125000962 [Google Scholar]
- Lima F.P, Queiroz N, Ribeiro P.A, Hawkins S.J. Recent changes in the distribution of a marine gastropod, Patella rustica Linnaeus, 1758, and their relationship to unusual climatic events. J. Biogeogr. 2006;33:812–822. doi:10.1111/j.1365-2699.2006.01457.x [Google Scholar]
- Lipps J.H, Krebs W.N, Temnikow N.K. Microbiota under Antarctic ice shelves. Nature. 1977;265:232–235. doi:10.1038/265232a0 [Google Scholar]
- Lipps J.H, Ronan T.E, DeLaca T.E. Life below the Ross Ice Shelf. Science. 1979;203:447–449. doi: 10.1126/science.203.4379.447. doi:10.1126/science.203.4379.447 [DOI] [PubMed] [Google Scholar]
- Littlepage J.L, Pearse J.S. Biological and oceanographic observations under an Antarctic ice shelf. Science. 1962;137:679–681. doi: 10.1126/science.137.3531.679. doi:10.1126/science.137.3531.679 [DOI] [PubMed] [Google Scholar]
- Liu J, Yuan X, Rind D, Martinson D.G. Mechanism study of the ENSO and southern high latitude climate teleconnections. Geophys. Res. Lett. 2002;29:1679. doi:10.1029/2002GL015143 [Google Scholar]
- Loeb V, Siegel V, Holm-Hansen O, Hewitt R.P, Fraser W.R, Trivelpiece W.Z, Trivelpiece S.G. Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature. 1997;387:897–900. doi:10.1038/43174 [Google Scholar]
- Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime J.P, Hector A, Hooper D.U, Huston M.A, Raffaelli D, Schmid B, Tilman D, Wardle D.A. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science. 2001;294:804–808. doi: 10.1126/science.1064088. [DOI] [PubMed] [Google Scholar]
- Lynch M, Lande R. Evolution and extinction in relation to environmental change. In: Kareiva P.M, Kingsolver J.G, Huey R.B, editors. Biotic interactions and global change. Sinauer Associates; Sunderland, MA: 1993. pp. 234–250. [Google Scholar]
- Lyons W.B, Bartek L.R, Mayewski P.A, Doran P.T. Climate history of the McMurdo Dry Valleys since the last glacial maximum: a synthesis. In: Lyons W.B, Howard-Williams C, Hawes I, editors. Ecosystem processes in Antarctic ice-free landscapes. Balkema; Rotterdam, The Netherlands: 1997. pp. 15–22. [Google Scholar]
- Marshall G.J, King J.C. Southern hemispheric circulation anomalies associated with extreme Antarctic Peninsula winter temperatures. Geophys. Res. Lett. 1998;25:2437–2440. doi:10.1029/98GL01651 [Google Scholar]
- Marshall G.J, Stott P.A, Turner J, Connolley W.M, King J.C, Lachlan-Cope T.A. Causes of exceptional atmospheric circulation changes in the Southern Hemisphere. Geophys. Res. Lett. 2004;31:L14205. doi:10.1029/2004GL019952 [Google Scholar]
- Marshall, G. J., Orr, A., van Lipzig, N. P. M. & King, J. C. 2006 The impact of a changing Southern Hemisphere Annular Mode on Antarctic Peninsula summer temperatures. J. Clim.20, 5388–5404.
- Martinez N.D. Scale-dependent constraints on food-web structure. Am. Nat. 1994;144:935–953. doi:10.1086/285719 [Google Scholar]
- Meredith M.P, King J.C. Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys. Res. Lett. 2005;32:L19604. doi:10.1029/2005GL024042 [Google Scholar]
- Meredith M.P, Renfrew I.A, Clarke A, King J.C, Brandon M.A. Impact of the 1997/98 ENSO on upper ocean characteristics in Marguerite Bay, western Antarctic Peninsula. J. Geophys. Res. 2004;109 C09013 (19pp). [Google Scholar]
- Mieszkowska, N., Kendall, M. A., Hawkins, S. J., Leaper, R., Williamson, P., Hardman-Mountford, N. J. & Southward, A. J. 2006 Changes in the range of some common rocky shore species in Britain—a response to climate change? Hydrobiologia555, 241–251.
- Mitchell B.G, Holm-Hansen O. Observations and modeling of the Antarctic phytoplankton crop in relation to mixing depth. Deep-Sea Res. 1991;38:981–1007. doi:10.1016/0198-0149(91)90093-U [Google Scholar]
- Moline M.A, Claustre H, Frazer T.K, Schofield O, Vernet M. Alteration of the food web along the Antarctic Peninsula in response to a warming trend. Global Change Biol. 2004;10:1973–1980. doi:10.1111/j.1365-2486.2004.00825.x [Google Scholar]
- Mosby H. The waters of the Atlantic Antarctic Ocean. Scientific Results of the Norwegian Antarctic Expedition, 1927–1928. 1936;11:1–131. [Google Scholar]
- Murphy E.J, Clarke A, Symon C, Priddle J.H. Temporal variation in Antarctic sea ice: analysis of a long-term fast-ice record from the South Orkney Islands. Deep-Sea Res. 1995;42:1945–1062. [Google Scholar]
- Murphy E.J, et al. Interannual variability of the South Georgia marine ecosystem: biological and physical sources of variation in the abundance of krill. Fish. Oceanogr. 1998;7:381–390. doi:10.1046/j.1365-2419.1998.00081.x [Google Scholar]
- Nicholls N, Gruza G, Jouzel J, Karl T, Ogallo I, Parker D. Observed climate variability and change. In: Houghton J.T, Meria Filho L.G, Callander B.A, Harris N, Kattenberg A, Maskell K, editors. Climate change 1995—The science of climate change. Cambridge University Press; Cambridge, UK: 1995. pp. 133–192. [Google Scholar]
- Palumbi S.R. What can molecular genetics contribute to marine biogeography? An urchin's tale. J. Exp. Mar. Biol. Ecol. 1996;203:75–92. doi:10.1016/0022-0981(96)02571-3 [Google Scholar]
- Palumbi S.R. Molecular biogeography of the Pacific. Coral Reefs. 1997;16:S47–S52. doi:10.1007/s003380050241 [Google Scholar]
- Parkinson C.L. Trends in the length of the Southern Ocean sea ice season. Ann. Glaciol. 2002;34:435–440. [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. doi:10.1038/nature01286 [DOI] [PubMed] [Google Scholar]
- Patterson D.L, Easter-Pilcher A, Fraser W.R. The effects of human activity and environmental variability on long-term changes in Adelie penguin populations at Palmer Station, Antarctica. In: Huiskes A.H.L, Gieskes W.W.C, Rozema J, Schorno R.M.L, van der Vries S.M, Wolff W.J, editors. Antarctic biology in a global context; Proc. VIII SCAR biology symposium. Backhuys Publishers; Leiden, The Netherlands: 2003. pp. 301–307. [Google Scholar]
- Pearson R.G, Dawson T.P. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecol. Biogeogr. 2003;12:361–371. [Google Scholar]
- Peat, H. J., Clarke, A, Convey, P. In press. Diversity and biogeography of the Antarctic flora. J. Biogeogr
- Peck L.S. Prospects for surviving climate change in Antarctic aquatic species. Front. Zool. 2005a;2 doi: 10.1186/1742-9994-2-9. doi: 10.1186/1742-9994-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L.S. Prospects for survival in the Southern Ocean: vulnerability of benthic species to temperature change. Antarctic Sci. 2005b;17:497–507. doi:10.1017/S0954102005002920 [Google Scholar]
- Peck L.S, Brey T. Bomb signals in old Antarctic brachiopods. Nature. 1996;380:207–208. doi:10.1038/380207b0 [Google Scholar]
- Peck L.S, Pörtner H.O, Hardewig I. Metabolic demand, oxygen supply, and critical temperatures in the Antarctic bivalve Laternula elliptica. Physiol. Biochem. Zool. 2002;75:123–133. doi: 10.1086/340990. doi:10.1086/340990 [DOI] [PubMed] [Google Scholar]
- Peck L.S, Ansell A.D, Webb K.E, Hepburn L, Burrows M. Burrowing in Antarctic bivalve molluscs. Polar Biol. 2004a;27:357–367. doi:10.1007/s00300-003-0588-7 [Google Scholar]
- Peck L.S, Webb K.E, Bailey D.M. Extreme sensitivity of biological function to temperature in Antarctic marine species. Funct. Ecol. 2004b;18:625–630. doi:10.1111/j.0269-8463.2004.00903.x [Google Scholar]
- Peck L.S, Clarke A, Chapman A.L. Metabolism and development in pelagic larvae of Antarctic gastropods with mixed reproductive strategies. Mar. Ecol. Prog. Ser. 2006;318:213–220. [Google Scholar]
- Petchey O.L, Gaston K.J. Extinction and the loss of functional diversity. Proc. R. Soc. B. 2002;269:1721–1727. doi: 10.1098/rspb.2002.2073. doi:10.1098/rspb.2002.2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy L.R. The ocean's food web: a changing paradigm. BioScience. 1974;24:499–504. doi:10.2307/1296885 [Google Scholar]
- Pomeroy L.R. Food web connections: links and sinks. In: Bell C.R, Brylinsky M, Johnson-Green P, editors. Microbial systems: new frontiers. Proc. 8th Int. Symp. on Microbial Ecology. Atlantic Canada Society for Microbial Ecology; Halifax, Canada: 2000. pp. 81–87. [Google Scholar]
- Pörtner H.O. Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften. 2001;88:137–146. doi: 10.1007/s001140100216. doi:10.1007/s001140100216 [DOI] [PubMed] [Google Scholar]
- Pörtner H.O, Peck L.S, Zielinski S, Conway L.Z. Temperature and metabolism in the highly stenothermal bivalve mollusc Limopsis marionensis from the Weddell Sea, Antarctica. Polar Biol. 1999;22:17–30. doi:10.1007/s003000050386 [Google Scholar]
- Poulin E, Palma A.T, Féral J.-P. Evolutionary versus ecological success in Antarctic benthic invertebrates. Trends Ecol. Evol. 2002;17:218–222. doi:10.1016/S0169-5347(02)02493-X [Google Scholar]
- Prezelin B.B, Hofmann E.E, Mengelt C, Klinck J.M. The linkage between Upper Circumpolar Deep Water (UCDW) and phytoplankton assemblages on the west Antarctic Peninsula continental shelf. J. Mar. Res. 2000;58:165–202. doi:10.1357/002224000321511133 [Google Scholar]
- Pudsey C.J, Evans J. First survey of Antarctic sub-ice shelf sediments reveals mid-Holocene ice shelf retreat. Geology. 2001;29:787–790. doi:10.1130/0091-7613(2001)029<0787:FSOASI>2.0.CO;2 [Google Scholar]
- Quetin L.B, Ross R.M. Episodic recruitment in Antarctic krill Euphausia superba in the Palmer LTER study region. Mar. Ecol. Prog. Ser. 2003;259:185–200. [Google Scholar]
- Ritchie P.A, Millar C.D, Gibb G.C, Baroni C, Lambert D.M. Ancient DNA enables timing of the Pleistocene origin and Holarctic expansion of two Adelie penguin lineages in Antarctica. Mol. Biol. Evol. 2004;21:240–248. doi: 10.1093/molbev/msh012. doi:10.1093/molbev/msh012 [DOI] [PubMed] [Google Scholar]
- Robertson R, Visbeck M, Gordon A.L, Fahrbach E. Long-term temperature trends in the deep waters of the Weddell Sea. Deep-Sea Res. II. 2002;49:4791–4806. doi:10.1016/S0967-0645(02)00159-5 [Google Scholar]
- Ross R.M, Quetin L.B, Kirsch E. Effect of temperature on developmental times and survival of early larval stages of Euphausia superba Dana. J. Exp. Mar. Biol. Ecol. 1988;121:55–71. doi:10.1016/0022-0981(88)90023-8 [Google Scholar]
- Ross R.M, Quetin L.B, Baker K.S, Vernet M, Smith R.C. Growth limitation in young Euphausia superba under field conditions. Limnol. Oceanogr. 2000;45:31–43. [Google Scholar]
- Ross, R. M., Quetin, L. B., Martinson, D. G., Iannuzzi, R. A., Stammerjohn, S. E. & Smith, R. C. In press. Palmer LTER: patterns of distribution of major zooplankton species west of the Antarctic Peninsula over a twelve year span. Deep-Sea Res. II
- Root T.L, Price J.T, Hall K.R, Schneider S.H, Rosenzweig C, Pounds J.A. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. doi:10.1038/nature01333 [DOI] [PubMed] [Google Scholar]
- Roy K, Jablonski D, Valentine J.W. Thermally anomalous assemblages revisited: patterns in the extraprovincial latitudinal range shifts of Pleistocene marine mollusks. Geology. 1995;23:1071–1074. doi:10.1130/0091-7613(1995)023<1071:TAARPI>2.3.CO;2 [Google Scholar]
- Roy K, Valentine J.W, Jablonski D, Kidwell S.M. Scales of climatic variability and time averaging in Pleistocene biotas: implications for ecology and evolution. Trends Ecol. Evol. 1996;11:458–463. doi: 10.1016/0169-5347(96)10054-9. doi:10.1016/0169-5347(96)10054-9 [DOI] [PubMed] [Google Scholar]
- Sagarin R.D, Barry J.P, Gilman S.E, Baxter C.H. Climate-related change in an intertidal community over short and long time scales. Ecol. Monogr. 1999;69:465–490. doi:10.2307/2657226 [Google Scholar]
- Sedwick P.N, DiTullio G.R, Mackey D.J. Iron and manganese in the Ross Sea, Antarctica: seasonal iron limitation in Antarctic shelf waters. J. Geophys. Res. 2000;105:11 321–11 336. doi:10.1029/2000JC000256 [Google Scholar]
- Seebacher F, Davison W, Lowe C.J, Franklin C.E. A falsification of the thermal specialization paradigm: compensation for elevated temperatures in Antarctic fishes. Biol. Lett. 2005;1:151–154. doi: 10.1098/rsbl.2004.0280. doi:10.1098/rsbl.2004.0280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedsrud L.H. Warming of the deep water in the Weddell Sea along the Greenwich meridian: 1977–2001. Deep-Sea Res. I. 2005;52:241–258. doi:10.1016/j.dsr.2004.10.004 [Google Scholar]
- Smetacek V, Nicol S. Polar ecosystems in a changing world. Nature. 2005;437:362–368. doi: 10.1038/nature04161. doi:10.1038/nature04161 [DOI] [PubMed] [Google Scholar]
- Smith D.A, Klinck J.M. Water properties on the west Antarctic Peninsula continental shelf: a model study of effects of surface fluxes and sea ice. Deep-Sea Res. II. 2002;49:4863–4886. doi:10.1016/S0967-0645(02)00163-7 [Google Scholar]
- Smith R.C, Stammerjohn S. Variations of surface air temperaure and sea ice extent in the Western Antarctic Peninsula (WAP) region. Ann. Glaciol. 2001;33:493–500. [Google Scholar]
- Smith R.C, Stammerjohn S.E, Baker K.S. Surface air temperature variations in the western Antarctic Peninsula region. In: Ross R.M, Hofmann E.E, Quetin L.B, editors. Foundations for ecological research west of the Antarctic Peninsula. Antarctic research series. vol. 70. American Geophysical Union; Washington, DC: 1996. pp. 105–121. [Google Scholar]
- Smith R.C, Baker K.S, Vernet M. Seasonal and interannual variability of phytoplankton biomass west of the Antarctic Peninsula. J. Mar. Syst. 1998;17:229–243. doi:10.1016/S0924-7963(98)00040-2 [Google Scholar]
- Smith R.C, Fraser W.R, Stammerjohn S.E. Climatic variability and ecological response of the marine ecosystem in the Western Antarctic Peninsula (WAP) region. In: Greenland D, Goodin D.G, Smith R.C, editors. Climate variability and ecosystem response at long-term ecological research sites. Oxford University Press; Oxford, UK: 2003. pp. 158–173. [Google Scholar]
- Somero G.N, DeVries A.L. Temperature tolerance in some Antarctic fishes. Science. 1967;156:257–258. doi: 10.1126/science.156.3772.257. doi:10.1126/science.156.3772.257 [DOI] [PubMed] [Google Scholar]
- Southward A.J, Hawkins S.J, Burrows M.T. 70 years observations of changes in distribution and abundance of zooplankton and intertidal organisms in the Western English Channel in relation to rising sea temperature. J. Therm. Biol. 1995;20:127–155. doi:10.1016/0306-4565(94)00043-I [Google Scholar]
- Southward A.J, et al. Long-term oceanographic and ecological research in the western English Channel. Adv. Mar. Biol. 2005;47:1–105. doi: 10.1016/S0065-2881(04)47001-1. doi:10.1016/S0065-2881(04)47001-1 [DOI] [PubMed] [Google Scholar]
- Stammerjohn S.E, Smith R.C. Opposing Southern Ocean climate patterns as revealed by trends in regional sea ice coverage. Clim. Change. 1997;37:617–639. doi:10.1023/A:1005331731034 [Google Scholar]
- Stanwell-Smith D.P, Peck L.S. Temperature and embryonic development in relation to spawning and field occurrence of larvae of 3 Antarctic echinoderms. Biol. Bull. 1998;194:44–52. doi: 10.2307/1542512. [DOI] [PubMed] [Google Scholar]
- Taylor J.D, Morris N.J, Taylor C.N. Food specialization and the evolution of predatory prosobranch gastropods. Palaeontology. 1980;23:375–409. [Google Scholar]
- Thompson D.W.J, Solomon S. Interpretation of recent southern hemisphere climate change. Science. 2002;296:895–899. doi: 10.1126/science.1069270. doi:10.1126/science.1069270 [DOI] [PubMed] [Google Scholar]
- Thomas D.W, Blondel J, Perret P, Lambrechts M.M, Speakman J.R. Energetic and fitness costs of mismatching resource supply and demand in seasonally breeding birds. Science. 2001;291:2598–2600. doi: 10.1126/science.1057487. doi:10.1126/science.1057487 [DOI] [PubMed] [Google Scholar]
- Turner J, Colwell S.R, Marshall G.J, Lachlan-Cope T.A, Carleton A.M, Jones P.D, Lagun V, Reid P.A, Iagovkina S. Antarctic climate change during the last 50 years. Int. J. Climatol. 2005;25:279–294. doi:10.1002/joc.1130 [Google Scholar]
- Turner J, Connolley W.M, Lachlan-Cope T.A, Marshall G.J. The performance of the Hadley Centre climate model (HadCM3) in high southern latitudes. Int. J. Climatol. 2006;26:91–112. doi:10.1002/joc.1260 [Google Scholar]
- Valentine J.W, Jablonski D. Biotic effects of sea level change: the Pleistocene change. J. Geophys. Res. 1991;96:6873–6878. [Google Scholar]
- Vaughan D.G. Recent trends in melting conditions on the Antarctic Peninsula and their implications for ice-sheet mass balance and sea level. Arctic Alpine Res. 2006;38:147–152. doi:10.1657/1523-0430(2006)038[0147:RTIMCO]2.0.CO;2 [Google Scholar]
- Vaughan D.G, Doake C.S.M. Recent atmospheric warming and retreat of ice shelves on the Antarctic Peninsula. Nature. 1996;379:328–331. doi:10.1038/379328a0 [Google Scholar]
- Vaughan D.G, Marshall G.J, Connolley W.M, Parkinson C.L, Mulvaney R, Hodgson D.A, King J.C, Pudsey C.J, Turner J. Recent rapid regional climate warming on the Antarctic Peninsula. Clim. Change. 2003;60:243–274. doi:10.1023/A:1026021217991 [Google Scholar]
- Visser M.E, Both C, Lambrechts M.M. Global climate change leads to mistimed avian reproduction. Adv. Ecol. Res. 2004;35:89–110. [Google Scholar]
- Waller, C., Barnes, D. K. A. & Convey, P. 2006. Ecological contrasts across an Antarctic land-sea interface. Austral Ecol 31, 656–666.
- Walsh J.J, Dieterle D.A, Lenes J. A numerical analysis of carbon dynamics of the Southern Ocean phytoplankton community: the roles of light and grazing in effecting both sequestration of atmospheric CO2 and food availability to larval krill. Deep-Sea Res. I. 2001;48:1–48. doi:10.1016/S0967-0637(00)00032-7 [Google Scholar]
- Warner N.R, Domack E.W. Millenial- to decadal-scale paleoenvironmental change during the Holocene in the Palmer Deep, Antarctica, as recorded by particle size analysis. Paleooceanography. 2002;42:8004. doi:10.1029/2000PA000602 [Google Scholar]
- Wolff E.W, et al. Southern Ocean sea-ice extent, productivity and iron flux over the past eight glacial cycles. Nature. 2006;440:491–496. doi: 10.1038/nature04614. doi:10.1038/nature04614 [DOI] [PubMed] [Google Scholar]
- Yuan X. ENSO-related impacts on Antarctic sea ice: a synthesis of phenomenon and mechanisms. Antarctic Sci. 2004;16:415–425. doi:10.1017/S0954102004002238 [Google Scholar]
- Yuan X, Martinson D.G. The Antarctic Dipole and its predictability. Geophys. Res. Lett. 2001;28:3609–3612. doi:10.1029/2001GL012969 [Google Scholar]
- Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. doi:10.1126/science.1059412 [DOI] [PubMed] [Google Scholar]
- Zachos J.C, Wara M.W, Boharty S, Delany M.L, Petrizzo M.R, Brill A, Bralower T.J, Permoli-Silva I. A transient rise in tropical sea surface temperature during the Paleocene–Eocene thermal maximum. Science. 2003;302:1551–1554. doi: 10.1126/science.1090110. doi:10.1126/science.1090110 [DOI] [PubMed] [Google Scholar]
- Zwally H.J, Comiso J.C, Parkinson C.L, Cavalieri D.J, Gloersen P. Variability of Antarctic sea ice 1979–1998. J. Geophys. Res. 2002;107 doi: 10.1029/2000JC000733 [Google Scholar]