Abstract
The marine ecosystem of the West Antarctic Peninsula (WAP) extends from the Bellingshausen Sea to the northern tip of the peninsula and from the mostly glaciated coast across the continental shelf to the shelf break in the west. The glacially sculpted coastline along the peninsula is highly convoluted and characterized by deep embayments that are often interconnected by channels that facilitate transport of heat and nutrients into the shelf domain. The ecosystem is divided into three subregions, the continental slope, shelf and coastal regions, each with unique ocean dynamics, water mass and biological distributions. The WAP shelf lies within the Antarctic Sea Ice Zone (SIZ) and like other SIZs, the WAP system is very productive, supporting large stocks of marine mammals, birds and the Antarctic krill, Euphausia superba. Ecosystem dynamics is dominated by the seasonal and interannual variation in sea ice extent and retreat. The Antarctic Peninsula is one among the most rapidly warming regions on Earth, having experienced a 2°C increase in the annual mean temperature and a 6°C rise in the mean winter temperature since 1950. Delivery of heat from the Antarctic Circumpolar Current has increased significantly in the past decade, sufficient to drive to a 0.6°C warming of the upper 300 m of shelf water. In the past 50 years and continuing in the twenty-first century, the warm, moist maritime climate of the northern WAP has been migrating south, displacing the once dominant cold, dry continental Antarctic climate and causing multi-level responses in the marine ecosystem. Ecosystem responses to the regional warming include increased heat transport, decreased sea ice extent and duration, local declines in ice-dependent Adélie penguins, increase in ice-tolerant gentoo and chinstrap penguins, alterations in phytoplankton and zooplankton community composition and changes in krill recruitment, abundance and availability to predators. The climate/ecological gradients extending along the WAP and the presence of monitoring systems, field stations and long-term research programmes make the region an invaluable observatory of climate change and marine ecosystem response.
Keywords: Palmer Station, LTER, climate change, Adélie penguin, Antarctic krill, Antarctic Circumpolar Current
1. Introduction
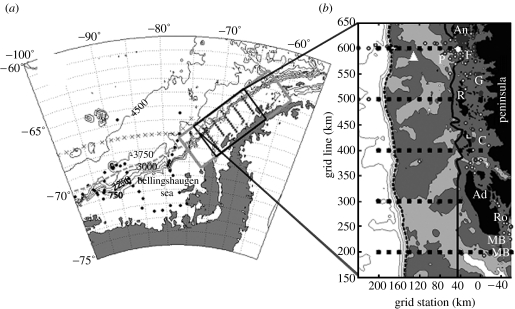
The marine ecosystem of the West Antarctic Peninsula (WAP) extends for approximately 1500 km from the Bellingshausen Sea near 75° S, 80° W to the northern tip of the peninsula near 63° S, 60° W, and from the mostly glaciated coast in the east across the continental shelf to the shelf break in the west. The shelf is about 200 km wide and averages 430 m in depth. The shelf break is defined by steep, rapidly deepening bathymetry between 750 and 3000 m (figure 1). The glacially sculpted (Anderson 2002) coastline along the peninsula is highly convoluted, cut with numerous islands, deeps, bays, fjords and a series of embayments often interconnected by channels, sometimes as deep as 900 m. The domain is divided into three subregions (continental slope, shelf and coastal regions of figure 1b) consistent with the bathymetry, ocean dynamics, water mass and biological distributions.
Figure 1.
(a) Palmer LTER study region along the WAP showing sampling grid (filled squares with labelled contoured bathymetry (750 m intervals) and climatological southern edge of Antarctic Circumpolar Current (ACC; dashed grey line). (b) The main sampling grid occupied each January since 1993 consists of stations (small squares 10 km apart) arranged in 10 onshore to offshore lines spaced 100 km apart, with line 000 to the south and 900 to the north along the peninsula (only lines 200–600 shown); stations proceed offshore from an arbitrary 0 line defining the peninsular coastline. Bathymetry shaded (white≥750 m, 750 m<light-grey≤450 m, dark-grey<450 m) and contoured (greater than or equal to 1500 m at 750 m intervals); white diamond, Palmer Station; white triangle, long-term sediment trap mooring; F, Faraday (Vernadsky) base; P, Palmer Deep region on shelf; Ro, Rothera Station; G and C, Grandidier Channel and Crystal Sound; MB, Marguerite Bay; An, R and Ad, Anvers, Renaud and Adelaide Islands, respectively; continental shelf break indicated by dashed bold line (slope to left); shelf-coastal subregions separated by solid bold line; and small white circles, various stations ‘inside’ the islands and channels with distinct hydrography influenced by glacial ice melt.
This region at its widest extent includes three of the four circumpolar Antarctic marine ecosystem types or biomes defined by Treguer & Jacques (1992): the Permanently Open Ocean Zone; Sea Ice Zone (SIZ) and, bordering the continent, the Coastal and Continental Shelf Zone. The Polar Front Zone per se is not in our study region but the Southern Antarctic Circumpolar Current Front (SACCF) impinges on the continental shelf (see below). Like other SIZs, the WAP system is very productive, supporting large stocks of marine mammals and birds, as well as the Antarctic krill, Euphausia superba (Ross et al. 1996). The dynamics of the ecosystem is dominated by the seasonal growth, extent and retreat of sea ice and their interannual variations. The Antarctic Peninsula (AP) is also one among the most rapidly warming regions on Earth, having experienced a 2°C increase in annual mean temperature since 1950. The surface ocean, west of the peninsula, has also warmed significantly. Meredith & King (2005) demonstrate warming of over 1°C since 1955–1964. In the past two decades, dramatic responses of the ecosystem to the climate and ocean warming have been documented. In this article, we synthesize the studies of rapid climate change and ecosystem responses to it, proposing the WAP marine system as a premier example of the more generalized phenomenon experienced globally. Results from the Palmer Antarctic Long-Term Ecological Research (Pal-LTER) project (Ross et al. 1996; Smith et al. 2003b), Research on Coastal Antarctic Ecosystem Rates (RACER; Huntley et al. 1991), Southern Ocean GLOBEC (Hofmann et al. 2004) and other studies are reviewed. This paper provides an observational context for the recent review of Southern Ocean ecology by Smetacek & Nicol (2005).
The life cycles of organisms in the Antarctic coastal marine ecosystem depend profoundly on the annual cycle and interannual variations in sea ice cover. We begin by discussing recent climate changes and their effects on the duration and extent of sea ice, then proceed to some of the principal components of the upper ocean pelagic ecosystem. Benthopelagic exchanges are of less importance because Antarctic continental shelves are greater than 300 m deep, well below the winter mixed layer. Benthic systems are reviewed by Clarke et al. (2007) and by Smith et al. (in press a).
2. Climate and ice
(a) Surface air temperature
Significant changes have occurred over the last half-century in the AP region, including the northwestern Weddell and southern Bellingshausen seas, as revealed by instrument records, station observations, satellite data and paleoenvironmental records (Domack et al. 2003). Surface air temperature records, in particular, reveal a warming in winter of 5–6°C over the past 50 years, a warming rate that exceeds any other observed globally (Vaughan et al. 2003). The paleo-records provide a longer-term history of change in the AP region and lend perspective for understanding the most recent (half century) warming trend, showing it to be unique within the last few millennia (Smith et al. 1999b; Domack et al. 2003; Vaughan et al. 2003).
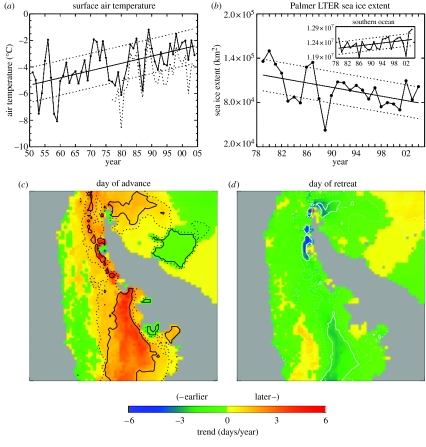
The British Antarctic Survey meteorological observations (http://www.antarctica.ac.uk/met/gjma/temps.html) at Faraday/Vernadsky station (65°15′ S, 64°16′ W) have been especially useful given their length (>5 decades), consistency and quality control. Here, we update and augment our earlier analyses of these data (Smith et al. 1996b; Smith & Stammerjohn 2001) with the addition of data through 2004. Figure 2a shows the Faraday/Vernadsky annual average air temperatures from 1951 to 2004 (N=54). The linear trend (solid line) determined by the least squares slope is 0.054±0.017°C yr−1 (p<0.01). Monthly and seasonal analyses show that the warming trend in Faraday/Vernadsky air temperatures is strongest during the midwinter months and peaks in July at 0.1165±0.0476 °C yr−1 (Neff=32.5, p=0.01). This represents a 6.3°C increase in July temperatures over the 54-year record. Spring and summer trends are not as pronounced. The record from Rothera (further south on the WAP, 67°34′ S, 68°08′ W) shows strong temporal coherence to Faraday/Vernadsky (figure 2), displaying similar trends but with mean annual temperatures averaging a few degrees cooler (King 1994; Smith et al. 1996b). Spatial coherence of surface air and sea surface temperature in the AP region is also displayed in infrared satellite observations (Comiso 2000). Changes in the annual progression of temperature and the amount of variability associated with those temperatures is suggestive of a climate shift along the WAP, where continental influences from the south are giving way to increasing maritime influences from the north (Smith et al. 1999).
Figure 2.
(a) Annual average air temperature recorded at Faraday/Vernadsky Station (65°15′ S, 64°16′ W) from 1951 to 2004. The linear regression fit (solid) and ±1 standard deviation (dotted) about this fit are included. Annual average air temperature recorded at Rothera Station (67°34′ S, 68°08′ W) from 1977 to 2004 is shown by the dotted curve. The standard error and significance were determined using the effective degrees of freedom (Neff=24.8) present in the regression residuals (see Smith et al. 1996a for methods). Also included are the ±1 standard deviation lines (dotted). (b) Annual average sea ice extent for the Palmer LTER region and for the Southern Ocean (inset) from 1979 to 2004. The linear regression fit (solid) and ±1 s.d. (dotted) about this fit are included. Spatial maps of linear trends (1979–2004) in (c) day of advance and (d) day of retreat in the greater AP region.
(b) Sea ice
Concurrently, various trends have been detected in Antarctic sea ice, showing that magnitude and direction are strongly dependent on the region and time-interval studied (Cavalieri et al. 1997; Stammerjohn & Smith 1997; Watkins & Simmonds 2000; Zwally et al. 2002; Parkinson 2004). Here, we analyse the trends during 1979–2004 (N=26), the time period for which reliable satellite observations (Comiso et al. 1997) are currently available. In contrast to the Southern Ocean as a whole, but consistent with the observed AP warming, the annual mean sea ice extent has trended down in the WAP region. Figure 2b shows the mean annual sea ice extent for the Southern Ocean (inset) and the Pal-LTER region. The Southern Ocean trend is weakly positive (11001±5982 km2 yr−1, Neff=20.4, p=0.04), representing a 2% increase relative to the mean (1.2×107 km2) over 26 years. In contrast, the trend in the Pal-LTER region is strongly negative (−1502±814 km2 yr−1, Neff=13.9, p=0.05), representing a 40% decrease relative to the mean (98 361 km2) over 26 years. As shown by Smith & Stammerjohn (2001), the decreasing trend in annually averaged sea ice extent in the Pal-LTER region is due to a decrease in the duration (not magnitude) of winter sea ice extent (i.e. winter sea ice still roughly extends as far equatorward as before but does not remain there for as long). This is in agreement with other studies that have shown a decrease in the winter sea ice season in the AP region (Parkinson 2002, 2004). Concurrently, winter sea ice concentration is decreasing (Vaughan et al. 2003; Liu et al. 2004).
Recent studies confirm that the decrease in the duration of winter sea ice extent is due to strong trends in the timing of sea ice advance and retreat, such that the advance is occurring later while the retreat is occurring earlier (Stammerjohn et al. in press a,b). Figure 2c,d also shows the spatial distribution of the trends in the advance and retreat in the greater AP region. The solid and dotted contours denote the 0.01 and 0.10 significance levels. Most of the WAP coastal region shows a strong trend towards a later advance and a somewhat weaker trend towards an earlier retreat; further to the south (i.e. southern Bellingshausen Sea) the magnitudes of the trends increase. Elsewhere in the Southern Ocean, the advance and retreat trends are weak except in the western Ross Sea region where winter sea ice duration is increasing (Parkinson 2002), concurrent with trends towards an earlier advance and later retreat (Stammerjohn et al. in press a), and overall increasing winter sea ice concentration (Liu et al. 2004).
(c) Climate covariability
Numerous studies have shown air temperature and sea ice in the AP region to be sensitive to variability in (i) the Southern Oscillation (Simmonds & Jacka 1995; Smith et al. 1996b; Kwok & Comiso 2002a), (ii) the El Niño/southern Oscillation (ENSO; Marshall & King 1998; Harangozo 2000; Rind et al. 2001; Yuan & Martinson 2000, 2001), and (iii) the Southern Annular Mode (SAM; Hall & Visbeck 2002; Thompson & Solomon 2002; Simmonds 2003; van den Broeke & Lipzig 2003; Lefebvre et al. 2004; Marshall et al. 2004). Other studies offer general reviews of climate covariability and the high latitude teleconnection in the Southern Ocean (Carleton 2003; Parkinson 2004; Simmonds & King 2004; Turner 2004; Yuan 2004). Yuan (2004) provides a thorough conceptualization (figures 5 and 8 in that study) of the potential mechanistic linkages between polar and lower latitude ocean and atmospheric processes. A few studies in particular have analysed the high latitude response in the Southeast Pacific to the combined effect of ENSO and SAM variability (Kwok & Comiso 2002b; Liu et al. 2004; Fogt & Bromwich 2005; Stammerjohn et al. in press a). In fact, Yuan (2004) notes that this region undergoes the largest extra-tropical surface temperature response to ENSO on Earth.
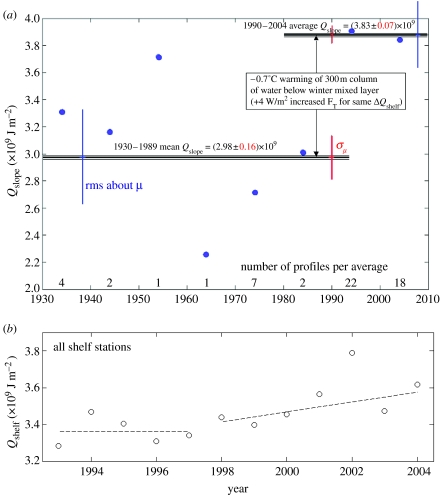
Figure 5.
(a) Heat content (relative to freezing) of ACC slope water that has direct access to LTER grid on continental shelf, serving as source of ocean heat on shelf (and shown in Martinson et al. (in press), to be linearly related to shelf heat flux through 2003). A considerable jump in this heat content occurs before 1990. Specifically, Qslope averages (2.98±0.16)×109 J m−2 for the 17 stations pre-1990 versus 40 (3.83±0.07)×109 J m−2 post-1990 stations (uncertainty in mean value shown about horizontal means as red lines; scatter about means given by blue vertical bars). This is equivalent to a uniform warming of the approximately 300 m thick layer by 0.7°C, comparable to a jump in heat flux (according to linear relationship between heat flux and heat content shown in Martinson et al. (in press)) of more than 3 W m−2. (b) More directly, heat content of this water on shelf, which has been shown to be linearly related to the ocean heat flux, shows a jump in 1998, comparable to a 3 W m−2 heat flux, followed by a linearly increasing trend of another 3 W m−2 per year (excluding 2002 which is an unusually large outlier).
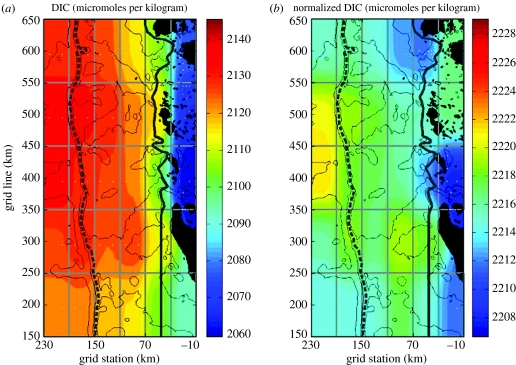
Figure 8.
(a) Total and (b) salinity-normalized (to salinity=35) mean dissolved inorganic carbon (DIC) concentrations in the surface layer in the Palmer LTER sampling region (figure 1) for 1993–2004.
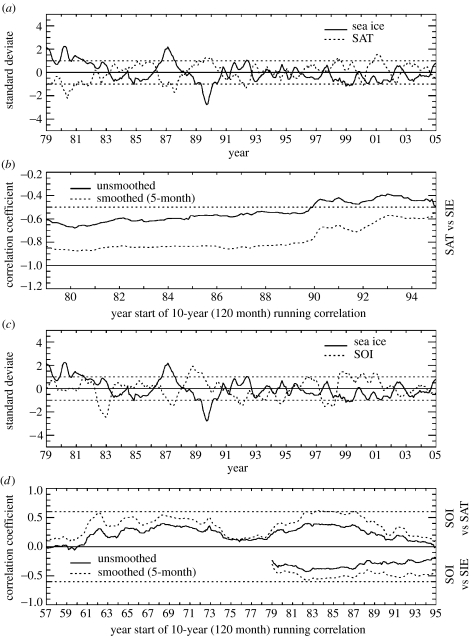
Figure 3 summarizes some of the highlights of climate covariability in the Pal-LTER region. (i) Pal-LTER sea ice extent negatively covaries with Faraday/Vernadsky air temperature (1979–2004 monthly, R=−0.79; annual, R=−0.94), (ii) 10-year running correlations show, however, that covariability has decreased since the 1990s, (iii) Pal-LTER sea ice extent and the Southern Oscillation Index (SOI; the standardized sea-level pressure difference between Tahiti and Darwin, Australia) negatively covary (1979–2004 monthly, R=−0.24; annual, R=−0.43), and (iv) 10-year running correlations show, however, that covariability between Pal-LTER sea ice extent and the SOI has decreased since the 1990s (unless the time-series are first smoothed, dotted lines), while the longer view provided by the Faraday/Vernadsky air temperature record shows that SOI covariability was also stronger in the 1960s to mid-1970s in addition to the 1980s. Similar results to (iii) and (iv) are obtained when an ENSO index (e.g. Nino3.4) is used. Additionally, Pal-LTER sea ice extent negatively covaries with SAM variability. However, correlations are in general weak unless examined on a month-to-month basis, November showing the most consistent and strongest correlations (e.g. 10-year running correlations range from −0.4 to −0.5).
Figure 3.
Monthly standard deviates (smoothed by 5-month running means) from January 1979 to December 2004. Monthly standard deviates were determined by dividing the anomaly (for the month and year in question) by the standard deviation of the anomaly (for the month in question). (a) Faraday/Vernadsky air temperature (dotted) and Palmer LTER sea ice extent (solid); (b) 10-year (120 month) running correlations between unsmoothed (solid) and smoothed (dotted) time-series of Faraday/Vernadsky air temperature and Palmer LTER sea ice extent: the smoothing was by 5-month running means; (c) Palmer LTER sea ice extent (solid) and the Southern Oscillation Index (SOI; dotted); and (d) 10-year (120 month) running correlations between unsmoothed (solid) and smoothed (dotted) time-series of Faraday/Vernadsky air temperature and SOI (positive correlations from 1957 to 1995), and between unsmoothed (solid) and smoothed (dotted) time-series of Palmer LTER sea ice extent and the SOI (negative correlations from 1979 to 1995).
Concurrent with decreased climate covariability with Pal-LTER sea ice extent in the 1990s is increased intra-seasonal variability in monthly sea ice extent (Smith et al. 1998a; Smith & Stammerjohn 2001; Stammerjohn et al. in press b). This is partially confirmed by the fact that correlations remain higher in the 1990s when using smoothed timeseries (dotted lines in figure 3b,d). The increase in intraseasonal variability between the 1980s and 1990s is captured by the degree of persistence in monthly sea ice extent anomalies, which decreased from 12 to 13 months in the 1980s to two months in the 1990s (based on autocorrelation analysis). Increased intraseasonal variability is largely a result of increased variability in the timing of sea ice advance and retreat in the 1990s, and this has direct implications for the marine ecosystem. The life histories of most polar marine species have evolved to be synchronized with the seasonality of sea ice (Smith et al. 1995; Ross et al. 1996). Therefore, the marine ecosystem may be more sensitive to changes in the seasonal timing of sea ice advance and retreat than to overall changes in magnitude of winter sea ice extent (Smith et al. 2003a,b), thus providing further impetus to understand the increased variability in sea ice advance and retreat.
As indicated by figure 2, we have analysed the variability and trends in the timing of sea ice advance and retreat (Stammerjohn et al. 2003, in press a). In contrast to results shown in figure 3 that were based on monthly sea ice extent, correlations between the timing of sea ice advance and the SOI are stronger and markedly increase in the 1990s (R=+0.72 for 1992–2004 compared with R=+0.38 for 1979–2004). The correlations between sea ice retreat and the SOI also show an increase in the 1990s (R=−0.46 for 1992–2004 compared with R=−0.38 for 1979–2004) and are somewhat weaker than for the advance but still stronger than for monthly sea ice extent. Again, similar results are obtained when an ENSO index (e.g. Nino3.4) is used. These results further confirm that the high latitude Southeast Pacific is sensitive to ENSO variability. However, given the increased intraseasonal variability of sea ice in the WAP region, monthly sea ice extent may not be the best variable for examining this relationship. As our most recent results show (Stammerjohn et al. in press a,b), we seem to better capture sea ice sensitivity to ENSO variability by restricting our focus to the periods of sea ice advance and retreat.
This is not surprising when given that the high-latitude response to ENSO variability is strongest during the austral spring–summer (especially in the 1990s due to a strengthening of the in-phase relationship with SAM during spring; Fogt & Bromwich 2005). Our studies, however, suggest that the sea ice response to ENSO variability is still evident well into austral autumn (Stammerjohn et al. in press a,b), given the high correlations we observed for sea ice advance. This may be due in part to the ice advance being more sensitive to climate variability than its retreat (Stammerjohn et al. in press b). The equatorward expansion of sea ice during advance is unconstrained physically (no continental boundary to the immediate north) and can quickly occur (relative to the retreat), given the ability to rapidly vent ocean heat, especially during cold air outbursts. In contrast, sea ice retreat is constrained physically by incoming warm air masses, by the physical presence of the Antarctic and also by increasing sea ice thickness; thus more time is needed to melt ice relative to growing ice. Further, Stammerjohn et al. (in press a) show that the negative impacts from individual La Nina and positive SAM events (both associated with earlier sea ice retreats and later sea ice advances) appear to outweigh the positive impacts from El Niño and negative SAM events, and thus strongly contribute to the overall trend of decreasing winter sea ice duration.
Within the context of the rapid warming of the AP region we summarize our current findings as follows. The strongest trends in surface air temperature are during midwinter months, peaking in July, with a 6.3°C increase since 1951. In contrast, the strongest trends in sea ice are occurring during the austral spring–summer when sea ice is retreating and during the subsequent austral autumn when sea ice is advancing. The trend towards a later advance and earlier retreat results in decreased winter sea ice duration. A shorter sea ice season implies less time for sea ice to thicken (both thermodynamically and mechanically). An overall thinner sea ice cover can be more mobile, thus less concentrated and more variable (both of which have been observed in the WAP region). Decreased winter sea ice duration, concentration and presumably thickness, are changes that would, perhaps quite dramatically, increase winter ocean heat flux, both to the overlying atmosphere through leads and other openings, as well as to the underside of sea ice and marine glaciers (see §3). In turn, the increased ocean heat flux would amplify the winter air temperature trend, the decrease in sea ice thickness (Stammerjohn et al. in press b) and the marine glacier retreat (Cook et al. 2005).
Therefore, the rapid warming in winter in the AP region may largely be due to changes occurring in the atmospheric circulation during austral spring, summer and autumn that are negatively affecting the advance and retreat such that winter sea ice duration, concentration and thickness are decreasing, and ocean winter heat flux is increasing.
3. Physical oceanography
The most voluminous source of ocean heat and nutrients in the Southern Ocean, Circumpolar Deep Water (CDW), is transported by the Antarctic Circumpolar Current (ACC). Research in the PAL-LTER (Martinson et al. in press) and throughout the Southern Ocean (Orsi et al. 1995) show that the climatological southern edge or boundary of the ACC (SBACC, defined in Orsi et al. 1995 as the southern limit of Upper CDW (UCDW) characteristics) lies along the continental shelf break in the WAP region. To the north is the SACCF (the southernmost current core of the ACC). The close proximity of the ACC to the broad continental shelves of the WAP (including the shelves of the Amundsen and Bellingshausen seas at the base of the WAP to the southwest) makes this region oceanographically unique in the Antarctic.
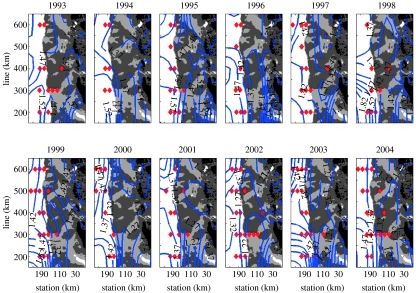
Fundamental to the WAP is the relationship of shelf water masses to those of the ACC. Key water masses as they appear in the WAP austral summer have been analysed and discussed in detail by Martinson et al. (in press) Gordon (1971) distinguishes between UCDW and Lower CDW (LCDW), noting that these are distinguished by temperature (UCDW) and salinity (LCDW) core layer maxima; the latter is absent over the shelf in the WAP. Martinson et al. (in press), wishing to relate shelf waters to those delivered to the region by the ACC, restricts the definition of UCDW to that as it occurs in the ACC immediately offshore of the WAP (hereafter referred to as ‘ACC-core UCDW’). When ACC-core UCDW is swept onto the shelf, mixing cools it to form modified UCDW (M-UCDW). Unmodified UCDW incursions occasionally survive short distances on the shelf (figure 4). Incursions most consistently move onto the shelf at the northern end of the large cross-shelf channel (Marguerite Trough) at the 300 cross-shelf line (figures 1 and 4). Incursions of UCDW are consistent with the dynamic topography (circulation), indicating interactions of the ACC with shelf bathymetry as the key physical mechanism driving the appearance of UCDW on the shelf.
Figure 4.
Annual dynamic topography as blue contours superimposed on a tripartite grey-scale bathymetry (dark grey≤450 m; 450 m<light grey≤750 m bathymetry and white>750 m). Locations where ACC-core UCDW appears anywhere in water column shown by red triangles. Note frequent occurrence of UCDW on shelf at the 300 line, in mouth of trough west of Adelaide Island (Marguerite Trough).
Winter water is prevalent throughout the Antarctic polar waters. This water is formed at or very near the freezing point—being the remnant winter mixed layer water—but here the summer values are well above freezing due to vertical mixing with the warmer waters above and below (Klinck 1998; Smith et al. 1999a; Martinson et al. in press). The most conspicuously absent Antarctic water masses on the WAP shelf are the low- and high-salinity shelf waters (LSSW, HSSW) found at depth in numerous shelf locations around the continent (Carmack 1977). These waters, near the freezing point, with 34.6 salinity delimiting LSSW from HSSW, are notable for their role in deep and bottom water formation (Gill 1973). This absence is consistent with the notion that bottom waters do not form in the WAP region today. LCDW is not commonly seen on the WAP shelf.
UCDW is quickly modified (cooled by mixing) as it moves across the shelf, cooling approximately linearly with distance from the slope (source) of the ACC-core UCDW. The significance of the cooling of this relatively warm water (3–4°C above the freezing point) on the continental shelf is that the heat is passed from the water either to the atmosphere through leads and other openings or to the underside of ice (both sea ice and marine glaciers) thus melting it. This is important given the role of glacial ice melt to rising sea level, and the ocean heat is the only source of enough heat to melt this ice (the heat content of water is 1000 times larger than that of a comparable volume of air at the same temperature above freezing). Recent research in the Pal-LTER region, using two different approaches for estimating the ocean heat flux suggests that the heat flux from the ocean has resulted in a substantial increase in the water temperature and associated heat flux beginning in the 1990s (figure 5; comparable with a number of other changes documented throughout the region for sea ice and other climate variables, Stammerjohn et al. in press a). Figure 5 shows that the increase in heat flux since 1990 is sufficient to cause a ∼0.7°C warming of the upper 300 m of the water column below the winter mixed layer—and indicates that the warming noted by Meredith & King (2005) extends well below the surface layer. There was a further jump in the heat flux after 1998, with an increasing trend since then (figure 5b). This increase is a profound change in the physical environment and underlines the role of ocean circulation as the principal driver translating climate warming into ecosystem changes on the WAP shelf. The heat flux is also a proxy for nutrient fluxes because UCDW is the primary imported source of these as well as heat; see §4a.
4. Nutrients and carbon
(a) Nutrients and UCDW intrusions
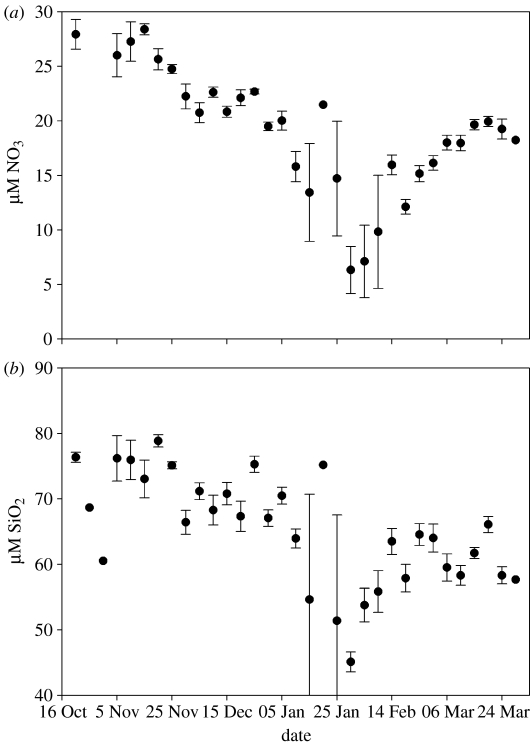
Surface macronutrient (nitrate and phosphate) concentrations generally persist at high levels in the Southern Ocean as a result of three factors: high concentrations in deep water, deep winter mixing that resupplies the surface layer following biological depletion, and micronutrient (iron) limitation. Nitrate and silicate concentrations below the main pycnocline average 33 and 95 μM, respectively, seaward of the shelf break in the Pal-LTER study region. Occasionally, in years with large phytoplankton blooms, or in areas of large phytoplankton accumulation, surface nitrate and phosphate may be nearly depleted. Surface concentrations across the WAP shelf in January (midsummer) are highly variable, ranging from near 0 to 33 μM, and from 32 to 110 μM, respectively. On average, surface nitrate and silicate are depleted from 30 to less than 10 μM and 80 to 50 μM, respectively, between November and February at inshore stations near Palmer Station (figure 6).
Figure 6.
Surface layer nutrient utilization. (a) Nitrate and (b) silicate depletion at inshore time-series station near Palmer Station, 1993–2004. Error bars show standard errors. The number of data points for each 5-day interval ranges from 1 (no bars) to 12.
The ACC is forced topographically to flow along the continental shelf break of the WAP, causing intrusions of UCDW onto the outer shelf where the flow impinges on canyon walls (see §3). These intrusions have been implicated as sources of nutrients for phytoplankton over the shelf region (Prézelin et al. 2000, 2004). From a comprehensive, multiseason, multiyear study of nutrients, hydrography and phytoplankton community composition throughout the WAP region including Marguerite Bay (MB; see below), Prézelin et al. (2004) concluded that shelf break upwelling of episodic, non-seasonal UCDW intrusions stimulated subsurface (i.e. below depths detected by remote sensing) diatom growth in the outer to midshelf region. In fact, upwelling is not necessary to bring nutrients onto the shelf: UCDW enters above the seafloor at the shelf break (figure 7), flooding the water column to the base of the pycnocline. Following the entry onto the shelf, UCDW-associated nutrients can be mixed into the surface layer by turbulent diffusive mixing, active erosion of the pycnocline and nutricline by surface mixing and upward elevation of the pycnocline by upwelling, and by active erosion of the pycnocline in winter by mixed layer expansion associated with destabilization following brine rejection during ice growth. The latter two processes greatly dominate the vertical fluxes by a factor of 4–20. Nutrient concentrations track temperature in the UCDW; therefore, it is likely that increased nutrient inputs accompanied the increase in heat flux onto the shelf since 1990 (figure 5). This remains to be demonstrated.
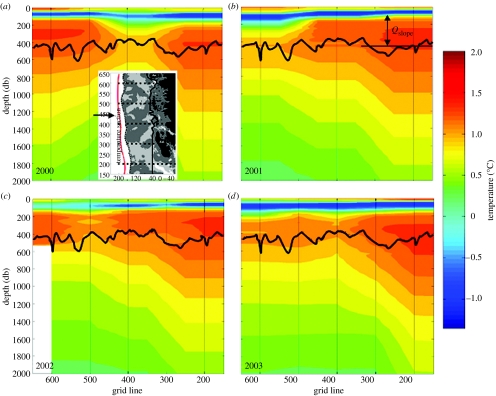
Figure 7.
Temperature sections along the red line shown in inset of (a) off Palmer LTER sampling region. Heavy black line shows average depth of shelf break. UCDW is the warmest (orange–red) water in each panel. Note that UCDW has direct access to shelf, providing considerable heat and nutrients.
Serebrennikova & Fanning (2004) investigated nutrient variability in the MB (figure 1) region during Southern Ocean-GLOBEC in 2001–2002. UCDW, supplied to the shelf by intrusions of the ACC (see §3) is characterized in this region by nitrate and silicate concentrations of 33–36 and 80–100 μM, respectively. They concluded from a detailed seasonal study of water mass properties, cross-shelf sections and a transect along the Marguerite Trough (figure 1), that UCDW intrusions had essentially the same nutrient concentrations as water already over the shelf, and that UCDW was not a net source of higher nitrate or silicate concentrations, at least in this region. In contrast to the findings of Prézelin et al. (2004), they suggested that UCDW intrusions resulted in dilution, rather than enrichment of high silicate concentrations already present over the shelf. They further suggested that the high (greater than 100 μM) concentrations of silicate encountered in bottom water were caused by dissolution of opal in the bottom sediments. The apparently conflicting results obtained by Serebrennikova & Fanning (2004) and by Prézelin et al. (2004) point to differences in nutrient distributions, supply and dynamics between MB and the WAP shelf to the north, and have not been resolved.
(b) Carbon cycle
Here, we focus on the roles played by the WAP marginal ice zone and coastal region in atmosphere–ocean CO2 exchange and particle sedimentation. These processes are linked through the action of physical–chemical and biological processes driving the solubility and biological carbon pumps (Volk & Hoffert 1985; Ducklow et al. 2001b) that transport dissolved inorganic carbon (DIC or TCO2) as well as dissolved organic carbon (DOC) against the vertical concentration gradient towards long-term storage in the deep ocean (Ducklow & McCallister 2004; Feely et al. 2001). The Southern Ocean below 50° S, with 10% of the total ocean area, is responsible for approximately 20% of the global ocean CO2 uptake (0.47 of 2.2 Pg C yr−1; Takahashi et al. 2002). Polar continental shelves covered by seasonal sea ice have been hypothesized to act as rectified (one-way) CO2 pumps, due to the phasing of sea ice cover and biological activity. Sea surface temperature is almost constant near Antarctica (relative to lower-latitude systems) and the CO2 partial pressure (pCO2) excursion in seawater governing CO2 gas exchange is almost entirely due to biological drawdown and respiration (Takahashi et al. 2002). In nearshore areas, dilution of seawater with high DIC by glacial meltwater with negligible DIC is also important. Yager et al. (1995) found that the northeast water polynya on the Greenland Shelf was strongly undersaturated in the summer, ice-free season. They put forward the ‘seasonal rectification hypothesis’, stating that in marginal ice zones, the ice-free season coincides with the main summer period of low pCO2, when the regions act as atmospheric sinks. At other times of the year, when pCO2 could be well above saturation, the water is covered by sea ice and gas exchange is prevented. In spring, primary production may consume excess DIC even before the ice cover recedes. Yager et al. (1995) and Miller et al. (2002) used estimates of CO2 exchange in the ice-free season as an annual average for air–sea gas exchange, leading to very areal high estimates of the air to sea flux. The Ross Sea polynya may function as such as a sink for atmospheric CO2 because it is strongly undersaturated in CO2 in summer in response to the Phaeocystis bloom (Takahashi et al. 2002) and covered by ice during the rest of the year.
Whether the WAP shelves act as rectified or unrectified net annual CO2 sinks is not established. The Pal-LTER grid (figure 1) has been surveyed for DIC each January since 1993 (Carrillo et al. 2004). The area is characterized by large spatial and temporal variability and by the co-occurrence of various biological (e.g. respiration and photosynthesis) and physical (e.g. heating, cooling, ice formation and ablation, melting, freshening and dilution) processes, all of which make understanding and budgeting very challenging. Carrillo et al. (2004) studied these variations in detail using high spatial resolution underway mapping of surface fCO2 and fO2 (f=fugacity, similar to pCO2) during cruises in January and July 1997. Different regions of the Pal-LTER grid showed different patterns of CO2 and O2 over- and undersaturation, resulting from spatial variation in dominance of physical or biological processes. Even in summer, dissolved CO2 was near atmospheric equilibrium in some regions, particularly offshore and towards the north part of the grid. This pattern leads to some doubt regarding the universality of the rectified sink hypothesis.
Positive net community production (NCP) is the dominant biological process in the inshore areas and especially in MB, leading to strong drawdown of DIC and undersaturation of dissolved CO2 (pCO2<200 p.p.m.). Serebrennikova & Fanning (2004) estimated NCP from total inorganic N and Si drawdown over the growing season. They found that NCP estimated from net N utilization was 3.8±1.9 and 2.8±1.3 mol C m−2 yr−1 in MB in 2001 and 2002. NCP estimated from Si utilization was 1.1 and 0.9 mol C m−2 yr−1, suggesting diatoms were responsible for about 30% of the annual NCP. The estimates for NCP in MB are comparable to the estimates made in the hyperproductive Ross Sea by Sweeney et al. (2000a,b; 3.9±0.9 mol C m−2 yr−1), but the range in MB was 0.6–9.6 mol C m−2 yr−1. Figure 8 shows the average distribution of DIC over the Pal-LTER grid for 1993–2005. Normalization of the DIC concentrations to salinity of 35 indicates the strong dilution by glacial meltwater in the nearshore zone. Higher concentrations offshore may reflect offshore inputs, and the drawdown south of Anvers Island reveals the effects of the phytoplankton bloom in the northern area.
(c) Dissolved organic carbon
There have been few measurements of DOC in the WAP shelf region. DOC concentrations in January range 45–50 μM, against a deepwater background concentration of 39 μM (H. W. Ducklow 2005, unpublished data). Carlson et al. (1998) showed that DOC accumulation was similarly low in the Ross Sea (cf. Bermuda summertime DOC of 60–70 μM and Hawaii, greater than 70 μM) and suggested that Antarctic plankton systems funnel most of the seasonal net primary production through the particulate, not dissolved carbon pools. This appears to be true in the WAP as well.
(d) Sedimentation
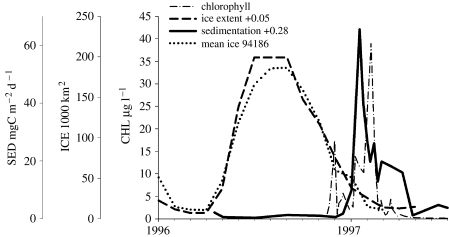
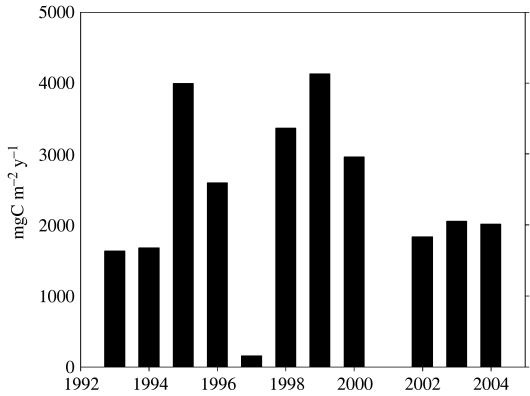
CO2 uptake in the WAP is strongly influenced by vertical sedimentation (Karl et al. 1991b). Palmer LTER has maintained a sediment trap moored at 64.5° S, 66° W since 1993, about 100 km from Palmer Station in 350 m depth (trap depth 150 m) and seaward of the Palmer Deep trough west of Anvers Island (figure 1). As elsewhere in Antarctic marginal ice zones (Wefer et al. 1988; Fischer et al. 2002), particle flux in the WAP exhibits extreme seasonality (figure 9), with a strong peak in the summer following the ice retreat and phytoplankton bloom (Ducklow et al. 2006). The timing of the annual sedimentation episode is remarkably consistent but the duration, amplitude and annual total flux all exhibit significant interannual variability (figure 10). In particular, the annual sedimentation varied by nearly an order of magnitude over the 11-year observation period. The annual sedimentation is not related to local annual primary production. Other biological factors besides primary production probably influence the patterns and magnitude of sedimentation. The annual sedimentation is weakly but significantly and inversely correlated with krill abundance (n=11, p=0.012, r2=0.51; Ducklow et al. 2006). Annual sedimentation is also significantly and directly correlated with salp abundance but only since 1998 (n=5, p=0.04, r2=0.79).
Figure 9.
Sea ice extent, chlorophyll at Palmer Station, and sedimentation rate for 1996–1997. Note that chlorophyll was high for about five months following the ice retreat, while the annual sedimentation was concentrated in a brief episode during January–February.
Figure 10.
Annual sedimentation at 150 m in the Pal-LTER study area, 64.5° S, 66° W (see figure 1). The annual integrals are based on 21 individual samples collected over the course of each year, with an interval ranging from 7 to 30 days, depending on season and expected flux (data for 1993–2000 collected by D. M. Karl and C. J. Carrillo, Univ. Hawaii for Pal-LTER).
The direct relationship between sedimentation and salp abundance is supported in the literature, which abundantly documents the large size and rapid sinking rates of salp pellets (Perissinotto & Pakhomov 1998). The inverse relationship between krill abundance and sedimentation is surprising. Krill produce large, rapidly sinking faecal pellets, but it may be that heavier sedimentation occurs when krill grazing is lower and more of the phytoplankton stock sinks as ungrazed diatoms (Smetacek & Nicol 2005), analogous to the prevailing condition in the Ross Sea. It is also possible that krill fragment sinking particles (coprohexy; coprophagy), slowing sedimentation velocities and allowing more time for decomposition during transport (Turner 2002). Antarctic microbial communities can rapidly decompose faecal pellets (Povero et al. 2003).
Interannual variations in krill abundance in the southwest Atlantic sector including the WAP are directly related to sea ice extent (Atkinson et al. 2004), and salp abundance is inversely related to ice retreat and the duration of ice cover (Atkinson et al. 2004; Ross et al. in press). Earlier studies (Loeb et al. 1997) established the concept of apparent variation in the dominance of salps versus krill in the Antarctic marine food web at the tip of the Peninsula, also tied to sea ice variations. In a period when sea ice duration and extent are declining rapidly along the WAP (see §2), the composition and dynamics of Antarctic zooplankton communities may also be changing, with only poorly understood biogeochemical consequences.
5. Primary production and phytoplankton ecology
The classic paradigm of the Southern Ocean, developed during the early expeditions more than 50 years ago, proposes a short food chain supported by diatom growth (Hart 1942). Later studies showed that nano- and picoplankton (cells less than 20 and less than 2 μm, respectively) are important components of the planktonic community (Hewes et al. 1990; Buma et al. 1991; Jacques & Panouse 1991; Villafañe et al. 1993), ubiquitous and dominant in oceanic waters. Large microplankton (cells greater than 20 μm) were thus considered rare and present only in coastal and restricted environments (Holm-Hansen & Mitchell 1991). Expeditions during the past decade have challenged this scenario as large diatoms are found also associated with fronts, such as the Polar Front (Smetacek et al. 1997) and the Southern edge of the Polar Front in the Bellingshausen Sea or SACCF (Savidge et al. 1995).
(a) Community composition and distribution
Diatoms and cryptomonads are the dominant taxa in the WAP in terms of biomass (chlorophyll a or cell carbon, Garibotti et al. 2003a). They are found in waters around Elephant Island (Villafañe et al. 1993), in the Bransfield and Gerlache straits (Holm-Hansen & Mitchell 1991; Rodriguez et al. 2002a), south of Anvers Island (Moline & Prezelin 1996; Ross et al. 2000), Grandidier Passage and MB (Garibotti et al. 2003b). Small unidentified flagellates (usually less than 5 μm) are always numerically dominant (Villafañe et al. 1993; Rodriguez et al. 2002a; Garibotti et al. 2003a). Phaeocystis cf. antarctica, a dominant Prymnesiophyte in the Ross Sea, is rare as a colonial form in the shelf waters of the WAP. It is commonly found in surface waters of the Bellingshausen Sea and slope waters of the WAP in late spring (Savidge et al. 1995; Bidigare et al. 1996; Rodriguez et al. 2002a) and can be dominant inshore in the Gerlache Strait (Rodriguez et al. 2002a) and MB (Vernet & Kozlowski 2001). Phaeocystis cf. antarctica often cooccurs with large microplanktonic diatoms and Pyramimonas sp. (Rodriguez et al. 2002b; Garibotti et al. 2003b).
Species distribution and community composition are tightly correlated with water masses, fronts and the ice edge. Large diatoms dominate frontal areas and at the ice edge, many times in combination with P. antarctica (Huntley et al. 1991; Prézelin et al. 2000; Anadon & Estrada 2002; Garibotti et al. 2003b). This community has a high rate of sedimentation (Anadon et al. 2002). Cryptomonads are frequently abundant after diatom blooms (Moline & Prezelin 1996); this is attributed to the presence of a well stratified water column, sometimes originating from glacial melt (Moline et al. 2004).
The seasonal progression, either associated with sea ice retreat, or marked by a spring bloom or a frontal bloom, is identified by the replacement of a diatom-dominated community by a flagellate or cryptomonad-dominated community. Coastal waters in the summer (January) are characterized by cryptomonads in the area south of Anvers Island, by microflagellates in the Grandidier Passage and by large chain-forming diatoms in Crystal Sound and MB. These assemblages are interpreted as late summer communities in the north, dominated by flagellates, and early ice edge communities in the south (Garibotti et al. 2003b). The transition from diatoms to flagellates has been attributed to sedimentation (Castro et al. 2002), advection (Moline & Prezelin 1996) and grazing (Garibotti et al. 2003b). Modelling indicates that if diatoms are the preferred food for krill (Haberman et al. 2003a) and young of krill (Ross et al. 2000), diatom communities initiated at the ice edge will transition to cryptomonads (Walsh et al. 2001).
Sea ice communities are characterized by similar composition as water column communities. Diatoms, flagellates and the colonial form of P. cf. antarctica are cited as more abundant, indicating a tight coupling between ice and water column, presumably by seeding of the water column during spring sea ice retreat (Ackley & Sullivan 1994) and during particle entrainment during sea ice formation in the autumn (Garrison & Mathot 1996). Different from other parts of Antarctica, sea ice communities in the WAP are characterized by infiltration (Arrigo et al. 1997; Massom et al. 2006). Flooding of sea ice at the layer between ice and snow replenishes nutrients and brings in cells from the water column. Once seeded, these communities grow. The layering of chlorophyll a in the ice is interpreted as remnants of successive flooding events (Massom et al. in press).
(b) Biomass and primary productivity
Primary production in the WAP presents a strong seasonal component dominated by day length. Phytoplankton growth in the water column, after sea ice ablation, starts as early as October and continues through early autumn (March/April; Moline & Prezelin 1996; Smith et al. 1998b, 2001). In coastal waters off Palmer Station, primary production averages 176 g C m−2 over the growing season (range 47–351 g C m−2 season−1). This is same as the annual primary production measured by similar techniques (14C incorporation) at subtropical sites like Bermuda and Hawaii, but is achieved in half the time. Similar seasonal production was observed in other ice edge areas of Antarctica, such as the Ross Sea in 1996–95 season (168 g C m−2 season−1; Smith et al. 2000). Primary production is closely correlated to phytoplankton biomass expressed as chlorophyll a (Dierssen & Smith 2000) such that biomass can represent production as well.
Oceanographic fronts, water masses and the ice edge are also the main contributors to spatial heterogeneity in biomass and primary production. The waters seaward of the shelf break (roughly 200–400 km from the coast) often display the highest spring phytoplankton biomass observed in the WAP, are seasonally swept by sea ice and are influenced by the SACCF (figure 11). Frontal blooms have been reported in the Bellingshausen Sea off Bransfield Strait (Smith et al. 1992; Savidge et al. 1995; Lorenzo et al. 2002) as well as further south (Smith et al. in press b). However, these blooms decline rapidly (several weeks) and only seldom are they followed by elevated values during the summer/autumn period. As the season progresses, the frontal bloom becomes subsurface and it is seen as a chlorophyll maximum over the slope off King George Island (Holm-Hansen et al. 2004), the Bransfield Strait (Castro et al. 2002) and Anvers Island (Garibotti et al. 2005a). The subsurface maximum is dominated by diatoms and it is believed to be an important feature for maintaining seasonal production in slope waters (Prézelin et al. 2000, 2004).
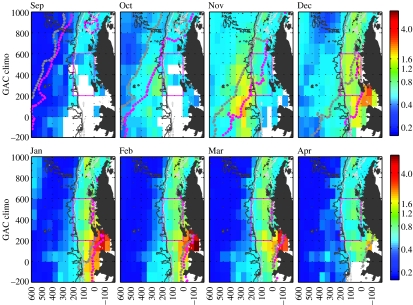
Figure 11.
Monthly mean chlorophyll a time-series (coloured areas, mg m−3, based on 7 years of SeaWiFS data with the Southern Ocean algorithm developed by Dierssen & Smith 2000) for the Palmer LTER study area (pink box). The grey and pink dotted lines show the mean ice edge position at the start and end of each month, respectively. White areas denote regions where cloud or ice cover precluded firm chlorophyll estimates.
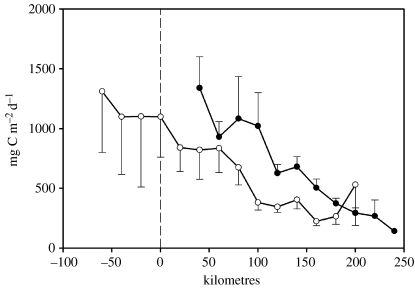
Biomass and primary production in the region over the shelf generally peak later in the season (December to January) and often show subsequent summer and/or autumn blooms, providing a more continuous source of food from late spring to autumn for the grazers (figure 11). These shelf waters show greater variability in the seasonal timing of peak values, with typically elevated biomass and production over a longer period, giving rise to higher integrated seasonal values. As the season progresses, shelf waters present a strong cross shelf gradient with higher production inshore (figure 12), as expected in coastal regions. These gradients are present in summer but not earlier in the season (figure 11). Large biomass accumulates in areas with shallow mixed layer depth (Mitchell & Holm-Hansen 1991; Garibotti et al. 2005b) and the associated stratification from sea ice melting (Smith & Nelson 1985), which are presumably protected from high winds. The decrease in biomass and production gradient is associated with a distinct change in vertical distribution. Other factors identified as key in maintaining high levels of primary production are topography-induced upwelling, as observed in 1993 at mid shelf south of Anvers Island (Prézelin et al. 2000, 2004) and stratification in late summer by freshwater input from glacier melt (Dierssen et al. 2002).
Figure 12.
Spatial variability in average January integrated production across the shelf. Estimated from 6 January cruises between 1994 and 2000 (mean and 95% confidence intervals). Solid circles, 600 line stations off Anvers Island; open circles, 200 line off Marguerite Bay (figure 1a).
Bloom demise has been attributed to several environmental and biological factors, although few studies have investigated the process (Savidge et al. 1995; Anadon & Estrada 2002). In frontal areas, biomass decrease can partly be attributed to the consumption of micronutrients, such as iron (Smith et al. in press b). At this time, a subsurface chlorophyll maximum is developed, and can be found between 40 and 80 m depth. Similarly, micronutrient limitation in surface waters after an ice edge bloom can evolve into a subsurface chlorophyll maximum. In both cases, the presence of a subsurface front, such as the SACCF, is necessary to maintain the chlorophyll maximum in slope waters (Garibotti et al. 2003a). The dominance of diatoms at the chlorophyll maximum further supports the notion of the SACCF as a source of fresh injection of micronutrients. As mentioned above, advection or intense mixing due to storms can remove coastal blooms (Moline & Prezelin 1996). On average, approximately 25% of the seasonal primary production is removed from the mixed layer (Karl et al. 1991b; Anadon et al. 2002) mainly by macrozooplankton grazers such as krill and salps (Ross et al. 1998).
Summer sea ice production in the WAP is low as multiyear sea ice is restricted to areas south of MB and the Amundsen Sea. No field studies have sampled south of Alexander Island. Remote sensing suggests first year sea ice to be the most productive. Average ice production rates are 4.4 g C m−2 season−1 (from October to April, Arrigo et al. 1997) or 2.5% of the seasonal production off Palmer Station, well below the expected 10% of the annual primary production (Legendre et al. 1992). Summer ice production is limited by nutrients while light is the main limitant at other times (Fritsen et al. 1998). Most of the production is attributed to diatoms (Prezelin et al. 1998) although flagellates and P. cf. antarctica are also abundant (Massom et al. 2006).
6. Microbial ecology
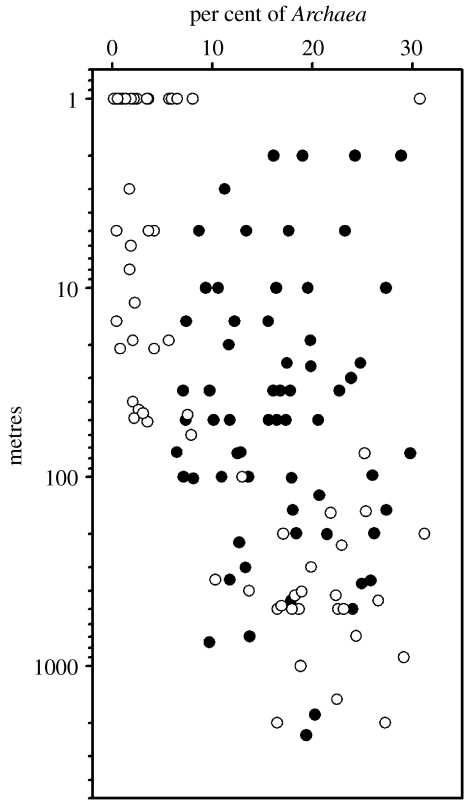
As in other marine systems (Ducklow 1999, 2000), bacterioplankton in Antarctic waters principally process dissolved organic matter (DOM), derived ultimately from phytoplankton production. Bacteria complete the microbial loop by converting the DOM into biomass, then being consumed by protozoan bacteriovores (Karl 1993; Karl et al. 1996). In the WAP, heterotrophic prokaryote stocks contain a significant portion of planktonic Archaea (Massana et al. 1998; Murray et al. 1998, 1999; Church et al. 2003), but as yet these organisms remain uncultivated and we do not know their modes of nutrition, metabolic and trophic status or roles in the plankton system. It is not yet even clear if they are autotrophs or heterotrophs. Church et al. (2003) showed seasonal and depth-related variations in the relative and absolute abundances of Archaea versus Bacteria in the LTER grid region (figure 13) with a greater proportion of the Archaea in deeper waters and during the winter. The Archaea increased in absolute abundance by approximately 40% from summer to winter in WAP surface waters, and also varied at depth, suggesting a dynamic and active population.
Figure 13.
Depth profiles from the LTER study grid (figure 1) showing the relative proportion of picoplankton cells identified as Archaea or Bacteria using poly-fluorescent in situ hybridization (FISH) probes (Church et al. 2003). Filled circles, winter samples (June–July, 1999); open circles, summer samples (January, 1999).
Total prokaryote (hereafter ‘bacteria’, including varying proportions of Archaea, with unknown metabolic identities; and heterotrophic Bacteria) abundance is greater in the summer than winter, but does not vary as conspicuously as in the Ross Sea, where bacterioplankton undergo an annual bloom (Ducklow et al. 2001a). Total abundance is generally less than 108 cells l−1 in winter (July) and reaches to about 109 cells l−1 in January (Church et al. 2003), that is, about the same range as in the temperate North Atlantic, but less than in the Ross Sea (Ducklow et al. 2001a).
Bacteria in Antarctic coastal waters must ultimately depend on phytoplankton production for organic matter (there are no terrestrial inputs of organic matter), so in some sense the two groups must be coupled by material flows in the plankton food web. In the RACER project, Karl and colleagues (Bird & Karl 1991, 1999; Karl et al. 1991a) carried out intensive seasonal (summer, December–March 1987; spring, November 1989) investigations of microbial processes in the northern AP and Drake Passage. They observed that bacteria were not correlated with chlorophyll during the spring phytoplankton bloom in the Gerlache Strait, with no bacterial response to increased chlorophyll greater than 2.5 μg l−1 (Bird & Karl 1999). Bacterial biomass was less than 2% of the total plankton biomass and bacterial production (BP) was approximately 3% of the co-occurring primary production (PP). They concluded that the bacterial response to the diatom bloom was suppressed by heterotrophic nanoplankton (HNAN) populations that consumed growing bacteria as the phytoplankton bloomed, and kept BP : PP low (see below), i.e. the HNAN exerted top-down control. Bird and Karl concluded that at least in their study area and during the spring bloom period, the microbial loop was uncoupled from primary producers, but they added that the uncoupling was not necessarily more widespread in space and time, and could be expressed more strongly in other seasons.
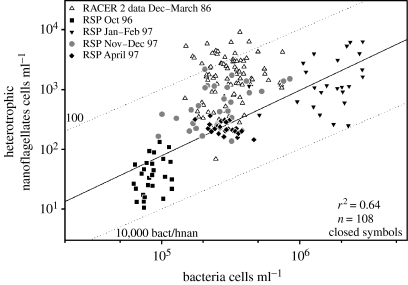
Bird & Karl (1999) diagnosed top-down control by computing the ratio of bacterial cells per individual HNAN in the standing stock of plankton samples taken at various times of the bloom cycle. Figure 14 reproduces the observations of Bird & Karl (1999) along with observations from the Ross Sea polynya. There were only about 100 bacteria per HNAN in the Gerlache Straits, and an order magnitude more in the Ross Sea over the full growth season. There were consistently fewer HNAN available to graze on bacterial cells in the Ross Sea than in the Gerlache Strait. The bacteria : HNAN ratio approached 10 000 in some samples in the Ross Sea. The striking contrast in predator–prey ratios between the two regions suggests fundamental differences in food-web structure. For example, the notable release of bacteria from predation by HNAN in the Ross Sea suggests that the bacteriovores (HNAN) are more heavily preyed upon than in the WAP region. In general, there are fewer krill in the central Ross Sea, an observation not consistent with the trophic cascade hypothesis. Salps or other mucus net feeders like pteropods could exert such top-down control on HNAN and initiate a trophic cascade favouring bacteria. The idea has not been tested.
Figure 14.
The ratio of bacterial to heterotrophic nanoflagellate (bacteriovore, HNAN) abundance in the Gerlache Strait, WAP (open symbols) and the Ross Sea (closed symbols). The dotted lines indicate fixed ratios of 100, 1000 and 10 000 bacteria per HNAN. Gerlache data courtesy of D. Bird, Université de Québec à Montréal.
Moran and colleagues studied phytoplankton–bacteria coupling in the Bransfield Strait (Moran et al. 2001, 2002). They provided a clear operational definition of phytoplankton–bacteria coupling by focusing specifically on the release of recently synthesized DOC from active phytoplankton (14% of total particulate plus dissolved primary production). In a series of carefully analysed time-series experiments, they showed that the released DOC met the metabolic requirement of bacteria in the same region studied in RACER and concluded that bacteria and phytoplankton were strongly coupled. They also concluded that BP was a very low fraction (mean 1.5±0.4%) of the total particulate plus dissolved production but termed the coupling ‘strong’ nonetheless.
Better understanding of the couplings and mechanisms of bacterial population dynamics will come when we can delve more specifically into the ecology of individual bacterial groups. This is now possible using a new battery of genomic approaches (Clark et al. 2004; Peck et al. in press). A. E. Murray (2005, personal communication) studied the annual cycle of bacterial community composition at an inshore site at Palmer Station in 2001–2002 using denaturing gradient gel electrophoresis (DGGE) of planktonic DNA. He observed different bacterial groups with distinctive seasonal distribution patterns. For example, a Polaribacter sp. constituted 20% of the total DGGE signal in winter (June–October) but less than 10% in December–January. In contrast, a Roseobacter sp. increased its apparent abundance by an order of magnitude in December–March. The crude reflections of population dynamics we see now by monitoring the total abundance are the net result of such species changes in response to similar complexity in DOM and nutrient availability caused by the interplay of physical forcing and plankton dynamics.
7. Krill and other zooplankton
(a) Zooplankton assemblages
Zooplankton, particularly those greater than 0.2 mm in length, provide the main trophic link between primary producers and apex predators in the Southern Ocean. Beginning with Macintosh (1936) and the Discovery expeditions, zooplankton assemblages in the Southern Ocean have been broadly associated with different water masses with different sea ice influences—the northern ‘oceanic’ zone with warm-water species, a zone with cold-water species influenced by seasonal pack ice, and a cold continental shelf zone close to the continent associated with permanent summer ice. Sea ice plays a role in the development of the distribution pattern of zooplankton because the marginal ice zone acts both as a frontal system with enhanced primary production and a delimiter of cold surface water. The waters west of the AP belong primarily to the seasonal pack-ice zone, but also contain species from other assemblages (Schnack-Schiel & Mujica 1994). Copepods and euphausiids, and in some years salps, dominate the zooplankton assemblages in the AP region. These major macroherbivores are often spatially segregated (Voronina 1998). In a recent large-scale study of the southwest Atlantic that extended partway down the AP (Ward et al. 2004), the relative numbers of copepods, euphausiids and salps varied depending on the distribution of ice-influenced water. In more oceanic realms that were relatively ice-free, copepods were orders of magnitude more abundant than in colder waters influenced by sea ice, particularly south of the SBACC. South of the SACCF, abundance of both salps and Antarctic krill doubled (Ward et al. 2004). In waters closer to the AP where schools of krill occur, the biomass of Antarctic krill is often higher than that of the copepods (Brinton & Antezana 1984; Hopkins 1985). Taxonomic groups other than copepods, euphausiids and salps (such as polychaetes, chaetognaths, pteropods) contribute only a small per cent (less than 10%) to the zooplankton biomass in the AP region (Schnack-Schiel & Mujica 1994).
Both oceanic (generally warmer water with depths greater than 2000 m) and neritic (shelf break and slope) zooplankton assemblages occur in the Pal-LTER study region (Siegel & Piatkowski 1990; Smith & Schnack-Schiel 1990). Both the oceanic and neritic assemblages occur in varying mixes year to year with no clear boundaries between zones. The oceanic assemblage inhabits the seaward edge of the study region, and includes herbivorous copepods, salps (Salpa thompsoni) and tomopterid polychaetes, whereas the neritic assemblage includes several species of euphausiids, including Antarctic krill (E. superba) and Thysanöessa macrura, and the herbivorous shelled pteropod, Limacina helicina. Close to the continent, usually in waters shallower than 350 m, two species (larval Antarctic silverfish, Pleuragramma antarcticum, and the ice krill, Euphausia crystallorophias) are generally considered indicator species for a zone of cold continental shelf water (Smith & Schnack-Schiel 1990).
Salps and Antarctic krill often dominate the total zooplankton biomass, accounting for over 50% of the volume in 62% of the tows conducted in summers 1993–2004, and for over 70% in 48% of the same tows. Catches varied in the presence or absence of krill and salps: 45% contained both krill and salps; 45%, krill and no salps; 4%, salps and no krill; and 3.3%, neither species. This large spatial scale co-occurrence of salps and Antarctic krill is not common. In most other regions there is a relatively distinct demarcation in the habitats of these two species (Loeb et al. 1997; Voronina 1998; Nicol et al. 2000; Atkinson et al. 2004), although in the recent large scale CCAMLR (Commission for the Conservation of Antarctic Marine Living Resources) 2000 study in the southwest Atlantic, considerable overlap was also found in the distributions of the two species (Kawaguchi et al. 2004). West of the AP, the SBACC comes closer to the shelf break than in most of the Southern Ocean, which may foster the mixing of the two assemblages.
(b) Trends or cycles
Some evidence exists for trends and/or cycles in zooplankton abundance, specifically salps and Antarctic krill. Atkinson et al. (2004), in a study of net tow data from around the Southern Ocean, suggested that salps increased and Antarctic krill decreased in the southwest Atlantic between 1976 and 2004. Smaller-scale (both time and space) studies of zooplankton west of the AP support the increase in salp numbers at the tip of the peninsula (Loeb et al. 1997) and in the Pal-LTER region (Ross et al. in press). For example, between 1993 and 1998 there were only 2 years with abundances of salps above the long-term mean on the mid-grid and southern shelf. However, from 1999 to the present, abundances have been above the long-term mean in most regions, suggesting that there has been a distinct shift in the frequency of occurrence of salps across the entire Palmer Shelf (Ross et al. in press). This shift is consistent with the observation of Pakhomov et al. (2002) that during the past two decades the region of dense concentrations of S. thompsoni has extended southwards to approximately 65° S, i.e. the latitude of the most northern transect line of the Palmer summer study region. Confirmation of the trend found by Atkinson et al. (2004) for Antarctic krill is more problematic (Smetacek & Nicol 2005). For example, a shorter acoustic time-series from the northern tip of the AP shows different patterns than the net-tow time-series (Hewitt et al. 2003). Some of the difficulties with studying trends in Antarctic krill are technical (patchiness, net avoidance, acoustic calibrations, etc.), but others lie in attempting to detect a linear trend in a species with a cycle in population abundance due to variability in recruitment (Siegel & Loeb 1995; Quetin & Ross 2003).
Fewer studies have investigated trends in other zooplankton species. In the Pal-LTER region, abundance and distribution of the two indicator species for cold continental shelf water (larvae of Antarctic silverfish and ice krill) have shown different patterns over the past 12 years. For larval Antarctic silverfish, abundance at the northern coastal stations decreased dramatically between 1997 and 1998, and none have been found at those stations since 1999. However, abundance of larval Antarctic silverfish at the southern coastal stations remained about the same throughout the time-series. The distribution and abundance of the ice krill, E. crystallorophias, has not shown any long-term trends, but is correlated in time and space with the day of ice retreat (Ross et al. in press), and thus would be expected to change if the trend in the day of retreat continues.
(c) Antarctic krill as a key species
Historically, Antarctic krill have been studied far more extensively than other Southern Ocean zooplankton (Huntley et al. 1991; El-Sayed 1994; Hofmann et al. 2004). Antarctic krill occupy a key position in the ecosystem, as they are both an important prey item for many of the seabirds and mammals in the region (Everson 2000), and a dominant grazer, particularly of the larger phytoplankton (Ross et al. 1998; Garibotti et al. 2003a), and produce fast-sinking faecal pellets that contribute to carbon flux. Studies have also been conducted to gather data on both Antarctic krill and the ecology of the ‘krill-based ecosystem’ (Watkins et al. 2004) to help in the management of its stocks by CCAMLR (Hewitt et al. 2004). Two long-term studies have been established in the waters west of the AP: initially the Antarctic Marine Living Resources (AMLR) programme was primarily sited at the tip of the AP, but it now currently includes prey and oceanography studies ranging from the tip of the peninsula through Bransfield Strait and outside the South Shetland Islands; the Pal-LTER study region is to the south of the AMLR study region in an area with comparatively little historical research on the zooplankton community. Shelf areas east and west of the AP have been suggested as the source region for populations of larvae and young krill for the high krill biomass regions in the Scotia Sea and its surrounding shelf areas with many of the major predator colonies of the Southern Ocean (Croxall et al. 1988; Murphy et al. 2004).
(d) Interface with primary production
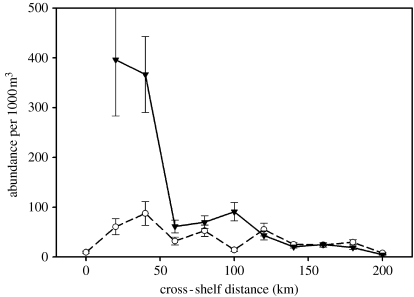
Historically, concentrations of Antarctic krill are coherent with phytoplankton concentrations at relatively long length-scales, but not coherent at length-scales below about 20 km (Weber & El-Sayed 1986). Within the Pal-LTER study region, north/south and on/offshore gradients in primary production are reflected in the gradients in abundance of Antarctic krill. Krill abundance is generally higher at inner shelf stations than offshore, and higher in the north than the south in January (figure 15, cf. figure 12). The same distribution will not hold in the autumn and winter when Antarctic krill are concentrated inshore at inner shelf stations and in the fjords (Siegel 1988; Ross et al. 1996; Lascara et al. 1999; Ashjian et al. 2004; Lawson et al. 2004). The main difference between the gradients for primary production and Antarctic krill abundance is the decrease in abundance at the innermost stations of the 200 line for krill compared to a continuing increase for primary production. What possible mechanism could underlie this difference? One possibility to explore is the role of phytoplankton community composition in controlling the distribution of krill. Adult Antarctic krill are known to select for diatoms when given mixtures of diatoms and prymnesiophytes or diatoms and cryptomonads (Haberman et al. 2003a,b). Higher growth rates of young krill in the field were also observed when phytoplankton communities were dominated by diatoms and not prymnesiophytes (Ross et al. 2000). Reproduction was also correlated with measures of primary production. The per cent of female krill reproducing during the reproductive season varied between 10 and 90% in the first 7 years of the Pal-LTER sampling, and was significantly correlated with annual primary production (Quetin & Ross 2001). Thus, krill are selective grazers, and potentially select the location with the most desirable phytoplankton community.
Figure 15.
Gradients in abundance of Euphausia superba within the Palmer LTER region in January. Mean abundance (1993–2004) with standard error for abundance within standard grid cells 100 km alongshore and 20 km on/offshore. Abundance estimates from the delta distribution (Ross et al. in press). Abundance data shown for the 600 line (inverted triangles) just south of Anvers Island, and the 200 line (open circles) which enters Marguerite Bay just south of Adelaide Island.
(e) Interface with seasonal sea ice
The concept that Antarctic krill populations are tightly coupled to seasonal sea ice dynamics stems from early observations that the distribution of Antarctic krill was coherent with the region affected by the seasonal advance and retreat of sea ice (Marr 1962; Laws 1985). Based on behavioural and physiological evidence from research in the 1980s (Daly 1990; Smetacek et al. 1990; Ross & Quetin 1991; Quetin et al. 1996), the initial LTER hypotheses regarding recruitment in Antarctic krill addressed the interaction of krill with seasonal sea ice both during the first winter for the larvae (a critical period, Ross & Quetin 1991) and during the austral spring prior to spawning. The potential mechanisms underlying interannual variability in recruitment success included, (i) higher young-of-the-year survival after winters of heavy sea ice extent because the under-ice habitat with its resident sea ice microbial communities plays an important role in winter growth rates and, potentially, survival of larval krill and (ii) higher reproductive effort (the number of larvae produced) after a spring of high sea ice extent based on the assumption that food levels in the austral spring and in the summer (when the ovary is developing and the oocytes mature prior to spawning) will determine reproductive output, and that the food levels are partly determined by seasonal sea ice dynamics. These hypotheses have expanded to include the dynamics of the sea ice cycle, the consequences of the warming trend west of the AP, and teleconnections to the rest of the world's ocean. The rapidly changing climate in the region west of the AP, including delay in the timing of advance of seasonal sea ice and decrease in the extent of summer sea ice (see §2b), suggest that krill, and in particular krill recruitment, may be a key indicator of interactions between climate change or migration and ecosystem response, including potential changes in the classical diatom–krill–higher predator food web.
(f) Time-series of recruitment success
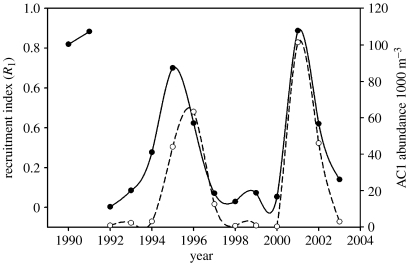
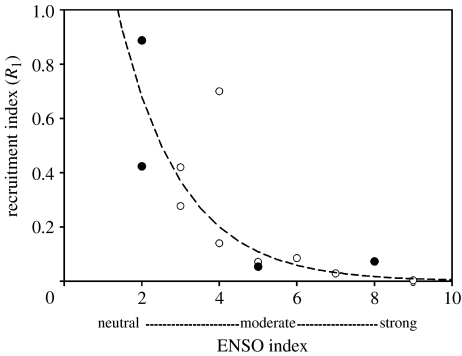
The time-series data from both AMLR and the Pal-LTER have been analysed for recruitment success (Siegel & Loeb 1995; Siegel et al. 1998, 2002; Quetin & Ross 2003). Recruitment success (R1, the proportion of the total population that is AC1 or 1 year old in January) is episodic in these long-lived animals. Two sequential successful year classes (YCs) have dominated the 5–6 year cycles in the Pal-LTER region, with strong YCs in 1990 and 1991, 1995 and 1996, and 2001 and 2002 (figure 16) (Quetin & Ross 2003). At the northern tip of the AP strong YCs have tended to occur only once every 5–6 years (Siegel et al. 2003). Basin-scale linkages and interactions are important to understanding the structure of the circumpolar ecosystem. Given the established linkage between sea-ice and the SOI (see §2c), recruitment success might also be linked to a seasonal index of ENSO that would summarize all times relevant to the life cycle of krill, i.e. spring (reproduction) through winter (larval survival). A correlation with the absolute value of summed ENSO seasonal rankings (Quetin & Ross 2003; figure 17) suggests that recruitment success is dependent on moderate, not extreme, ENSO conditions, and in turn more average sea ice conditions.
Figure 16.
Time-series of recruitment index (R1, filled circles, solid line) and abundance of AC1s (open circles, dashed line) calculated from length-frequency distributions of Euphausia superba with the maximum likelihood fitting procedure of de la Mare (1994) as used in the program CMIX. Krill were sampled in the full summer Pal-LTER study region in January of the years 1993–2004, and in a restricted sampling region in November 1991. The year class is identified from the year of the January of the spawning season. Series extended from that presented in Quetin & Ross (2003). The two estimates of R1 for year class 1990 and 1991 were estimated either from R2 (YC1990) or from a restricted sampling region (YC1991). Strong year classes are denoted either by high R1s (greater than 0.4) and/or high abundance of AC1s (greater than 40×1000 m−3).
Figure 17.
Euphausia superba. Relationship between the recruitment index (R1) and the absolute value of a seasonal index of the ENSO cycle based on the three months running mean of ERSST.v2 SST anomalies (1971–2000 base period) in the Nino3.4 region. See http://www.cpc.ncep.noaa.gov/products/analysis_monitoring/ensostuff/ensoyears.html. The temperature anomalies were used to categorize three-month periods (JFM, AMJ, JAS and OND) as neutral (0) or as a strong (3), moderate (2) or weak (1). Sequential spring, summer (spawning), autumn and winter indices were summed. Open circles are dominated by El Niño and filled circles by La Niña. Series extended from that presented in Quetin & Ross (2003). R2=0.775.
Predicting the effects of climate warming on krill populations is complex. Cycles in krill abundance, intensity of reproduction and recruitment success are correlated with different aspects of sea ice. Unlike the northern AP, the optimal conditions in the mid-AP region appear to be the mean conditions, not the extremes (Siegel & Loeb 1995; Loeb et al. 1997; Quetin & Ross 2003). Thus, in the Pal-LTER study region (figure 1), conditions similar to the 23-year average of sea ice extent in spring were best for reproductive output of the population (Quetin & Ross 2001), and a pattern of sea ice advance and retreat similar to or greater than the climatological mean for five months or longer was correlated with good overwinter survival of the larvae (Quetin & Ross 2003). Thus, deviations from average conditions in the timing, duration and extent of sea ice will adversely impact krill recruitment and ultimately availability to predators. In the WAP region, ice advance is becoming later in the autumn and the duration of summer ice is decreasing (Smith & Stammerjohn 2001). Both these changes will adversely impact the winter-over survival of the larvae and the reproductive output of female krill, leading to predictions that if those trends in the sea ice persist, regional abundance of krill will decline in the future.
8. Penguins
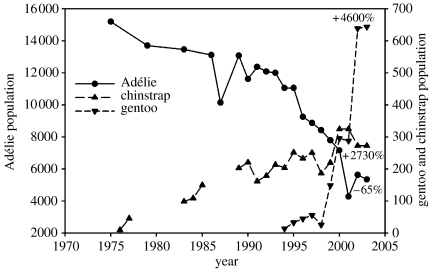
The western AP region harbours breeding populations of five of the world's 17 recognized penguin species (Williams 1995). Among these, emperor (Apdenodytes forsteri) and Adélie (Pygoscelis adeliae) penguins are considered true Antarctic species, and exhibit life histories that are closely linked to the presence of sea ice (Fraser et al. 1992; Williams 1995; Ainley 2002). The three remaining penguins, gentoo (Pygoscelis papua), chinstrap (P. antarctica) and macaroni (Eudyptes chrysolophus) are regarded as sub-Antarctic species, with life histories that are characterized by ice avoidance (Fraser et al. 1992; Williams 1995). Although the historical record indicates that emperor and macaroni penguins were never abundant in the WAP (less than a few hundred breeding pairs of each), the combined total population of the three other species numbers close to 1.5 million breeding pairs (Woehler 1993; Fraser & Trivelpiece 1996). The dominant component of WAP regional avian biomass is thus represented by the populations of these three penguin species, an axiom that holds true even in winter due to their propensity to remain associated with their preferred polar or subpolar habitats (Fraser et al. 1992; Fraser & Trivelpiece 1996).
Penguins in the WAP are important top consumers of marine resources, a trophic position they also hold in virtually all sectors of Antarctica due to their relatively high abundances and clear dominance of local and regional avian biomass. However, unlike the situation in other regions of Antarctica, and particularly in the case of Adélie penguins, their diets are almost exclusively represented by one prey species, E. superba, the Antarctic krill (Volkman et al. 1980; Ainley 2002; Fraser & Hofmann 2003). Due to their local abundance in the Palmer Station area, accessibility of their colonies and their affinity for winter sea ice, Adélie penguins were selected as the focal top predator at the inception of Pal-LTER research programme (Smith et al. 1995). As the LTER matured, the two ice intolerant species, gentoo and chinstrap penguins, were added, although detailed research on these two species lags the effort on Adélies.
As mobile, long-lived, upper-trophic level predators, penguins and other seabirds integrate the effects of variability in aspects of the physical and biological environment over large spatial and temporal scales (Fraser & Trivelpiece 1996). As indicated previously, the marine environment of the WAP is experiencing some of the most rapid and significant warming on Earth, with the loss of sea ice perhaps representing one of the most dramatic manifestations of change in a key physical variable (figure 2). Research on penguins whose life histories exhibit opposing affinities to sea ice not surprisingly provided some of the first evidence linking these changes in the physical environment to the biological responses of top predators (Fraser et al. 1992). More importantly, this research established the importance of understanding the role of life-history strategies within the context of the overall marine ecosystem response to climate variability. This focus underpins the formulation of hypotheses that guide the design of experiments and the interpretation of all aspects of the Pal-LTER penguin data (Fraser & Trivelpiece 1996; Fraser & Patterson 1997; Smith et al. 1999; Fraser & Hofmann 2003; Patterson et al. 2003).
One of the mechanisms by which climate warming induces change in ecosystem structure and function is by disrupting the evolved life-history strategies of key component species (Rhodes & Odum 1996). Certainly one of the most striking trends observed in the penguin population data is the change in community composition during the past three decades as ice-dependent Adélie penguins have decreased and ice-intolerant chinstrap and gentoo penguins have increased (figure 18). Indeed, the latter are the product of founder populations only recently established (1976 and 1994, respectively), and which may signal a unique event in the Palmer Station area given paleoecological evidence indicating that these two sub-Antarctic species have not been present locally for at least the past 700 years (Emslie et al. 1998). This implies that the environmental conditions promoting these population increases are unprecedented within the temporal limits of this record.
Figure 18.
Population trends for three penguin species in the Anvers Island vicinity, 1975–2003. The numbers on the graph indicate percentage change from initial sampling year for each species.
Although the precise causal mechanisms associated with these population trends remain equivocal (Fraser & Trivelpiece 1996), analyses focused especially on the longer-term Adélie penguin data suggest that interactions between at least two scales of processes, local and regional, that can be linked directly to the effects of rapid climate warming. As previously intimated, and particularly in view of recent analyses of several WAP penguin populations (Woehler et al. 2001), there is wide concurrence that regional-scale trends are forced by a gradual decrease in the availability of winter sea ice (Fraser et al. 1992). However, based on work at Palmer Station specifically, a more local source of population forcing has also been identified. This appears to be related to increasing snow precipitation in the WAP (Thompson et al. 1994), which affects Adélie penguin colonies breeding on landscapes where snow accumulations are enhanced by landscape aspect and prevailing winds during spring storms. These colonies have over the past 30 years decreased significantly faster than colonies where wind-scour abates snow accumulations (Fraser & Patterson 1997; Patterson et al. 2003). Interestingly, Palmer populations of the ice-intolerant chinstrap and gentoo penguins have maintained their sub-Antarctic breeding chronologies (Williams 1995); hence by breeding approximately three weeks later than Adélies, chinstrap and gentoo penguins in effect permit spring melt to circumvent the negative effects of snow accumulation.
These two scales of processes operate by producing a spatial and/or temporal mismatch between needed resources and critical aspects of a species' life history. What remains a key challenge, however, is integrating a food web perspective within the context of this dynamics. Changes in the abundance and availability of prey must surely have a role in altering the threshold states that lead to optimal habitat conditions for one species but suboptimal conditions for another (Fraser et al. 1992; Fraser & Trivelpiece 1996), yet integrating these factors into a model in which sea ice seems quite well established as a key determinant of changes in predator populations has been problematic (Smith et al. 1999). Palmer Station Adélie penguin responses to changes in krill abundance are temporally coherent with those of other krill-dependent predators over spatial scales that include the northern WAP and much of the southwest Atlantic sector of the Southern Ocean (Fraser & Hofmann 2003). The response variables, moreover, are diverse, encompassing a range of factors from changes in foraging trip durations to population trajectories, and involve other predator groups besides penguins. A key conclusion to be drawn from these findings, given the large spatial scales over which changes in krill abundance affect predator responses, is that the causal mechanisms that determine how the presence or absence of sea ice tips the balance in favour of one life-history strategy over another may actually operate over much smaller scales than previously thought. These scales may encompass, for example, the factors that determine access to breeding sites or traditional wintering areas (Fraser & Trivelpiece 1996), and incorporate predator responses that result from species-specific competitive abilities for local prey resources (Lynnes et al. 2002, 2004). Continuing Pal-LTER research on penguins is currently starting to focus on the possible relevance of these small-scale processes within the scope of understanding regional demographic responses to climate change.
9. Marine mammals
The marine mammals of the WAP include five pinniped species and at least nine species of cetaceans. Although these megafauna are one of the most conspicuous features of the WAP marine ecosystem, they are also among the least well known, a characteristic that holds true for the Antarctic in general. Indeed, it is probably not even possible to give firm order-of-magnitude estimates of standing stocks of any of these organisms along the WAP (Costa & Crocker 1996), a region where survey and other basic data are still addressing the possible presence of new species and new populations of known species (Pitman & Ensor 2003; Sirovic et al. 2004).
Not unlike the penguins previously discussed, both the pinnipeds and cetaceans are composed of species whose life histories also exhibit varying affinities to sea ice. Thus, the pack ice seals, crabeater (Lobodon carcinophagus), Weddell (Leptonychotes weddellii), leopard (Hydrurga leptonyx) and Ross (Ommatophoca rossii ), are ice-obligate species whose distribution, abundance, reproduction and foraging ecology are closely tied to the presence of sea ice, while southern elephant (Mirounga leonina) and fur (Arctocephalus gazella) seals tend to winter and forage in open water and marginal ice zones, but reproduce on land (Costa & Crocker 1996; Burns et al. 2004; Gales et al. 2004). Among cetaceans, minke (Balaenoptera bonaerensis) and killer (Orcinus orca) whales exhibit life histories with affinities to sea ice, while the other known species tend to be ice-avoiding, feeding in the WAP during the summer, but migrating north during austral winter to reproduce (Bonner 1998; Pitman & Ensor 2003; Sirovic et al. 2004).
What seems most clear about the role of the WAP in the ecology of these marine mammals is its significance as a feeding ground. Apart from the ice-dependent species that are tied to the region year-round, the other species clearly migrate into the region for the sole purpose of feeding during austral summer (most of the whales) or in autumn following reproduction in the sub-Antarctic (fur and southern elephant seals; Bonner 1998; Parmelee & Parmelee 1987; W. R. Fraser 1978–2006, unpublished data). Although fish and squid are fed upon by all these species to varying degrees, Antarctic krill is by far the most important single component in the diets of these marine mammals. Thus, in light of the better documented population responses of penguins to changes in factors such as sea ice and krill availability (see §8), it is not inconceivable that WAP marine mammal populations that are similarly associated with these variables through life history have responded in kind. Early surveys seem to support the existence of such changes, including dramatic increases in fur seal populations, an ice-avoiding species, and possible decreases in the pack ice seals, especially crabeater (Bonner 1985; Laws 1985; Erickson & Hanson 1990; Parmelee & Parmelee 1987; W. R. Fraser 1978–2006, unpublished data). Fur seals at South Georgia (north of the WAP) responded in a nonlinear fashion to climate-driven anomalies in sea surface temperature and food availability (Forcada et al. 2005). Atkinson et al. (2004), moreover, have shown convincingly that declining sea ice has resulted in a significant decrease in krill abundance in the regions where the surveys were undertaken, the WAP and southwest Atlantic sector of the Southern Ocean. However, although climate-related population changes are suspected for marine mammals in other Antarctic sectors (e.g. fur and southern elephant seals (Weimerskirch et al. 2003; Hucke-Gaete et al. 2004; McMahon et al. 2005) and minke whales (Branch & Butterworth 2001)), data interpretation is potentially confounded by the fact that many of these species are recovering from massive population declines induced by human harvest. As indicated by Smetacek & Nicol (2005), disentangling the effects of human exploitation, climate change and changing modes of top-down control exerted by large predators is a major scientific and societal challenge facing Antarctic science.
10. Conclusion
The marine pelagic ecosystem WAP, dominated by diatom primary producers, krill and a great variety of upper level vertebrate consumers, is similar in its structure and dynamics to other Antarctic shelf regions, with the exception of the Ross Sea system (Smith et al. 2007). The Ross Sea is dominated by P. antarctica, and has fewer krill than the WAP (Daniels et al. 2006). However, the WAP differs from all other Antarctic systems in one important respect: it is experiencing the most rapid warming of any marine ecosystem on the planet. Recently resolved changes in the regional climate and sea ice are now understood to affect all levels of the food web, from top predators whose life histories exhibit different affinities to sea ice to fish, krill, phytoplankton and bacteria. Changes in these ecosystem components appear to be modulated by global teleconnections with ENSO and other modes of climate variability. Clarke et al. (2007) present a schematic view of a Southern Ocean food web in which primary production is channelled through krill, salps and other zooplankton towards three general fates: passage to higher predators, sinking to benthic food webs and transfer into the microbial food web. In the WAP, most production appears to move up through the food chain to the higher predators or into bacteria. Only a few per cent of the primary production sinks through the deep (300–700 m) water column to the benthos. Whether these modes of ecosystem function will change in importance with further warming (or indeed, if they have already changed) is unknown.
A major challenge for Antarctic scientists involves not only documenting ecosystem responses at all levels of biotic organization (genome to planetary), but also establishing a mechanistic understanding of the linkages between climate, sea ice, biogeochemical processes and lower to upper trophic levels. The WAP is fortuitously characterized by a relatively simplified marine ecosystem (though one still demonstrating complex dynamics and feedbacks), rapid climate warming and a well-populated scientific contingent. These factors present the international community of Antarctic scientists and policymakers with an unparalleled opportunity for observing and understanding the interactions between climate change and marine ecosystem response.
Acknowledgments
Preparation of this article was supported by US NSF grant 0217282 from the Office of Polar Programs Antarctic Biology and Medicine Program and the US Antarctic Program.
Footnotes
One contribution of 8 to a Theme Issue ‘Antarctic ecology: from genes to ecosystems. I’.
References
- Ackley S.F, Sullivan C.W. Physical controls on the development and characteristics of Antarctic sea ice biological communities—a review and synthesis. Deep-Sea Res. I. 1994;41:1583–1604. doi:10.1016/0967-0637(94)90062-0 [Google Scholar]
- Ainley D.G. Columbia University Press; New York, NY: 2002. The Adélie penguin: bellwether of climate change. [Google Scholar]
- Anadon R, Estrada M. The Fruela cruises: a carbon flux study in productive areas of the Antarctic Peninsula (December 1995–February 1996) Deep-Sea Res. II. 2002;49:567–583. doi:10.1016/S0967-0645(01)00112-6 [Google Scholar]
- Anadon R, Alvarez-Marques F, Fernandez E, Varela M, Zapata M, Gasol J.M, Vaque D. Vertical biogenic particle flux during austral summer in the Antarctic Peninsula area. Deep-Sea Res. II. 2002;49:883–901. doi:10.1016/S0967-0645(01)00129-1 [Google Scholar]
- Anderson J.B.Antarctic marine geology2002Cambridge University Press; Cambridge, UK [Google Scholar]
- Arrigo K.R, Worthen D.L, Lizotte M.P, Dixon P, Dieckmann G. Primary production in Antarctic sea ice. Science. 1997;276:394–397. doi: 10.1126/science.276.5311.394. doi:10.1126/science.276.5311.394 [DOI] [PubMed] [Google Scholar]
- Ashjian C.J, Rosenwaks G.A, Wiebe P.H, Davis C.S, Gallager S.M, Copley N.J, Lawson G.L, Alatalo P. Distribution of zooplankton on the continental shelf off Marguerite Bay, Antarctic Peninsula, during austral fall and winter, 2001. Deep-Sea Res. II. 2004;51:2073–2098. doi:10.1016/j.dsr2.2004.07.025 [Google Scholar]
- Atkinson A, Siegel V, Pakhomov E, Rothery P. Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature. 2004;432:100–103. doi: 10.1038/nature02996. doi:10.1038/nature02996 [DOI] [PubMed] [Google Scholar]
- Bidigare R.R, Iriarte J.L, Kang S, Karentz D, Ondrusek M.E, Fryxell G.A. Phytoplankton: quantitative and qualitative assessments. In: Ross R.M, Hofmann E.E, Quetin L.B, editors. Foundations for ecological research west of the Antarctic Peninsula. vol. 70. American Geophysical Union; Washington, DC: 1996. pp. 173–198. [Google Scholar]
- Bird D.F, Karl D.M. Spatial patterns of glutamate and thymidine assimilation in the Bransfield Strait, Antarctica, during and following the austral spring bloom. Deep-Sea Res. 1991;38:1057–1075. doi:10.1016/0198-0149(91)90096-X [Google Scholar]
- Bird D.F, Karl D.M. Uncoupling of bacteria and phytoplankton during the austral spring bloom in Gerlache Strait, Antarctic Peninsula. Aquat. Microb. Ecol. 1999;19:13–27. [Google Scholar]
- Bonner W.N. Impact of fur seals on the terrestrial environment at South Georgia. In: Siegfried W.R, Condy P.R, Laws R.M, editors. Antarctic nutrient cycles and food webs. Springer; Berlin, Germany; New York, NY: 1985. pp. 641–646. [Google Scholar]
- Bonner N. Blandford Press; London, UK: 1998. Whales of the world. [Google Scholar]
- Branch T.A, Butterworth D.S. Southern hemisphere minke whales: standardized abundance estimates from the 1978/79 to 1997/98 IDCR-SOWER surveys. J. Cetacean Res. Manag. 2001;3:143–174. [Google Scholar]
- Brinton E, Antezana T. Structures of swarming and dispersed populations of krill (Euphausia superba) in Scotia Sea and South Shetland waters during January–March 1981, determined by bongo nets. J. Crust. Biol. 1984;4:45–66. [Google Scholar]
- Buma A.G.J, de Baar Nolting H.J.W, van Bennekom R.F. Metal enrichment experiments in the Weddell–Scotia Seas: effects of Fe and Mn on various plankton communities. Limnol. Oceanogr. 1991;36:1865–1878. [Google Scholar]
- Burns J.M, Costa D.P, Fedak M.A, Hindell M.A, Bradshaw C.J.A, Gales N.J, McDonald B, Trumble S.J, Crocker D.E. Winter habitat use and foraging behavior of crabeater seals along the Western Antarctic Peninsula. Deep-Sea Res. II. 2004;51:2279–2303. doi:10.1016/j.dsr2.2004.07.021 [Google Scholar]
- Carleton A.M. Atmospheric teleconnections involving the Southern Ocean. J. Geophys. Res. 2003;108:8080. doi:10.1029/2000JC000379 [Google Scholar]
- Carlson C.A, Ducklow H.W, Hansell D.A, Smith W.O., Jr Organic carbon partitioning during spring phytoplankton blooms in the Ross Sea polynya and the Sargasso Sea. Limnol. Oceanogr. 1998;43:375–386. [Google Scholar]
- Carmack E.C. Water characteristics of the Southern Ocean south of the polar front. In: Angel E, editor. A voyage of discovery. Pergamon Press; New York, NY: 1977. pp. 15–42. [Google Scholar]
- Carrillo C.J, Smith R.C, Karl D.M. Processes regulating oxygen and carbon dioxide in surface waters west of the Antarctic Peninsula. Mar. Chem. 2004;84:161–179. doi:10.1016/j.marchem.2003.07.004 [Google Scholar]
- Castro C.G, Rios A.F, Doval M.D, Perez F.F. Nutrient utilisation and chlorophyll distribution in the Atlantic sector of the Southern Ocean during austral summer 1995–96. Deep-Sea Res. II. 2002;49:623–641. doi:10.1016/S0967-0645(01)00115-1 [Google Scholar]
- Cavalieri D.J, Gloersen P, Parkinson C.L, Comiso J.C, Zwally H.J. Observed hemispheric asymmetry in global sea ice changes. Science. 1997;278:1104–1106. doi:10.1126/science.278.5340.1104 [Google Scholar]
- Church M.J, DeLong E.F, Ducklow H.W, Karner M.B, Preston C.M, Karl D.M. Abundance and distribution of planktonic Archaea and Bacteria in the waters west of the Antarctic Peninsula. Limnol. Oceanogr. 2003;48:1893–1902. [Google Scholar]
- Clark M.S, et al. Antarctic genomics. Comp. Funct. Genom. 2004;5:230–238. doi: 10.1002/cfg.398. doi:10.1002/cfg.398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A, Murphy E.J, Meredith M.P, King J.C, Peck L.S, Barnes D.K.A, Smith R.C. Climate change and the marine ecosystem of the western Antarctic Peninsula. Phil. Trans. R. Soc. B. 2007;362:149–166. doi: 10.1098/rstb.2006.1958. doi:10.1098/rstb.2006.1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comiso J.C. Variability and trends in Antarctic surface temperatures from in situ and satellite infrared measurements. J. Clim. 2000;13:1674–1696. doi:10.1175/1520-0442(2000)013<1674:VATIAS>2.0.CO;2 [Google Scholar]
- Comiso J.C, Cavalieri D, Parkinson C, Gloersen P. Passive microwave algorithms for sea ice concentration—a comparison of two techniques. Remote Sens. Environ. 1997;60:357–384. doi:10.1016/S0034-4257(96)00220-9 [Google Scholar]
- Cook A.J, Fox A.J, Vaughan D.J, Ferrigno J.G. Retreating glacier fronts on the Antarctic Peninsula over the past half-century. Science. 2005;308:541–544. doi: 10.1126/science.1104235. doi:10.1126/science.1104235 [DOI] [PubMed] [Google Scholar]
- Costa D.P, Crocker D.E. Marine mammals of the Southern Ocean. In: Ross R.M, Hofmann E.E, Quetin L.B, editors. Foundations for ecological research west of the Antarctic Peninsula. American Geophysical Union; Washington, DC: 1996. pp. 287–301. [Google Scholar]
- Croxall J.P, McCann T.S, Prince P.A, Rothery P. Reproductive performance of seabirds and seals at South Georgia and Signy Island, South Orkney Islands, 1976–1987: implications for Southern Ocean monitoring studies. In: Sahrhage D, editor. Antarctic ocean and resources variability. Springer; Berlin, Germany: 1988. pp. 261–285. [Google Scholar]
- Daly K.L. Overwintering development, growth, and feeding of larval Euphausia superba in the Antarctic marginal ice zone. Limnol. Oceanogr. 1990;35:1564–1576. [Google Scholar]
- Daniels, R. M., Ducklow, H. W. & Richardson, T. L. 2006. Food web structure and biogeochemical processes during oceanic phytoplankton blooms: an inverse model analysis. Deep-Sea Res. II53, 532–554.
- De la Mare W.K. Estimating krill recruitment and its variability. CCAMLR Sci. 1994;1:55–69. [Google Scholar]
- Dierssen H.M, Smith R.C. Bio-optical properties and remote sensing ocean color algorithms for Antarctic Peninsula waters. J. Geophys. Res. 2000;105:26 301–26 312. doi:10.1029/1999JC000296 [Google Scholar]
- Dierssen H.M, Smith R.C, Vernet M. Glacial meltwater dynamics in coastal waters west of the Antarctic Peninsula. Proc. Natl Acad. Sci. USA. 2002;99:1790–1795. doi: 10.1073/pnas.032206999. doi:10.1073/pnas.032206999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domack E, Leventer A, Burnett A, Bindschadler R, Convey P, Kirby M. American Geophysical Union; Washington, DC: 2003. Antarctic Peninsula climate variability: historical and paleoenvironmental perspectives. [Google Scholar]
- Ducklow H.W. Minireview: the bacterial content of the oceanic euphotic zone. FEMS Microbiol. Ecol. 1999;30:1–10. doi:10.1111/j.1574-6941.1999.tb00630.x [Google Scholar]
- Ducklow H.W. Bacterioplankton production and biomass in the oceans. In: Kirchman D.L, editor. Microbial ecology of the oceans. Wiley; New York, NY: 2000. pp. 85–120. [Google Scholar]
- Ducklow H.W, McCallister S.L. The biogeochemistry of carbon dioxide in the coastal oceans. In: Robinson A.R, Brink K, Rothschild B.J, editors. The sea. The global coastal ocean: multiscale interdisciplinary processes. vol. 13. Harvard University Press; Cambridge, MA: 2004. pp. 269–315. ch. 9. [Google Scholar]
- Ducklow H.W, Carlson C.A, Church M, Kirchman D, Smith D, Steward G. The seasonal development of the bacterioplankton bloom in the Ross Sea, Antarctica, 1994–1997. Deep-Sea Res. II. 2001a;48:4199–4221. doi:10.1016/S0967-0645(01)00086-8 [Google Scholar]
- Ducklow H.W, Steinberg D.K, Buesseler K.O. Upper ocean carbon export and the biological pump. Oceanography. 2001b;14:50–58. [Google Scholar]
- Ducklow, H. W., Fraser, W., Karl, D. M., Quetin, L. B., Ross, R. M., Smith, R. C., Stammerjohn, S. E., Vernet, M. & Daniels, R. M. 2006. Water column processes in the West Antarctic Peninsula and the Ross Sea: foodweb structure and interannual variability. Deep-Sea Res. II53, 834–852.
- El-Sayed S.Z. History, organization and accomplishments of the BIOMASS programme. In: El-Sayed S.Z, editor. Southern Ocean ecology. Cambridge University Press; Cambridge, UK: 1994. pp. 1–8. [Google Scholar]
- Emslie S.D, Fraser W.R, Smith R.C, Walker W. Abandoned penguin colonies and environmental change in the Palmer Station area, Anvers Island, Antarctic Peninsula. Antarctic Sci. 1998;10:257–268. [Google Scholar]
- Erickson A.W, Hanson M.B. Continental estimates and population trends in Antarctic seals. In: Kerry K.R, Hempel G, editors. Ecological change and the conservation of Antarctic ecosystems. Springer; Berlin, Germany; New York, NY: 1990. pp. 253–254. [Google Scholar]
- Everson I. Role of krill in marine food webs: the Southern Ocean. In: Everson I, editor. Krill: biology, ecology and fisheries. Blackwell Science; Oxford, UK: 2000. pp. 194–201. [Google Scholar]
- Feely R.A, Sabine C.L, Takahashi T, Wanninkhof R. Oceanography. vol. 14. 2001. Uptake and storage of carbon dioxide in the ocean. pp. 18–32. [Google Scholar]
- Fischer G, Gersonde R, Wefer G. Organic carbon, biogenic silica and diatom fluxes in the marginal winter sea-ice zone and in the Polar Front Region: interannual variations and differences in composition. Deep-Sea Res. II. 2002;49:1721–1745. [Google Scholar]
- Fogt R.L, Bromwich D.H. Decadal variability of the ENSO teleconnection to the high latitude South Pacific governed by coupling with the southern annular mode. J. Clim. 2005;19:979–997. doi:10.1175/JCLI3671.1 [Google Scholar]
- Forcada J, Trathan P.N, Reid K, Murphy E.J. The effects of global climate variability in pup production of Antarctic fur seals. Ecology. 2005;86:2408–2417. [Google Scholar]
- Fraser W.R, Hofmann E.E. A predator's perspective on causal links between climate change, physical forcing and ecosystem response. Mar. Ecol. Prog. Ser. 2003;265:1–15. [Google Scholar]
- Fraser W.R, Patterson D.L. Human disturbance and long-term changes in Adelie penguin populations: a natural experiment at Palmer Station, Antarctic Peninsula. In: Battaglia B, Valencia J, Walton D.W.H, editors. Antarctic communities: species, structure and survival, scientific committee for Antarctic research (SCAR), sixth biological symposium. Cambridge University Press; New York, NY: 1997. pp. 445–452. [Google Scholar]
- Fraser W.R, Trivelpiece W.Z. Factors controlling the distribution of seabirds: winter–summer heterogeneity in the distribution of Adelie penguin populations. In: Ross R.M, Hofmann E.E, Quetin L.B, editors. Foundations for ecological research west of the Antarctic Peninsula. vol. 70. American Geophysical Union; Washington, DC: 1996. pp. 257–272. [Google Scholar]
- Fraser W.R, Trivelpiece W.Z, Ainley D.G, Trivelpiece S.G. Increases in Antarctic penguin populations: reduced competition with whales or a loss of sea ice due to environmental warming? Polar Biol. 1992;11:525–531. doi:10.1007/BF00237945 [Google Scholar]
- Fritsen C.H, Ackley S.F, Kremer J.N, Sullivan C.W. Flood–freeze cycles and microalgal dynamics in Antarctic pack ice. In: Lizotte M.L, Arrigo K.R, editors. Antarctic sea ice: biological processes, interactions, and variability. American Geophysical Union; Washington, DC: 1998. pp. 1–21. [Google Scholar]
- Gales N.J, Fraser W.R, Costa D.P, Southwell C. Do crabeater seals forage cooperatively? Deep-Sea Res. II. 2004;51:2305–2310. doi:10.1016/j.dsr2.2004.07.006 [Google Scholar]
- Garibotti I.A, Vernet M, Ferrario M.E, Smith R.C, Ross R.M, Quetin L.B. Phytoplankton spatial distribution patterns along the western Antarctic Peninsula (Southern Ocean) Mar. Ecol. Prog. Ser. 2003a;261:21–39. [Google Scholar]
- Garibotti I.A, Vernet M, Kozlowski W.A, Ferrario M.E. Composition and biomass of phytoplankton assemblages in coastal Antarctic waters: a comparison of chemotaxonomic and microscopic analyses. Mar. Ecol. Prog. Ser. 2003b;247:27–42. [Google Scholar]
- Garibotti I.A, Vernet M, Ferrario M.E. Annually recurrent phytoplanktonic assemblages during summer in the seasonal ice zone west of the Antarctic Peninsula (Southern Ocean) Deep-Sea Res. II. 2005a;52:1823–1841. doi:10.1016/j.dsr.2005.05.003 [Google Scholar]
- Garibotti, I. A., Vernet, M., Smith, R. C. & Ferrario, M. E. 2005b Marine phytoplankton distribution during three summers in the seasonal sea-ice zone west of the Antarctic Peninsula. J. Plankton Res 27, 825–843.
- Garrison D.L, Mathot S. Pelagic and sea ice microbial communities. In: Ross R.M, Hofmann E.E, Quetin L.B, editors. Foundations for ecological research west of the Antarctic Peninsula. vol. 70. American Geophysical Union; Washington, DC: 1996. pp. 155–172. [Google Scholar]
- Gill A.E. Circulation and bottom water production in the Weddell Sea. Deep-Sea Res. 1973;20:111–140. [Google Scholar]
- Gordon A.L. Oceanography of Antarctic Waters. Antarctic Res. Ser. 1971;15:169–203. [Google Scholar]
- Haberman K.L, Quetin L.B, Ross R.M. Diet of the Antarctic krill (Euphausia superba Dana) I. Comparisons of grazing on Phaeocystis antarctica (Karsten) and Thalassiosira antarctica (Comber) J. Exp. Mar. Biol. Ecol. 2003a;283:79–95. doi:10.1016/S0022-0981(02)00466-5 [Google Scholar]
- Haberman K.L, Ross R.M, Quetin L.B. Diet of the Antarctic krill (Euphausia superba Dana) II. Selective grazing on mixed phytoplankton assemblages. J. Exp. Mar. Biol. Ecol. 2003b;283:97–113. doi:10.1016/S0022-0981(02)00467-7 [Google Scholar]
- Hall A, Visbeck M. Synchronous variability in the southern hemisphere atmosphere, sea ice and ocean resulting from the annular mode. J. Clim. 2002;15:3043–3057. doi:10.1175/1520-0442(2002)015<3043:SVITSH>2.0.CO;2 [Google Scholar]
- Harangozo S.A. A search for ENSO teleconnections in the West Antarctic Peninsula climate in austral winter. Int. J. Climatol. 2000;20:663–679. doi:10.1002/(SICI)1097-0088(200005)20:6<663::AID-JOC493>3.0.CO;2-I [Google Scholar]
- Hart T.J. Phytoplankton periodicity in Antarctic surface waters. Discov. Rep. 1942;21:261–356. [Google Scholar]
- Hewes C.D, Sakshaug E, Reid F.M.H, Holm-Hansen O. Microbial autotrophic and heterotrophic eucaryotes in Antarctic waters: relationships between biomass and chlorophyll, adenosine triphosphate and particulate organic carbon. Mar. Ecol. Prog. Ser. 1990;63:27–35. [Google Scholar]
- Hewitt R.P, Demer D.A, Emery J.H. An 8-year cycle in krill biomass density inferred from acoustic surveys conducted in the vicinity of the South Shetland Islands during the austral summers of 1991/92 through 2001/2002. Aquat. Living Resour. 2003;16:205–213. doi:10.1016/S0990-7440(03)00019-6 [Google Scholar]
- Hewitt R.P, et al. Biomass of Antarctic krill in the Scotia Sea in January/February 2000 and its use in revising an estimate of precautionary yield. Deep-Sea Res. II. 2004;51:1215–1236. doi:10.1016/j.dsr2.2004.06.011 [Google Scholar]
- Hofmann E.E, Wiebe P.H, Costa D.P, Torres J.J. An overview of the Southern Ocean global ocean ecosystems dynamics program. Deep-Sea Res. II. 2004;51:1921–1924. doi:10.1016/j.dsr2.2004.08.007 [Google Scholar]
- Holm-Hansen O, Mitchell B.G. Spatial and temporal distribution of phytoplankton and primary production in the western Bransfield Strait region. Deep-Sea Res. 1991;38:961–980. doi:10.1016/0198-0149(91)90092-T [Google Scholar]
- Holm-Hansen O, et al. Factors influencing the distribution, biomass, and productivity of phytoplankton in the Scotia Sea and adjoining waters. Deep-Sea Res. II. 2004;51:1333–1350. doi:10.1016/j.dsr2.2004.06.015 [Google Scholar]
- Hopkins T.L. The zooplankton community of Croker passage, Antarctic Peninsula. Polar Biol. 1985;4:161–170. doi:10.1007/BF00263879 [Google Scholar]
- Hucke-Gaete R, Osman L.P, Moreno C.A, Torres D. Examining natural population growth from near extinction: the case of the Antarctic fur seal at the South Shetlands, Antarctica. Polar Biol. 2004;27:304–311. doi:10.1007/s00300-003-0587-8 [Google Scholar]
- Huntley M.E, Karl D.M, Niiler P.P, Holm-Hansen O. Research on Antarctic coastal ecosystem rates (RACER): an interdisciplinary field experiment. Deep-Sea Res. 1991;38:911–941. doi:10.1016/0198-0149(91)90090-3 [Google Scholar]
- Jacques G, Panouse M. Biomass and composition of size fractionated phytoplankton in the Weddell Sea confluence area. Polar Biol. 1991;11:315–328. doi:10.1007/BF00239024 [Google Scholar]
- Karl D.M. Microbial processes in the Southern Oceans. In: Friedman I.E, editor. Antarctic microbiology. Wiley-Liss; New York, NY: 1993. pp. 1–63. [Google Scholar]
- Karl D.M, Holm-Hansen O, Taylor G.T, Tien G, Bird D.F. Microbial biomass and productivity in the western Bransfield Strait, Antarctica during the 1986–87 austral summer. Deep-Sea Res. 1991a;38:1029–1055. doi:10.1016/0198-0149(91)90095-W [Google Scholar]
- Karl D.M, Tilbrook B.D, Tien G. Seasonal coupling of organic matter production and particle flux in the western Bransfield Strait, Antarctica. Deep-Sea Res. 1991b;38:1097–1126. doi:10.1016/0198-0149(91)90098-Z [Google Scholar]
- Karl D.M, Christian J.R, Dore J.E, Letelier R.M. Microbiological oceanography in the region west of the Antarctic Peninsula: microbial dynamics, nitrogen cycle and carbon flux. In: Ross R.M, Hofmann E.E, Quetin L.B, editors. Foundations for ecological research west of the Antarctic Peninsula. vol. 70. American Geophysical Union; Washington, DC: 1996. pp. 303–332. [Google Scholar]
- Kawaguchi S, Siegel V, Litvinov F, Loeb V, Watkins J. Salp distribution and size composition in the Atlantic sector of the Southern Ocean. Deep-Sea Res. II. 2004;51:1369–1381. doi:10.1016/j.dsr2.2004.06.017 [Google Scholar]
- King J.C. Recent climate variability in the vicinity of the Antarctic Peninsula. Int. J. Climatol. 1994;14:357–369. [Google Scholar]
- Klinck J.M. Heat and salt changes on the continental shelf west of the Antarctic Peninsula between January ’93 and January ’94. J. Geophys. Res. 1998;103b:7617–7636. doi:10.1029/98JC00369 [Google Scholar]
- Kwok R, Comiso J.C. Southern Ocean climate and sea ice anomalies associated with the Southern Oscillation. J. Clim. 2002a;15:487–501. doi:10.1175/1520-0442(2002)015<0487:SOCASI>2.0.CO;2 [Google Scholar]
- Kwok R, Comiso J.C. Spatial patterns of variability in Antarctic surface temperature: connections to the southern hemisphere annular mode and the Southern Oscillation. Geophys. Res. Lett. 2002b;29:1705. doi:10:1029/2002GL015415 [Google Scholar]
- Lascara C.M, Hofmann E.E, Ross R.M, Quetin L.B. Seasonal variability in the distribution of Antarctic krill, Euphausia superba, west of the Antarctic Peninsula. Deep-Sea Res. 1999;46:951–984. doi:10.1016/S0967-0637(98)00099-5 [Google Scholar]
- Laws R.M. The ecology of the Southern Ocean. Am. Sci. 1985;73:26–40. [Google Scholar]
- Lawson G.L, Wiebe P.H, Ashjian C.J, Gallager S.M, Davis C.S, Warren J.D. Acoustically-inferred zooplankton distribution in relation to hydrography west of the Antarctic Peninsula. Deep-Sea Res. II. 2004;51:2041–2072. doi:10.1016/j.dsr2.2004.07.022 [Google Scholar]
- Lefebvre W, Goosse H, Timmermann R, Fichefet T. Influence of the southern annular mode on the sea ice-ocean system. J. Geophys. Res. 2004;109 doi:101029/2004JC002403 [Google Scholar]
- Legendre L, et al. Ecology of sea ice biota 2: global significance. Polar Biol. 1992;12:429–444. [Google Scholar]
- Liu J, Curry J.A, Martinson D.G. Interpretation of recent Antarctic sea ice variability. Geophys. Res. Lett. 2004;31:L02205. doi:10.1029/2003GL018732 [Google Scholar]
- Loeb V, Siegel V, Holm-Hansen O, Hewitt R, Fraser W.R, Trivelpiece W.Z, Trivelpiece S.G. Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature. 1997;387:897–900. doi:10.1038/43174 [Google Scholar]
- Lorenzo L.M, Arbones B, Figueiras F.G, Tilstone G.H, Figueroa F.L. Photosynthesis, primary production and phytoplankton growth rates in Gerlache and Bransfield Straits during austral summer: cruise Fruela 95. Deep-Sea Res. II. 2002;49:707–721. doi:10.1016/S0967-0645(01)00120-5 [Google Scholar]
- Lynnes A.S, Reid K, Croxall J.P, Trathan P.N. Conflict or coexistence? Foraging distribution and competition for prey between Adélie and chinstrap penguins. Mar. Biol. 2002;141:1165–1174. doi:10.1007/s00227-002-0899-1 [Google Scholar]
- Lynnes A.S, Reid K, Croxall J.P. Diet and reproductive success of Adélie and chinstrap penguins: linking response of predators to prey population dynamics. Polar Biol. 2004;27:544–554. doi:10.1007/s00300-004-0617-1 [Google Scholar]
- Macintosh N.S. Distribution of the macroplankton in the Atlantic sector of the Antarctic. Discov. Rep. 1936;9:65–160. [Google Scholar]
- Marr J.W.S. The natural history and geography of the Antarctic krill (Euphausia superba Dana) Discov. Rep. 1962;32:33–464. [Google Scholar]
- Marshall G.J, King J.C. Southern hemisphere circulation anomalies associated with extreme Antarctic Peninsula winter temperatures. Geophys. Res. Lett. 1998;25:2437–2440. doi:10.1029/98GL01651 [Google Scholar]
- Marshall G.J, Stott P.A, Turner J, Connolley W.M, King J.C, Lachlan-Cope T.A. Causes of exceptional atmospheric circulation changes in the southern hemisphere. Geophys. Res. Lett. 2004;31:L14205. doi:101029/2004GL019952 [Google Scholar]
- Martinson, D. G., Stammerjohn, S. E., Smith, R. C. & Iannuzzi, R. A. In press. Palmer, Antarctica, long-term ecological research program first 12 years: physical oceanography, spatio-temporal variability. Deep-Sea Res. II
- Massana R, Taylor L.T, Murray A.E, Wu K.Y, Jeffrey W.H, DeLong E.F. Distribution of marine planktonic archaea in the Gerlache Strait, Antarctic Peninsula, during early spring. Limnol. Oceanogr. 1998;43:607–617. [Google Scholar]
- Massom, R. A. et al 2006 Extreme anomalous atmospheric circulation in the West Antarctic Peninsula region in austral spring and summer 2001/02, and its profound impact on sea ice and biota. J. Clim 19, 3544–3571.
- McMahon C.R, Bester M.N, Burton H.R, Hindell M.A, Bradshaw C.J.A. Population status, trends and a re-examination of the hypotheses explaining the recent declines of the southern elephant seal Mirounga leonina. Mammal Rev. 2005;35:82–100. doi:10.1111/j.1365-2907.2005.00055.x [Google Scholar]
- Meredith M.P, King J.C. Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys. Res. Lett. 2005;32:L19604. doi:101029/2005GL024042 [Google Scholar]
- Miller L.A, et al. Carbon distributions and fluxes in the North Water, 1998 and 1999. Deep-Sea Res. II. 2002;49:5151–5170. doi:10.1016/S0967-0645(02)00183-2 [Google Scholar]
- Mitchell B.G, Holm-Hansen O. Observations and modeling of the Antarctic phytoplankton crop in relation to mixing depth. Deep-Sea Res. 1991;38:981–1007. [Google Scholar]
- Moline M.A, Prezelin B.B. Palmer LTER 1991-1994: long-term monitoring and analyses of physical factors regulating variability in coastal Antarctic phytoplankton biomass, in situ productivity and taxonomic composition over subseasonal, seasonal and interannual time scales phytoplankton dynamics. Mar. Ecol. Prog. Ser. 1996;145:143–160. [Google Scholar]
- Moline M.A, Claustre H, Frazer T.K, Schofield O, Vernet M. Alteration of the food web along the Antarctic Peninsula in response to a regional warming trend. Global Change Biol. 2004;10:1973–1980. doi:10.1111/j.1365-2486.2004.00825.x [Google Scholar]
- Moran X.A.G, Gasol J.M, Pedros-Alio C, Estrada M. Dissolved and particulate primary production and bacterial production in offshore Antarctic waters during Austral summer: coupled or uncoupled? Mar. Ecol. Prog. Ser. 2001;222:25–39. [Google Scholar]
- Moran X.A.G, Estrada M, Gasol J.M, Pedros-Alio C. Dissolved primary production and the strength of phytoplankton–bacterioplankton coupling in contrasting marine regions. Microb. Ecol. 2002;44:217–223. doi: 10.1007/s00248-002-1026-z. [DOI] [PubMed] [Google Scholar]
- Murphy E.J, Thorpe S.E, Watkins J.L, Hewitt R. Modeling the krill transport pathways in the Scotia Sea: spatial and environmental connections generating the seasonal distribution of krill. Deep-Sea Res. II. 2004;51:1435–1456. doi:10.1016/j.dsr2.2004.06.019 [Google Scholar]
- Murray A.E, Preston C.M, Massana R, Taylor L.T, Blakis A, Wu K, DeLong E.F. Seasonal and spatial variability of bacterial and archaeal assemblages in the coastal waters off Anvers Island, Antarctica. Appl. Environ. Microb. 1998;64:2585–2595. doi: 10.1128/aem.64.7.2585-2595.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A.E, Wu K.Y, Moyer C.L, Karl D.M, DeLong E.F. Evidence for circumpolar distribution of planktonic Archaea in the Southern Ocean. Aquat. Microb. Ecol. 1999;18:263–273. [Google Scholar]
- Nicol S, Constable A, Pauly T. Estimates of circumpolar Antarctic krill abundance based on recent acoustic density measurements. CCAMLR Sci. 2000;7:87–99. [Google Scholar]
- Orsi A.H, Whitworth T, III, Nowlin W.D. On the meridional extent and fronts of the Antarctic circumpolar current. Deep-Sea Res. 1995;42:641–673. doi:10.1016/0967-0637(95)00021-W [Google Scholar]
- Pakhomov E.A, Froneman P.W, Perissinotto R. Salp/krill interactions in the Southern Ocean: spatial segregation and implications for the carbon flux. Deep-Sea Res. II. 2002;49:1881–1907. doi:10.1016/S0967-0645(02)00017-6 [Google Scholar]
- Parkinson C.L. Trends in the length of the Southern Ocean sea ice season. Ann. Glaciol. 2002;34:435–440. [Google Scholar]
- Parkinson C.L. Southern Ocean sea ice and its wider linkages: insights revealed from models and observations. Antarctic Sci. 2004;16:387–400. doi:10.1017/S0954102004002214 [Google Scholar]
- Parmelee D.F, Parmelee J.M. Updated penguin distribution for Anvers Island. Antarctica. Br. Antarctic Surv. Bull. 1987;76:65–74. [Google Scholar]
- Patterson D.L, Easter-Pilcher A, Fraser W.R. The effects of human activity and environmental variability on long-term changes in Adelie penguin populations at Palmer Station, Antarctica. In: Huiskes A.H.L, Gieskes W.W.C, Rozema J, Schorno R.M.L, van der Vies S.M, Wolff W.J, editors. Antarctic biology in a global context. Backhuys Publishers; Leiden, The Netherlands: 2003. pp. 301–307. [Google Scholar]
- Peck, L. S. et al 2005 Genomics: applications to Antarctic ecosystems. Polar Biol 28, 351–365.
- Perissinotto R, Pakhomov E.A. Contribution of salps to carbon flux of marginal ice zone of the Lazarev Sea, Southern Ocean. Mar. Biol. 1998;131:25–32. doi:10.1007/s002270050292 [Google Scholar]
- Pitman R.L, Ensor P. Three different forms of killer whales in Antarctic waters. J. Cetacean Res. Manag. 2003;5:131–139. [Google Scholar]
- Povero P, Misic C, Ossola C, Castellano M, Fabiano M. The trophic role and ecological implications of oval faecal pellets in Terra Nova Bay (Ross Sea) Polar Biol. 2003;26:302–310. [Google Scholar]
- Prezelin B.B, Moline M.A, Matlick H.A. Icecolors 1993: spectral UV radiation effects on Antarctic frazil ice algae. In: Lizotte M.P, Arrigo K.R, editors. Antarctic sea ice: biological processes, interactions and variability. vol. 73. American Geophysical Union; Washington, DC: 1998. pp. 45–83. [Google Scholar]
- Prézelin B.B, Hofmann E.E, Mengelt C, Klinck J.M. The linkage between upper circumpolar deep water (UCDW) and phytoplankton assemblages on the West Antarctic Peninsula continental shelf. J. Mar. Res. 2000;58:165–202. doi:10.1357/002224000321511133 [Google Scholar]
- Prézelin B.B, Hofmann E.E, Moline M, Klinck J.M. Physical forcing of phytoplankton community structure and primary production in continental shelf waters of the Western Antarctic Peninsula. J. Mar. Res. 2004;62:419–460. doi:10.1357/0022240041446173 [Google Scholar]
- Quetin L.B, Ross R.M. Environmental variability and its impact on the reproductive cycle of Antarctic krill. Am. Zool. 2001;41:74–89. doi:10.1668/0003-1569(2001)041[0074:EVAIIO]2.0.CO;2 [Google Scholar]
- Quetin L.B, Ross R.M. Episodic recruitment in Antarctic krill, Euphausia superba, in the Palmer LTER study region. Mar. Ecol. Prog. Ser. 2003;259:185–200. [Google Scholar]
- Quetin, L. B., Ross, R. M., Frazer, T. K., Haberman & K. L. 1996 Factors affecting distribution and abundance of zooplankton, with an emphasis on Antarctic krill, Euphausia superba In Foundations for ecological research west of the Antarctic Peninsula, vol. 70 (ed. R. M. Ross, E. E. Hofmann & L. B. Quetin), pp. 357–371. Washington, DC: American Geophysical Union.
- Rhodes O.E, Odum E.P. Spatiotemporal approaches in ecology and genetics: the road less traveled. In: Rhodes O.E, Chesser R.K, Smith M.H, editors. Population dynamics in ecological space and time. University of Chicago Press; Chicago, IL: 1996. pp. 1–8. [Google Scholar]
- Rind D, Chandler M, Lerner J, Martinson D.G, Yuan X. Climate response to basin-specific changes in latitudinal temperature gradients and implications for sea ice variability. J. Geophys. Res. 2001;106:20 161–20 173. doi:10.1029/2000JD900643 [Google Scholar]
- Rodriguez F, Varela M, Zapata M. Phytoplankton assemblages in the Gerlache and Bransfield Straits (Antarctic Peninsula) determined by light microscopy and CHEMTAX analysis of HPLC pigment data. Deep-Sea Res. II. 2002a;49:723–747. doi:10.1016/S0967-0645(01)00121-7 [Google Scholar]
- Rodriguez J, Jimenez-Gomez F, Blanco J.M, Figueroa F.L. Physical gradients and spatial variability of the size structure and composition of phytoplankton in the Gerlache Strait (Antarctica) Deep-Sea Res. II. 2002;49:693–706. doi:10.1016/S0967-0645(01)00119-9 [Google Scholar]
- Ross R.M, Quetin L.B. Ecological physiology of larval euphausiids, Euphausia superba (Euphausiacea) Mem. Queensl. Mus. 1991;31:321–333. [Google Scholar]
- Ross R.M, Hofmann E.E, Quetin L.B, editors. Foundations for ecological research west of the Antarctic Peninsula. AGU Antarctic research series. vol. 70. American Geophysical Union; Washington, DC: 1996. [Google Scholar]
- Ross R.M, Quetin L.B, Haberman K.L. Interannual and seasonal variability in short-term grazing impact of Euphausia superba in nearshore and offshore waters west of the Antarctic Peninsula. J. Mar. Syst. 1998;17:261–273. doi:10.1016/S0924-7963(98)00042-6 [Google Scholar]
- Ross R.M, Quetin L.B, Baker K.S, Vernet M, Smith R.C. Growth limitation in young Euphausia superba under field conditions. Limnol. Oceanogr. 2000;45:31–43. [Google Scholar]
- Ross, R. M., Quetin, L. B., Martinson, D. G., Iannuzzi, R. A., Stammerjohn, S. E. & Smith, R. C. In press. Palmer LTER: patterns of distribution of major zooplankton species west of the Antarctic Peninsula over a twelve year span. Deep-Sea Res. II
- Savidge G, Harbour D.S, Gilpin L.C, Boyd P.W. Phytoplankton distributions and production in the Bellingshausen Sea, Austral spring 1992. Deep-Sea Res. II. 1995;42:1201–1224. doi:10.1016/0967-0645(95)00062-U [Google Scholar]
- Schnack-Schiel S.B, Mujica A. The zooplankton of the Antarctic Peninsula region. In: El-Sayed S.Z, editor. Southern Ocean ecology: the BIOMASS perspective. Cambridge University Press; Cambridge, UK: 1994. pp. 79–92. [Google Scholar]
- Serebrennikova Y.M, Fanning K.A. Nutrients in the Southern Ocean GLOBEC region: variations, water circulation, and cycling. Deep-Sea Res. II. 2004;51:1981–2002. doi:10.1016/j.dsr2.2004.07.023 [Google Scholar]
- Siegel V. A concept of seasonal variation of krill (Euphausia superba) distribution and abundance west of the Antarctic Peninsula. In: Sarhage D, editor. Antarctic ocean and resources variability. Springer; Berlin, Germany: 1988. pp. 220–230. [Google Scholar]
- Siegel V, Loeb V. Recruitment of Antarctic krill Euphausia superba and possible causes for its variability. Mar. Ecol. Prog. Ser. 1995;123:45–56. [Google Scholar]
- Siegel V, Piatkowski U. Variability in the macrozooplankton community off the Antarctic Peninsula. Polar Biol. 1990;10:373–386. [Google Scholar]
- Siegel V, Loeb V, Gröger J. Krill (Euphausia superba) density, proportional and absolute recruitment and biomass in the Elephant Island region (Antarctic Peninsula) during the period 1977 to 1997. Polar Biol. 1998;19:393–398. doi:10.1007/s003000050264 [Google Scholar]
- Siegel V, Bergström B, Mühlenhardt-Siegel U, Thomasson M. Demography of krill in the Elephant Island area during summer 2001 and its significance for stock recruitment. Antarctic Sci. 2002;14:162–170. doi:10.1017/S095410200200072X [Google Scholar]
- Siegel V, Ross R.M, Quetin L.B. Krill (Euphausia superba) recruitment indices from the western Antarctic Peninsula: are they representative of larger regions? Polar Biol. 2003;26:672–679. doi:10.1007/s00300-003-0537-5 [Google Scholar]
- Simmonds I. Modes of atmospheric variability over the Southern Ocean. J. Geophys. Res. 2003;108 doi:101029/2000JC000542 [Google Scholar]
- Simmonds I, Jacka T.H. Relationships between the interannual variability of Antarctic sea ice and the Southern Oscillation. J. Clim. 1995;8:637–647. doi:10.1175/1520-0442(1995)008<0637:RBTIVO>2.0.CO;2 [Google Scholar]
- Simmonds I, King J.C. Global and hemispheric climate variations affecting the Southern Ocean. Antarctic Sci. 2004;16:401–413. doi:10.1017/S0954102004002226 [Google Scholar]
- Sirovic A, Hildebrand J.A, Wiggins S.M, McDonald M.A, Moore S.E, Thiele D. Seasonality of blue and fin whale calls and the influence of sea ice in the Western Antarctic Peninsula. Deep-Sea Res. II. 2004;51:2327–2344. doi:10.1016/j.dsr2.2004.08.005 [Google Scholar]
- Smetacek V, Nicol S. Polar ocean ecosystems in a changing world. Nature. 2005;437:362–368. doi: 10.1038/nature04161. doi:10.1038/nature04161 [DOI] [PubMed] [Google Scholar]
- Smetacek V, Scharek R, Nothig E.M. Seasonal and regional variation in the pelagial and its relationship to the life history of krill. In: Kerry K.R, Hempel G, editors. Antarctic ecosystems: ecological change and conservation. Springer; Berlin, Germany: 1990. pp. 103–114. [Google Scholar]
- Smetacek V, De Baar H.J.W, Bathmann U.V, Lochte K, Rutgers van der Loeff M.M. Ecology and biogeochemistry of the Antarctic circumpolar current during austral spring: a summary of Southern Ocean JGOFS cruise ANT X/6 of R.V. Polarstern. Deep-Sea Res. II. 1997;44:1–21. doi:10.1016/S0967-0645(96)00100-2 [Google Scholar]
- Smith W.O, Jr, Nelson D.M. Phytoplankton bloom produced by a receding ice edge in the Ross Sea: spatial coherence with the density field. Science. 1985;227:163–166. doi: 10.1126/science.227.4683.163. doi:10.1126/science.227.4683.163 [DOI] [PubMed] [Google Scholar]
- Smith S.L, Schnack-Schiel S.B. Polar Zooplankton. In: Smith W.O Jr, editor. Polar oceanography. Academic Press; San Diego, CA: 1990. pp. 527–598. [Google Scholar]
- Smith R.C, Stammerjohn S.E. Variations of surface air temperature and sea ice extent in the Western Antarctic Peninsula (WAP) region. Ann. Glaciol. 2001;33:493–500. [Google Scholar]
- Smith R.C, et al. Ozone depletion: ultraviolet radiation and phytoplankton biology in Antarctic waters. Science. 1992;255:952–959. doi: 10.1126/science.1546292. doi:10.1126/science.1546292 [DOI] [PubMed] [Google Scholar]
- Smith R.C, et al. The Palmer LTER: a long-term ecological research program at Palmer Station, Antarctica. Oceanography. 1995;8:77–86. [Google Scholar]
- Smith, R. C., Dierssen, H. M. & Vernet, M. 1996a Phytoplankton biomass and productivity in the western Antarctic peninsula region. In Foundations for ecological research west of the Antarctic Peninsula, vol. 70 (R. M. Ross, E. E. Hofmann & L. B. Quetin), pp. 333–356. Washington, DC: American Geophysical Union.
- Smith R.C, Stammerjohn S.E, Baker K.S. Surface air temperature variations in the western Antarctic peninsula region. In: Ross R.M, Hofmann E.E, Quetin L.B, editors. Foundations for ecological research west of the Antarctic Peninsula. vol. 70. American Geophysical Union; Washington, DC: 1996b. pp. 105–121. [Google Scholar]
- Smith R.C, Baker K.S, Stammerjohn S.E. Exploring sea ice indexes for polar ecosystem studies. BioScience. 1998a;48:83–93. doi:10.2307/1313133 [Google Scholar]
- Smith R.C, Baker K.S, Vernet M. Seasonal and interannual variability of phytoplankton biomass west of the Antarctic Peninsula. J. Mar. Syst. 1998b;17:229–243. doi:10.1016/S0924-7963(98)00040-2 [Google Scholar]
- Smith D.A, E E, Hofmann J.M, Klinck C.M. Lascara hydrography and circulation of the west Antarctic Peninsula continental shelf. Deep-Sea Res. I. 1999a;46:925–949. doi:10.1016/S0967-0637(98)00103-4 [Google Scholar]
- Smith R.C, et al. Marine ecosystems sensitivity to historical climate change: Antarctic Peninsula. BioScience. 1999b;49:393–404. doi:10.2307/1313632 [Google Scholar]
- Smith W.O, Jr, Marra J, Hiscock M.R, Barber R.T. The seasonal cycle of phytoplankton biomass and primary productivity in the Ross Sea, Antarctica. Deep-Sea Res. II. 2000;47:3119–3140. doi:10.1016/S0967-0645(00)00061-8 [Google Scholar]
- Smith R.C, Baker K.S, Dierssen H.M, Stammerjohn S.E, Vernet M. Variability of primary production in an Antarctic marine ecosystem as estimated using a multi-scale sampling strategy. Am. Zool. 2001;41:40–56. doi:10.1668/0003-1569(2001)041[0040:VOPPIA]2.0.CO;2 [Google Scholar]
- Smith R.C, Fraser W.R, Stammerjohn S.E. Climate variability and ecological response of the marine ecosystem in the western Antarctic Peninsula (WAP) region. In: Greenland D, Goodin D.G, Smith R.C, editors. Climate variability and ecosystem response at long-term ecological research sites. Oxford University Press; New York, NY: 2003a. pp. 158–173. [Google Scholar]
- Smith R.C, Fraser W.R, Stammerjohn S.E, Vernet M. Palmer long-term ecological research on the Antarctic marine ecosystem. In: Domack E, Leventer A, Burnett A, Bindschadler R, Convey P, Kirby M, editors. Antarctic Peninsula climate variability: historical and paleoenvironmental perspective. American Geophysical Union; Washington, DC: 2003b. pp. 131–144. [Google Scholar]
- Smith W.O, Ainley D.G, Cattaneo-Vietti R. Trophic interactions within the Ross Sea Continental shelf ecosystem. Phil. Trans. R. Soc. B. 2007;362:95–111. doi: 10.1098/rstb.2006.1956. doi:10.1098/rstb.2006.1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, C. R., Mincks, S. & DeMaster, D. J. In press a A synthesis of bentho-pelagic coupling on the Antarctic Shelf: food banks, ecosystem inertia and global climate change. Deep-Sea Res. II
- Smith, R. C., Martinson, D. G., Stammerjohn, S. E., Iannuzzi, R. A. & Ireson, K. In press b Bellingshausen and Western Antarctic Peninsula region: pigment biomass and sea ice spatial/temporal distributions and interannual variability. Deep-Sea Res. I.
- Stammerjohn S.E, Smith R.C. Opposing Southern Ocean climate patterns as revealed by trends in regional sea ice coverage. Clim. Change. 1997;37:617–639. doi:10.1023/A:1005331731034 [Google Scholar]
- Stammerjohn S.E, Drinkwater M.R, Smith R.C, Liu X. Ice–atmosphere interactions during sea-ice advance and retreat in the western Antarctic Peninsula region. J. Geophys. Res. 2003;108 doi:101029/2002JC001543 [Google Scholar]
- Stammerjohn, S. E., Martinson, D. G., Smith, R. C. & Yuan, X. In press a Trends in sea ice retreat and subsequent advance in response to ENSO and SAM variability at high southern latitudes. J. Climatol
- Stammerjohn, S. E., Martinson, D. G., Smith, R. C. & Iannuzzi, R. A. In press b Sea ice in the Palmer LTER region: spatio-temporal variability from ecological and climate change perspectives. Deep Sea Res. II
- Sweeney C, Hansell D.A, Carlson C.A, Codispoti L.A, Gordon L.I, Marra J, Millero F.J, Smith W.O, Takahashi T. Biogeochemical regimes, net community production and carbon export in the Ross Sea, Antarctica. Deep-Sea Res. II. 2000a;47:3369–3394. doi:10.1016/S0967-0645(00)00072-2 [Google Scholar]
- Sweeney C, et al. Nutrient and carbon removal ratios and fluxes in the Ross Sea, Antarctica. Deep-Sea Res. II. 2000b;47:3395–3421. doi:10.1016/S0967-0645(00)00073-4 [Google Scholar]
- Takahashi T, et al. Global sea–air CO2 flux based on climatological surface ocean pCO2, and seasonal biological and temperature effects. Deep-Sea Res. II. 2002;49:1601–1622. doi:10.1016/S0967-0645(02)00003-6 [Google Scholar]
- Thompson D.W.J, Solomon S. Interpretation of recent southern hemisphere climate change. Science. 2002;296:895–899. doi: 10.1126/science.1069270. doi:10.1126/science.1069270 [DOI] [PubMed] [Google Scholar]
- Thompson L.G, Peel D.A, Mosley-Thompson E, Mulvaney R, Dai J, Lin P.N, Davis M.E, Raymond C.F. Climate since A.D. 1510 on Dyer Plateau, Antarctic Peninsula: evidence for recent climate change. Ann. Glaciol. 1994;20:420–426. [Google Scholar]
- Treguer P, Jacques G. Dynamics of nutrients and phytoplankton and fluxes of carbon, nitrogen and silicon in the Antarctic Ocean. Polar Biol. 1992;12:149–162. doi:10.1007/BF00238255 [Google Scholar]
- Turner J.T. Zooplankton fecal pellets, marine snow and sinking phytoplankton blooms. Aquat. Microb. Ecol. 2002;27:57–102. [Google Scholar]
- Turner J. Review: the El Niño-Southern Oscillation and Antarctica. Int. J. Clim. 2004;24:1–31. doi:10.1002/joc.965 [Google Scholar]
- van den Broeke M.R, van Lipzig N. Response of wintertime Antarctic temperatures to the Antarctic Oscillation: results of a regional climate model. In: Domack E, Leventer A, Burnett A, Bindschadler R, Convey P, Kirby M, editors. Antarctic Peninsula climate variability: historical and paleoenvironmental perspectives. American Geophysical Union; Washington, DC: 2003. pp. 43–58. [Google Scholar]
- Vaughan D.G, Marshall G.J, Connolley W.M, Parkinson C, Mulvaney R, Hodgson D.A, King J.C, Pudsey C.J, Turner J. Recent rapid regional climate warming on the Antarctic Peninsula. Clim. Change. 2003;60:243–274. doi:10.1023/A:1026021217991 [Google Scholar]
- Vernet M, Kozlowski W.A. Ultraviolet radiation and the Antarctic coastal marine ecosystem. In: Cockell C.S, Blaustein A.R, editors. Ecosystems and ultraviolet radiation. Springer; New York, NY: 2001. pp. 170–194. [Google Scholar]
- Villafañe V, Helbling E.W, Holm-Hansen O. Phytoplankton around Elephant Island, Antarctica. Polar Biol. 1993;13:183–191. [Google Scholar]
- Volk T, Hoffert M.I. Ocean carbon pumps: analysis of relative strengths and efficiencies in ocean-driven atmospheric CO2 changes. In: Sundquist E.T, Broecker W.S, editors. The carbon cycle and atmospheric CO2: natural variations Archean to present. vol. 32. American Geophysical Union; Washington, DC: 1985. pp. 99–110. [Google Scholar]
- Volkman N.J, Presler P, Trivelpiece W.Z. Diets of pygoscelid penguins at King George Island, Antarctica. Condor. 1980;82:373–378. [Google Scholar]
- Voronina N.M. Comparative abundance and distribution of major filter-feeders in the Antarctic pelagic zone. J. Mar. Syst. 1998;17:375–390. doi:10.1016/S0924-7963(98)00050-5 [Google Scholar]
- Walsh J.J, Dieterle D.A, Lenes J. A numerical analysis of carbon dynamics of the Southern Ocean phytoplankton community: the roles of light and grazing in effecting both sequestration of atmospheric CO2 and food availability to krill. Deep-Sea Res. I. 2001;48:1–48. doi:10.1016/S0967-0637(00)00032-7 [Google Scholar]
- Ward P, Grant S, Brandon M, Siegel V, Sushin V, Loeb V, Griffiths H. Mesozooplankton community structure in the Scotia Sea during the CCAMLR 2000 survey: January–February 2000. Deep-Sea Res. II. 2004;51:1351–1367. doi:10.1016/j.dsr2.2004.06.016 [Google Scholar]
- Watkins A.B, Simmonds I. Current trends in Antarctic sea ice: the 1990s impact on a short climatology. J. Clim. 2000;13:4441–4451. doi:10.1175/1520-0442(2000)013<4441:CTIASI>2.0.CO;2 [Google Scholar]
- Watkins J.L, Hewitt R, Naganobu M, Sushin V. The CCAMLR 2000 survey: a multinational, multi-ship biological oceanography survey of the Atlantic sector of the Southern Ocean. Deep-Sea Res. II. 2004;51:1205–1213. doi:10.1016/j.dsr2.2004.06.010 [Google Scholar]
- Weber L.H, El-Sayed S.Z. The variance spectra of phytoplankton, krill and water temperature in the Antarctic Ocean south of Africa. Deep-Sea Res. I. 1986;33:1327–1343. doi:10.1016/0198-0149(86)90039-7 [Google Scholar]
- Wefer G, Fischer G, Futterer D, Gersonde R. Seasonal particle flux in the Bransfield Strait. Antarctica. Deep-Sea Res. 1988;35:891–898. [Google Scholar]
- Weimerskirch H, Inchausti P, Guinet C, Barbraud C. Trends in bird and seal populations as indicators of a system shift in the Southern Ocean. Antarctic Sci. 2003;15:249–256. doi:10.1017/S0954102003001202 [Google Scholar]
- Williams T.D. Oxford University Press; New York, NY: 1995. The penguins. [Google Scholar]
- Woehler E.J. Hobart, Scientific Committee on Antarctic Research; Australia: 1993. The distribution and abundance of Antarctic and Subantarctic penguins. [Google Scholar]
- Woehler, E. J., et al 2001 A statistical assessment of the status and trends of Antarctic and Subantarctic seabirds Hobart, Australia: Scientific Committee on Antarctic Research.
- Yager P.L, Wallace D.W.R, Johnson K.M, Smith W.O, Jr, Minnett P.J, Deming J.W. The Northeast Water Polynya as an atmospheric CO2 sink: a seasonal rectification hypothesis. J. Geophys. Res. C. 1995;100:4389–4398. doi:10.1029/94JC01962 [Google Scholar]
- Yuan X. ENSO-related impacts on Antarctic sea ice: a synthesis of phenomenon and mechanisms. Antarctic Sci. 2004;16:415–425. doi:10.1017/S0954102004002238 [Google Scholar]
- Yuan X, Martinson D.G. Antarctic sea ice extent variability and its global connnectivity. J. Clim. 2000;13:1697–1717. doi:10.1175/1520-0442(2000)013<1697:ASIEVA>2.0.CO;2 [Google Scholar]
- Yuan X, Martinson D.G. The Antarctic dipole and its predictability. Geophys. Res. Lett. 2001;28:3609–3612. doi:10.1029/2001GL012969 [Google Scholar]
- Zwally J.H, Comiso J.C, Parkinson C.L, Cavalieri D.J, Gloersen P. Variability of Antarctic sea ice 1979−1998. J. Geophys. Res. 2002;107 doi:101029/2000JC000733 [Google Scholar]