Abstract
Sexual reproduction strategies vary both between and within species in the level of investment in offspring. Life-history theories suggest that the rate of sexual maturation is critically linked to reproductive strategy, with high investment being associated with few offspring and delayed maturation. For humans, age of puberty and age of first sex are two developmental milestones that have been associated with reproductive strategies. Stress during early development can retard or accelerate sexual maturation and reproduction. Early age of menarche is associated with absence of younger siblings, absence of a father figure during early life and increased weight. Father absence during early life is also associated with early marriage, pregnancy and divorce.
Choice of partner characteristics is critical to successful implementation of sexual strategies. It has been suggested that sexually dimorphic traits (including those evident in the face) signal high-quality immune function and reproductive status. Masculinity in males has also been associated with low investment in mate and offspring. Thus, women's reproductive strategy should be matched to the probability of male investment, hence to male masculinity.
Our review leads us to predict associations between the rate of sexual maturation and adult preferences for facial characteristics (enhanced sexual dimorphism and attractiveness). We find for men, engaging in sex at an early age is related to an increased preference for feminized female faces. Similarly, for women, the earlier the age of first sex the greater the preference for masculinity in opposite-sex faces. When we controlled sexual dimorphism in male faces, the speed of sexual development in women was not associated with differences in preference for male facial attractiveness.
These developmental influences on partner choice were not mediated by self-rated attractiveness or parental relationships. We conclude that individuals assort in preferences based on the rapidity of their sexual development. Fast developing individuals prefer opposite-sex partners with an increased level of sexually dimorphic facial characteristics.
Keywords: face, attraction, development, masculinity, mate value, assortative mating
1. Introduction
Sexual maturation is a key milestone in human development, and much research has focused on multiple factors influencing its timing. These include psychosocial factors (Belsky & Draper 1987; Belsky et al. 1991; MacDonald 1999; Ellis & Garber 2000; Ellis et al. 2003), hormones (Nottlemann et al. 1987; Tremblay et al. 1998) and genetics (Comings et al. 2002). Timing of puberty, independent of the factors mediating it, influences the social environment of adolescence. Whether one matures early, late or ‘on-time’ will shape individual experiences in interactions with peers as well as adults. The outcome of these interactions contributes to the overall psychosocial well-being of the individual during adolescence. Our interest is to go beyond the immediate influences of pubertal timing and sexual maturation on adolescent behaviour and question the outcome of sexual maturational timing on adult mate choice and strategies.
We speculate that adults who were early sexual maturers, e.g. in terms of both puberty and initiation of sexual intercourse, will differ from late maturers when making judgements of opposite-sex facial attractiveness. This over-arching hypothesis is framed by alternative reproductive strategies that are responses to the developmental environment. These strategy differences are reflected in individual preference judgements for particular facial characteristics. We consider three issues: (i) early maturation as a negative outcome owing to stress; (ii) early maturation as a positive influence on social status; and (iii) mate choice preferences as a product of learning through peer social interactions.
(a) Alternative reproductive strategies
Alternative tactics to maximize reproductive fitness are found across a wide variety of species, including humans (Gross 1985, 1996; Henson & Warner 1997). The theoretical framework offered by both game theory (Maynard Smith 1982) and evolutionarily stable strategy (Maynard Smith & Price 1973) offers a platform from which investigations into humans' use of alternative reproductive strategies can be launched. Beginning with the most basic assumptions of human female/male differences: females invest heavily in offspring, through pregnancy and lactation, males by contrast need only to contribute sperm for successful reproduction. To maximize reproductive success (by producing as many offspring as possible), males are thought to take the approach of capitalizing on as many mating opportunities as possible, but are constrained by female choice and demands for investment (Trivers 1972). Females, on the other hand, should seek out mates who are willing to provide, but they are forced to make trade-offs between good paternal investment and genetic fitness of the male (immunocompetence; Møller & Thornhill 1998; Perrett et al. 1999; Scheib et al. 1999; Scheib 2001). In a competitive market, high-quality males (with a healthy immune system) may find that they are in high demand and can potentially contribute less paternal investment, especially when considering partnership with a low-quality female. If a low-quality female wishes to secure a more genetically fit male's genes for her offspring, she could opt to settle for a short-term sexual relationship with him and sacrifice other benefits such as long-term financial support and paternal care for her offspring (Little & Hancock 2002; Penton-Voak et al. 2003).
Research on attraction has successfully used this theoretical framework to explain individual differences in female preferences for symmetric and sexually dimorphic facial characteristics in opposite-sex faces. High-quality individuals have a greater ability to attract high-quality mates and thus procure higher reproductive advantages. A more attractive male can adopt a mating strategy of multiple mating partners, investing less in each partner (Thornhill & Gangestad 1994) with less risk to his offspring (Burley 1986; Gowaty 1996; Sheldon 2000; Badyael & Hill 2002). Females of high-quality can not only attract high-quality mates, but can also enforce demands for paternal investment and thereby circumvent the trade-off between good genes (for immunity) and high investment. Individuals of lesser quality cannot successfully employ these strategies, despite their desire to do so, and this gives rise to variation in mate choice strategies. Humans offer us a unique opportunity to investigate the influence of self-assessed quality on mate choice and strategies.
(b) r and K strategies
Robert MacArthur (1962) incorporated aspects of R. A. Fisher and J. B. S. Haldane's theorems to account for varying degrees of inbreeding and effects of population density in terms of fitness. From this, as well as later work (MacArthur & Wilson 1967), arose r and K selection theories. The names come from two parameters of standard population dynamic theory. K-strategists are said to live close to K, the carrying capacity of the environment; and r-strategists are said to maximize r, the intrinsic rate of increase of the population.
The general premise is that organisms must adapt to their environment to maximize their fitness, and environments vary in stability. In unstable environments, the best strategy would be to produce large numbers of offspring, many of which will die but a few are likely to survive. In stable environments, the better strategy is to have fewer offspring, but invest more in each so that offspring survival chances are increased. Thus, r-selected species are short-lived, reproduce rapidly, take advantage of open niches, and are prone to boom or bust populations depending on the vagaries of the environment. K-selection refers to species that are longer-lived, reproduce slower, and are more immune to environmental swings. Compared with r-strategists, K-strategists are larger, the energy to produce one offspring is high, few offspring are produced, life expectancy is long, individuals can reproduce multiple times, sexual maturity is slow to arrive, and survival of offspring should be fairly high—with most offspring living a full-maximum lifespan.
Humans lie near the K end of the continuum if we go by our long lives, slow maturation, few offspring and good offspring survival rates (Mace 2000). But, some scientists have suggested that even within a species there is variation of strategy, and have employed the ideas of r and K strategies to characterize human mating strategies, reproduction and parental investment (Draper & Harpending 1982; Belsky et al. 1991; MacDonald 1997; Bereczkei & Csanaky 2001). The idea is that in unstable environments, humans may opt to increase their rate of reproduction, investing less in each individual offspring, and that offspring will reach sexual maturity earlier and begin their own reproduction earlier than humans raised in stable environments. Unstable environments during development could affect reproductive strategies, including mate choice. Indeed, girls who experienced longer duration of father absence (e.g. fathers left the family earlier) were more likely to engage in sexual intercourse earlier than girls whose fathers left later or remained ‘faithful’ (Ellis et al. 2003). Quinlan (2003) looked at retrospective data for 10 847 US women to examine the effects of divorce and separation of parents, including any effects related to the age of the child when divorce or separation took place. He found that when women's parents divorced or separated early during her childhood (before birth up to 5 years of age), the women were more likely to reach menarche earlier, engage in sexual intercourse earlier, become pregnant earlier and their marriages were shorter in duration when compared with women whose parents' separation occurred later or not at all. Additionally, Quinlan found that if parents divorced or separated during the women's adolescence, these women were likely to have more sexual partners than women whose parents did not separate or divorce.
The original work by MacArthur & Wilson (1967) was not intended to explain mating strategies, but to account for varying degrees of inbreeding and effects of population density in terms of fitness. Certainly, it was not developed to explain the individual differences of mate choice within a species. Consideration of the home environment during human development is important, as many factors can affect adult behaviour. These include attachment to parents, parenting styles, presence of siblings, size of extended family, moving house, illness, and home and community (e.g. level of neighbourhood violence). Whether these factors can be fitted into r and K selection is perhaps debatable.
(c) Life-history theory
Life-history theory has been particularly useful for exploring pubertal timing from an evolutionary-developmental perspective (for review see Geary 2002; Ellis 2004). Unlike r and K strategies, life-history theory specifically relates to within-species differences and explores environmental (e.g. stress, nutrition and father absence) and genetic influences that could influence maturation rates and sexual strategies. Phenotypic plasticity and ability to adapt to environmental factors contribute to the decision process in individual organisms in terms of trade-offs. This is not to imply that decision processes affecting choice of strategies are conscious. For example, by ‘choosing’ to delay maturation and reproduction, individuals may reduce their overall number of offspring compared with individuals with accelerated maturation and onset of reproduction, but slow maturers gain by producing healthier, higher quality offspring (Black & DeBlassie 1985; Overpeck et al. 1998; Elfenbein & Felice 2003). Thus, increased fitness is measured through multiple generations rather than the number of immediate offspring.
Ellis (2004) argues that selection for adaptive responses, i.e. plasticity, of the individual was favoured during our evolutionary history. While there are several competing and complementary hypotheses within life-history theory, the central questions are: when is it optimal for an individual to cease expending energy in growth and redirect it towards reproductive efforts and what are the critical determinants of timing (Ellis 2004)? Contributing factors influencing maturation include: (i) poor nutrition which is associated with late maturation and decreased fertility (Miller 1994; MacDonald 1999); (ii) negative physical or social conditions delaying reproductive maturation (stress-suppression theory, e.g. Miller 1994; MacDonald 1999), or accelerating reproductive maturation (Draper & Harpending 1982; Belsky et al. 1991; Chisholm 1993, 1996; Wilson & Daly 1997); and (iii) father absence which can speed up development in females (Ellis & Garber 2000). In essence, all life-history theories suggest that early environmental factors affect the developmental profile of the individual. This in turn will also have effects on later adult behaviours, including reproductive strategies and mate choice.
(d) Timing of puberty and reproductive strategies
The possible environmental factors mediating pubertal timing have been studied since the 1930s, and family, economic, physical and nutritional stressors have been indicated as having effects on sexual maturation. Despite the amount of research expended, there remains a great deal of controversy as to which particular stressors accelerate and which decelerate puberty timing (Hoier 2003; Romans et al. 2003). Some psychologists have examined the role of environmental stressors and their possible influence on reproductive strategies, including the timing of sexual maturation and mate choice. Humans, it is argued, have been selected to respond to environmental cues by adopting a reproductive tactic most suited to enhance fitness; furthermore, the choice of tactic is sensitive to the environmental cues experienced during development (Jones et al. 1972; Belsky & Draper 1987; Surbey 1990; Belsky et al. 1991; Moffitt et al. 1992; MacDonald 1999; Ellis & Garber 2000; Ellis et al. 2003). It has been asserted that more precocious sexual behaviours indicate a strategy of early reproduction, more offspring, but less investment; whereas later sexual maturity and conservative sexual activity may reflect an investment-biased reproductive strategy with fewer offspring, but heavier investment. Adopting either of these strategies may reflect the environment to which the individual was exposed at specific times during development or throughout development.
(e) Parental influences
Puberty is the key developmental milestone towards achieving adult sexual status, and its timing has been linked to strong hormonal and genetic influences. There remains debate concerning the contribution of genetic (Pickles et al. 1998) and hormonal influences on development (Dorn et al. 2003a,b). One factor, mother's age of menarche, has been found to be the best predictor of daughter's age of menarche (Kirk et al. 2001). Environmental factors also mediate age of menarche, with stressful family situations such as father absence accelerating menarche (Jones et al. 1972; Belsky & Draper 1987; Surbey 1990; Belsky et al. 1991; Moffitt et al. 1992), while having younger siblings decelerates it (Jones et al. 1972; Hoier 2003). Less work has been done on the effect of father absence on puberty in males, but father absence for 1 year or longer during childhood is significantly associated with earlier spermarche (Kim et al. 1997; Kim & Smith 1998). Early spermarche and puberty have been associated with increased number of romantic partners, sexual partners, earlier onset of sexual interest (dating) and earlier first intercourse (Kim & Smith 1998; Edgardh 2002). In contrast, good relationships with parents, especially between girls and their mothers, can decrease the likelihood of early sexual intercourse (McNeely et al. 2002).
Since poor relations between parent and offspring are thought to accelerate sexual maturation and negatively affect mate quality (Boothroyd & Perrett 2006), they must be taken into account when investigating the association between sexual development and mate choice preferences. Parental relationships may affect mate choice preferences in a way that is independent of maturation effects, such as through an effect on self-esteem and psychological well-being (McNeely et al. 2002; Spencer et al. 2002; Berg 2003). We therefore investigate the effect of maturation and relationship with parents on adult partner preference.
(f) Peer interactions
Adolescence is a time for individuals to explore and come to terms with peer group social hierarchy and their rank within it (Harris 1995; Hawley 1999, 2003; Hawley & Vaughn 2003). If adolescence is a particularly sensitive time for determining reproductive strategies, then social status and adolescent sexual behaviour should be of particular importance. While there is evidence that early puberty can have negative psychological, social and behavioural effects both during and after adolescence, there is also evidence for its positive effects (Dorn et al. 2003a,b; Weichold et al. 2003; Weisfeld & Woodward 2004). Reaching puberty slightly ahead of peers may give distinct advantages in terms of social status, and these advantages may in fact continue on into adulthood. Higher levels of testosterone during early puberty in boys have also been associated with social success (Schaal et al. 1996). Boys who mature earlier are often looked up to by their same sex peers (Peterson & Crockett 1985), and have greater opportunity to affiliate romantically with females (Susman et al. 1987; Halpern et al. 1998). Such affiliations increase the potential for earlier initiation of sexual activity compared with their slower developing peers (Stattin & Magnusson 1990). Girls who mature earlier are more likely to procure the attention of older, more physically mature boys (Magnusson et al. 1985; Stattin & Magnusson 1990; Weichold & Silbereisen 2001; Gowen et al. 2004), and such girls find older boys to be more attractive than boys of peer age (Kracke 1993). Associating with older boys may give early maturing girls access to social activities and the trappings of higher social status not afforded to slower developing peers, as well as increase the likelihood of engaging in romantic and/or sexual activity (Silbereisen & Kracke 1997; Prokopèáková 1998). These early affiliations with earlier like-developing opposite-sex peers may enhance preferences for more sexually mature characteristics. Faster developing girls, for example, may learn positive associations with more masculine-looking boys (also faster developers) and in turn, boys may relate more feminine characteristics in female faces with early sexual rewards. These preferences could continue on into adulthood, and thus associations between early maturation and preferences for exaggerated sexually dimorphic features would be expected in mate choice for those with early maturation. Another possible influence of sexual development on mate choice is that both early maturing girls and boys may gain social status within their peer groups, and thus enhance self-perceived attractiveness and mate value. If self-perceptions established during development continue into adulthood, early maturers are likely to perceive themselves as high-status and high-quality adults. Effects of self-perceived quality have been found to influence adult partner choice. For example, high-quality individuals prefer partners of similar quality. This is reflected in their increased preferences for quality markers such as symmetry and exaggerated sexually dimorphic facial characteristics (Little et al. 2001; Penton-Voak et al. 2003). For these reasons, we examine the influence of self-rated attractiveness on preferences for sexual dimorphism in opposite-sex faces.
(g) Mate quality signals
(i) Sexually dimorphic facial traits
Symmetry is considered a positive characteristic for both sexes, as it indicates good immunocompetence during the difficulties of the developmental process (Perrett et al. 1999; Jones et al. 2001). By contrast, the particular growth patterns mediated by sex hormones resulting in epigamic traits are thought to signal both positive and negative mate characteristics (Perrett et al. 1998). Characteristics more typical of the female face include full lips, large eyes, small nose and delicate features, which are thought to be associated with higher levels of oestrogen. Feminine facial characteristics may signal fecundity in women (Enlow 1990) and immunocompetence (Seli & Arici 2002). Faces of women with higher levels of oestrogen are rated as more feminine looking than faces of women with lower levels (Law Smith et al. 2006). A feminine female face shape is found attractive by both sexes, and confers personality merits such as warmth and nurturing (Perrett et al. 1998). Thus, enhanced sexually dimorphic features are attractive in female faces.
The more classic male facial features include square jaw, heavier brow and thinner lips, which are related to testosterone levels during development. Faces of males with higher levels of testosterone were rated as looking more masculine than faces of males with lower levels (Penton-Voak & Chen 2004). Testosterone is known to depress the immune system (Ahmed & Talal 1990), and Folstad & Karter (1992) argue that only the healthiest males with the best genes for immunocompetence are capable of displaying such epigamic traits. Testosterone is also related to male–male competition, and it is reasoned that male characteristics may enhance signals related to male dominance (Mazur & Booth 1998). Masculine features simultaneously suggest both positive and negative signals, including personality attributes such as dominance, high risk taking, aggression, sexual impulsivity, spousal abuse, inability to commit to a relationship and anti-social behaviour (Olweus et al. 1988; Mazur & Booth 1998; Perrett et al. 1998). Therefore, masculine features are of contrary desirability, and women must resolve trade-offs between (masculine) males with genes signalling high immunocompetence and (feminine) males signalling affable personality traits and high paternal investment. As we review above, such trade-offs will depend on sexual strategies: high-quality women will seek and retain high-quality sexually dimorphic males. In essence, this claim points towards assortment in mate quality; (high-quality) feminine women and (high-quality) masculine men are most likely to form partnerships. Moreover, women following high investment reproductive strategies and who desire high paternal investment might seek out less masculine male partners.
(ii) Attractiveness beyond sexual dimorphism
What is ‘attractiveness’? In the context of mate preferences, it should mean that one individual is ‘attracted to’ or ‘drawn-in’ by another individual as a potential sexual partner. It is also used in more general terms, as a sort of rating system. For example, compared to asymmetrical faces, symmetrical faces are generally preferred; thus, symmetrical faces are described as being more ‘attractive’.
Researchers studying mating strategies need to understand whether attractiveness means the same thing across a variety of individuals. In the last section, we noted that partnership and even attraction to sexually dimorphic males could vary with female reproductive strategy. Thus, it may be that attraction to particular individuals is not universal but can be strategic.
In a meta-analysis, Langlois et al. (2000) found strong agreement between raters on judgements of facial attractiveness, both within and across cultures. Still, while individuals may agree in general who is or is not attractive, there still might be disagreement on what faces individuals prefer. We see variation of this sort when examining the influence of hormonal markers on facial preferences. Women's preferences for masculine facial characteristics have been far from consistent across a range of studies. Women have been found to prefer more masculinized male faces in some studies (Grammer & Thornhill 1994; Scheib et al. 1999; Penton-Voak & Perrett 2001), and to prefer more feminized male faces in others (Perrett et al. 1998; Penton-Voak et al. 1999, 2003). Women are not the only ones who appear fickle, as male preferences for feminine facial characteristics also vary among individuals (Cunningham et al. 1995; Swaddle & Reierson 2002; Cornwell et al. 2004). So, if women and men concur on facial attractiveness but differ on preferences of sexual dimorphism, is there an aesthetic quality in the human face that we do not yet fully understand? And if so, what is its role in mate choice?
Masculine and feminine facial characteristics are signals of mate quality, but strong indicators of sexual dimorphism do not automatically confer attractiveness. For example, Arnold Schwarzenegger's ‘Terminator’ would certainly be judged as a ‘highly masculinized’ male, but not all women would judge him as facially attractive. On the other hand, the character Everett, as played by the actor George Clooney in the film ‘O Brother, Where Art Thou?’, is both masculine and to many women very attractive. Likewise, feminine facial characteristics are not the only feature contributing to a woman's attractiveness. Both Sigourney Weaver and Meg Ryan are highly attractive, and yet Ms Ryan would likely be judged as being much more feminine looking than Ms Weaver.
The point we are making is that there are aesthetic qualities that alter our judgements of attractiveness outside of or in addition to feminine or masculine facial characteristics. We assert that these ‘attractiveness’ characteristics are a signal to mate value, but whether these signals suggest the same meanings as epigamic facial characteristics is unknown. To investigate whether there is an ‘attractiveness’ component to the face, we have attempted to isolate it from variations of facial masculinity or femininity by creating a new range of facial images. These images attempt to keep constant sexually dimorphic characteristics and vary in a characteristic we shall at this time refer to as ‘attractiveness’. Reciprocally, we created images that vary in masculinity and femininity, while attempting to keep attractiveness characteristics constant.
If epigamic traits and the attractiveness component convey distinct information to the receiver, then there should be independent variation in preferences for these characteristics. While we are attempting to understand the variation found in the literature regarding female preferences for male facial appearance and how these variations relate to mate qualities, for the sake of parity we created comparable female face images attempting to manipulate attractiveness and femininity independently and conducted parallel research on male preferences.
(iii) Timing of puberty and predictions for facial preferences
Based on our review of prior research, and theories relating to reproductive strategies and assortative mating, we offer three predictions for the effects of sexual maturation on preferences for facial epigamic traits.
If early timing of sexual maturity is associated with high stress and therefore producing low-quality individuals, then early maturing men should prefer low-quality female faces, i.e. less feminine and less attractive faces, while later maturing men should indicate preferences for high-quality female faces. Early developing women should indicate preference for low-quality males by choosing less masculine and less attractive male faces. However, it should be noted that owing to the use of short-term strategies, low-quality women may indicate a preference for high-quality males if they are considering short-term relationships.
If learning occurs, that is to say early developing adolescents have learned to associate increased sexually dimorphic characteristics with potential mates, then we would expect to see early maturers preferring increased sexual dimorphism but not necessarily indicating a preference for higher facial attractiveness.
If early maturers have higher social status and consider themselves to be higher quality mates owing to social success through puberty, then early maturing men and women should choose high-quality mates on both facial dimensions, i.e. more sexually dimorphic and more attractive opposite-sex faces.
2. Material and methods
(a) Rating original images
We began with a collection of 701 original face images (more than 90% Caucasian; 456 female: age mean=20.21, s.d.=3.18 years; 245 male: age mean 21.21, s.d.=3.58 years). Seventeen participants (11 females) rated attractiveness and 14 participants (7 females) rated facial femininity of female faces and masculinity of male faces. Images were masked (to exclude hair and clothing) and presented in random order. Participants were Caucasian and aged 18–29 years. Each image was assessed on scales of 1–7 for (i) attractiveness for both female and male faces; (ii) masculinity on male faces; and (iii) femininity on female faces. Initial correlation analyses revealed that the female rating of male facial attractiveness and facial masculinity were significantly correlated (r196=0.202, p=0.005), and correlations between the male ratings of female facial attractiveness and facial femininity were even stronger (r345=0.592, p<0.001).
To create our new images, we first matched the facial images on one dimension, and then from within the matched group we selected the high and low faces on the second dimension. For the attractiveness images, we averaged shape, colour and texture of those male Caucasian faces (n=26–30) that had been rated either high or low on the dimension of attractiveness, while rated similarly on the second dimension of male masculinity (Tiddeman et al. 2001). This effectively created high and low attractiveness male face prototypes that were matched on the dimension of masculinity (scale: 1–7; mean attractiveness ratings 3.42 versus 2.43; mean masculinity ratings 4.38 versus 3.93). The same process was then used to create two male prototypes of high and low masculinity matched on attractiveness (mean masculinity ratings 5.18 versus 2.88; mean attractiveness ratings 2.85 versus 2.75), two female prototypes of high and low femininity while controlling for attractiveness (mean femininity ratings 4.98 versus 2.92; mean attractiveness 3.10 versus 2.97), and two female prototypes of high and low attractiveness while controlling for femininity (mean attractiveness ratings 4.22 versus 2.26; mean attractiveness 4.26 versus 4.44).
(b) Composite image calibration
The male face prototype images were rated on both attractiveness and masculinity, and the female faces were rated for attractiveness and femininity. The raters were recruited through an introductory psychology class at the University of Colorado at Colorado Springs for course credit. For our analyses, we included only female raters under 25 years of age (N=38, mean age=18.7±1.2, range 17–23 years) and not taking hormonal contraceptives or pregnant. Male raters were under 25 years of age (N=39, mean age=19.6±1.4, range 17–23 years). Analyses are based on opposite-sex ratings. Images were presented individually and in random order among filler items, and rated on a 7-point scale.
Paired t-test analyses revealed that the high masculine male face prototype was judged more masculine than the low masculine male face prototype, t(37)=7.3, p<0.001, η2=0.59. When judged on attractiveness, the high and low masculinity face prototypes were found not to be significantly different, t(33)=−0.114, p=0.91.
For the high and low attractive male images, we found that the high attractive male face was rated significantly more attractive than the low attractive face, t(33)=4.112, p<0.001, η2=0.34. When rated on masculinity, the two were not significantly different t(37)=1.02, p=0.31. These data show that the intended manipulation of male face prototypes along one dimension while controlling a second dimension was successful.
However, for the female images, the calibration results indicate that segregating attractiveness and femininity in female faces was not successful. The high and low feminine face pairs were rated differently on femininity, t(28)=3.92, p=0.001, η2=0.354, but the same images were also judged differently on attractiveness, t(20)=3.25, p=0.004, η2=0.346.
The high and low attractiveness female faces were rated differently on attractiveness, t(20)=3.301, p=0.004, η2=0.353, but also differed on rated femininity, t(28)=3.111, p=0.004, η2=0.257. These results, though disappointing, are not surprising owing to the strong positive relationship found between men's attractiveness ratings and female facial femininity in previous research (Perrett et al. 1998; Cornwell et al. 2004). Given the ambiguity of female facial stimuli, we restricted further analysis to the female composites based on sexual dimorphism which are unambiguous for this trait, since this was the dimension we set out to investigate.
(c) Experimental images
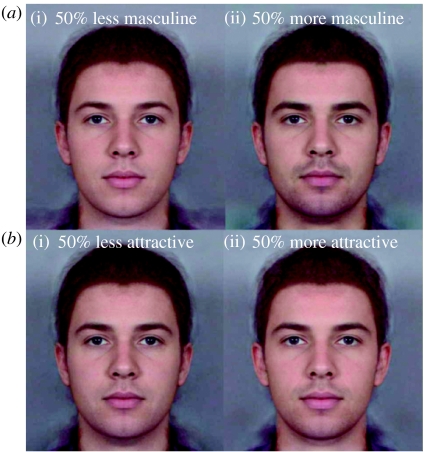
Three composite ‘base’ male faces were made by averaging eight randomly chosen Caucasian male face images, aged between 18 and 24 years. Three ‘base’ female faces were similarly created. These base faces differing in apparent identity, were then transformed by ±115% of the difference in face shape, colour and texture between the high and low sexually dimorphic and high/low attractive prototypes (Tiddeman et al. 2001). Finally, a sequence of 25 images was created by interpolating between the +115 and −115% end-point images. This effectively created three face continua (of 25 images) for each sex that differed along one dimension but were matched in other respects (i.e. different in apparent masculinity but matched in identity and attractiveness). For illustration see figures 1 and 2. For analysis, the mean value for each of the three examples for each participant was correlated with questionnaire responses.
Figure 1.
Example stimuli used in the studies. (a) Masculinity lowered (i) and raised (ii) while attempting to keep attractiveness constant. (b) Attractiveness lowered (i) and raised (ii) while attempting to keep masculinity constant.
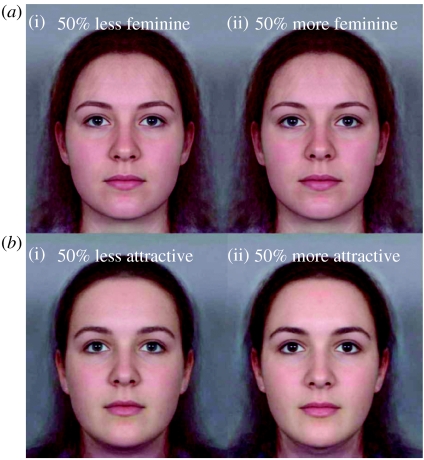
Figure 2.
Example stimuli used in the studies. (a) Femininity lowered (i) and raised (ii) while attempting to keep attractiveness constant. (b) Attractiveness lowered (i) and raised (ii) while attempting to keep femininity constant.
These continua were used to create three interactive sequences, with 25 individual images in each sequence. Participants were asked to choose the image that they considered to be the most attractive from the range available (Perrett et al. 1998; Little et al. 2001).
(d) Participants
Heterosexual undergraduate students were recruited from the University of St Andrews: 46 women not taking hormonal contraceptives or reporting pregnancy (age range 18–23 years, mean 19.50±1.36) and 52 men (age range 18–24 years, mean 20.62±1.60).
3. Materials
To assess preferences for facial masculinity and attractiveness, interactive face-sequence trials were used, consisting of three male and three female Caucasian faces.
Participants were also asked to complete a questionnaire, which included life-history questions relating to age of menarche/puberty and age of first sex. For individuals reporting not having had sex or sexual partners (9 males and 16 females), current age was used as age of first sex.
Additionally, family relationship questions included warmth towards father and mother, quality of parents' relationship with one another which used a 9-point Likert-type scale and current age of parents. Also relevant to this study was self-rated attractiveness, which used a 7-point Likert-type scale. Father absence was assessed with questions relating to the participant's age at the time of parents' separation.
(a) Procedures
After reading and signing a consent form, participants were asked to complete an on-line questionnaire. Participants were presented with two conditions of interactive face-sequence trials, sexual dimorphism and attractiveness for opposite-sex faces. Both the conditions and the example faces within each condition were randomized. For each face, the participants were asked to select the face they found most attractive by moving the cursor over the image to scroll through the continua of sequenced faces. By clicking on the computer mouse, the participant chose the face he or she found most attractive as well as moving them on to the next trial.
4. Results
(a) Women
We performed initial Spearman's rank correlations and found that age of first sex significantly correlated with preference for masculine facial characteristics (r39=−0.427, p=0.007) and showed a trend to correlate with male facial attractiveness (r39=0.292, p=0.071). Women with early sexual experience preferred more masculine-looking males, yet showed a reduced tendency to prefer attractive male faces. Interestingly, we did not find a significant correlation between age of menarche and any facial characteristic preferences (all r42, p>0.314). In our sample, we did not find a relationship between our two developmental markers, age of first sex and age of menarche (r42=0.047, p=0.768). Additionally, face preferences for attractiveness and masculinity were not significantly correlated (r42=−0.047, p=0.769).
(b) Control variables and partial correlations
Our Spearman's rank correlations revealed that both warmth towards father (r44=0.297, p=0.050) and warmth towards mother (r44=0.313, p=0.036) significantly correlated with parents' relationship, as well as with one another (r44=0.585, p<0.001). Thus, we chose quality of parents' relationship as a control variable. Our analysis also revealed that self-rated attractiveness was positively correlated with preferences for more attractive male faces (r42=0.307, p=0.048), indicating that as self-perceived attractiveness increased, so did a preference for more attractive male faces. None of our other control variables were found to significantly relate to preferences for facial characteristics (all p>0.180).
To assess our hypothesis that timing of developmental milestones influenced women's preferences for male facial characteristics, partial correlations were used to control the possibility of other factors known to influence mate choice preferences. We found that the relationship between age of first sex and preferences for male facial masculinity remained significant after controlling for current age, self-rated attractiveness, dad's age, and quality of parents' relationship (r31=−0.423, p=0.014). The other correlations between sexual developmental markers (age of first sex and age of menarche) and face preferences remained non-significant (all p>0.18). The relationship between age of menarche and male face masculinity was non-significant (r29=0.173, p=0.35). We did not find any other significant correlations with masculinity preferences among our control variables (all p>0.22).
To investigate further the relationship of self-rated attractiveness and our dependent variable male facial attractiveness, we ran a partial correlation with age of first sex, age of menarche, current age, dad's age and quality of parents' relationship as control variables. The relationship between self-rated attractiveness and preference for male facial attractiveness remained significant (r29=0.431, p=0.016), while the relationship between self-rated attractiveness and masculinity remained non-significant (r29=0.185, p=0.320).
(c) Men
We hypothesized that timing of developmental markers would influence adult mate choice preferences. Correlations indicate that both age of puberty (Spearman's r49=−0.331, p=0.020) and age of first sex (r51=−0.286, p=0.042) related to preferences for facial femininity. Thus, early male sexual development was associated with increased preference for feminized characteristics in women's faces.
Despite findings in previous research linking age of puberty with age of first sex, we found only a positive but non-significant correlation (r50=0.204, p=0.156), perhaps owing to the small sample size.
(d) Control variables and partial correlations
We used partial correlations to determine whether other known effects (i.e. family background, own age and attractiveness) contributed to the current finding of a relationship between male sexual maturation and preference for female facial femininity.
Spearman's rank correlations revealed that both warmth towards mother (r52=0.419, p=0.002) and warmth towards father (r52=0.465, p=0.001) were positively correlated with quality of parents' relationship, and with one another (r52=0.587, p<0.001). We therefore opted to use quality of parents' relationship as a control variable.
Our partial correlations indicated that the relationship between male sexual maturation and femininity preference in women's faces remained significant (after controlling quality of parents' relationship, current age, mother's age and self-rated attractiveness) with age of puberty (r43=−0.302, p=0.044), although the relationship with age of first sex was only marginally significant (r44=−0.285, p=0.055). Additionally, none of the control variables (quality of parents' relationship, current age, mother's age and self-rated attractiveness) related to face preferences in zero-order Spearman's correlation (all p>0.23).
5. Discussion
(a) Stimuli
For this experiment, we used novel stimuli in an attempt to differentiate between sexual dimorphism and another factor we labelled ‘attractiveness’. We made explicit predictions concerning preferences for these new stimulus dimensions. Our calibration study indicated that the male attractive faces were judged to vary in attractiveness but not masculinity, and the male masculine faces were judged to vary in masculinity but not in attractiveness. This suggests, as Langlois et al. (2000) implied, that there is a general agreement about attractiveness. Moreover, there is also an agreement as to what constitutes facial masculinity in males, and that these two dimensions for the male face are not necessarily the same. For the female face, we were unsuccessful at separating facial attractiveness from feminine facial characteristics; thus, we looked only at the high and low feminine faces and found that the timing of men's sexual development was associated with preferences for more feminine female faces.
(b) Facial preferences and timing of sexual maturation
As predicted, the timing of sexual developmental markers was found to influence both women and men's mate preferences, and earlier maturers preferred increased sexual dimorphism in opposite-sex faces. Men who had experienced earlier puberty and earlier initial sexual intercourse were found to prefer more feminized female faces compared with those males who matured later. Women who experienced earlier first sex preferred more masculine male faces while those who experienced initial sex later, or remained virgins, preferred less masculinized faces. We did not find, as predicted, that age of menarche was associated with facial preferences. Other factors known to influence preferences for mate facial characteristics, self-rated attractiveness, parental relationships, age of opposite-sex parent or own age, could not explain the developmental differences in preferences for facial sexual dimorphism.
In addition to our general prediction that timing of puberty and age of first sex would be associated with facial preferences, we considered three specific explanations regarding how mating strategies may have been influenced by sexual development.
The third explanation that early maturing adolescents would view themselves as having higher social status than their peers was not supported by the data. High status individuals should show increased preference for high-quality individuals, and therefore should prefer both sexual dimorphism and attractiveness. We found support for the former but not the latter.
Our first prediction had mixed results. We suggested that if early developers are low-quality individuals, then as per an assortative mating strategy these individuals should seek low-quality mates. Based on this inference, our data suggest that early maturers are high- and not low-quality individuals. We did not ask participants to choose the most attractive face based on either long- or short-term relationships, so we cannot exclude the possibility that low-quality women were selecting for short- and not long-term mates. It has been suggested that low-quality women will seek out high-quality males for a short-term opportunistic mating in order to obtain better genes for immunocompetence. To use this explanation to interpret our data, we do need to make unsubstantiated assumptions that (i) the early maturing women were employing a short-term mating strategy and (ii) a ‘condition-dependent’ preference (Little et al. 2001) is exclusive to sexually dimorphic traits. Our findings suggest that early sexually maturing men select high-quality females because they themselves are high-quality. Thus, the results indicate then the early sexual maturation of both sexes is associated with ‘high’ quality. We note that it is best to consider early and late maturers as having different types of characteristics of mate quality rather than categorizing them as high and low levels of condition. We therefore suggest that individuals varying in rates of maturation emphasize different qualities and seek self-similar qualities in others.
The observation that earlier sexual development was associated with preferences for sexual dimorphism but not for attractiveness characteristics in the face supports our second explanation that learning plays a role in adult mate choice. We reasoned that early maturing adolescents were more likely to receive positive feedback from early maturing opposite-sex adolescents in their early forays into sexual behaviour. Adolescents who matured later would more likely be spurned by early maturing opposite-sex adolescents and could possibly associate negative feedback with these interactions. The signals of early maturation would be associated with exaggerated sexually dimorphic characteristics in the face, and these characteristics would be associated with either positive or negative experiences during adolescence. These preferences for epigamic traits would continue on into adulthood. The facial characteristics we have labelled as attractive would not be associated with pubertal timing, and therefore we would not expect to see a strong preference for these characteristics associated with sexual maturation rate.
(c) Self-rated attractiveness and condition dependence
Among women, we found that as ratings of self-perceived attractiveness increased, so too did preferences for our ‘attractive’ male faces but not preferences for the masculine male faces. Previous work examining condition dependence found that women rated by others as more attractive preferred more masculine-looking males (Penton-Voak et al. 2003). We attribute these contrary findings to the differences between stimuli. Penton-Voak and his colleagues varied their images along a continuum of masculinity in face shape. Our stimuli varied in three ways, in colour and texture as well as shape.
We also went a step further to refine facial dimensions by separating epigamic traits from other facial characteristics signalling mate quality. What we have found is an answer to the question if there is something other than facial masculinity which contributes to mate choice preferences. The answer is positive. However, questions remain as to what information our masculine and attractive male faces stimuli suggest to women, and why women who rate themselves as attractive prefer one and not the other.
The results may be a manifestation of assortative mating or matching on self-similar qualities (Berscheid et al. 1971; Feingold 1988, 1990). In other words, early maturing individuals prefer early maturing (sexually dimorphic) partners, while attractive individuals prefer attractive partners. Prior research has not separated maturation and attractiveness in stimuli or observers.
6. Closing considerations
Accelerated sexual maturation is associated with preferences for exaggerated sexually dimorphic features in opposite-sex faces in both men and women. We suggest that these preferences are due to learning influences during adolescence. It is possible that early maturers are higher quality; however, this conclusion is speculative and requires further investigation. A more parsimonious explanation is that early maturing men and women are seeking out similar individuals in much the same way as more attractive individuals seek out partners with similar attractiveness. Signs of early maturation are most likely to be enhanced sexually dimorphic characteristics in the face and body shape, and seeking out self-similar opposite-sex partners would fit in with the ‘matching-hypothesis’ (Berscheid et al. 1971; Feingold 1988). We asked individuals to select those faces they found most attractive, without any type of interpersonal or social feedback; this method is perhaps akin to how we might decide whom to approach in social situation. Our research suggests that people initially seek out individuals who are more like themselves on the dimension of sexual dimorphism. Feingold (1988) found that men and women do initially seek out partners who are self-similarly attractive, and there is a mild correlation in terms of attractiveness between partners in long-term relationships; however, other components such as socio-economic status, within-group desirability, and interpersonal similarity become much more important in long-term partnerships. Initial attraction is only a small part of the picture, and it is not surprising that we use facial appearance to sort out initial likes and dislikes.
Acknowledgments
Special thanks to Lesley Ferrier, Robert Pitman, Susie Whiten, Bill Calderhead, Carolyn Cheetham, Fiona Elder, Jen Hardingham, Laura Johnson, Anne Marie Morgan, Anne Perrett and Lisa DeBruine.
Footnotes
One contribution of 14 to a theme issue ‘The neurobiology of social recognition, attraction and bonding’.
References
- Ahmed S.A, Talal N. Sex-hormones and the immune-system. Part 2. Animal data. Baillieres Best Pract. Res. Clin. Rheumatol. 1990;4:13–31. doi: 10.1016/s0950-3579(05)80241-9. [DOI] [PubMed] [Google Scholar]
- Badyael A.V, Hill G.E. Paternal care as a conditional strategy: distinct reproductive tactics associated with elaboration of plumage ornamentation in the house finch. Behav. Ecol. 2002;13:591–597. doi:10.1093/beheco/13.5.591 [Google Scholar]
- Belsky J, Draper P. Reproductive strategies and radical solutions. Society. 1987;24:20–24. [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy—an evolutionary theory of socialization. Child Dev. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. doi:10.2307/1131166 [DOI] [PubMed] [Google Scholar]
- Bereczkei T, Csanaky A. Stressful family environment, mortality, and child socialisation: life history strategies among adolescents and adults from unfavourable social circumstances. Int. J. Behav. Dev. 2001;25:501–508. doi:10.1080/01650250042000573 [Google Scholar]
- Berg E.C. The effects of perceived closeness to custodial parents, stepparents and non-resident parents on adolescent self-esteem. J. Divorce Remarriage. 2003;40:69–86. doi:10.1300/J087v40n01_05 [Google Scholar]
- Berscheid E, Dion K, Walster E, Walster G.W. Physical attractiveness and dating choice: a test of the matching hypothesis. J. Exp. Social Psychol. 1971;7:173–189. doi:10.1016/0022-1031(71)90065-5 [Google Scholar]
- Black C, DeBlassie R.R. Adolescent pregnancy: contributing factors, consequences, treatment, and plausible solutions. Adolescence. 1985;20:281–290. [PubMed] [Google Scholar]
- Boothroyd L.G, Perrett D.I. Facial and bodily correlates of father absence. Proc. R. Soc. B. 2006;273:2375–2380. doi: 10.1098/rspb.2006.3579. doi:10.1098/rspb.2006.3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley N.T. Sexual selection for aesthetic traits in species with biparental care. Am. Nat. 1986;127:415–445. doi:10.1086/284493 [Google Scholar]
- Chisholm J.S. Death, hope, and sex: life-history theory and the development of reproductive strategies. Curr. Anthropol. 1993;34:1–24. doi:10.1086/204131 [Google Scholar]
- Chisholm J.S. The evolutionary ecology of attachment organization. Hum. Nat. 1996;7:1–37. doi: 10.1007/BF02733488. [DOI] [PubMed] [Google Scholar]
- Comings D.E, Muhleman D, Johnson J.P, MacMurray J.P. Parent–daughter transmission of the androgen receptor gene as an explanation of the effect of father absence on age of menarche. Child Dev. 2002;73:1046–1051. doi: 10.1111/1467-8624.00456. doi:10.1111/1467-8624.00456 [DOI] [PubMed] [Google Scholar]
- Cornwell R.E, Boothroyd L, Burt D.M, Feinberg D.R, Jones B.C, Little A.C, Pitman R, Whiten S, Perrett D.I. Concordant preferences for opposite-sex signals? Human pheromones and facial characteristics. Proc. R. Soc. B. 2004;271:635–640. doi: 10.1098/rspb.2003.2649. doi:10.1098/rspb.2003.2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M.R, Roberts A.R, Barbee A.P, Druen R.B, Wu C.-H. “Their ideas of beauty are, on the whole, the same as ours”: consistency and variability in the cross-cultural perception of female physical attractiveness. J. Pers. Soc. Psychol. 1995;68:261–279. doi:10.1037/0022-3514.68.2.261 [Google Scholar]
- Dorn L.D, Dahl R.E, Williamson D.E, Birmaher B, Axelson D, Perel J, Stull S.D, Ryan N.D. Developmental markers in adolescence: implications for studies of pubertal processes. J. Youth Adolesc. 2003a;32:315–324. doi:10.1023/A:1024945113763 [Google Scholar]
- Dorn L.D, Susman E.J, Ponirakis A. Pubertal timing and adolescent adjustment and behavior: conclusions vary by rater. J. Youth Adolesc. 2003b;32:157–167. doi:10.1023/A:1022590818839 [Google Scholar]
- Draper P, Harpending H. Father absence and reproductive strategy: an evolutionary perspective. J. Anthropol. Res. 1982;38:255–273. [Google Scholar]
- Edgardh K. Sexual behaviour and early coitarche in a national sample of 17-year old Swedish boys. Acta Paediatr. 2002;91:985–991. doi: 10.1080/080352502760272704. doi:10.1080/080352502760272704 [DOI] [PubMed] [Google Scholar]
- Elfenbein D.S, Felice M.E. Adolescent pregnancy. Pediatr. Clin. North Am. 2003;50:781. doi: 10.1016/s0031-3955(03)00069-5. doi:10.1016/S0031-3955(03)00069-5 [DOI] [PubMed] [Google Scholar]
- Ellis B.J. Timing of pubertal maturation in girls: an integrated life history approach. Psychol. Bull. 2004;130:920–958. doi: 10.1037/0033-2909.130.6.920. doi:10.1037/0033-2909.130.6.920 [DOI] [PubMed] [Google Scholar]
- Ellis B.J, Garber J. Psychosocial antecedents of variation in girls' pubertal timing: maternal depression, stepfather presence, and marital and family stress. Child Dev. 2000;71:4585–4501. doi: 10.1111/1467-8624.00159. [DOI] [PubMed] [Google Scholar]
- Ellis B.J, Bates J.E, Dodge K.A, Fergusson D.M, Horwood L.J, Pettit G.S, Woodward L.J. Does father absence place daughters at special risk for early sexual activity and teenage pregnancy? Child Dev. 2003;74:801–821. doi: 10.1111/1467-8624.00569. doi:10.1111/1467-8624.00569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enlow D.H. Harcourt Brace Javanovich; Philadelphia, PA: 1990. Facial growth. [Google Scholar]
- Feingold A. Matching for attractiveness in romantic partners and same-sex friends: a meta-analysis and theoretical critique. Psychol. Bull. 1988;104:226–235. doi:10.1037/0033-2909.104.2.226 [Google Scholar]
- Feingold A. Gender differences in effects of physical attractiveness on romantic attraction: a comparison across five research paradigms. J. Pers. Soc. Psychol. 1990;59:981–993. doi:10.1037/0022-3514.59.5.981 [Google Scholar]
- Folstad I, Karter A.J. Parasites, bright males and the immunocompetence handicap. Am. Nat. 1992;139:603–622. doi:10.1086/285346 [Google Scholar]
- Geary D.C. Sexual selection and human life history. Adv. Child Dev. Behav. 2002;30:41–101. doi: 10.1016/s0065-2407(02)80039-8. [DOI] [PubMed] [Google Scholar]
- Gowaty P.A. Field studies of parental care in birds. Adv. Study Behav. 1996;25:477–531. [Google Scholar]
- Gowen L.K, Feldman S.S, Diaz R, Somera Yisrael D. A comparison of the sexual behaviors and attitudes of adolescent girls with older vs. similar-aged boyfriends. J. Youth Adolesc. 2004;33:167–176. doi:10.1023/B:JOYO.0000013428.41781.a0 [Google Scholar]
- Grammer K, Thornhill R. Human (Homo sapiens) facial attractiveness and sexual selection: the role of symmetry and averagness. J. Comp. Psychol. 1994;108:233–242. doi: 10.1037/0735-7036.108.3.233. doi:10.1037/0735-7036.108.3.233 [DOI] [PubMed] [Google Scholar]
- Gross M.R. Disruptive selection for alternative life histories in salmon. Nature. 1985;313:47–48. doi:10.1038/313047a0 [Google Scholar]
- Gross M.R. Alternative reproductive strategies and tactics: diversity within the sexes. Trends Ecol. Evol. 1996;11:92–98. doi: 10.1016/0169-5347(96)81050-0. doi:10.1016/0169-5347(96)81050-0 [DOI] [PubMed] [Google Scholar]
- Halpern C.T, Udry J.R, Suchindran C. Monthly measures of salivary testosterone predict sexual activity in adolescent males. Arch. Sex. Behav. 1998;27:445–465. doi: 10.1023/a:1018700529128. doi:10.1023/A:1018700529128 [DOI] [PubMed] [Google Scholar]
- Harris J.R. Where is the child's environment? A group socialization theory of development. Psychol. Rev. 1995;102:458–489. doi:10.1037/0033-295X.102.3.458 [Google Scholar]
- Hawley P.H. The ontogenesis of social dominance: a strategy based evolutionary perspective. Dev. Rev. 1999;19:97–132. doi:10.1006/drev.1998.0470 [Google Scholar]
- Hawley P.H. Prosocial and coercive configurations of resource control in early adolescence: a case for the well-adapted Machiavellian. Merrill-Palmer Q. J. Dev. Psychol. 2003;49:279–309. [Google Scholar]
- Hawley P.H, Vaughn B.E. Aggression and adaptive functioning: the bright side to bad behavior. Merrill-Palmer Q. J. Dev. Psychol. 2003;49:239–242. [Google Scholar]
- Henson S.A, Warner R.R. Male and female alternative reproductive behaviors in fishes: a new approach using intersexual dynamics. Annu. Rev. Ecol. Syst. 1997;28:571–592. doi:10.1146/annurev.ecolsys.28.1.571 [Google Scholar]
- Hoier S. Father absence and age at menarche—a test of four evolutionary models. Hum. Nat. Interdisciplinary Biosoc. Perspect. 2003;14:209–233. doi: 10.1007/s12110-003-1004-2. [DOI] [PubMed] [Google Scholar]
- Jones B.C, Leeton J, McLeod I, Wood C. Factors influencing the age of menarche in a lower socieconomic group in Melbourne. Med. J. Aust. 1972;21:533–535. doi: 10.5694/j.1326-5377.1972.tb47460.x. [DOI] [PubMed] [Google Scholar]
- Jones B.C, Little A.C, Penton-Voak I.S, Tiddeman B.P, Burt D.M, Perrett D.I. Facial symmetry and judgements of apparent health. Support for a “good genes” explanation of the attractiveness—symmetry relationship. Evol. Hum. Behav. 2001;22:417–429. doi:10.1016/S1090-5138(01)00083-6 [Google Scholar]
- Kim K, Smith P.K. Retrospective survey of parental marital relations and child reproductive development. Int. J. Behav. Dev. 1998;22:729–751. doi:10.1080/016502598384144 [Google Scholar]
- Kim K, Smith P.K, Palermiti A.-L. Conflict in childhood and reproductive development. Evol. Hum. Behav. 1997;18:109–142. doi:10.1016/S1090-5138(96)00114-6 [Google Scholar]
- Kirk K.M, Blomberg S.P, Duffy D.L, Heath A.C, Owens I.P.F, Martin N.G. Natural selection and quantitative genetics of life-history traits in western women: a twin study. Evolution. 2001;55:423–435. doi: 10.1111/j.0014-3820.2001.tb01304.x. doi:10.1554/0014-3820(2001)055[0423:NSAQGO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kracke B. Beltz PVU; Weinheim, Germany: 1993. Pubertaet und Problemverhalten bei Jungen [Puberty and behavior problems of male adolescents] [Google Scholar]
- Langlois J.H, Kalakanis L, Rubenstein A.J, Larson A, Hallam M, Smoot M. Maxims or myths of beauty? A meta-analytic and theoretical review. Psychol. Bull. 2000;126:390–423. doi: 10.1037/0033-2909.126.3.390. doi:10.1037/0033-2909.126.3.390 [DOI] [PubMed] [Google Scholar]
- Law Smith M.J, et al. Facial appearance is a cue to oestrogen levels in women. Proc. R. Soc. B. 2006;273:135–140. doi: 10.1098/rspb.2005.3296. doi:10.1098/rspb.2005.3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little A.C, Hancock P.J. The role of masculinity and distinctiveness in judgments of human male facial attractiveness. Br. J. Psychol. 2002;93:451–464. doi: 10.1348/000712602761381349. doi:10.1348/000712602761381349 [DOI] [PubMed] [Google Scholar]
- Little A.C, Burt D.C, Penton-Voak I, Perrett D.I. Self-perceived attractiveness influences human female preferences for sexual dimorphism and symmetry in male faces. Proc. R. Soc. B. 2001;268:39–44. doi: 10.1098/rspb.2000.1327. doi:10.1098/rspb.2000.1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur R.H. Some generalized theorems of natural selection. Genetics. 1962;48:1893–1897. doi: 10.1073/pnas.48.11.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur R.H, Wilson E.O. Princeton University Press; Princeton, NJ: 1967. The theory of island biogeography. [Google Scholar]
- MacDonald K. Life history theory and human reproductive behavior—environmental/contextual influences and heritable variation. Hum. Nat. Interdisciplinary Biosoc. Perspec. 1997;8:327–359. doi: 10.1007/BF02913038. [DOI] [PubMed] [Google Scholar]
- MacDonald K. An evolutionary perspective on human fertility. Popul. Environ. 1999;21:223–246. [Google Scholar]
- Mace R. Evolutionary ecology of human life history. Anim. Behav. 2000;59:1–10. doi: 10.1006/anbe.1999.1287. doi:10.1006/anbe.1999.1287 [DOI] [PubMed] [Google Scholar]
- Magnusson D, Stattin H, Allen V.L. Biological maturation and social development: a longitudinal study of some adjustment processes from midadolescence to adulthood. J. Youth Adolesc. 1985;14:267–283. doi: 10.1007/BF02089234. doi:10.1007/BF02089234 [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. Cambridge University Press; Cambridge, UK: 1982. Evolution and the theory of games. [Google Scholar]
- Maynard Smith J, Price G.R. The logic of animal conflict. Nature. 1973;246:15–18. doi:10.1038/246015a0 [Google Scholar]
- Mazur A, Booth A. Testosterone and dominance in men. Behav. Brain Sci. 1998;21:353–397. doi:10.1017/S0140525X98001228 [PubMed] [Google Scholar]
- McNeely C, Shew M.L, Beuhrink T, Sieving R, Miller B.C, Blum R.W.M. Mothers' influence on the timing of first sex among 14- and 15-year olds. J. Adolesc. Health. 2002;31:256–265. doi: 10.1016/s1054-139x(02)00350-6. doi:10.1016/S1054-139X(02)00350-6 [DOI] [PubMed] [Google Scholar]
- Miller E.M. Optimal adjustment of mating effort to environmental-conditions—a critique of Chisholms application of life-history theory with comments on race differences in male paternal investment strategies. Mankind Q. 1994;34:297–316. [Google Scholar]
- Moffitt T.E, Caspi A, Belsky J, Silva P.A. Childhood experience and the onset of menarche—a test of a sociobiological model. Child Dev. 1992;63:47–58. doi: 10.1111/j.1467-8624.1992.tb03594.x. doi:10.2307/1130900 [DOI] [PubMed] [Google Scholar]
- Møller A.P, Thornhill R. Male parental care, differential parental investment by females and sexual selection. Anim. Behav. 1998;55:1507–1515. doi: 10.1006/anbe.1998.0731. doi:10.1006/anbe.1998.0731 [DOI] [PubMed] [Google Scholar]
- Nottlemann E.D, Susman E.J, Dorn L.D, Inoff-Germain G, Loriaux D.L, Cutler G.B, Jr, Chrousos G.P. Developmental processes in early adolescence. J. Adolesc. Health Care. 1987;8:246–260. doi: 10.1016/0197-0070(87)90428-1. doi:10.1016/0197-0070(87)90428-1 [DOI] [PubMed] [Google Scholar]
- Olweus D, Mattsson D, Schalling D, Löw H. Circulating teststerone levels and aggression in adolescent males: a causal analysis. Psychosom. Med. 1988;50:261–272. doi: 10.1097/00006842-198805000-00004. [DOI] [PubMed] [Google Scholar]
- Overpeck M.D, Brenner R.A, Trumble A.C, Trifiletti L.B, Berendes H.W. Risk factors for infant homicide in the United States. New Engl. J. Med. 1998;339:1211–1216. doi: 10.1056/NEJM199810223391706. doi:10.1056/NEJM199810223391706 [DOI] [PubMed] [Google Scholar]
- Penton-Voak I.S, Chen J.Y. High salivary testosterone is linked to masculine male facial appearance in humans. Evol. Hum. Behav. 2004;25:229–241. doi:10.1016/j.evolhumbehav.2004.04.003 [Google Scholar]
- Penton-Voak I.S, Perrett D.I. Male facial attractiveness: perceived personality and shifting female preferences for male traits across the menstrual cycle. Adv. Study Behav. 2001;30:219–259. doi:10.1016/S0065-3454(01)80008-5 [Google Scholar]
- Penton-Voak I.S, Perrett D.I, Castles D.L, Burt D.M, Kobayashi T, Murray L.K, Minamisawa R. Menstrual cycle alters face preferences. Nature. 1999;399:714–742. doi: 10.1038/21557. doi:10.1038/21557 [DOI] [PubMed] [Google Scholar]
- Penton-Voak I.S, Little A.C, Jones B.C, Burt D.M, Tiddeman B.P, Perrett D.I. Female condition influences preferences for sexual dimorphism in faces of male Homo sapiens. Comp. Psychol. 2003;117:264–271. doi: 10.1037/0735-7036.117.3.264. doi:10.1037/0735-7036.117.3.264 [DOI] [PubMed] [Google Scholar]
- Perrett D.I, Lee K.J, Penton-Voak I.S, Rowland D.R, Yoshikawa S, Burt D.M, Henzi S.P, Castles D.L, Akamatsu S. Effects of sexual dimorphism on facial attractiveness. Nature. 1998;394:884–887. doi: 10.1038/29772. doi:10.1038/29772 [DOI] [PubMed] [Google Scholar]
- Perrett D.I, Burt D.M, Penton-Voak I.S, Lee K.J, Rowland D.A, Edwards R. Symmetry and human facial attractiveness. Evol. Hum. Behav. 1999;20:295–307. doi:10.1016/S1090-5138(99)00014-8 [Google Scholar]
- Peterson J.C, Crockett L.J. Pubertal timing and grade effects on adjustment. J. Youth Adolesc. 1985;14:191–206. doi: 10.1007/BF02090318. doi:10.1007/BF02090318 [DOI] [PubMed] [Google Scholar]
- Pickles A, Pickering K, Simonoff E, Silberg J, Meyer J, Maes H. Genetic ‘clocks’ and ‘soft’ events: a twin model for pubertal development and other recalled sequences of developmental milestones, transitions, or ages at onset. Behav. Genet. 1998;28:243–253. doi: 10.1023/a:1021615228995. doi:10.1023/A:1021615228995 [DOI] [PubMed] [Google Scholar]
- Prokopèáková A. Drug experimenting and pubertal maturation in girls. Studia Psychol. 1998;40:287–290. [Google Scholar]
- Quinlan R.J. Father absence, parental care, and female reproductive development. Evol. Hum. Behav. 2003;24:376–390. doi:10.1016/S1090-5138(03)00039-4 [Google Scholar]
- Romans S.E, Martin J.M, Gendall K, Herbison G.P. Age of menarche: the role of some psychosocial factors. Psychol. Med. 2003;33:933–939. doi: 10.1017/s0033291703007530. doi:10.1017/S0033291703007530 [DOI] [PubMed] [Google Scholar]
- Schaal B, Tremblay R.E, Soussignan R, Susman E.J. Male testosterone linked to high social dominance but low physical aggression in early adolescence. J. Acad. Child Adolesc. Psychiatry. 1996;35:1322–1331. doi: 10.1097/00004583-199610000-00019. doi:10.1097/00004583-199610000-00018 [DOI] [PubMed] [Google Scholar]
- Scheib J.E. Context-specific mate choice criteria: women's trade-offs in the contexts of long-term and extra-pair mateships. Pers. Relat. 2001;8:371–389. doi:10.1111/j.1475-6811.2001.tb00046.x [Google Scholar]
- Scheib J.E, Gangestad S.W, Thornhill R. Facial attractiveness, symmetry and cues of good genes. Proc. R. Soc. B. 1999;266:1913–1917. doi: 10.1098/rspb.1999.0866. doi:10.1098/rspb.1999.0866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seli E, Arici A. Sex steroids and the immune system. Immunol. Allergy Clin. North Am. 2002;22:407. doi:10.1016/S0889-8561(02)00017-6 [Google Scholar]
- Sheldon B.C. Differential allocation: tests, mechanisms and implications. Trends Ecol. Evol. 2000;15:397–402. doi: 10.1016/s0169-5347(00)01953-4. doi:10.1016/S0169-5347(00)01953-4 [DOI] [PubMed] [Google Scholar]
- Silbereisen R.K, Kracke B. Self-reported maturational timing and adaptation in adolescence. In: Schulenberg J, Maggs J.L, Hurrelmann K, editors. Health risks and developmental transitions during adolescence. Cambridge University Press; Cambridge, UK: 1997. pp. 85–109. [Google Scholar]
- Spencer J.M, Zimet G.D, Aalsma M.C, Orr D.P. Self-esteem as a predictor of initiation of coitus in early adolescents. Pediatrics. 2002;109:581–584. doi: 10.1542/peds.109.4.581. doi:10.1542/peds.109.4.581 [DOI] [PubMed] [Google Scholar]
- Stattin H, Magnusson D. Paths through life. vol. 2. Lawrence Erlbaum Associates; Hillsdale, NJ; Oxford, UK: 1990. Pubertal maturation in female development. [Google Scholar]
- Surbey M.K. Family composition, stress, and the timing of human menarche. In: Bercovitch F.B, editor. Sociendocrinology of primate reproduction. Monographs in primatology. vol. XIII. Wiley; New York, NY: 1990. pp. 11–32. [Google Scholar]
- Susman E.J, Inoff-Germain G, Nottlemann E.D, Loriaux D.L, Cutler G.B, Jr, Chousos G.P. Hormones, emotional dispositions, and aggressive attributes in young adolescents. Child Dev. 1987;58:1114–1134. doi:10.2307/1130551 [PubMed] [Google Scholar]
- Swaddle J.P, Reierson G.W. Testosterone increases perceived dominance but not attractiveness in human males. Proc. R. Soc. B. 2002;269:2285–2289. doi: 10.1098/rspb.2002.2165. doi:10.1098/rspb.2002.2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornhill R, Gangestad S.W. Human fluctuating asymmetry and sexual behavior. Psychol. Sci. 1994;5:297–302. doi:10.1111/j.1467-9280.1994.tb00629.x [Google Scholar]
- Tiddeman B.P, Burt D.M, Perrett D.I. Prototyping and transforming facial texture for perception research. IEEE Comput. Graph. Appl. 2001;21:42–50. doi:10.1109/38.946630 [Google Scholar]
- Tremblay R.E, Schaal B, Boulerice B, Arseneault L, Soussignan R.G, Paquette D, Laurent D. Testosterone, physical aggression, dominance, and physical development in early adolescence. Int. J. Behav. Dev. 1998;22:753–777. doi:10.1080/016502598384153 [Google Scholar]
- Trivers R. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man: 1871–1971. Aldine; Chicago, IL: 1972. pp. 136–179. [Google Scholar]
- Weichold, K. & Silbereisen, R. K. 2001 Pubertal timing and substance use: the role of peers and leisure context. In Seventh European Congress of Psychology, London.
- Weichold K, Sibereisen R.K, Schmitt-Rodermund E. Short-term and long-term consequences of early versus late maturation in adolescents. In: Hayward C, editor. Gender differences at puberty (International studies on child & adolescent health) Cambridge University Press; Cambridge, UK: 2003. [Google Scholar]
- Weisfeld G.E, Woodward L. Current evolutionary perspectives on adolescent romantic relations and sexuality. J. Am. Acad. Child Adolesc. Psychiatry. 2004;41:11–19. doi: 10.1097/00004583-200401000-00010. doi:10.1097/00004583-200401000-00010 [DOI] [PubMed] [Google Scholar]
- Wilson M, Daly M. Life expectancy, economic inequality, homicide, and reproductive timing in Chicago neighbourhoods. Br. Med. J. 1997;314:1271–1274. doi: 10.1136/bmj.314.7089.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]