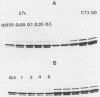
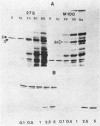
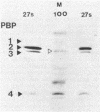
Abstract
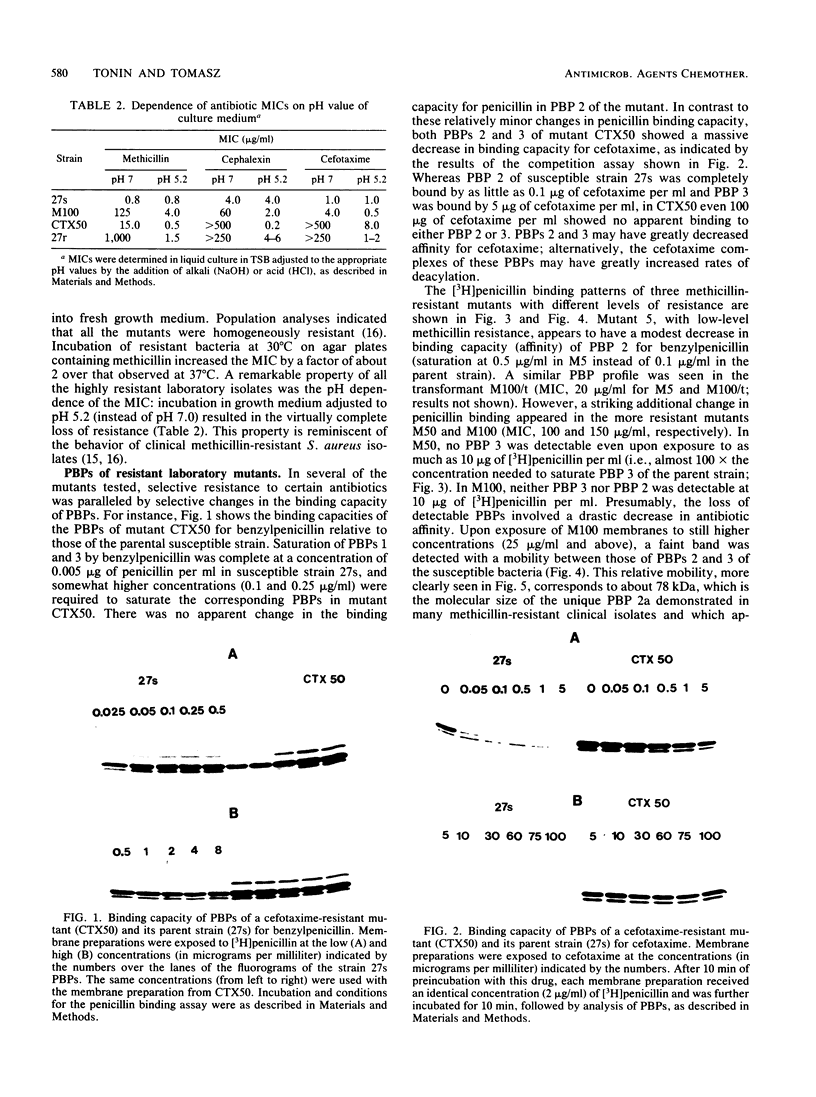
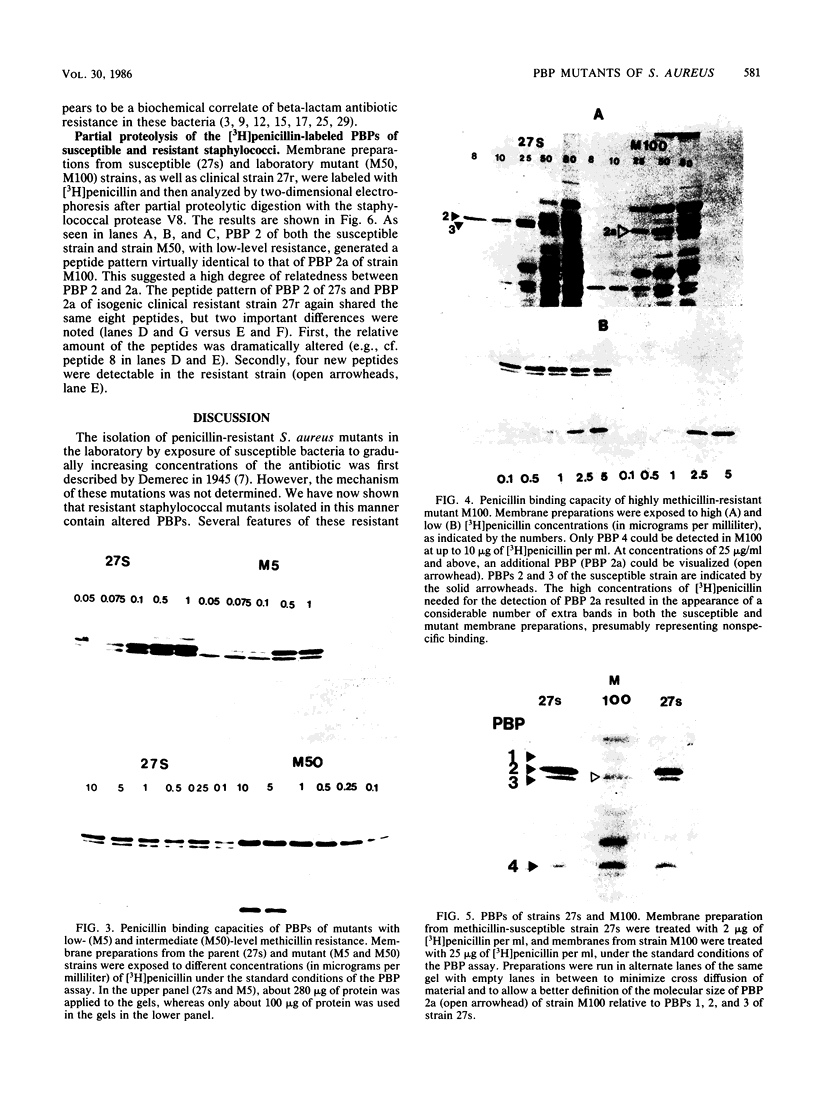
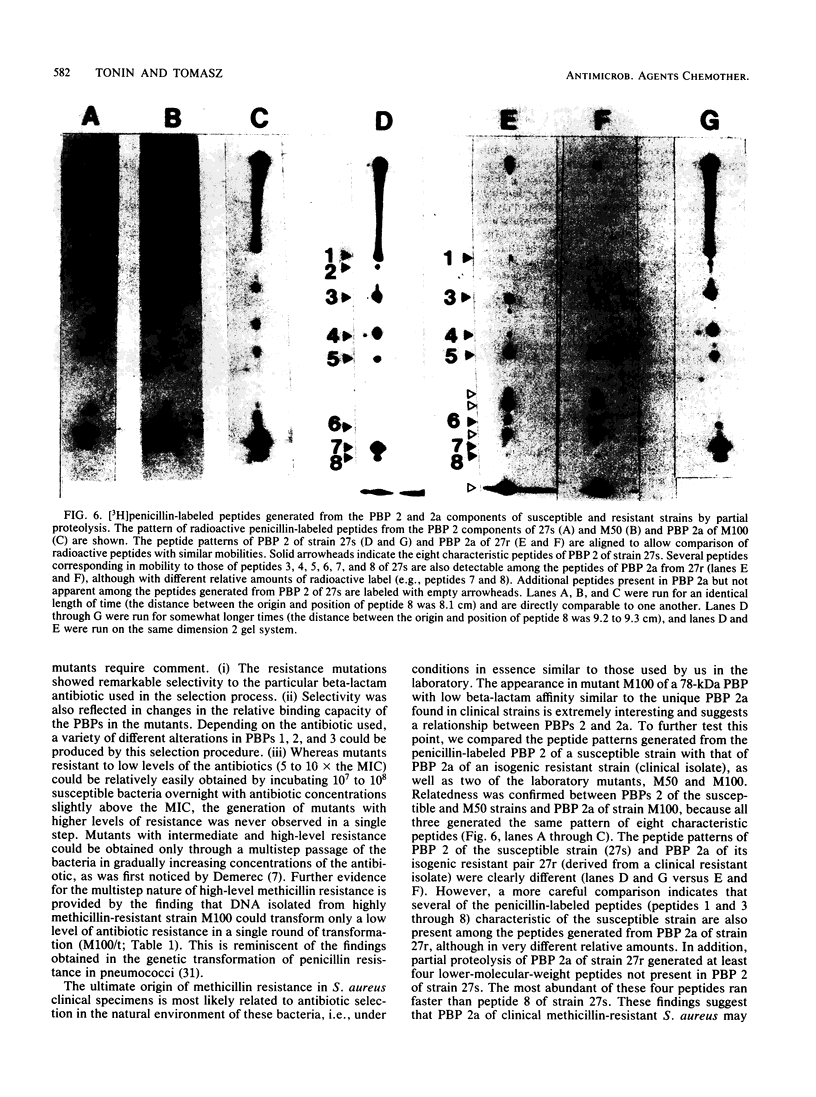
In an approach to understanding the origin of methicillin resistance in clinical isolates of staphylococci, a series of Staphylococcus aureus mutants resistant to various beta-lactam antibiotics were isolated in the laboratory by antibiotic selection. Mutants with low- and intermediate-level resistance showed considerable specificity for the particular antibiotic used in the selection process (methicillin, cefotaxime, cephalexin, and amdinocillin), and resistance in such mutants also showed alterations in the antibiotic binding capacities of penicillin-binding proteins (PBPs). In each case the isolation of mutants resistant to high concentrations of antibiotics required sequential passage in gradually increasing concentrations of the drug. The acquisition of increasing levels of methicillin resistance was paralleled by a gradual decrease in the binding capacities of PBPs 2, 3, and, possibly, 1. In a highly methicillin-resistant mutant (MIC, 150 micrograms/ml), PBPs 2 and 3 were no longer detectable by the penicillin binding assay. Instead, a new PBP of poor binding capacity and anomalous molecular size (about 78 kilodaltons [kDa]) appeared in these cells. This corresponds to the molecular size of PBP 2a, the unique PBP that appears to be the biochemical correlate of resistance in clinical isolates of methicillin-resistant S. aureus. Also, similar to the case of resistant clinical isolates, high-level beta-lactam resistance was highly pH dependent in the laboratory mutants. We compared the patterns of radioactive peptides generated by partial proteolysis from the penicillin-labeled PBP 2 of antibiotic-susceptible staphylococci and from the 78-kDa PBP 2a of a resistant clinical strain. Although the patterns were clearly different, seven of the eight characteristic peptides generated from PBP 2 of the susceptible strain were also detectable among the peptides released from PBP 2a. The results suggest that the 78-kDa PBP 2a of the resistant clinical strain evolved from PBP 2 of antibiotic-susceptible staphylococci and that in PBP 2a of the clinical isolate mutational changes have resulted in extensive alterations near the beta-lactam binding site.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G. Properties of penicillin-binding proteins in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1981 Feb;19(2):316–322. doi: 10.1128/aac.19.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown D. F., Reynolds P. E. Intrinsic resistance to beta-lactam antibiotics in Staphylococcus aureus. FEBS Lett. 1980 Dec 29;122(2):275–278. doi: 10.1016/0014-5793(80)80455-8. [DOI] [PubMed] [Google Scholar]
- Cafferkey M. T., Hone R., Keane C. T. Antimicrobial chemotherapy of septicemia due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985 Dec;28(6):819–823. doi: 10.1128/aac.28.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F., Hartman B. J., Tomasz A. Increased amounts of a novel penicillin-binding protein in a strain of methicillin-resistant Staphylococcus aureus exposed to nafcillin. J Clin Invest. 1985 Jul;76(1):325–331. doi: 10.1172/JCI111965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Demerec M. Production of Staphylococcus Strains Resistant to Various Concentrations of Penicillin. Proc Natl Acad Sci U S A. 1945 Jan;31(1):16–24. doi: 10.1073/pnas.31.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty T. J., Koller A. E., Tomasz A. Penicillin-binding proteins of penicillin-susceptible and intrinsically resistant Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1980 Nov;18(5):730–737. doi: 10.1128/aac.18.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana R., Cerini R., Longoni P., Grossato A., Canepari P. Identification of a streptococcal penicillin-binding protein that reacts very slowly with penicillin. J Bacteriol. 1983 Sep;155(3):1343–1350. doi: 10.1128/jb.155.3.1343-1350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana R., Grossato A., Rossi L., Cheng Y. R., Satta G. Transition from resistance to hypersusceptibility to beta-lactam antibiotics associated with loss of a low-affinity penicillin-binding protein in a Streptococcus faecium mutant highly resistant to penicillin. Antimicrob Agents Chemother. 1985 Nov;28(5):678–683. doi: 10.1128/aac.28.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Dix B. A., Mauriz Y. R. Possible physiological functions of penicillin-binding proteins in Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Feb;29(2):333–336. doi: 10.1128/aac.29.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Smith S. A., Bonner D. P. Penicillin-binding proteins in a Staphylococcus aureus strain resistant to specific beta-lactam antibiotics. Antimicrob Agents Chemother. 1982 Jul;22(1):172–175. doi: 10.1128/aac.22.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley R. W., Hightower A. W., Khabbaz R. F., Thornsberry C., Martone W. J., Allen J. R., Hughes J. M. The emergence of methicillin-resistant Staphylococcus aureus infections in United States hospitals. Possible role of the house staff-patient transfer circuit. Ann Intern Med. 1982 Sep;97(3):297–308. doi: 10.7326/0003-4819-97-3-297. [DOI] [PubMed] [Google Scholar]
- Handwerger S., Tomasz A. Alterations in penicillin-binding proteins of clinical and laboratory isolates of pathogenic Streptococcus pneumoniae with low levels of penicillin resistance. J Infect Dis. 1986 Jan;153(1):83–89. doi: 10.1093/infdis/153.1.83. [DOI] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Expression of methicillin resistance in heterogeneous strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Jan;29(1):85–92. doi: 10.1128/aac.29.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mendelman P. M., Chaffin D. O., Stull T. L., Rubens C. E., Mack K. D., Smith A. L. Characterization of non-beta-lactamase-mediated ampicillin resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1984 Aug;26(2):235–244. doi: 10.1128/aac.26.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr T. R., Jr, Bryan L. E. Mechanism of resistance of an ampicillin-resistant, beta-lactamase-negative clinical isolate of Haemophilus influenzae type b to beta-lactam antibiotics. Antimicrob Agents Chemother. 1984 Jun;25(6):747–753. doi: 10.1128/aac.25.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattee P. A., Neveln D. S. Transformation analysis of three linkage groups in Staphylococcus aureus. J Bacteriol. 1975 Oct;124(1):201–211. doi: 10.1128/jb.124.1.201-211.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi L., Tonin E., Cheng Y. R., Fontana R. Regulation of penicillin-binding protein activity: description of a methicillin-inducible penicillin-binding protein in Staphylococcus aureus. Antimicrob Agents Chemother. 1985 May;27(5):828–831. doi: 10.1128/aac.27.5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Lindberg M., Philipson L. Competence for transfection in Staphylococcus aureus. J Bacteriol. 1973 Feb;113(2):576–585. doi: 10.1128/jb.113.2.576-585.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend D. E., Ashdown N., Pearman J. W., Annear D. I., Grubb W. B. Genetics and epidemiology of methicillin-resistant Staphylococcus aureus isolated in a Western Australian hospital. Med J Aust. 1985 Jan 21;142(2):108–111. doi: 10.5694/j.1326-5377.1985.tb133045.x. [DOI] [PubMed] [Google Scholar]
- Ubukata K., Yamashita N., Konno M. Occurrence of a beta-lactam-inducible penicillin-binding protein in methicillin-resistant staphylococci. Antimicrob Agents Chemother. 1985 May;27(5):851–857. doi: 10.1128/aac.27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R., le Bouguénec C., Gutmann L., Horaud T. One or two low affinity penicillin-binding proteins may be responsible for the range of susceptibility of Enterococcus faecium to benzylpenicillin. J Gen Microbiol. 1985 Aug;131(8):1933–1940. doi: 10.1099/00221287-131-8-1933. [DOI] [PubMed] [Google Scholar]
- Zighelboim S., Tomasz A. Penicillin-binding proteins of multiply antibiotic-resistant South African strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):434–442. doi: 10.1128/aac.17.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]