Abstract
Genomic imprinting refers to the parent-of-origin-specific epigenetic marking of a number of genes. This epigenetic mark leads to a bias in expression between maternally and paternally inherited imprinted genes, that in some cases results in monoallelic expression from one parental allele. Genomic imprinting is often thought to have evolved as a consequence of the intragenomic conflict between the parental alleles that occurs whenever there is an asymmetry of relatedness. The two main examples of asymmetry of relatedness are when there is partiality of parental investment in offspring (as is the case for placental mammals, where there is also the possibility of extended postnatal care by one parent), and in social groups where there is a sex-biased dispersal. From this evolutionary starting point, it is predicted that, at the behavioural level, imprinted genes will influence what can broadly be termed bonding and social behaviour. We examine the animal and human literature for examples of imprinted genes mediating these behaviours, and divide them into two general classes. Firstly, mother–offspring interactions (suckling, attachment and maternal behaviours) that are predicted to occur when partiality in parental investment in early postnatal offspring occurs; and secondly, adult social interactions, when there is an asymmetry of relatedness in social groups. Finally, we return to the evolutionary theory and examine whether there is a pattern of behavioural functions mediated by imprinted genes emerging from the limited data, and also whether any tangible predictions can be made with regards to the direction of action of genes of maternal or paternal origin.
Keywords: imprinted genes, evolution, behaviour, cognition, X-chromosome
1. Introduction
As our knowledge of genes and genomes has grown, it has become increasingly clear that in some cases the epigenetic status of genes is as important as the information encoded by DNA sequence itself. One class of genes that falls under the ‘epigenetics’ umbrella is imprinted genes. Here, the epigenetic marking, which consists of DNA methylation and chromatin modification, occurs in a parent-of-origin-specific manner (Delaval & Feil 2004). This epigenetic mark is established in the developing embryo by early post-implantation and distinguishes the maternal and paternal genomes, leading to a bias in expression between maternally and paternally inherited imprinted genes that in some cases results in expression solely from one parental allele. This is in contrast to normal (non-imprinted) autosomal gene expression which is generally biallelic, or indifferent to the parental origin of the allele being expressed.
Although the number of known imprinted genes is relatively small (at present approximately 80 coding genes and 37 non-coding RNAs), their very existence contravenes Mendel's third law of inheritance (that maternal and paternal genomes are functionally equivalent). Furthermore, despite their relatively small number, the first studies suggesting the existence of imprinted genes, in which androgenetic (diploid for paternally derived chromosomes only) and parthenogenetic (diploid for maternally derived chromosomes only) mouse embryos failed to survive beyond mid-gestation (Barton et al. 1984; McGrath & Solter 1984; Surani et al. 1984), demonstrated that they have important developmental functions. Since these discoveries, the genomic imprinting field has expanded rapidly, with many efforts focused on understanding the underpinning epigenetic control mechanisms and the physiological role of imprinted genes.
(a) Kinship and evolution of imprinted genes
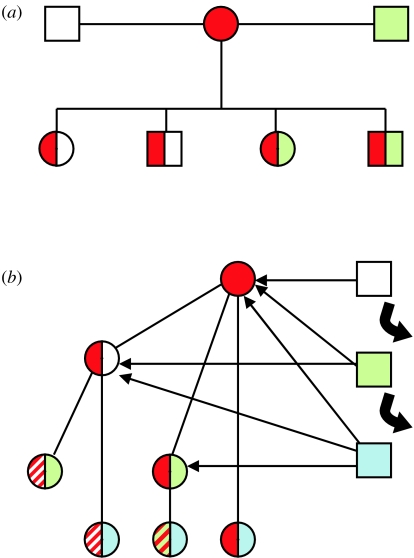
Imprinted genes often show monoallelic expression, meaning an individual is effectively haploid at this locus, thus negating any benefits of diploidy and sexual reproduction (Orr 1995). This has led to an extensive debate as to why genomic imprinting has evolved (Hurst 1997). Although many theories have been put forward, the most resilient, in terms of predictions and explaining the existing data, is the kinship (or conflict) theory (Haig & Westoby 1989; Moore & Haig 1991). This theory is an extension of the parent–offspring conflict theory developed by Trivers (1974) which suggested that, with regard to resource provisioning, the interests of the offspring do not necessarily equate to the interests of the parents (or mother) who have to take into account their lifetime reproductive output. The conflict theory in relation to imprinted genes takes this thinking to the genomic level, and suggests that, owing to relatedness asymmetries, maternally and paternally derived genomes may be selected to favour differential outcomes in the offspring. There are a number of presumptions that underlie this thinking; critically, a male would not anticipate fathering all a given female's offspring; either within her lifetime and/or within a given brood. This would lead to an asymmetry of relatedness between the maternal and the paternal genomes in the offspring: maternal genomes in a given female's offspring would be shared with the mother and among all her other offspring; however, paternal genomes are not shared with the mother, and are possibly shared with some, but not all her other offspring (figure 1a). It is this asymmetry of relatedness that gives rise to the differential interests of the maternal and paternal genomes of an individual.
Figure 1.
A diagram showing how asymmetries of relatedness can occur through multiple paternity of a female's offspring, and in a simple matrilineal society with sex-biased dispersal. (a) With multiple paternity either within a brood or, as is more likely, across a female's reproductive lifetime, offspring from the same mother differ in their relatedness owing to the presence of different paternal genomes. (b) Schematic of a simple matrilineal society as described in the text. Males, coming in from outside the group, hold short breeding tenure (matings with females are indicated by thin arrows). Male offspring (not shown) leave the group when sexually mature. What can be seen is that all females share, to differing degrees, maternally derived genes (in red)—this would of course also be true for male offspring. However, paternally derived genes are only present in the strata of the social group and are not shared as widely.
The possibility of intragenomic conflict is particularly obvious during the development of mammalian offspring in utero, where the system is possibly easiest to manipulate (Moore & Haig 1991). In this situation, paternally derived genes in the offspring would be expected to maximize nutrient resource acquisition from the mother via the placenta, given that the paternal genome is not shared with the mother and may not be shared with any of her subsequent offspring. However, this strategy may compromise the mother's overall lifetime fitness. The maternal genome is shared with the mother, and will be shared with all the offspring she has throughout her reproductive lifetime. Consequently, it is in the maternal genome's interest to redress the balance so that the mother can provide equal resources for all of her offspring, thus maximizing the overall lifetime fitness of the maternally derived genes. This prediction received strong support as the first few imprinted genes to be identified consisted of growth factors and their antagonistic counterparts acting mainly in utero, with paternally derived genes increasing, and maternally derived genes limiting, foetal growth (Dechiara et al. 1990; Barlow et al. 1991; Leighton et al. 1995).
The conflict between parental genomes with regards to in utero resource allocation has often been used as an evolutionary framework in which to place observed functions of many imprinted genes (Haig & Graham 1991; Itier et al. 1998; Lefebvre et al. 1998; Li et al. 1999). However, the original synthesis of the conflict hypothesis merely suggested that in utero may be one of the potential battlegrounds. Certainly, it is easy to see that the critical period between birth and weaning may provide an additional area where intragenomic conflict, with regard to resources allocation between the mother and the offspring, can arise. However, a number of researchers have also shown in theoretical models that imprinting is unlikely to be confined to resource allocation between parents and offspring, and may evolve whenever there are asymmetries of relatedness (Haig 1997; Trivers & Burt 1999; Haig 2000); the two classic scenarios when this occurs are multiple paternity of a female's offspring (either within-brood or over a reproductive lifetime), and when there is sex-biased dispersal from a social group. The latter scenario, although common in mammals (Greenwood 1980; Pusey 1987), has rarely been invoked when discussing the evolution of imprinted genes, and yet it provides an excellent starting point for discussion of those imprinted genes/parent-of-origin effects that influence postnatal functions extending into the adult. The main group of postnatal functions for which there is a growing body of evidence of imprinted gene/parent-of-origin effects from both mouse work and studies of human mental disease is brain and behavioural functioning (Isles & Wilkinson 2000; Davies et al. 2001). A large number of imprinted genes are expressed in the brain (Davies et al. 2005b), and yet very few have questioned why imprinting should persist in the adult brain or why imprinted genes have evolved to affect brain functioning at all.
In this article, we argue that, from the evolutionary standpoint, it is predicted that imprinted genes will influence what can broadly be termed social behaviours. We expand on the reasons for this, and examine the animal and human literature for examples of imprinted genes mediating social behaviour, and divide these into two general classes. Firstly, mother–offspring interactions that are predicted to occur when partiality in parental investment of offspring occurs; and secondly, adult social interactions, where there is an asymmetry of relatedness in social groups.
2. The pre-weaning period and mother–offspring bonding
There is ample evidence from a number of mouse studies regarding imprinted genes and resource allocation in utero that appears to support the predictions made by the kinship theory (Reik et al. 2003). However, as discussed previously, there is also predicted to be selective pressure for the involvement of imprinted genes in the pre-weaning period, when the offspring are still dependent on their mother for resources (Constancia et al. 2004; Isles & Holland 2005). Support for this general idea is increasing, and there are a number of examples of imprinted genes affecting growth in the pre-weaning period (Itier et al. 1998; Curley et al. 2004; Plagge et al. 2004).
(a) Suckling
Within the context of nutrient supply during the pre-weaning period, evidence from animal models (Curley et al. 2004; Plagge et al. 2004), and clinical conditions (Holm et al. 1993; Haig & Wharton 2003), suggests that imprinted genes may impact on suckling behaviour, with paternally expressed genes enhancing resource acquisition as they do in utero. A case in point is the paternally expressed gene Gnasxl, which encodes an isoform of the stimulatory G-protein subunit Gsα (XLαs). Gnasxl is expressed (among other places) in the facial, hypoglossal and trigeminal motor nuclei (Plagge et al. 2004), the key areas of the brain that provide innervation of the orofacial muscles controlling the jaw and the tongue (Lund et al. 1998; Sawczuk & Mosier 2001). Mice carrying a targeted deletion of this gene show a postnatal phenotype that includes deficits in suckling behaviour; they have substantially reduced milk content in their stomachs and postnatal weight gain is much less when compared with wild-type littermates (Plagge et al. 2004). Although not demonstrated directly, these suckling deficits in the targeted mutant mice are presumably owing to impaired activity in the orofacial motor neurons and the muscles they innervate.
(b) Emotional cues and positive affect signals
However, in many animals, mother–offspring interactions are not just about the supply and demand of food. Intragenomic conflict over resource allocation may also include aspects of care (licking and grooming) and emotional cues. A good example of where imprinted genes impact on this kind of function is provided by the neurogenetic disorder Angelman syndrome (AS). AS, which is characterized by abnormalities in neurological, motor and intellectual functioning, is caused by genetic anomalies involving a cluster of imprinted genes on chromosome 15. These causal anomalies include paternal chromosome 15 uniparental disomy, maternal deletion of the critical region (15q11–13) and mutations in the maternally expressed/paternally imprinted gene UBE3A (Clayton-Smith & Laan 2003). A key characteristic of individuals with AS is their unusually sociable disposition and frequent laughter and smiling, with reduced displays of negative affect signals such as crying and tantrums (Summers et al. 1995; Brown & Consedine 2004). These positive affect behaviours, although originally considered to occur inappropriately (Clayton-Smith & Laan 2003), are increasingly thought to occur specifically in a social context (Yamada & Volpe 1990; Oliver et al. 2002). This suggests that one or both of the two known maternally expressed genes in the critical region (UBE3A and ATP10C) normally act as brake-limiting positive affect signals, and that this function is lost in AS when these gene products are absent.
This idea is further supported by evidence from the clinical condition caused by the reciprocal mutation at 15q11–13, Prader–Willi syndrome (PWS). The main PWS characteristics are caused by an absence of paternally expressed gene product from this critical region (Goldstone 2004). However, two genetic sub-types, maternal chromosome 15 uniparental disomy and PWS-imprinting centre mutations, also have an overdosage of the maternally expressed UBE3A and ATP10C gene products owing to the presence of two maternal copies and a relaxation of the silencing of the paternal copy, respectively. In addition to the classic PWS symptoms, these individuals show increased negative affect signals (stubbornness and temper tantrums) and a much increased propensity to develop an affective psychosis (Boer et al. 2002; Vogels et al. 2003). In these cases of PWS, the overdosage of maternal gene products presumably leads to a greater than normal application on the brake that acts to limit positive affect signals.
Taken together, these clinical data have led to the proposal that one or both of these maternally expressed genes influence attachment between offspring and mother, in that they limit the display of positive affect signalling (Isles & Holland 2005). This idea is predicted by the kinship theory (Brown & Consedine 2004), the suggestion being that these positive affect signals manipulate the sensory systems of receivers with respect to the allocation of social ‘resources’. In the same manner as manipulating nutrient resources, it is in the ‘interest’ of the paternal genome to maximize the amount of social resources received from the mother; whereas it is in the ‘interest’ of the maternal genome to equalize the amount of these resources across all maternally related kin.
(c) Maternal behaviour
So far, the focus has been on situations where imprinted genes impact on the ability of the offspring to extract ‘resources’ from the mother in the pre-weaning period. However, two classic studies examining mice with targeted mutations demonstrate that imprinted genes also influence maternal behaviours (Lefebvre et al. 1998; Li et al. 1999). Peg1 and Peg3 are both paternally expressed imprinted genes that were identified in a screen for novel imprinted genes (Kanekoishino et al. 1995; Kuroiwa et al. 1996). Both have enhancing effects on foetal growth and are highly expressed in the adult brain (Lefebvre et al. 1998; Li et al. 1999). Females who carry a targeted (null) copy of Peg1 or Peg3 inherited from their fathers show similar gross deficits in maternal care that are independent of any possible effects in the pups (Lefebvre et al. 1998; Li et al. 1999; Curley et al. 2004). Peg1 mutant females were deficit in normal aspects of maternal behaviour such as placentophagia, retrieval of pups, nest building and crouching (suckling). Needless to say, the consequence of these behavioural deficits is a reduced survivability of the offspring.
Similarly, Peg3 mutant females also displayed deficits in aspects of normal maternal care (retrieval, nest building and crouching). Like the Peg1 mutant females (Lefebvre et al. 1998), this maternal care deficit was not due to olfactory dysfunction, and furthermore the Peg3 mutant females showed normal reaction to newly introduced pups (Li et al. 1999). Additionally, Peg3 mutant females showed reduced milk letdown, despite having histologically normal mammary glands. This suggested a deficit in the neurobiological control of lactation, and investigations demonstrated a reduced number of oxytocin-positive neurons in the hypothalamus of Peg3 mutant females (Li et al. 1999). Given the central role played by oxytocin in both maternal behaviour and milk letdown, the indication is that this is the neurobiological pathway via which Peg3 is exerting an effect (Keverne 2001), an idea that sits nicely with its involvement in the tumour necrosis factor signalling pathway affecting NFκB phosphorylation, apoptosis and cell survival (Relaix et al. 1998, 2000).
At first glance, the effect of imprinted genes on maternal behaviour seems to fit quite nicely with the idea of intragenomic conflict over food resources, care and emotional bonds in the pre-weaning period (Constancia et al. 2004). However, this aspect of Peg1 and Peg3 functions is inconsistent with a kinship/intragenomic conflict explanation, as relatedness asymmetries between parent and offspring do not carry over between generations (Hurst et al. 2000). Nevertheless, the gross physiological effects of mutant Peg1 and Peg3 on foetal growth (lack of paternally expressed product produces smaller offspring) are consistent with intragenomic conflict, leading to the suggestion that this is the main selective force behind the evolution of imprinting at these loci (Wilkins & Haig 2003). Additionally, Peg3 has recently been shown to have a role regulating suckling behaviour in pups in a similar manner to Gnasxl (Curley et al. 2004). This mutant phenotype also fits with intragenomic conflict as it relates to resource acquisition during the pre-weaning period, and may be due to disruption of similar hypothalamic processes that underlie maternal behaviour (Curley et al. 2004). Whether or not the effect of these two imprinted genes on maternal behaviour can be reconciled within the kinship hypothesis, or whether this function of these genes is subservient to their effects on in utero and pre-weaning resource acquisition remains to be seen.
3. Sex-biased dispersal, intragenomic conflict and social behaviour
Asymmetries of relatedness can also be established in animal societies in which individuals of one or other of the sexes move away from the natal group (sex-biased dispersal). Take an animal social group that consists of maternally related females: sisters, maternal half-sisters, maternal cousins, maternal aunties, etc. Male offspring leave the group on becoming sexually mature, and the dominant/reproductive males come from outside of the group, holding tenure for only a few breeding cycles before being replaced by others. When a given female gives birth to an offspring, owing to the asymmetric relatedness of the group, this individual, regardless of gender, will share a large proportion of its maternally derived alleles with the other members of the group (figure 1b). Consequently, owing to this asymmetry of relatedness, the overall fitness benefit to these maternally derived alleles may, for example, be increased if this individual delays its own reproduction for a period in order to help the other members of the group raise their offspring. However, as a breeding male only holds tenure for a certain period, paternally derived alleles are less likely to be shared between individuals within the group. As a result, delaying reproduction to help the other female members of the group raise their offspring will be of no benefit and will in fact incur a cost to paternally derived alleles (unless the benefit can be tailored towards paternal relatives only—see later). Obviously, we can see that this scenario leads to conflict between the paternally and the maternally derived genomes within the offspring, and yet equally it does not necessarily involve growth or food allocation between mother and offspring. What is clear is that such an internal conflict would be manifest at the behavioural level, impacting on how individuals interact socially within the group.
In social/family groups, as in our example, where relatedness asymmetries occur owing to sex-biased dispersal, imprinted genes are predicted to influence those behaviours that relate to how an individual interacts with the other members of a social group to which they belong (Trivers & Burt 1999). Unlike the asymmetry of relatedness situation in utero, where the predicted mode of action of imprinted genes is limited to the supply and demand for nutrient resources (Moore & Haig 1991), the potential functions of imprinted genes in social behaviours are far more expansive and complex. For instance, these could include one or many functions such as kin recognition, alarm calls, risk taking, grooming, communal nursing and aggressive behaviour (Hurst 1997; Trivers & Burt 1999; Isles et al. 2002; Roulin & Hager 2003). There are tentative suggestions from mouse studies that some of these functions are indeed subject to the action of imprinted genes.
(a) Kin recognition
Kin recognition is obviously a key factor in the cohesion of social groups, and in mice, like in many mammals, this is olfactory system based (Brennan 2004). Using reciprocal crosses between inbred strains of mice, Isles et al. (2001) demonstrated that there is a parent-of-origin effect on olfactory-based kin-recognition mechanisms. Specifically, reciprocal F1 mice were more sensitive to, and avoided, female urinary odour from their genetic maternal strain (Isles et al. 2002). As the F1 mice had never been exposed to these maternal odours previously (all the animals were embryo transferred to foster mothers of a separate strain), the most parsimonious explanation for this behavioural parent-of-origin effect was that it was genetic in basis, and is therefore probably due to imprinted genes. Further investigations into the neurobiological basis of this behaviour suggested that this was not due to self-referent phenotype matching (Mateo & Johnston 2000), as the urinary odours produced by the reciprocal F1 mice themselves were indistinguishable (Isles et al. 2001, 2002). Therefore, the action of this imprinted gene effect is most probably exerted via the neural systems controlling olfactory cue perception and/or information processing.
(b) Exploratory behaviour and risk taking
Another behavioural output that comes under the umbrella of social behaviour is risk taking, either in terms of alarm calling, looking for food or defending the group. Although caution needs to be used when discussing a behavioural construct as complex as ‘risk-taking’, a recent study by us suggests that imprinted genes may impact on one aspect of this behaviour, namely exploration of a novel environment (Plagge et al. 2005). Mice carrying a maternally derived targeted allele of the gene Nesp showed a reduced propensity to explore a novel environment. Nesp is expressed only maternally, and is found in discrete locations in the brain including the noradrenergic locus coeruleus (Plagge et al. 2005), a key brain area in the control of reactivity to novel stimuli (Sara et al. 1995; Cole et al. 1988). In mice at least, it appears that the paternal interest may be to limit risk-taking (by silencing the paternal copy of Nesp), while it is in the maternal interest to promote these behaviours.
(c) Imprinted genes and adult cognition
Nevertheless, despite these examples, direct evidence for imprinted gene effects on adult social behaviours is limited. However, the fact that there are many examples of genes that are still subject to genomic imprinting in the adult brain (Davies et al. 2005b) suggests that not only is there a role for these genes in the brain, but that their imprinted status is also important. Consequently, further progress may be made by examining the role of imprinted genes in adult brain functions such as discrete aspects of cognition. This has been reviewed elsewhere (Isles & Wilkinson 2000), but in summary animal studies have so far demonstrated that imprinted genes impact on behavioural flexibility (Davies et al. 2005a) and several different aspects of memory functioning, including emotional (Brambilla et al. 1997), context dependent (Jiang et al. 1998) and spatial (Muscatelli et al. 2000). Studies on human mental dysfunction indicate a similar array of possible roles for imprinted genes in cognitive functioning (reviewed in Davies et al. 2001), including behavioural flexibility (Skuse et al. 1997), spatial memory (Curfs et al. 1991) and mental rotation (Bishop et al. 2000), and what can be broadly described as ‘social cognition’ (Cook et al. 1997; Skuse et al. 1997; Boer et al. 2002). Such ‘cold’ cognitive functions may seem remote from social behaviours such as grooming and alarm calls, but there is an accumulating body of evidence elucidating the neural basis of social interactions that suggests behaviours even as complex as deception, result as a consequence of the concerted action of a number of basic psychological functions (Blakemore & Frith 2004). Consequently, knockout experiments in mice and studies on human mental dysfunction may shed light on the function of imprinted genes in distinct aspects of cognition and psychology that underpin how individuals interact socially.
A good example of this is the case of E6-AP ubiquitin ligase encoded by the gene Ube3a. Imprinting of this gene is maintained in discrete regions of the adult brain (Albrecht et al. 1997; Rougeulle et al. 1997; Vu & Hoffman 1997), and as we have seen previously, this gene is involved in aspects of social functioning in the young (Oliver et al. 2002), so may well have a similar function in the adult. This idea is supported by the fact that UBE3A has been implicated in autism-spectrum disorders (Cook et al. 1997; Samaco et al. 2005). A study of mice carrying a maternally derived null Ube3a allele demonstrated the importance of E6-AP ubiquitin ligase in the degradation of certain effector proteins such as p53 in neurons, long-term potentiation in the hippocampus, and consequently context-dependent learning (Jiang et al. 1998). Clearly, it may be these neural and cognitive functions of E6-AP ubiquitin ligase underlying its role in social behaviour, particularly given the importance of contextual learning in social groups (Kamil 2004). The challenge is to make similar such links for those imprinted genes that are known to have a function in cognitive processes.
(d) The special case of the X-chromosome
Although limited, there is evidence for the existence of a number of X-linked imprinted genes in mice and humans (Skuse et al. 1997; Davies et al. 2005a; Raefski & O'Neill 2005). Interestingly, so far the vast majority of this evidence is brain based: brain-expressed imprinted genes (Davies et al. 2005a; Raefski & O'Neill 2005); parent-of-origin effects on structural changes and cognition in Turner syndrome (TS; Skuse et al. 1997; Bishop et al. 2000; Kesler et al. 2003); and cognitive functioning in mice (Davies et al. 2005a). These X-linked imprinted genes pose interesting questions in terms of the consequences of their expression and, interwoven with this, their evolution. What effect would an X-linked imprinted gene have on the hypothetical matrilineal society described above? An important factor to consider here is that recombination of genetic material between the sex chromosomes during meiosis in males is limited to the small pseudo-autosomal region, which constitutes less than 1% of the genes on the X. As a dominant male sires all the offspring during his tenure, it means that there will be strata of females in the group who are all effectively clonal for their paternally derived X-chromosomes (see figure 1b). In this case, the kinship theory would predict that X-linked paternally derived genes may lead to increased social interaction—restricted, however, to paternal female relatives.
There is evidence from a number of social animals that individuals can recognize paternal sibs (Holmes 1986; Alberts 1999; Wahaj et al. 2004), suggesting that the mechanisms are in place for specific discrimination between maternal and paternal relatives. Furthermore, two separate primate studies have shown that this kin recognition can lead to altered behaviour, in that within groups females do bias their social behaviour towards their female paternal sisters (Widdig et al. 2001; Smith et al. 2003). These studies, one of rhesus macaques (Widdig et al. 2001) and the other of baboons (Smith et al. 2003), both showed that females demonstrated more approach, cohesion and grooming-related behaviours to paternal kin than non-kin. In both cases (although to varying degrees), this was present against a backdrop of strong maternal-kin bias as is found among matrilineal primate groupings such as these. Clearly, imprinted genes expressed from the paternally derived X could be a genetic mechanism that contributes to this bias in social behaviour, possibly via kin-recognition mechanisms alone or by influencing the behavioural output upon encountering paternal kin (i.e. increasing social behaviour). Conversely, genes expressed from the maternally derived X, which are shared much more widely within the group, would be expected to limit such a bias in behaviour towards paternal kin specifically.
(e) Genetic sexual dimorphism
Nevertheless, although it is clear that there are X-linked imprinted genes, some researchers have expressed dissatisfaction with a kinship explanation for their evolution. Instead, they argue that X-linked imprinting has evolved as a genetic mechanism to generate sexual dimorphisms, as male mammals (XY) always inherit their sole X from their mother, and therefore lack any paternally derived X-linked genes (provided there are no Y-linked homologues), and only females (XX) inherit a paternally derived X (Iwasa & Pomiankowski 1999; Iwasa & Pomiankowski 2001). Although gonadal hormones are a strong factor in generating sexually dimorphic traits, there is increasing evidence that genetic factors may also have an independent role (Arnold & Burgoyne 2004). A number of recognized sex differences, particularly in the brain, are observed before the advent of sex differences in gonadal hormones (Sibug et al. 1996; Dewing et al. 2003), and indeed occur independently of gonadal steroids (Carruth et al. 2002). These differences in brain development/function could be explained, for instance, by the action of X-linked imprinted genes. Indeed, this theory for the existence of X-linked imprinted genes has been used to explain the TS data in terms of generating the sexual dimorphism in social cognition (Skuse 1999), and possibly the differential vulnerability of males to mental diseases such as autism (Skuse 2000).
4. Concluding remarks
In this review, we have attempted to give an overview of the role played by imprinted genes in social behaviour. In line with the predictions from the most robust evolutionary hypothesis for genomic imprinting, the kinship theory, we have been able to classify imprinted gene effects on the social brain into mother–offspring bonding and adult interactions in social groups. The main idea behind the kinship theory is that there is an asymmetry of relatedness which leads to intragenomic conflict owing to the differential interests of the paternal and maternal genome. With regard to mother–offspring interactions, this asymmetry is generated by a father's uncertain paternity of all of the mother's offspring; while in social groups, an asymmetry of relatedness can become established when there is sex-biased dispersal from the group leading to matrilineal or patrilineal social groupings.
With regards to the former (mother–offspring interactions), the predictive actions of maternally and paternally imprinted genes are relatively straightforward, being an extension of previously established effects in utero (Reik et al. 2003). However, whereas in utero where the differential interest regards the distribution of nutrient resources across the placenta, in the early postnatal period the definition of resources can be extended to include aspects of maternal care and mother–infant bonding. Classically, it would be in the paternal genome's interest to increase resource acquisition from the mother by influencing suckling and consequently nutrient transfer; but acquisition of other resources such as care and emotional cues via affective signalling may also be affected (Brown & Consedine 2004). Conversely, it is in the maternal genome's interest to reduce these effects, equalizing resource distribution across all the offspring in her lifetime.
The second situation in which asymmetries of relatedness occur, and therefore imprinted genes are expected to evolve, is in social groups with sex-biased dispersal. Sex-biased dispersal of this kind is certainly not a theoretical convenience, and in fact is widespread among vertebrates (Pusey 1987). Indeed, although there are examples of female dispersal, it is mainly males that disperse in mammalian societies (Dobson 1982; Pusey 1987), particularly in primates (Wrangham 1980), mirroring the structure of the hypothetical society described above. This fact has led to the suggestion that the expansion of the forebrain regions in primates is related to increased sociality and that developmentally this brain evolution may have been mediated in part by imprinted genes (Keverne et al. 1996). Therefore, it is possible to make a tentative suggestion that in adults autosomal genes predominantly expressed from the maternally derived allele will generally promote social, cooperative behaviour among all group members, whereas those genes predominantly expressed from the paternally derived allele may suppress or inhibit such behaviour. However, these predictions may not hold up universally, and are very much dependent on the exact relatedness dynamics of the social group in question.
Generally, there is increasing evidence, from both animal and human studies, that imprinted genes impact on social functioning, both in terms of affecting the mother–infant bond, and by influencing adult social behaviour and cognition. Overall, these data appear to support the theoretical standpoint, particularly with regards to mother–infant bonding. However, there are notable exceptions (for instance, the affect of Peg1 and Peg3 on maternal behaviour), and it is important to bear in mind that as there is an inherent flexibility in conflict-based theories to explain data (Hurst 1997), caution is needed when using the theory to explain data post hoc. Nevertheless, regardless of the evolutionary arguments, what is clear is that imprinted genes do have a role in brain functioning, and their effects are often felt via actions on social behaviour, both in early life and in the adult.
Acknowledgments
A.R.I. is supported by the Beebe Trust and the Health Foundation. A.R.I., W.D. and L.S.W. are supported by the Biotechnology and Biological Sciences Research Council (Babraham Institute Synergy award to L.S.W.). L.S.W. is a member of the Medical Research Council (UK) Co-Operative on Imprinting in Health and Disease.
Footnotes
One contribution of 14 to a Theme Issue ‘The neurobiology of social recognition, attraction and bonding’.
References
- Alberts S.C. Paternal kin discrimination in wild baboons. Proc. R. Soc. B. 1999;266:1501–1506. doi: 10.1098/rspb.1999.0807. doi:10.1098/rspb.1999.0807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht U, Sutcliffe J.S, Cattanach B.M, Beechey C.V, Armstrong D, Eichele G, Beaudet A.L. Imprinted expression of the murine Angelman syndrome gene, Ube3a, in hippocampal and Purkinje neurons. Nat. Genet. 1997;17:75–78. doi: 10.1038/ng0997-75. doi:10.1038/ng0997-75 [DOI] [PubMed] [Google Scholar]
- Arnold A.P, Burgoyne P.S. Are XX and XY brain cells intrinsically different? Trends Endocrinol. Metab. 2004;15:6–11. doi: 10.1016/j.tem.2003.11.001. doi:10.1016/j.tem.2003.11.001 [DOI] [PubMed] [Google Scholar]
- Barlow D.P, Stoger R, Herrmann B.G, Saito K, Schweifer N. The mouse insulin-like growth-factor type 2 receptor is imprinted and closely linked to the Tme locus. Nature. 1991;349:84–87. doi: 10.1038/349084a0. doi:10.1038/349084a0 [DOI] [PubMed] [Google Scholar]
- Barton S.C, Surani M.A, Norris M.L. Role of paternal and maternal genomes in mouse development. Nature. 1984;311:374–376. doi: 10.1038/311374a0. doi:10.1038/311374a0 [DOI] [PubMed] [Google Scholar]
- Bishop D, Canning E, Elgar K, Morris E, Jacobs P, Skuse D. Distinctive patterns of memory function in subgroups of females with Turner syndrome: evidence for imprinted loci on the X-chromosome affecting neurodevelopment. Neuropsychologica. 2000;38:712–721. doi: 10.1016/s0028-3932(99)00118-9. doi:10.1016/S0028-3932(99)00118-9 [DOI] [PubMed] [Google Scholar]
- Blakemore S.J, Frith U. How does the brain deal with the social world? Neuroreport. 2004;15:119–128. doi: 10.1097/00001756-200401190-00024. doi:10.1097/00001756-200401190-00024 [DOI] [PubMed] [Google Scholar]
- Boer H, Holland A, Whittington J, Butler J, Webb T, Clarke D. Psychotic illness in people with Prader–Willi syndrome due to chromosome 15 maternal uniparental disomy. Lancet. 2002;359:135–136. doi: 10.1016/S0140-6736(02)07340-3. doi:10.1016/S0140-6736(02)07340-3 [DOI] [PubMed] [Google Scholar]
- Brambilla R, et al. A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature. 1997;390:281–286. doi: 10.1038/36849. doi:10.1038/36849 [DOI] [PubMed] [Google Scholar]
- Brennan P.A. The nose knows who's who: chemosensory individuality and mate recognition in mice. Horm. Behav. 2004;46:231–240. doi: 10.1016/j.yhbeh.2004.01.010. doi:10.1016/j.yhbeh.2004.01.010 [DOI] [PubMed] [Google Scholar]
- Brown W.M, Consedine N.S. Just how happy is the happy puppet? An emotion signaling and kinship theory perspective on the behavioral phenotype of children with Angelman syndrome. Med. Hypotheses. 2004;63:377–385. doi: 10.1016/j.mehy.2004.05.010. doi:10.1016/j.mehy.2004.05.010 [DOI] [PubMed] [Google Scholar]
- Carruth L.L, Reisert I, Arnold A.P. Sex chromosome genes directly affect brain sexual differentiation. Nat. Neurosci. 2002;5:933–934. doi: 10.1038/nn922. doi:10.1038/nn922 [DOI] [PubMed] [Google Scholar]
- Clayton-Smith J, Laan L. Angelman syndrome: a review of the clinical and genetic aspects. J. Med. Genet. 2003;40:87–95. doi: 10.1136/jmg.40.2.87. doi:10.1136/jmg.40.2.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B.J, Robbins T.W, Everitt B.J. Lesions of the dorsal noradrenergic bundle simultaneously enhance and reduce responsivity to novelty in a food preference test. Brain Res. 1988;472:325–349. doi: 10.1016/0006-8993(88)91225-5. doi:10.1016/0006-8993(88)91225-5 [DOI] [PubMed] [Google Scholar]
- Constancia M, Kelsey G, Reik W. Resourceful imprinting. Nature. 2004;432:53–57. doi: 10.1038/432053a. doi:10.1038/432053a [DOI] [PubMed] [Google Scholar]
- Cook E.H, Lindgren V, Leventhal B.L, Courchesne R, Lincoln A, Shulman C, Lord C, Courchesne E. Autism or atypical autism in maternally but not paternally derived proximal 15q duplication. Am. J. Hum. Genet. 1997;60:928–934. [PMC free article] [PubMed] [Google Scholar]
- Curfs L.M, Wiegers A.M, Sommers J.R, Borghgraef M, Fryns J.P. Strengths and weaknesses in the cognitive profile of youngsters with Prader–Willi syndrome. Clin. Genet. 1991;40:430–434. doi: 10.1111/j.1399-0004.1991.tb03114.x. [DOI] [PubMed] [Google Scholar]
- Curley J.P, Barton S, Surani A, Keverne E.B. Coadaptation in mother and infant regulated by a paternally expressed imprinted gene. Proc. R. Soc. B. 2004;271:1303–1309. doi: 10.1098/rspb.2004.2725. doi:10.1098/rspb.2004.2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies W, Isles A.R, Wilkinson L.S. Imprinted genes and mental dysfunction. Ann. Med. 2001;33:428–436. doi: 10.3109/07853890108995956. [DOI] [PubMed] [Google Scholar]
- Davies W, et al. Xlr3b is a new imprinted candidate for X-linked parent-of-origin effects on cognitive function in mice. Nat. Genet. 2005a;37:625–629. doi: 10.1038/ng1577. doi:10.1038/ng1577 [DOI] [PubMed] [Google Scholar]
- Davies W, Isles A.R, Wilkinson L.S. Imprinted gene expression in the brain. Neurosci. Biobehav. Rev. 2005b;29:421–430. doi: 10.1016/j.neubiorev.2004.11.007. doi:10.1016/j.neubiorev.2004.11.007 [DOI] [PubMed] [Google Scholar]
- Dechiara T.M, Efstratiadis A, Robertson E.J. A growth-deficiency phenotype in heterozygous mice carry an insulin-like growth factor-II gene disrupted by targeting. Nature. 1990;345:78–80. doi: 10.1038/345078a0. doi:10.1038/345078a0 [DOI] [PubMed] [Google Scholar]
- Delaval K, Feil R. Epigenetic regulation of mammalian genomic imprinting. Curr. Opin. Genet. Dev. 2004;14:188–195. doi: 10.1016/j.gde.2004.01.005. doi:10.1016/j.gde.2004.01.005 [DOI] [PubMed] [Google Scholar]
- Dewing P, Shi T, Horvath S, Vilain E. Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Brain Res. Mol. Brain Res. 2003;118:82–90. doi: 10.1016/s0169-328x(03)00339-5. doi:10.1016/S0169-328X(03)00339-5 [DOI] [PubMed] [Google Scholar]
- Dobson F.S. Competition for mates and predominant juvenile male dispersal in mammals. Anim. Behav. 1982;30:1183–1192. doi:10.1016/S0003-3472(82)80209-1 [Google Scholar]
- Goldstone A.P. Prader–Willi syndrome: advances in genetics, pathophysiology and treatment. Trends Endocrinol. Metab. 2004;15:12–20. doi: 10.1016/j.tem.2003.11.003. doi:10.1016/j.tem.2003.11.003 [DOI] [PubMed] [Google Scholar]
- Greenwood P.J. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 1980;28:1140–1162. doi:10.1016/S0003-3472(80)80103-5 [Google Scholar]
- Haig D. Parental antagonism, relatedness asymmetries, and genomic imprinting. Proc. R. Soc. B. 1997;264:1657–1662. doi: 10.1098/rspb.1997.0230. doi:10.1098/rspb.1997.0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig D. Genomic imprinting, sex-biased dispersal, and social behavior. Ann NY Acad. Sci. 2000;907:149–163. doi: 10.1111/j.1749-6632.2000.tb06621.x. [DOI] [PubMed] [Google Scholar]
- Haig D, Graham C. Genomic imprinting and the strange case of the insulin-like growth-factor II receptor. Cell. 1991;64:1045–1046. doi: 10.1016/0092-8674(91)90256-x. doi:10.1016/0092-8674(91)90256-X [DOI] [PubMed] [Google Scholar]
- Haig D, Westoby M. Parent-specific gene-expression and the triploid endosperm. Am. Nat. 1989;134:147–155. doi:10.1086/284971 [Google Scholar]
- Haig D, Wharton R. Prader–Willi syndrome and the evolution of human childhood. Am. J. Hum. Biol. 2003;15:320–329. doi: 10.1002/ajhb.10150. doi:10.1002/ajhb.10150 [DOI] [PubMed] [Google Scholar]
- Holm V.A, Cassidy S.B, Butler M.G, Hanchett J.M, Greenswag L.R, Whitman B.Y, Greenberg F. Prader–Willi syndrome: consensus diagnostic criteria. Pediatrics. 1993;91:398–402. [PMC free article] [PubMed] [Google Scholar]
- Holmes W.G. Identification of paternal half-siblings by captive belding ground-squirrels. Anim. Behav. 1986;34:321–327. doi:10.1016/S0003-3472(86)80099-9 [Google Scholar]
- Hurst L. Evolutionary theories of genomic imprinting. In: Surani M, Reik W, editors. Genomic imprinting. vol. 18. Oxford University Press; Oxford, UK: 1997. pp. 211–237. [Google Scholar]
- Hurst L.D, et al. Peg3 and the conflict hypothesis. Science. 2000;287:1167. doi:10.1126/science.287.5456.1167a [Google Scholar]
- Isles A.R, Holland A.J. Imprinted genes and mother–offspring interactions. Early Hum. Dev. 2005;81:73–77. doi: 10.1016/j.earlhumdev.2004.10.006. doi:10.1016/j.earlhumdev.2004.10.006 [DOI] [PubMed] [Google Scholar]
- Isles A.R, Wilkinson L.S. Imprinted genes, cognition and behaviour. Trends Cogn. Sci. 2000;4:309–318. doi: 10.1016/s1364-6613(00)01504-7. doi:10.1016/S1364-6613(00)01504-7 [DOI] [PubMed] [Google Scholar]
- Isles A.R, Baum M.J, Ma D, Keverne E.B, Allen N.D. Urinary odour preferences in mice. Nature. 2001;409:783–784. doi: 10.1038/35057323. doi:10.1038/35057323 [DOI] [PubMed] [Google Scholar]
- Isles A.R, Baum M.J, Ma D, Szeto A, Keverne E.B, Allen N.D. A possible role for imprinted genes in inbreeding avoidance and dispersal from the natal area in mice. Proc. R. Soc. B. 2002;269:665–670. doi: 10.1098/rspb.2001.1911. doi:10.1098/rspb.2001.1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier J.M, et al. Imprinted gene in postnatal growth role. Nature. 1998;393:125–126. doi: 10.1038/30120. doi:10.1038/30120 [DOI] [PubMed] [Google Scholar]
- Iwasa Y, Pomiankowski A. Sex specific X chromosome expression caused by genomic imprinting. J. Theor. Biol. 1999;197:487–495. doi: 10.1006/jtbi.1998.0888. doi:10.1006/jtbi.1998.0888 [DOI] [PubMed] [Google Scholar]
- Iwasa Y, Pomiankowski A. The evolution of X-linked genomic imprinting. Genetics. 2001;158:1801–1809. doi: 10.1093/genetics/158.4.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.H, Armstrong D, Albrecht U, Atkins C.M, Noebels J.L, Eichele G, Sweatt J.D, Beaudet A.L. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;21:799–811. doi: 10.1016/s0896-6273(00)80596-6. doi:10.1016/S0896-6273(00)80596-6 [DOI] [PubMed] [Google Scholar]
- Kamil A.C. Sociality and the evolution of intelligence. Trends Cogn. Sci. 2004;8:195–197. doi: 10.1016/j.tics.2004.03.002. doi:10.1016/j.tics.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Kanekoishino T, et al. Peg1/Mest imprinted gene on chromsome-6 identified by cDNA subtactive-hybridisation. Nat. Genet. 1995;11:52–59. doi: 10.1038/ng0995-52. doi:10.1038/ng0995-52 [DOI] [PubMed] [Google Scholar]
- Kesler S.R, Blasey C.M, Brown W.E, Yankowitz J, Zeng S.M, Bender B.G, Reiss A.L. Effects of X-monosomy and X-linked imprinting on superior temporal gyrus morphology in Turner syndrome. Biol. Psychiatry. 2003;54:636–646. doi: 10.1016/s0006-3223(03)00289-0. doi:10.1016/S0006-3223(03)00289-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keverne E.B. Genomic imprinting, maternal care, and brain evolution. Horm. Behav. 2001;40:146–155. doi: 10.1006/hbeh.2001.1685. doi:10.1006/hbeh.2001.1685 [DOI] [PubMed] [Google Scholar]
- Keverne E.B, Martel F.L, Nevison C.M. Primate brain evolution: genetic and functional considerations. Proc. R. Soc. B. 1996;263:689–696. doi: 10.1098/rspb.1996.0103. [DOI] [PubMed] [Google Scholar]
- Kuroiwa Y, et al. Peg3 imprinted gene on proximal chromosome 7 encodes for a zinc finger protein. Nat. Genet. 1996;12:186–190. doi: 10.1038/ng0296-186. doi:10.1038/ng0296-186 [DOI] [PubMed] [Google Scholar]
- Lefebvre L, Viville S, Barton S.C, Ishino F, Keverne E.B, Surani M.A. Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nat. Genet. 1998;20:163–169. doi: 10.1038/2464. doi:10.1038/2464 [DOI] [PubMed] [Google Scholar]
- Leighton P.A, Ingram R.S, Eggenschwiler J, Efstratiadis A, Tilghman S.M. Disruption of imprinting caused by deletion of the H19 gene region in mice. Nature. 1995;375:34–39. doi: 10.1038/375034a0. doi:10.1038/375034a0 [DOI] [PubMed] [Google Scholar]
- Li L.L, Keverne E.B, Aparicio S.A, Ishino F, Barton S.C, Surani M.A. Regulation of maternal behavior and offspring growth by paternally expressed Peg3. Science. 1999;284:330–333. doi: 10.1126/science.284.5412.330. doi:10.1126/science.284.5412.330 [DOI] [PubMed] [Google Scholar]
- Lund J.P, Kolta A, Westberg K.G, Scott G. Brainstem mechanisms underlying feeding behaviors. Curr. Opin. Neurobiol. 1998;8:718–724. doi: 10.1016/s0959-4388(98)80113-x. doi:10.1016/S0959-4388(98)80113-X [DOI] [PubMed] [Google Scholar]
- Mateo J.M, Johnston R.E. Kin recognition and the ‘armpit effect’: evidence of self-referent phenotype matching. Proc. R. Soc. B. 2000;267:695–700. doi: 10.1098/rspb.2000.1058. doi:10.1098/rspb.2000.1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Solter D. Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell. 1984;37:179–183. doi: 10.1016/0092-8674(84)90313-1. doi:10.1016/0092-8674(84)90313-1 [DOI] [PubMed] [Google Scholar]
- Moore T, Haig D. Genomic imprinting in mammalian development—a parental tug-of-war. Trends Genet. 1991;7:45–49. doi: 10.1016/0168-9525(91)90230-N. [DOI] [PubMed] [Google Scholar]
- Muscatelli F, Abrous D.N, Massacrier A, Boccaccio I, Le Moal M, Cau P, Cremer H. Disruption of the mouse Necdin gene results in hypothalamic and behavioral alterations reminiscent of the human Prader–Willi syndrome. Hum. Mol. Genet. 2000;9:3101–3110. doi: 10.1093/hmg/9.20.3101. doi:10.1093/hmg/9.20.3101 [DOI] [PubMed] [Google Scholar]
- Oliver C, Demetriades L, Hall S. Effects of environmental events on smiling and laughing behavior in Angelman syndrome. Am. J. Ment. Retard. 2002;107:194–200. doi: 10.1352/0895-8017(2002)107<0194:EOEEOS>2.0.CO;2. doi:10.1352/0895-8017(2002)107<0194:EOEEOS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Orr H.A. Somatic mutation favors the evolution of diploidy. Genetics. 1995;139:1441–1447. doi: 10.1093/genetics/139.3.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagge A, Gordon E, Dean W, Boiani R, Cinti S, Peters J, Kelsey G. The imprinted signaling protein XLalphas is required for postnatal adaptation to feeding. Nat. Genet. 2004;36:818–826. doi: 10.1038/ng1397. doi:10.1038/ng1397 [DOI] [PubMed] [Google Scholar]
- Plagge A, Isles A.R, Gordon E, Humby T, Dean W, Gritsch S, Fischer-Colbrie R, Wilkinson L.S, Kelsey G. Imprinted nesp55 influences behavioral reactivity to novel environments. Mol. Cell. Biol. 2005;25:3019–3026. doi: 10.1128/MCB.25.8.3019-3026.2005. doi:10.1128/MCB.25.8.3019-3026.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusey A.E. Sex-biased dispersal and inbreeding avoidance in birds and mammals. Trends Ecol. Evol. 1987;2:295–299. doi: 10.1016/0169-5347(87)90081-4. doi:10.1016/0169-5347(87)90081-4 [DOI] [PubMed] [Google Scholar]
- Raefski A.S, O'Neill M.J. Identification of a cluster of X-linked imprinted genes in mice. Nat. Genet. 2005;37:620–624. doi: 10.1038/ng1567. doi:10.1038/ng1567 [DOI] [PubMed] [Google Scholar]
- Reik W, Constancia M, Fowden A, Anderson N, Dean W, Ferguson-Smith A, Tycko B, Sibley C. Regulation of supply and demand for maternal nutrients in mammals by imprinted genes. J. Physiol. 2003;547:35–44. doi: 10.1113/jphysiol.2002.033274. doi:10.1113/jphysiol.2002.033274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Wei X.J, Wu X.W, Sassoon D.A. Peg3/Pw1 is an imprinted gene involved in the TNF-NF kappa B signal transduction pathway. Nat. Genet. 1998;18:287–291. doi: 10.1038/ng0398-287. doi:10.1038/ng0398-287 [DOI] [PubMed] [Google Scholar]
- Relaix F, Wei X, Li W, Pan J, Lin Y, Bowtell D.D, Sassoon D.A, Wu X. Pw1/Peg3 is a potential cell death mediator and cooperates with Siah1a in p53-mediated apoptosis. Proc. Natl Acad. Sci. USA. 2000;97:2105–2110. doi: 10.1073/pnas.040378897. doi:10.1073/pnas.040378897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougeulle C, Glatt H, Lalande M. The Angelman syndrome candidate gene, UBE3A/E6-AP, is imprinted in brain. Nat. Genet. 1997;17:14–15. doi: 10.1038/ng0997-14. doi:10.1038/ng0997-14 [DOI] [PubMed] [Google Scholar]
- Roulin A, Hager R. Indiscriminate nursing in communal breeders: a role for genomic imprinting. Ecol. Lett. 2003;6:165–166. doi:10.1046/j.1461-0248.2003.00421.x [Google Scholar]
- Samaco R.C, Hogart A, Lasalle J.M. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum. Mol. Genet. 2005;14:483–492. doi: 10.1093/hmg/ddi045. doi:10.1093/hmg/ddi045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara S.J, Dyon-Laurent C, Herve A. Novelty seeking behavior in the rat is dependent upon the integrity of the noradrenergic system. Brain Res. Cogn. Brain Res. 1995;2:181–187. doi: 10.1016/0926-6410(95)90007-1. doi:10.1016/0926-6410(95)90007-1 [DOI] [PubMed] [Google Scholar]
- Sawczuk A, Mosier K.M. Neural control of tongue movement with respect to respiration and swallowing. Crit. Rev. Oral Biol. Med. 2001;12:18–37. doi: 10.1177/10454411010120010101. [DOI] [PubMed] [Google Scholar]
- Sibug R, Kuppers E, Beyer C, Maxson S.C, Pilgrim C, Reisert I. Genotype-dependent sex differentiation of dopaminergic neurons in primary cultures of embryonic mouse brain. Brain Res. Dev. Brain Res. 1996;93:136–142. doi: 10.1016/0165-3806(96)00024-7. doi:10.1016/0165-3806(96)00024-7 [DOI] [PubMed] [Google Scholar]
- Skuse D.H. Genomic imprinting of the X chromosome: a novel mechanism for the evolution of sexual dimorphism. J. Lab. Clin. Med. 1999;133:23–32. doi: 10.1053/lc.1999.v133.a94575. doi:10.1053/lc.1999.v133.a94575 [DOI] [PubMed] [Google Scholar]
- Skuse D. Imprinting, the X-chromsome, and the male brain: explaining sex differences in the liability to autism. Pediatr. Res. 2000;47:9–16. doi: 10.1203/00006450-200001000-00006. [DOI] [PubMed] [Google Scholar]
- Skuse D.H, et al. Evidence from Turner's syndrome of an imprinted X-linked locus affecting cognitive function. Nature. 1997;387:705–708. doi: 10.1038/42706. doi:10.1038/42706 [DOI] [PubMed] [Google Scholar]
- Smith K, Alberts S.C, Altmann J. Wild female baboons bias their social behaviour towards paternal half-sisters. Proc. R. Soc. B. 2003;270:503–510. doi: 10.1098/rspb.2002.2277. doi:10.1098/rspb.2002.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J.A, Allison D.B, Lynch P.S, Sandler L. Behaviour problems in Angelman syndrome. J. Intellect. Disabil. Res. 1995;39(Pt 2):97–106. doi: 10.1111/j.1365-2788.1995.tb00477.x. [DOI] [PubMed] [Google Scholar]
- Surani M.A, Barton S.C, Norris M.L. Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature. 1984;308:548–550. doi: 10.1038/308548a0. doi:10.1038/308548a0 [DOI] [PubMed] [Google Scholar]
- Trivers R.L. Parent–offspring conflict. Am. Zool. 1974;14:249–264. [Google Scholar]
- Trivers R, Burt A. Kinship and genomic imprinting. In: Ohlsson R, editor. Genomic imprinting. Springer; Berlin, Heidelberg, Germany; New York, NY: 1999. pp. 1–21. [Google Scholar]
- Vogels A, Matthijs G, Legius E, Devriendt K, Fryns J.P. Chromosome 15 maternal uniparental disomy and psychosis in Prader–Willi syndrome. J. Med. Genet. 2003;40:72–73. doi: 10.1136/jmg.40.1.72. doi:10.1136/jmg.40.1.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T.H, Hoffman A.R. Imprinting of the Angelman syndrome gene, UBE3A, is restricted to brain. Nat. Genet. 1997;17:12–13. doi: 10.1038/ng0997-12. doi:10.1038/ng0997-12 [DOI] [PubMed] [Google Scholar]
- Wahaj S.A, Van Horn R.C, Van Horn T.L, Dreyer R, Hilgris R, Schwarz J, Holekamp K.E. Kin discrimination in the spotted hyena (Crocuta crocuta): nepotism among siblings. Behav. Ecol. Sociobiol. 2004;56:237–247. doi:10.1007/s00265-004-0783-8 [Google Scholar]
- Widdig A, Nurnberg P, Krawczak M, Streich W.J, Bercovitch F.B. Paternal relatedness and age proximity regulate social relationships among adult female rhesus macaques. Proc. Natl Acad. Sci. USA. 2001;98:13 769–13 773. doi: 10.1073/pnas.241210198. doi:10.1073/pnas.241210198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins J.F, Haig D. What good is genomic imprinting: the function of parent-specific gene expression. Nat. Rev. Genet. 2003;4:359–368. doi: 10.1038/nrg1062. doi:10.1038/nrg1062 [DOI] [PubMed] [Google Scholar]
- Wrangham R.W. An ecological model of female-bonded primate groups. Behaviour. 1980;75:262–300. [Google Scholar]
- Yamada K.A, Volpe J.J. Angelman's syndrome in infancy. Dev. Med. Child Neurol. 1990;32:1005–1011. doi: 10.1111/j.1469-8749.1990.tb08124.x. [DOI] [PubMed] [Google Scholar]