Abstract
The neural basis of social cognition has been the subject of intensive research in both human and non-human primates. Exciting, provocative and yet consistent findings are emerging. A major focus of interest is the role of efferent and afferent connectivity between the amygdala and the neocortical brain regions, now believed to be critical for the processing of social and emotional perceptions. One possible component is a subcortical neural pathway, which permits rapid and preconscious processing of potentially threatening stimuli, and it leads from the retina to the superior colliculus, to the pulvinar nucleus of the thalamus and then to the amygdala. This pathway is activated by direct eye contact, one of many classes of potential threat, and may be particularly responsive to the ‘whites of the eyes’. In humans, autonomic arousal evoked by this stimulus is associated with the activity in specific cortical regions concerned with processing visual information from faces. The integrated functioning of these pathways is modulated by one or more X-linked genes, yet to be identified. The emotional responsiveness of the amygdala, and its associated circuits, to social threat is also influenced by functional polymorphisms in the promoter of the serotonin transporter gene. We still do not have a clear account of how specific allelic variation, in candidate genes, increases susceptibility to developmental disorders, such as autism, or psychiatric conditions, such as anxiety or depressive illness. However, the regulation of emotional responsiveness to social cues lies at the heart of the problem, and recent research indicates that we may be nearing a deeper and more comprehensive understanding.
Keywords: social cognition, amygdala, X-chromosome, emotion, candidate genes, serotonin transporter
1. Introduction
Social interactions with other people are distinctively coloured by emotions, in ways that are both obvious and subtle. Neuroscientists are making rapid progress in understanding the interface between the neural processing of emotions, feelings and social cognition—that set of rules and responses which makes for a well-adjusted individual. Research on emotion processing, by both cortical and subcortical mechanisms, is illuminating our understanding of how social competence develops and how it is maintained. Emotions represent ‘complex psychological and physiological states that, to a greater or lesser degree, index occurrences of value’ (Dolan 2002). Psychological and physiological states influence our behaviour by making some activities more desirable, and hence more likely to be rewarding. On the other hand, they may also render other activities less desirable and unlikely to be associated with reward, or alternatively associated with an adverse and unpleasant outcome. The range of emotions an organism experiences will reflect the complexity of its adaptive niche. Higher-order primates, in particular humans, live in a complex social world. For that reason, in them, emotional regulation is closely linked to social behaviour, and any perturbation in the ability to regulate our emotions will have adverse consequences for our social adaptation.
Unlike most psychological states, emotions are embodied and manifested in uniquely recognizable, and stereotyped, behavioural patterns of facial expression, comportment and autonomic arousal (Dolan 2002). Most of us can ‘read’ other people's emotional states without effort. Our ability to respond appropriately to such states has a huge significance on our ability to successfully rear our young and to find a mate with whom to reproduce ourselves. When the ability to read another's emotional states is significantly impaired, we appear at the very least socially gauche, with difficulty responding appropriately in any social situation, a characteristic feature of autistic conditions (Schultz 2005).
From studies of fear and anxiety, there is considerable evidence that the amygdala is a central component in the processing of threatening stimuli in human and non-human primates, as well as other animals (Adolphs & Tranel 2000; LeDoux 2000). Stimuli with different objective levels of threat lead to variable activation of the amygdala. During interpersonal interactions, such threat may be posed by facial expression, by tone of voice or by body posture. Fearful facial expressions are particularly potent signals of danger (Thomas et al. 2001). Presentation of negative facial expressions in full consciousness activates, in particular, the amygdala and produces a neurophysiological arousal response (Phan et al. 2002; Wager et al. 2003).
Selective responsiveness by the amygdala to negative facial expressions encompasses not only fearful, but also angry faces. Adams et al. (2003) followed up the idea that the amygdala's response may not only be important for detecting threat, but also interpreting whether the threat is direct or indirect. On the other hand, meeting the direct gaze of someone looking angry is also potentially seriously threatening. Angry faces with direct gaze and fearful faces with averted gaze are recognized more quickly and accurately than either of the alternatives. Adams et al. (2003) hypothesized that the more ambiguous the stimulus, the greater the amygdala response would be. In other words, a longer period of ambiguity implies greater activation of the neural circuits recruited by the facial expression and gaze combination. They confirmed that angry faces with indirect gaze and fearful faces with direct gaze did indeed elicit greater activation in the left (but not the right) amygdala. Their observation is consistent with the evidence that the left amygdala is more consistently activated by facial stimuli that require cognitive processing (e.g. Singer et al. 2004; Das et al. 2005). This is an important dissociation, which will be discussed in greater detail. The left amygdala is involved not only in discerning facially communicated threat, but is also activated by the cortical and subcortical circuits that process that threat, especially when gaze perception is involved. On the other hand, the right amygdala arousal may be linked to the intensity of the affective response (measured by autonomic arousal; Anderson & Sobel 2003); the amygdala participates in autonomic activity, such as skin conductance responses (SCRs; Critchley et al. 2002; Sah et al. 2003). Efferent projections from the central nucleus include pathways to brainstem regions controlling motor and visceromotor responses, and hypothalamic areas that control hormonal release (Davis 1997). Co-activation of amygdala and arousal systems is thought to enable the cortex to distinguish fear signals from other arousal responses to novel stimuli (Damasio 2000).
2. Face processing and social adjustment
Critically important information can be gained about how to respond appropriately in social encounters by monitoring the expression on another's face. This provides information about the other person's emotional state and disposition. In certain circumstances, someone else's emotional expressions can evoke that same emotion in oneself—disgust, happiness and sadness are obvious examples. Haxby et al. (2000) proposed that there are dedicated systems for processing emotion expressions in other's faces, in which the amygdala and the insula play a crucial role.
The interpretation of the emotional content of a face takes into account a wide range of visual cues. These include, first, whether we know the individual or not (face recognition memory), the facial configuration (e.g. whether the mouth is wide open or shut, whether the eyes are wide open or narrowed) and, in particular, eye gaze (is this person looking at me or at something/someone else?). There are developmental trends in the ability to recognize emotions accurately, with some emotions being more readily recognized in early childhood, whereas others are not associated with adult levels of recognition until after puberty (Wade et al. 2005). There are also developmental trends in face recognition memory, and in the capacity to interpret direction of gaze as a social cue (is this person looking at me? Campbell et al. 2005, 2006).
3. Face processing and the amygdala
Studies from humans with congenital or acquired damage to the amygdala (Calder et al. 2001), and from primates in which lesions have been induced (Amaral 2002), show that this subcortical structure influences our ability to gain and to maintain socially appropriate behaviour by affecting face recognition memory, facial expression interpretation and eye-gaze monitoring. Whether its functional integrity is critical for normal social cognitive development in humans is still an open question (Amaral et al. 2003). However, there is a growing evidence to indicate that functional deficits in the integrated system that links amygdala with other neural centres, in what has been termed the ‘social brain’ (Adolphs et al. 2000), may be associated with autistic symptomatology.
Schultz (2005) has argued that an abnormality early in the development in the amygdala can give rise to later social perceptual deficits in face identity and facial expression perception. Because the visual cortices that are involved in face perception are also involved in representing semantic knowledge about people, aberrations in face perception may not only affect social perception, but also create deficits in the social knowledge system, with impaired social skills. Modulation of amygdala activity by reciprocal connections from anterior cingulate/medial prefrontal regions may affect our evaluation of fear-related signals (Davis & Whalen 2001). Das et al. (2005) argue that there is a dynamic interplay between the anterior cingulate cortex (ACC) and thalamo-amygdala pathways, and that a breakdown in their functional differentiation could lead to neuropsychiatric disorders, including paranoid schizophrenia and post-traumatic stress syndrome.
4. Eye contact, amygdala arousal and fear perception
Fearful faces are often considered to be processed automatically and independent of attention (Vuilleumier et al. 2001, 2002; Williams et al. 2005b) and awareness (Anderson & Sobel 2003; Pasley et al. 2004). Note, contrary evidence has been reported (e.g. Pessoa et al. 2002). Functional MRI studies that record the amygdala blood oxygen level dependent (BOLD) response to the presentation of facial expressions find a greater activation when we perceive fear compared with other emotional faces (Morris et al. 1998a). If the amygdala is bilaterally ablated, or degenerates, the perception of fear is selectively impaired (Adolphs et al. 1999). We do not fully understand why this is so, but recent evidence suggests that the amygdala responds specifically to eye contact in adults, and that it is maximally activated by exaggerated wide-open eyes, such as are associated with a fearful expression (Morris et al. 2002). This response occurs because direct gaze can be threatening (Nahm 1997). A simple stare is often the most effective stimulus in evoking a fight or flight response in non-human primates (Emery 2000), and in humans too in certain social situations—especially between males.
Which cortical circuits modulate, and are modulated by, amygdala activity that is evoked by eye contact? The amygdala is an essential and central component of a threat-detection system. It has extensive neocortical and subcortical connections that are crucial for the automatic non-conscious responses to a threatening stimulus (e.g. fight and flight). Appropriate social responses require complex cortical processing of potential threatening stimuli (Davidson et al. 2000; Hariri et al. 2000, 2003; Phan et al. 2002). How we respond to direct eye contact is critically dependent upon the social context in which it occurs. We must evaluate the stimulus, by means of complex neocortical connections.
In humans, a crucial component of the modulating circuitry is the recruitment of language centres, and the conscious processing of a ‘feeling’ (or visceral) response, which is important especially in social interactions with strangers (Kim et al. 2004). As adults, we find it harder to maintain eye contact with a stranger than with a familiar individual. There is an additional level of complexity, depending on the relative sex of the people concerned. While there are limits to the modulating ability of these higher cortical circuits, humans are in general able to tolerate direct eye contact for longer than other primates (Kleinke 1986). Eye contact evokes amygdala activation, in tandem with conscious (explicit) and non-conscious (implicit) neural mechanisms. Processing of direct eye contact necessitates the appropriate functioning of these pathways, which is essential for the development and maintenance of social cognitive skills.
5. Subcortical processing of threatening stimuli
Implicit processing of visual stimuli that could constitute a threat, including fearful expressions and other fear-evoking stimuli, is hypothesized to engage subcortical visual pathways that are routed directly to the amygdala, without passing through the visual cortex first (Morris 1998a,b, 2001). Consequently, threatening visual percepts can evoke a very rapid physiological response—before the neocortex has had time to consider the information and decide on an appropriate course of action (Morris et al. 2001). Normally, the main visual perception pathway to the amygdala brings information that has been processed by early visual cortical areas (e.g. V1, V4; Stefanacci & Amaral 2002), and it provides a hierarchically processed and detailed representation of objects. However, responses elicited by the activation of this pathway are at least 100–200 ms after stimulus presentation. If the object is threatening, it has been argued that this response time is too lengthy for an appropriate adaptive reaction. Some amygdala neurons in non-human primates have response latencies to particular classes of visual threat (e.g. snakes) that are as short as 60 ms. Because these latencies are too rapid to be compatible with prior cortical processing, there must be an alternative visual route to the amygdala. This could convey crude, but critical, visual material to the ‘threat-detection system’ and evoke very rapid reactions (Nakamura et al. 2000).
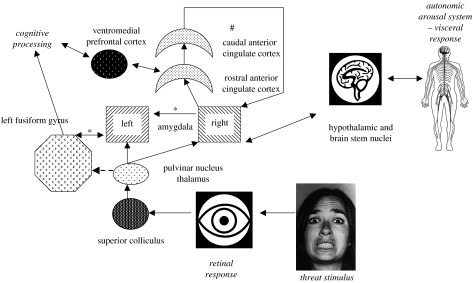
One proposed course of the subcortical visual pathway leads from the retina to the superior colliculus (a phylogenetically ancient visual processing module), to the pulvinar nuclei of the thalamus and then to the amygdala (Morris et al. 1999, 2001; figure 1). Evidence that this pathway does exist comes from a variety of sources. In non-human primates, there is anatomical evidence (Stepniewska et al. 2000). In humans, supporting evidence has come from the study of patients with ‘blindsight’, meaning that a lesion in primary visual (striate) cortex (V1), or in the connections between the lateral geniculate nucleus and V1, has prevented the conscious perception of visual material. Humans with blindsight claim that they can see nothing at all in their blind visual field or hemi-field, yet certain classes of stimulus do evoke neural responses. Blindsight implies the existence of alternative pathways, including the superior colliculus pathway that runs via the pulvinar (in thalamus) to V5 (the visual motion area at the temporal–parietal–occipital junction) with feedback to V4 (lingual and fusiform gyri of the inferior temporal cortex, a region activated by facial cues).
Figure 1.
A representation of the proposed neural systems outlined in the article, which respond to potentially threatening stimuli such as a fearful face. The ventral visual pathway is not shown. The subcortical pathway (e.g. Pasley et al. 2004) is shown, linking superior colliculus with amygdala bilaterally. Amygdala activation on left and right is linked with cognitive processing networks and autonomic responses, respectively. Feedback from the somatic response to threat enhances cognitive processing, possibly mediated by intra-amygdala connectivity (Adolphs et al. 2005). X-linked genes influence the functional integrity of pathways marked by asterisk symbols (Skuse et al. 2005). Pathways influenced by allelic variation in the serotonin transporter gene promoter are indicated by the hash symbol (Heinz et al. 2005; Pezawas et al. 2005). There is a positive functional coupling between the amygdala, the rostral anterior cingulate cortex (ACC) and the ventromedial prefontal cortex, and inhibitory feedback from the caudal ACC. The pathway marked in dashed line is a putative link, which could confound studies of non-conscious response to threat that focus on amygdala-mediated neural activity.
Because blindsight patients may have amygdala activation to emotionally salient stimuli (e.g. fearful faces) that are presented to their ‘blind’ visual cortices, Morris et al. (2001) concluded that some processing must be going on subcortically by the alternative visual pathway. On the other hand, for a variety of reasons (summarized by Pasley et al. 2004), this inference was not indisputable, owing to the evidence that there are links between the superior colliculus and inferior temporal regions of the cortex. Pasley et al. (2004) decided to measure the ability of the amygdala to discriminate complex emotionally salient objects in isolation from the inferior temporal neural representation of those objects. They aimed to show that highly processed information from the inferior temporal region is not required to support visual discrimination of certain emotionally salient cues in the amygdala. Previous studies aimed at isolating subcortical visual processing have not managed to avoid getting some target-related neural activity in the inferior temporal region (Whalen et al. 1998; Morris et al. 2001).
Pasley et al. (2004) used the ‘binocular rivalry’ technique in which each eye is presented with a different, incompatible image. The observer does not fuse such images, but suppresses one and experiences alternating perceptual dominance—the suppressed image is not consciously perceived. It appears from both monkey neurophysiology (Sheinberg & Logothetis 1997) and human MRI studies (Cohen & Tong 2001) that suppressed visual information does not reach inferior temporal cortex. They presented subjects with images that were either fearful faces (and hence potentially of interest to the amygdala threat-detection system) or chairs (of no interest to the amygdala, being emotionally neutral), in conditions of complete perceptual suppression. If the subcortical circuit did detect threats, beyond conscious awareness, there should be differential neural activity in the suppressed image conditions, with increased neural activity—especially in the amygdala—to fearful faces rather than to chairs.
This prediction was supported, with increased activity in the left amygdala to suppressed fearful faces, but no increased signal for images of chairs. There was no increase in inferior temporal lobe activation in response to suppressed faces or chairs, suggesting that this cortical site could not be influencing differential amygdala responsiveness. Pasley et al. (2004) confirmed earlier reports of significant correlations between the subcortical visual structures, which represent way stations in the pathway, including the left amygdala, the left superior colliculus and the left dorsal–posterior–lateral region of the thalamus. This set of activations is consistent with the ‘low-road’ to amygdala activation hypothesis of LeDoux (2000). Further evidence in support of the ‘subcortical threat-detection system’ is provided by Hamm et al. (2003), who discuss a patient with total cortical blindness. He could be conditioned to a visually presented stimulus (a line drawing of an aeroplane) in a fear-conditioning paradigm, in which the unconditioned stimulus was an aversive electric shock. The key finding was that startle responses (eyeblink) were enhanced when elicited in the presence of the conditioned visual stimulus. The presented stimulus was not biologically meaningful and the authors (Hamm et al. 2003) suggest that using more biologically relevant (threat) cues, like pictures of snakes or fearful faces, might increase the stimulus specificity of the subcortical threat detection system, although no such study has yet been published.
The subcortical pathway linking superior colliculus, putamen and amygdala can only respond to low-resolution displays (Vuilleumier et al. 2003). High spatial frequencies are necessary to identify facial features and some facial emotions. On the other hand, low spatial frequencies are sufficient to get the gist (gestalt) of a facial perception (Schyns & Oliva 1999). The superior colliculus is thought to have a phylogenetically ancient ability to process simple forms within a narrow range of low spatial frequencies (Emery 2000; Lomber 2002). Nevertheless, if the amygdala can be activated by this visual circuit (as in the Pasley et al. (2004) experiment), what information about the ‘threatening’ face is passed to the amygdala from the superior colliculus? Morris et al. (2002) proposed that the key percept was ‘fearful eyes’ (figure 1). Using an ingenious fMRI-based investigation, they found that wide-open eyes alone are sufficient to evoke increased neural responses in this non-conscious circuit (Morris et al. 1999). The existence of a subcortical circuit that supported a primitive representation of high-contrast elements, relating to the location of the eyes and the mouth, had been predicted by Morton & Johnson (1991).
Therefore, there is accumulating evidence that the amygdala is not only generally responsive to certain facial expressions, but that the eye region of the face is a critical source of information about the potential threat posed by another person (Morris et al. 2002).
In bilateral lesions of the amygdala, failure to address the information contributed by the eyes in a test of facial expression recognition renders it very difficult for a subject to identify accurately the associated facial expression of fear (Adolphs et al. 2005). It is worth remarking that the facial expression of surprise is also marked by wide-open eyes, and this is the very expression that is most commonly confused with fear in experiments in which subjects are presented with static facial expressions. The key difference between the two is in the mouth; this implies that making that emotion differentiation could require rather longer cortical processing time than, say, differentiating sadness from happiness. There are data to show that the amygdala is particularly responsive to the ‘wide-eyed’ expressions of both fear and surprise, and it seems a reasonable hypothesis that what the amygdala is responding to is the relative proportions of sclera to iris in the observed face. Whalen et al. (2004) tested this hypothesis by measuring whether the larger the size of ‘fearful’ sclera, the greater the response of the amygdala, irrespective of any other visual information. They modified standardized fearful and happy face stimuli by removing all the information from the face, with the exception of the whites of the eyes. Images were presented in a backward masking paradigm, in which the larger ‘wide’ eyes such as are found in association with fear and surprise alternated with the smaller eye whites that are associated with happy expressions. The signal intensity within the ventral amygdala was greater to fearful than to happy eye whites, even though all subjects reported being unaware of the presence of the masked stimuli. The authors conclude that responsiveness of the amygdala appears to be driven by the size of the white scleral field (not by the outline of the eyes), which is consistent with the hypothesis discussed previously that the amygdala is more responsive to low spatial frequency stimuli (Vuilleumier et al. 2003).
6. Amygdala–cortical functional connectivity and paranoia
Threatening or traumatic stimuli are probably processed in parallel by two distinct neural systems (Bechara et al. 1995; LeDoux 2000). The subjective experience of fear relies upon amygdala–medial frontal activity (as well as autonomic arousal). Setting threats into context depends upon hippocampal–lateral frontal activity. In order to confirm this differentiation, Williams et al. (2001) studied healthy individuals by means of functional magnetic resonance imaging (fMRI) and simultaneous SCR measures of phasic arousal (disentangling overlapping SCRs in a short interstimulus interval paradigm), while they viewed fearful and neutral faces. The fMRI activity was subaveraged according to whether or not there was an arousal SCR to each discrete face stimulus (fearful faces would evoke arousal). The fMRI responses to fearful faces associated with arousal were differentiated from those without associated arousal. This contrast differentiated left amygdala and hippocampal networks. Amygdala–medial frontal activity was observed only in association with stimuli that evoked SCRs, whereas right hippocampus–lateral frontal activity occurred only in the absence of SCRs. The authors concluded that amygdala and hippocampal networks differentiated visceral experience from declarative fact processing of fear-inducing stimuli.
Both tonic and phasic autonomic arousal abnormalities have been observed in association with schizophrenia (Kring & Neale 1996; Salem et al. 1996). The salience of fearful faces to the symptoms experienced by paranoid schizophrenics has also been a subject of some interesting investigations (Phillips et al. 1999; Williams et al. 2004a–c). Normally, amygdala and correlated medial prefrontal activity is associated with the subjective appraisal of threat and autonomic arousal, as measured by SCR. In contrast, the system for setting emotionally significant events in context is normally linked to hippocampal–lateral prefrontal activity, not associated consistently with SCR. Williams et al. (2004a) studied paranoid schizophrenic patients. Using fearful faces as the arousing stimuli, they found emotion recognition accuracy was impaired, coupled with significantly greater SCR than comparisons to both fearful and neutral facial expressions. Patients lacked integrated activity in amygdala and prefrontal circuits. The authors speculate that a lack of feedback from the medial prefrontal region, which does not respond to threat in the usual way in paranoid schizophrenia, leads to a perseveration and exacerbation of arousal (SCR) responses. Cognitive theories of persecutory delusions in schizophrenia include increased attention to threat and impaired decision-making in the interpretation of potential threats. Phillips et al. (2000) studied visual scan paths in schizophrenic patients with persecutory delusions, using black-and-white photographs of social scenes rated as depicting either neutral, ambiguous or overtly threatening activity. As anticipated, the schizophrenic patients did perceive potential threats in inappropriate places. However, to date there has not been a specific study of perceived threat in paranoid individuals as manifested by their response to direct social gaze.
7. Amygdala and gaze monitoring
The ability to follow and respond to the direction of gaze of a conspecific is a crucial skill in humans, shared with some, but not all, primate species (Perrett et al. 1985; Jellema et al. 2000). Our ability to meet and to follow another's gaze is present during early infancy and is associated with a growing appreciation of salient events in a socially structured world (Allison et al. 2000). The perception of direct gaze from a face that is neither threatening nor fearful also elicits an amygdala response in humans (Kawashima et al. 1999). In monkeys too, there are cells in the amygdala that respond selectively to eye gaze (Sato & Nakamura 2001). Neural interactions between the amygdala and the neocortical regions that are engaged by visual stimuli of faces are enhanced if those faces have direct gaze orientations (George et al. 2001). Kobayashi & Kohshima (2001) pointed out that human eyes have a widely exposed white sclera surrounding the darker coloured iris, making it easy to discern the direction in which they are looking. They compared the external morphology of primate eyes in nearly half of all primate species, and showed that this feature is uniquely human. Humans have the largest ratio of exposed sclera in the eye outline, which itself is elongated horizontally. They suggested that these are adaptations to extend the visual field by allowing greater eye movement, especially in the horizontal direction, and to enhance the ease of detecting the gaze direction of another individual.
There are two components to the developed skill of eye-gaze monitoring, which we have termed ‘allocentric’ and ‘egocentric’ (Elgar et al. 2002). In allocentric gaze monitoring, the directional aspect of perceiving and processing gaze becomes recruited for the purpose of following the intentional gaze of another, and it is thus critical for the development of joint attention (Slaughter & McConnell 2003). Allocentric gaze means paying attention to the salience and significance of events extrinsic to the viewer; to things happening ‘out there’. It enables the viewer to engage effectively with external and potentially distant events, orienting the observer to the appropriate location, and it uses extrinsic spatial coordinates. Its development normally occurs during the first few years of post-natal life, and we become increasingly accurate at determining where someone else is looking with experience (Langton et al. 2000).
Allocentric gaze skill can be contrasted with egocentric or direct engagement of gaze with the onlooker (Elgar et al. 2002). In contrast to the relatively slow development of allocentric gaze sensitivity, infants show egocentric gaze sensitivity from birth (Farroni et al. 2002). The young infant responds actively to being looked at, exhibiting a range of teasing and smiling behaviours of increasing complexity, suggesting an early developing ability to engage with conspecifics by facial acts involving direct gaze with the interactant. This interest in the gaze of others upon herself is accompanied by increasing precision in the child's ability to detect when she is being looked at (Lee et al. 1998). The main function of egocentric gaze relates primarily to the viewpoint of the perceiver and the onlooker, rather than to the spatial relations of the extrinsic world. Its goal is to engage the viewer and to control interaction.
Neural circuitry that involves the amygdala, the orbito-frontal cortices and the superior temporal sulcus constitutes a probable basis for the development of gaze monitoring, which is critically involved in the perceptual processing of a range of social behaviours (Brothers 1990). This network is preferentially activated when viewing faces and especially eye regions (Allison et al. 2000; Calder et al. 2002). Individuals with bilateral disruption to amygdala-related circuits typically have impairments of gaze monitoring (Adolphs et al. 2005). Failure of a social partner to make a direct eye contact, when in dyadic communication, has profound consequences for our interpretation of their mental health or trustworthiness. The importance that different cultures place on the appropriate role of eye contact in social interactions (Kleinke 1986) does not detract from that conclusion—rather, it emphasizes its validity.
8. Amygdala and fear conditioning
The amygdala plays a critical role in fear conditioning, which is a variant of Pavlovian classical conditioning (LeDoux 2000). The conditioned stimulus in a fear-conditioning experiment with humans might involve a loud noise (the unconditioned stimulus), a negative facial expression (the conditioned stimulus) and an autonomic response, such as an increased skin conductance (the conditioned response). Conditioned fear learning occurs very quickly in normal people, and it can result in persistent associations. On the other hand, repeated exposure to the conditioned stimulus in the absence of the unconditioned stimulus usually leads to ‘extinction’, and the conditioned response ‘habituates’ and diminishes in magnitude. Bechara et al. (1995) discuss a patient with ablation of the amygdala bilaterally, who had a complete blockage of conditioned SCRs, despite retaining conscious awareness of the stimulus contingencies. They contrast him with another with bilateral hippocampal damage, who had retained aversive skin conductance conditioning, but who had no such declarative memory for the contingencies.
9. Functional dissociation between roles of left and right amygdala
Left and right amygdala play distinct, but complementary, roles in the somatic and cognitive responses to facial expressions. Damage to the left amygdala leaves autonomic responses intact, but this is associated with a severe cognitive deficit (Glascher & Adolphs 2003; figure 1). Damage to the right amygdala can lead to autonomic arousal impairment, with a modest influence on cognitive evaluation. It is plausible that cognitive appraisal of negative facial expressions by the left amygdala is enhanced by concomitant somatic arousal, as measured by SCR (Glascher & Adolphs 2003; Williams et al. 2005a). The right amygdala serves to facilitate the cognitively mediated recognition of negative emotions by the left amygdala (Morris et al. 1997; Phillips et al. 1998; Dubois et al. 1999; Lane et al. 1999). All studies of the functional consequences for humans of bilateral or unilateral amygdala damage have inevitably involved damage to the associated temporal lobes as well as neural fibres that pass through the amygdala. Even in relatively circumscribed degeneration of the amygdala in the rare case of Urbach–Wiethe disease (Siebert et al. 2003), there is calcification of surrounding and more posterior temporal lobe. This contrasts with studies of induced and focussed lesions in primates, particularly the meticulous work of Amaral and colleagues (e.g. Amaral et al. 2003). Therefore, it is not possible to state with certainty the relative roles of the amygdala and the overlying cortex in regulating social behaviour, in humans.
Summarizing data from lesion studies, we are, however, led to the conclusion that the right amygdala is generally responsive to arousing stimuli (e.g. facial expressions) independent of their valence (Williams et al. 2004c). Left and right amygdala may work in concert, to produce an orchestrated behavioural response to threat, which guides cognition and behaviour (Hariri et al. 2003). An intact (right amygdala mediated) autonomic response enhances cognitive evaluation, but it is neither sufficient nor necessary for an accurate appraisal of a threatening stimulus. Preserved cognitive evaluation requires efferent and afferent connectivity between the left amygdala and the cortical regions associated with face processing, such as the fusiform gyrus (Kanwisher 2000; Kanwisher & Moscovitch 2000). While a dissociation between the cognitive and the autonomic processing of threatening stimuli by the amygdala has been hinted at in a previous literature, the interpretation of findings has been complicated by the fact that all subjects have had extensive damage to the amygdala, usually as a result of surgery for intractable epilepsy, which often impacts on other medial temporal structures such as the hippocampus. These regions are also reported as responsive to negative facial emotions (Surguladze et al. 2003). Until recently (Skuse et al. 2005), there were no reports of intact autonomic responses to facial expressions of fear, in the absence of accurate cognitive evaluation, other than in instances of physical damage to the amygdala.
10. X-linked genes and the modulation of amygdala activation
The autonomic and cognitive functions of the amygdala have been shown to be dissociated in X-monosomic females, in whom brain structure is essentially normal. Specific deficits in the cognitive appraisal of threat can have functional origins, as well as being related to structural damage to the amygdala (Skuse et al. 2005). These functional mechanisms may reflect an abnormality of dosage regulation in genes that escape X-inactivation and are required in two copies for normal female neural development (Jacobs et al. 1997; Clement-Jones et al. 2000; Carrel & Willard 2005). Gene dosage is haploinsufficient in females, who only have a single X-chromosome (Turner syndrome, 45, X). In X-monosomy, the form and severity of deficits in the cognitive appraisal of facial expressions and social emotions are virtually identical to those seen in patients who have had a bilateral amygdalectomy (Adolphs et al. 1994; Calder et al. 1996; Broks et al. 1998; Lawrence et al. 2003). Difficulties are found in the recognition of negative facial emotions, primarily in the recognition of fear (Sato et al. 2002; Hariri et al. 2003), but also in their recognition of anger (Adams et al. 2003). Turner syndrome females have difficulty in recognizing complex emotions and linking complex emotional expressions to emotional labels (Lawrence et al. 2003). This deficit implicates an abnormality of the functions of the left amygdala in particular; this circuit is linked to the cognitive appraisal of potentially threatening stimuli as discussed earlier (Killgore et al. 2000; Williams et al. 2005a,b), although it cannot mediate entirely normal responses in the absence of the right amygdala, which enhances cognitive performance by inducing arousal signalled by the threatening stimulus (Glascher & Adolphs 2003). The subjective experience of fear must be linked to the functional integration of the arousal response with cognitive appraisal; therefore, it is very interesting to discover that in X-monosomy, the consciously perceived visceral response to threat is much reduced (Good et al. 2003). This implies that there is a functional dissociation in the syndrome between the correlates, at a cortical level, of left and right amygdala-related activities.
Lateralization of amygdala activation could reflect verbal and non-verbal hemispheric asymmetries, typically ascribed to higher cortical functions in humans (Anderson & Phelps 2000). Left lateralized amygdala responses have been previously reported to explicit (i.e. unmasked) presentations of fearful faces (Breiter et al. 1996; Morris et al. 1996; Thomas et al. 2001; Wright et al. 2001; Vuilleumier et al. 2002). Others have found evidence, from lesion studies, that right amygdala ablation is associated with a deficit in the recognition of fearful faces (Adolphs et al. 2001), or with a more general deficit in attributing emotional intensity ratings accurately (Anderson et al. 2000).
We examined the integrity of amygdala-related functions in Turner syndrome by means of a behavioural study of fear conditioning, in which angry faces were used as the conditioned stimulus. Patients with bilateral amygdala lesions have impaired autonomic responsiveness in a fear-conditioning paradigm (Bechara et al. 1995). We used an aversive loud sound as the unconditioned stimulus (Morris & Dolan 2004) and measured SCRs. Despite their very poor performance on the cognitive task requiring the accurate identification of negative facial expressions, females with Turner syndrome showed normal acquisition of fear conditioning, indicating that in this respect they had normal amygdala function (LeDoux 2000). There is evidence from this experiment that amygdala-related cognitive performance could be impaired, in the presence of intact autonomic responsiveness to a threatening stimulus. Previous work (Glascher & Adolphs 2003; Hariri et al. 2003) had shown the converse; cognitive performance can be intact, in the presence of an amygdala lesion, despite the absence of normal autonomic responsiveness to threat.
We tested the dissociation hypothesis in a study of 12 X-monosomic (maternal origin) and 12 control females who participated in fMRI, during which simultaneous skin conductance recordings were acquired (Skuse et al. 2005). Faces depicting fear or neutral emotions were presented to both case and control subjects, in random order. Arousal to the contrast (fearful–neutral) faces was associated with transiently increased SCRs and bilateral amygdala activation in both groups. However, X-monosomic females had a proportionately greater and more persistent right amygdala activation than controls and arousal (SCR) was intact, in both Turner syndrome and normal 46,XX females. The fact that arousal-related functions of the right amygdala were intact was consistent with the preliminary behavioural studies we had conducted, using a fear-conditioning paradigm. In the comparison group, the greater the SCR, the better the performance on the cognitive task, supporting the hypothesis that in normal individuals there is functional integration between amygdala-mediated autonomic responses and cognitive evaluation of a threatening stimulus. Neocortical sensory processing of emotionally salient stimuli (e.g. in left inferior temporal neocortex) seems to be enhanced by the autonomic feedback emanating ultimately from the activity of the right amygdala (Williams et al. 2004b).
We found that there was a severe deficit in recognizing fearful facial expressions in about one-third of the X-monosomic sample. Evaluation of the aetiology of this deficit indicated that it originated in a lack of coordination between activity in the left amygdala and activation of the left fusiform cortex. Activation of the left (but not the right) fusiform gyrus was positively correlated with explicit cognitive performance on the task in both groups. This is consistent with the previous research indicating that cognitive appraisal of threat is left-biased. However, in X-monosomy, the magnitude of that left fusiform response was diminished, whereas left amygdala responsiveness to the stimulus, measured simply as a subtraction (BOLD response, fearful–neutral faces), did not differentiate the groups. A psychophysiological interaction analysis confirmed this lack of coordinated activity in the left amygdala/left fusiform gyrus of the 45,X females. This implied that the core deficit included a functional dissociation between activity in the left amygdala and the left fusiform gyrus (figure 1).
In summary, the left fusiform cortex receives prominent feedback projections from the amygdala (Amaral 1992), and these are associated with cognitive processing of (negative) emotional stimuli (Morris et al. 1998a). Threatening visual stimuli co-activate the left amygdala and the fusiform gyrus; therefore, it is activated more by fearful than by neutral faces (Lane et al. 1999; Vuilleumier & Schwartz 2001; Hadjikhani & de Gelder 2003). In broader terms, there is a suggestion that failure of integrated functional connectivity between these regions is associated with failure to recognize accurately the broader classes of negative/aversive emotional stimuli, such as emotionally salient oddball words (Strange et al. 2000; Anderson & Phelps 2001).
Taken together, these findings show that in the condition of X-monosomy, there are several deficits associated with the impaired cognitive response to the presentation of a fearful face (i.e. the inability to classify accurately the emotion). First, there is an absence of a significant correlation between cognitive performance and activation of either amygdala. Second, activation of the left amygdala is functionally dissociated from that of the left fusiform activation. Third, autonomic arousal (reflecting right amygdala activation) is present, but it is dissociated from left fusiform responsiveness.
Specific deficits in the cognitive appraisal of threat can have functional origins, as well as being related to structural damage. In this investigation, we showed that those functional origins appear to be related to dosage–regulation problems in a certain class of X-linked genes that escape X-inactivation and are required in two copies for normal female neural development (Skuse 2005). There may be additional modifying effects of insufficient sex steroid exposure, but these are currently speculative. Accordingly, we provide the first evidence that the binding of somatic (bodily arousal) responses to cognitive appraisal of emotional stimuli (Damasio 2000) has a genetic substrate. Our earlier research (Good et al. 2003) indicates that the genetic mechanism responsible is associated with one or more X-linked genes lying in a 5 Mb region on the short arm of the X-chromosome at Xp11.3-4. The candidate gene that is responsible for the normal association between left amygdala and cortical responsiveness to fearful faces has yet to be identified. There are likely to be several genetic mechanisms regulating these pathways, and recent evidence suggests that emotional responsiveness to fearful faces could also be influenced by the serotonin transporter gene on chromosome17q11-12 and allelic variants in the promoter region of that gene. These, in turn, may influence susceptibility disorders of emotion, such as depression and anxiety.
11. Genetic influences on amygdala function and susceptibility to psychiatric disorder
Affective disorders, such as generalized anxiety and depression, exhibit considerable variability between individuals (Davidson 2002). The genetic substrates underlying these individual differences are for the large part unknown, but in the past few years attention has been drawn to the serotonin transporter gene (5-HTT). Common variants in the promoter region of the gene have been associated with susceptibility to pathological anxiety and depression, and influence the effectiveness of selective serotonin reuptake inhibitors (SSRIs) in treating these conditions (Nemeroff & Owens 2003). Two alleles have attracted particular attention: the short (s) and the long (l) versions of a particular variable repeat sequence in the promoter of the gene. In general, possession of at least one copy of the short variant is associated with increased risk of depression and poorer response to SSRI treatment; the short allele is associated with reduced serotonin availability compared with the long allele (Hamann 2005). The physiological influence of these variants is likely to be in the prefrontal cortex and amygdala. Hariri et al. (2002) showed that normal individuals with one or two copies of the short allele of the 5-HTT promoter polymorphism had greater right amygdala neuronal activity, measured by the BOLD response, to faces showing threatening expressions (anger/fear) than those who were homozygous for the long version of the allele. Right posterior fusiform gyrus also showed a greater activity when presented with the threatening stimuli, in individuals with an ‘s’ than individuals who were homozygous for an ‘l’ allele. The findings of this investigation were subsequently replicated (Heinz et al. 2005) and are consistent with the hypothesis that feedback from the amygdala to the fusiform gyrus improves recognition, and refines behavioural responses, to aversive/threatening cues such as faces showing negative emotions (Armony et al. 1998; figure 1).
Individuals with an ‘s’ allele in the promoter of the 5-HTT gene therefore appear to have a hyper-responsive amygdala to threat. This observation was followed up in a subsequent study by the same group (Pezawas et al. 2005). This latter investigation studied healthy individuals again, this time with a view to studying potential mechanisms by which carriers of an ‘s’ allele of the 5-HTT promoter region (5-HTTLPR) polymorphism could be more prone to depression. There is considerable evidence to show that serotonin is an important modulator of emotion, and that depressed patients have abnormalities in their regulation of serotonin-related neural circuitry. Their subjects were free from any significant psychiatric history (both a strength and a weakness of the study, which sought clinical relevance). Those who were ‘s’ carriers had substantially reduced grey matter in the perigenual cingulate (pACC)—maximally in the rostral region, to a lesser extent in the caudal region—and in the amygdala (maximal on right). It is notable that reduced activity of the rostral ACC has been reported in both depression and induced sadness, and that this reduction can be reversed by SSRIs. The pACC has the greatest density of 5-HTT terminals within the human cortex, and it is a major target for projections from the amygdala with an excitatory circuit leading to the rostral ACC, which is in turn linked to the caudal region of the ACC, and weakly to the ventromedial prefrontal cortex. From the caudal ACC, inhibitory projections return to the amygdala (figure 1). In fMRI studies, homozygotes for the ‘l’ allele showed strong functional interactions between ventromedial prefrontal cortex, perigenual anterior cingulate and amygdala. This circuit was involved in extinguishing negative affect evoked by threat stimuli. However, the coupling was much diminished in people with at least one ‘s’ allele, implying that they were likely to become disproportionately aroused in response to threats. Scores on a self-rated scale of temperamental anxiety correlated well with the magnitude of cingulate–amygdala interaction; nearly 30% of the variance in harm avoidance scores on a standardized personality questionnaire were predicted by the measure of amygdala–p(rostral)ACC functional connectivity.
Pezawas et al. (2005) speculate that there may be a compensatory overactivity in the ventromedial prefrontal cortex in ‘s’ carriers. This is an ingenious proposal, which links developmental differences in 5-HT-dependent neuronal pathways to impaired interactions in a regulatory network mediating emotional reactivity. Genetically driven variability in 5-HT signalling therefore shapes the connectivity of the amygdala with the rostral anterior cingulate cortex, and in ‘s’ carriers, it is associated with persistent and inappropriate overactivity in response to threat, which in turn leads to the symptoms of anxiety and depression. The circuit could be responsive, in this way, to life events. Inadequate regulation of amygdala arousal, aggregated over lifetime experiences, will eventually result in different susceptibilities to depression/anxiety depending on the intensity and negative quality of those experiences (Hariri et al. 2005).
12. Conclusions
The amygdala lies at the confluence of widely distributed neural circuits that constitute components of the network known as the ‘social brain’ (Adolphs 2001). Deficits in the development of this network during childhood can lead to conditions, such as autistic spectrum disorders, in which social–cognitive competence is seriously compromised. Anomalies in the functioning of these circuits in adulthood can lead, on the one hand, to anxiety or depressive disorders and, on the other hand, to paranoid symptoms. It is probable that the functional integrity of the networks that constitute the social brain is critically linked to inter-individual genetic variation, but that the relationship is likely to be complex. Individual genetic influences on the functioning of the social brain, in the sense of the impact on susceptibility due to any single gene, are likely to be small. To date, it has not been shown conclusively that the genes which influence normal variation in responsiveness of the neural circuits to socially salient stimuli (e.g. fearful faces) are also responsible for dysregulation and a susceptibility to psychiatric disorder. Progress is nevertheless being made. Recent work has implicated vulnerability due to allelic variants in the promoter of the serotonin transporter gene in environmentally induced anxiety and depression, although this finding requires replication in different samples, both clinical and non-clinical. There is growing evidence that X-linked genetic mechanisms may also have a role to play in the functional integrity of the social brain, although no candidate genes have yet been identified. Nevertheless, the mechanisms by which X-linked genes could influence the development of social and emotional responsiveness are increasingly well understood (Carrel & Willard 2005; Skuse 2005) and rapid progress is likely to be made.
The regulation of emotional reactivity to social cues may occur by means of both cortical and subcortical pathways. Subcortical, non-conscious pathways are thought to have evolved as a rapid response system, sensitive to low-resolution representations of threatening stimuli. Afferent tracts leading to the amygdala via this route pass through the superior colliculus and the putamen, carrying poorly specified but rapidly conveyed visual information. Interestingly, such threats include—in a social context—direct eye contact. Recent evidence implies that the circuit is susceptible to alerting when the eyes are wide open and showing a high scleral–iris ratio. Such eye displays are likely to be clearly differentiated even by a crude system that cannot process detailed information on context. Complementary efferent connectivity passing from the amygdala to primary striate visual processing centres (e.g. V4) may alert the visual system to any potential threat, and facilitate the extraction of more detailed information from high-resolution perceptions. When this further information is available, the potential social threat can be set in into context, in light of previous experience (e.g. do I know this person? Are they liable to attack me? Do they find me attractive?).
Because a critical ‘threat stimulus’ is direct eye contact with a conspecific, this is handled in a unique way by the human neocortex. The arousal that is associated with face-to-face contact of this type, in which the eyes clearly show we are the centre of another's attention, appears to be harnessed in human species for a variety of purposes. Although, phylogenetically, it appears that the appropriate course of action faced with such attention was ‘fight or flight’, and this is still the case with primate species, humans have adapted the system for purposes that are arguably, in the appropriate context, critical to the survival of our species for other reasons. These include the attachment between parent and infant, and pair-bonding between adults.
In order to achieve this relatively recent (in evolutionary terms) adaptation of a phylogenetically ancient neural system, we have developed systems of reciprocal control over amygdala activity—exercised, in particular, by fronto-cortical circuits involving the ventromedial prefrontal cortex, the anterior cingulate cortex and the insular cortex (Critchley et al. 2004). Uniquely among all other species, we are able to control this complex ‘survival system’—and we do so by means of a variety of inhibitory pathways, linked with memory and language centres of considerable complexity. Possibly owing to their relatively recent evolutionary origins, the inhibitory systems are liable to dysfunction—and when they are dysfunctional, one possible outcome is a negative impact on quintessentially human traits of social cognition.
Footnotes
One contribution of 14 to a Theme Issue ‘The neurobiology of social recognition, attraction and bonding’.
References
- Adams R.B, Jr, Gordon H.L, Baird A.A, Ambady N, Kleck R.E. Effects of gaze on amygdala sensitivity to anger and fear faces. Science. 2003;300:1536. doi: 10.1126/science.1082244. doi:10.1126/science.1082244 [DOI] [PubMed] [Google Scholar]
- Adolphs R. The neurobiology of social cognition. Curr. Opin. Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. doi:10.1016/S0959-4388(00)00202-6 [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D. Emotion recognition and the human amygdala. In: Aggleton J.P, editor. The amygdala. A functional analysis. Oxford University Press; New York, NY: 2000. pp. 587–630. [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. doi:10.1038/372669a0 [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Hamann S, Young A.W, Calder A.J, Phelps E.A, Anderson A, Lee G.P, Damasio A.R. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37:1111–1117. doi: 10.1016/s0028-3932(99)00039-1. doi:10.1016/S0028-3932(99)00039-1 [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Denburg N. Impaired emotional declarative memory following unilateral amygdala damage. Learn. Mem. 2000;7:180–186. doi: 10.1101/lm.7.3.180. doi:10.1101/lm.7.3.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H. Emotion recognition from faces and prosody following temporal lobectomy. Neuropsychology. 2001;15:396–404. doi: 10.1037//0894-4105.15.3.396. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan T.W, Tranel D, Schyns P, Damasio A.R. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. doi:10.1038/nature03086 [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn. Sci. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. doi:10.1016/S1364-6613(00)01501-1 [DOI] [PubMed] [Google Scholar]
- Amaral D.G. Anatomical organization of the primate amygdaloid complex. In: Aggleton J.P, editor. The amygdala: neurobiological aspects of emotion, memory and mental dysfunction. Wiley-Liss; New York, NY: 1992. pp. 1–66. [Google Scholar]
- Amaral D.G. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol. Psychiatry. 2002;51:11–17. doi: 10.1016/s0006-3223(01)01307-5. doi:10.1016/S0006-3223(01)01307-5 [DOI] [PubMed] [Google Scholar]
- Amaral D.G, Capitanio J.P, Jourdain M, Mason W.A, Mendoza S.P, Prather M. The amygdala: is it an essential component of the neural network for social cognition? Neuropsychologia. 2003;41:235–240. doi: 10.1016/s0028-3932(02)00154-9. doi:10.1016/S0028-3932(02)00154-9 [DOI] [PubMed] [Google Scholar]
- Anderson A.K, Phelps E.A. Perceiving emotion: there's more than meets the eye. Curr. Biol. 2000;10:R551–R554. doi: 10.1016/s0960-9822(00)00612-6. doi:10.1016/S0960-9822(00)00612-6 [DOI] [PubMed] [Google Scholar]
- Anderson A.K, Phelps E.A. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. doi:10.1038/35077083 [DOI] [PubMed] [Google Scholar]
- Anderson A.K, Sobel N. Dissociating intensity from valence as sensory inputs to emotion. Neuron. 2003;39:581–583. doi: 10.1016/s0896-6273(03)00504-x. doi:10.1016/S0896-6273(03)00504-X [DOI] [PubMed] [Google Scholar]
- Anderson A.K, Spencer D.D, Fulbright R.K, Phelps E.A. Contribution of the anteromedial temporal lobes to the evaluation of facial emotion. Neuropsychology. 2000;14:526–536. doi: 10.1037//0894-4105.14.4.526. doi:10.1037/0894-4105.14.4.526 [DOI] [PubMed] [Google Scholar]
- Armony J.L, Quirk G.J, LeDoux J.E. Differential effects of amygdala lesions on early and late plastic components of auditory cortex spike trains during fear conditioning. J. Neurosci. 1998;18:2592–2601. doi: 10.1523/JNEUROSCI.18-07-02592.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio A.R. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Breiter H.C, Etcoff N.L, Whalen P.J, Kennedy W.A, Rauch S.L, Buckner R.L, Strauss M.M, Hyman S.E, Rosen B.R. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. doi:10.1016/S0896-6273(00)80219-6 [DOI] [PubMed] [Google Scholar]
- Broks P, et al. Face processing impairments after encephalitis: amygdala damage and recognition of fear. Neuropsychologia. 1998;36:59–70. doi: 10.1016/s0028-3932(97)00105-x. doi:10.1016/S0028-3932(97)00105-X [DOI] [PubMed] [Google Scholar]
- Brothers L. The social brain: a project for integrating primate behaviour and neurophysiology in a new domain. Concept. Neurosci. 1990;1:27–51. [Google Scholar]
- Calder A.J, Young A.W, Rowland D, Perrett D.I, Hodges J.R, Etcoff N.L. Facial emotion recognition after bilateral amygdala damage: differentially severe impairment of fear. Cogn. Neuropsychol. 1996;13:699–745. doi:10.1080/026432996381890 [Google Scholar]
- Calder A.J, Lawrence A.D, Young A.W. Neuropsychology of fear and loathing. Nat. Rev. Neurosci. 2001;2:352–363. doi: 10.1038/35072584. doi:10.1038/35072584 [DOI] [PubMed] [Google Scholar]
- Calder A.J, Lawrence A.D, Keane J, Scott S.K, Owen A.M, Christoffels I, Young A.W. Reading the mind from eye gaze. Neuropsychologia. 2002;40:1129–1138. doi: 10.1016/s0028-3932(02)00008-8. doi:10.1016/S0028-3932(02)00008-8 [DOI] [PubMed] [Google Scholar]
- Campbell R, Lawrence K, Mandy W, Mitra C, Jeyakuma L, Skuse D. Meanings in motion and faces: developmental associations between the processing of intention from geometrical animations and gaze detection accuracy. Dev. Psychopathol. 2005;18:99–118. doi: 10.1017/S0954579406060068. doi:10.1017/S0954579406060068 [DOI] [PubMed] [Google Scholar]
- Campbell R, Lawrence K, Mandy W, Mitra C, Jeyakuma L, Skuse D. Meanings in motion and faces: developmental associations between the processing of intention from geometrical animations and gaze detection accuracy. Dev. Psychopathol. 2006;18:99–118. doi: 10.1017/S0954579406060068. [DOI] [PubMed] [Google Scholar]
- Carrel L, Willard H.F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. doi:10.1038/nature03479 [DOI] [PubMed] [Google Scholar]
- Clement-Jones M, et al. The short stature homeobox gene SHOX is involved in skeletal abnormalities in Turner syndrome. Hum. Mol. Genet. 2000;9:695–702. doi: 10.1093/hmg/9.5.695. doi:10.1093/hmg/9.5.695 [DOI] [PubMed] [Google Scholar]
- Cohen J.D, Tong F. Neuroscience. The face of controversy. Science. 2001;293:2405–2407. doi: 10.1126/science.1066018. doi:10.1126/science.1066018 [DOI] [PubMed] [Google Scholar]
- Critchley H.D, Mathias C.J, Dolan R.J. Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron. 2002;33:653–663. doi: 10.1016/s0896-6273(02)00588-3. doi:10.1016/S0896-6273(02)00588-3 [DOI] [PubMed] [Google Scholar]
- Critchley H.D, Wiens S, Rotshtein P, Ohman A, Dolan R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. doi:10.1038/nn1176 [DOI] [PubMed] [Google Scholar]
- Damasio A.R. Heinemann; London, UK: 2000. The feeling of what happens: body and emotion in the making of consciousness. [Google Scholar]
- Das P, Kemp A.H, Liddell B.J, Brown K.J, Olivieri G, Peduto A, Gordon E, Williams L.M. Pathways for fear perception: modulation of amygdala activity by thalamo-cortical systems. Neuroimage. 2005;26:141–148. doi: 10.1016/j.neuroimage.2005.01.049. doi:10.1016/j.neuroimage.2005.01.049 [DOI] [PubMed] [Google Scholar]
- Davidson R.J. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol. Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. doi:10.1016/S0006-3223(01)01328-2 [DOI] [PubMed] [Google Scholar]
- Davidson R.J, Putnam K.M, Larson C.L. Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. doi:10.1126/science.289.5479.591 [DOI] [PubMed] [Google Scholar]
- Davis M. Neurobiology of fear responses: the role of the amygdala. J. Neuropsychiatry Clin. Neurosci. 1997;9:382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen P.J. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. doi:10.1038/sj.mp.4000812 [DOI] [PubMed] [Google Scholar]
- Dolan R.J. Emotion, cognition, and behavior. Science. 2002;298:1191–1194. doi: 10.1126/science.1076358. doi:10.1126/science.1076358 [DOI] [PubMed] [Google Scholar]
- Dubois S, Rossoin B, Schiltz C, Bodart J.M, Michel C, Bruyer R, Crommelinck M. Effect of familiarity on the processing of human faces. Neuroimage. 1999;9:278–289. doi: 10.1006/nimg.1998.0409. doi:10.1006/nimg.1998.0409 [DOI] [PubMed] [Google Scholar]
- Elgar K, Campbell R, Skuse D. Are you looking at me? Accuracy in processing line-of-sight in Turner syndrome. Proc. R. Soc. B. 2002;269:2415–2422. doi: 10.1098/rspb.2002.2173. doi:10.1098/rspb.2002.2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery N.J. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci. Behav. Rev. 2000;24:581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- Farroni T, Csibra G, Simion F, Johnson M.H. Eye contact detection in humans from birth. Proc. Natl Acad. Sci. USA. 2002;99:9602–9605. doi: 10.1073/pnas.152159999. doi:10.1073/pnas.152159999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George N, Driver J, Dolan R.J. Seen gaze-direction modulates fusiform activity and its coupling with other brain areas during face processing. Neuroimage. 2001;13:1102–1112. doi: 10.1006/nimg.2001.0769. doi:10.1006/nimg.2001.0769 [DOI] [PubMed] [Google Scholar]
- Glascher J, Adolphs R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J. Neurosci. 2003;23:10 274–10 282. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C.D, Lawrence K, Thomas N.S, Price C.J, Ashburner J, Friston K.J, Frackowiak S.J, Oreland L, Skuse D.H. Dosage sensitive X-linked locus influences the development of amygdala and orbito-frontal cortex, and fear recognition in humans. Brain. 2003;126:2431–2446. doi: 10.1093/brain/awg242. doi:10.1093/brain/awg242 [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, de Gelder B. Seeing fearful body expressions activates the fusiform cortex and amygdala. Curr. Biol. 2003;13:2201–2205. doi: 10.1016/j.cub.2003.11.049. doi:10.1016/j.cub.2003.11.049 [DOI] [PubMed] [Google Scholar]
- Hamm A.O, Weike A.I, Schupp H.T, Treig T, Dressel A, Kessler C. Affective blindsight: intact fear conditioning to a visual cue in a cortically blind patient. Brain. 2003;126:267–275. doi: 10.1093/brain/awg037. doi:10.1093/brain/awg037 [DOI] [PubMed] [Google Scholar]
- Hamann S. Blue genes: wiring the brain for depression. Nat. Neurosci. 2005;8:701–702. doi: 10.1038/nn0605-701. doi:10.1038/nn0605-701 [DOI] [PubMed] [Google Scholar]
- Hariri A.R, Bookheimer S.Y, Mazziotta J.C. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri A.R, Mattay V.S, Tessitore A, Kolachana B, Fera F, Goldman D, Egan M.F, Weinberger D.R. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. doi:10.1126/science.1071829 [DOI] [PubMed] [Google Scholar]
- Hariri A.R, Mattay V.S, Tessitore A, Fera F, Weinberger D.R. Neocortical modulation of the amygdala response to fearful stimuli. Biol. Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. doi:10.1016/S0006-3223(02)01786-9 [DOI] [PubMed] [Google Scholar]
- Hariri A.R, Drabant E.M, Munoz K.E, Kolachana B.S, Mattay V.S, Egan M.F, Weinberger D.R. A susceptibility gene for affective disorders and the response of the human amygdala. Arch. Gen. Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. doi:10.1001/archpsyc.62.2.146 [DOI] [PubMed] [Google Scholar]
- Haxby J.V, Hoffman E.A, Gobbini M.I. The distributed human neural system for face perception. Trends Cogn. Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. doi:10.1016/S1364-6613(00)01482-0 [DOI] [PubMed] [Google Scholar]
- Heinz A, et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat. Neurosci. 2005;8:20–21. doi: 10.1038/nn1366. doi:10.1038/nn1366 [DOI] [PubMed] [Google Scholar]
- Jacobs P, Dalton P, James R, Mosse K, Power M, Robinson D, Skuse D. Turner syndrome: a cytogenetic and molecular study. Ann. Hum. Genet. 1997;61:471–483. doi: 10.1046/j.1469-1809.1997.6160471.x. doi:10.1017/S0003480097006507 [DOI] [PubMed] [Google Scholar]
- Jellema T, Baker C.I, Wicker B, Perrett D.I. Neural representation for the perception of the intentionality of actions. Brain Cogn. 2000;44:280–302. doi: 10.1006/brcg.2000.1231. doi:10.1006/brcg.2000.1231 [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Domain specificity in face perception. Nat. Neurosci. 2000;3:759–763. doi: 10.1038/77664. doi:10.1038/77664 [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Moscovitch M. The cognitive neuroscience of face processing: an introduction. Cogn. Neuropsychol. 2000;17:1–11. doi: 10.1080/026432900380454. doi:10.1080/026432900380454 [DOI] [PubMed] [Google Scholar]
- Kawashima R, Sugiura M, Takashi K, Nakamura A, Hatano K, Ito K, Fukuda H, Kojima S, Nakamura K. The human amygdala plays an important role in gaze monitoring: a PET study. Brain. 1999;122:779–783. doi: 10.1093/brain/122.4.779. doi:10.1093/brain/122.4.779 [DOI] [PubMed] [Google Scholar]
- Killgore W.D, Casasanto D.J, Yurgelun-Todd D.A, Maldjian J.A, Detre J.A. Functional activation of the left amygdala and hippocampus during associative encoding. Neuroreport. 2000;11:2259–2263. doi: 10.1097/00001756-200007140-00039. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville L.H, Johnstone T, Polis S, Alexander A.L, Shin L.M, Whalen P.J. Contextual modulation of amygdala responsivity to surprised faces. J. Cogn. Neurosci. 2004;16:1730–1745. doi: 10.1162/0898929042947865. doi:10.1162/0898929042947865 [DOI] [PubMed] [Google Scholar]
- Kleinke C.L. Gaze and eye contact: a research review. Psychol. Bull. 1986;100:78–100. doi:10.1037/0033-2909.100.1.78 [PubMed] [Google Scholar]
- Kobayashi H, Kohshima S. Unique morphology of the human eye and its adaptive meaning: comparative studies on external morphology of the primate eye. J. Hum. Evol. 2001;40:419–435. doi: 10.1006/jhev.2001.0468. doi:10.1006/jhev.2001.0468 [DOI] [PubMed] [Google Scholar]
- Kring A.M, Neale J.M. Do schizophrenic patients show a disjunctive relationship among expressive, experiential, and psychophysiological components of emotion? J. Abnorm. Psychol. 1996;105:249–257. doi: 10.1037//0021-843x.105.2.249. doi:10.1037/0021-843X.105.2.249 [DOI] [PubMed] [Google Scholar]
- Lane R.D, Chua P.M, Dolan R.J. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia. 1999;37:989–997. doi: 10.1016/s0028-3932(99)00017-2. doi:10.1016/S0028-3932(99)00017-2 [DOI] [PubMed] [Google Scholar]
- Langton S.R, Watt R.J, Bruce I.I. Do the eyes have it? Cues to the direction of social attention. Trends Cogn. Sci. 2000;4:50–59. doi: 10.1016/s1364-6613(99)01436-9. doi:10.1016/S1364-6613(99)01436-9 [DOI] [PubMed] [Google Scholar]
- Lawrence K, Campbell R, Swettenham J, Terstegge J, Akers R, Coleman M, Skuse D. Interpreting gaze in Turner syndrome: impaired sensitivity to intention and emotion, but preservation of social cueing. Neuropsychologia. 2003;41:894–905. doi: 10.1016/s0028-3932(03)00002-2. doi:10.1016/S0028-3932(03)00002-2 [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. doi:10.1146/annurev.neuro.23.1.155 [DOI] [PubMed] [Google Scholar]
- Lee K, Eskritt M, Symons L.A, Muir D. Children's use of triadic eye gaze information for “mind reading”. Dev. Psychol. 1998;34:525–539. doi: 10.1037//0012-1649.34.3.525. doi:10.1037/0012-1649.34.3.525 [DOI] [PubMed] [Google Scholar]
- Lomber S.G. Learning to see the trees before the forest: reversible deactivation of the superior colliculus during learning of local and global visual features. Proc. Natl Acad. Sci. USA. 2002;99:4049–4054. doi: 10.1073/pnas.062551899. doi:10.1073/pnas.062551899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.S, Dolan R.J. Dissociable amygdala and orbitofrontal responses during reversal fear conditioning. Neuroimage. 2004;22:372–380. doi: 10.1016/j.neuroimage.2004.01.012. doi:10.1016/j.neuroimage.2004.01.012 [DOI] [PubMed] [Google Scholar]
- Morris J.S, Frith C.D, Perrett D.I, Rowland D, Young A.W, Calder A.J, Dolan R.J. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. doi:10.1038/383812a0 [DOI] [PubMed] [Google Scholar]
- Morris J.S, Friston K.J, Dolan R.J. Neural responses to salient visual stimuli. Proc. R. Soc. B. 1997;264:769–775. doi: 10.1098/rspb.1997.0109. doi:10.1098/rspb.1997.0109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.S, Friston K.J, Buchel C, Frith C.D, Young A.W, Calder A.J, Dolan R. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998a;121:47–57. doi: 10.1093/brain/121.1.47. doi:10.1093/brain/121.1.47 [DOI] [PubMed] [Google Scholar]
- Morris J.S, Ohman A, Dolan R.J. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998b;393:467–470. doi: 10.1038/30976. doi:10.1038/30976 [DOI] [PubMed] [Google Scholar]
- Morris J.S, Ohman A, Dolan R.J. A subcortical pathway to the right amygdala mediating “unseen” fear. Proc. Natl Acad. Sci. USA. 1999;96:1680–1685. doi: 10.1073/pnas.96.4.1680. doi:10.1073/pnas.96.4.1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.S, Buchel C, Dolan R.J. Parallel neural responses in amygdala subregions and sensory cortex during implicit fear conditioning. Neuroimage. 2001;13:1044–1052. doi: 10.1006/nimg.2000.0721. doi:10.1006/nimg.2000.0721 [DOI] [PubMed] [Google Scholar]
- Morris J.S, deBonis M, Dolan R.J. Human amygdala responses to fearful eyes. Neuroimage. 2002;17:214–222. doi: 10.1006/nimg.2002.1220. doi:10.1006/nimg.2002.1220 [DOI] [PubMed] [Google Scholar]
- Morton J, Johnson M.H. CONSPEC and CONLERN: a two-process theory of infant face recognition. Psychol. Rev. 1991;98:164–181. doi: 10.1037/0033-295x.98.2.164. doi:10.1037/0033-295X.98.2.164 [DOI] [PubMed] [Google Scholar]
- Nahm F.K. Heinrich Kluver and the temporal lobe syndrome. J. Hist. Neurosci. 1997;6:193–208. doi: 10.1080/09647049709525702. [DOI] [PubMed] [Google Scholar]
- Nakamura K, et al. Functional delineation of the human occipito-temporal areas related to face and scene processing. A PET study. Brain. 2000;123:1903–1912. doi: 10.1093/brain/123.9.1903. doi:10.1093/brain/123.9.1903 [DOI] [PubMed] [Google Scholar]
- Nemeroff C.B, Owens M.J. Neuropharmacology of paroxetine. Psychopharmacol. Bull. 2003;37(Suppl. 1):8–18. [PubMed] [Google Scholar]
- Pasley B.N, Mayes L.C, Schultz R.T. Subcortical discrimination of unperceived objects during binocular rivalry. Neuron. 2004;42:163–172. doi: 10.1016/s0896-6273(04)00155-2. doi:10.1016/S0896-6273(04)00155-2 [DOI] [PubMed] [Google Scholar]
- Perrett D.I, Smith P.A, Potter D.D, Mistlin A.J, Head A.S, Milner A.D, Jeeves M.A. Visual cells in the temporal cortex sensitive to face view and gaze direction. Proc. R. Soc. B. 1985;223:293–317. doi: 10.1098/rspb.1985.0003. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider L.G. Neural processing of emotional faces requires attention. Proc. Natl Acad. Sci. USA. 2002;99:11 458–11 463. doi: 10.1073/pnas.172403899. doi:10.1073/pnas.172403899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat. Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. doi:10.1038/nn1463 [DOI] [PubMed] [Google Scholar]
- Phan K.L, Wager T, Taylor S.F, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. doi:10.1006/nimg.2002.1087 [DOI] [PubMed] [Google Scholar]
- Phillips M.L, et al. Neural responses to facial and vocal expressions of fear and disgust. Proc. R. Soc. B. 1998;265:1809–1817. doi: 10.1098/rspb.1998.0506. doi:10.1098/rspb.1998.0506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L, Williams L, Senior C, Bullmore E.T, Brammer M.J, Andrew C, Williams S.C, David A.S. A differential neural response to threatening and non-threatening negative facial expressions in paranoid and non-paranoid schizophrenics. Psychiatry Res. 1999;92:11–31. doi: 10.1016/s0925-4927(99)00031-1. [DOI] [PubMed] [Google Scholar]
- Phillips M.L, Senior C, David A.S. Perception of threat in schizophrenics with persecutory delusions: an investigation using visual scan paths. Psychol. Med. 2000;30:157–167. doi: 10.1017/s0033291799001397. doi:10.1017/S0033291799001397 [DOI] [PubMed] [Google Scholar]
- Sah P, Faber E.S, Lopez D.A, Power J. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Salem J.E, Kring A.M, Kerr S.L. More evidence for generalized poor performance in facial emotion perception in schizophrenia. J. Abnorm. Psychol. 1996;105:480–483. doi: 10.1037//0021-843x.105.3.480. doi:10.1037/0021-843X.105.3.480 [DOI] [PubMed] [Google Scholar]
- Sato N, Nakamura K. Detection of directed gaze in rhesus monkeys (Macaca mulatta) J. Comp. Psychol. 2001;115:115–121. doi: 10.1037/0735-7036.115.2.115. doi:10.1037/0735-7036.115.2.115 [DOI] [PubMed] [Google Scholar]
- Sato W, Kubota Y, Okada T, Murai T, Yoshikawa S, Sengoku A. Seeing happy emotion in fearful and angry faces: qualitative analysis of facial expression recognition in a bilateral amygdala-damaged patient. Cortex. 2002;38:727–742. doi: 10.1016/s0010-9452(08)70040-6. [DOI] [PubMed] [Google Scholar]
- Schultz R.T. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int. J. Dev. Neurosci. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. doi:10.1016/j.ijdevneu.2004.12.012 [DOI] [PubMed] [Google Scholar]
- Schyns P.G, Oliva A. Dr. Angry and Mr. Smile: when categorization flexibly modifies the perception of faces in rapid visual presentations. Cognition. 1999;69:243–265. doi: 10.1016/s0010-0277(98)00069-9. doi:10.1016/S0010-0277(98)00069-9 [DOI] [PubMed] [Google Scholar]
- Sheinberg D.L, Logothetis N.K. The role of temporal cortical areas in perceptual organization. Proc. Natl Acad. Sci. USA. 1997;94:3408–3413. doi: 10.1073/pnas.94.7.3408. doi:10.1073/pnas.94.7.3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert M, Markowitsch H.J, Bartel P. Amygdala, affect and cognition: evidence from 10 patients with Urbach-Wiethe disease. Brain. 2003;126:2627–2637. doi: 10.1093/brain/awg271. doi:10.1093/brain/awg271 [DOI] [PubMed] [Google Scholar]
- Singer T, Kiebel S.J, Winston J.S, Dolan R.J, Frith C.D. Brain responses to the acquired moral status of faces. Neuron. 2004;41:653–662. doi: 10.1016/s0896-6273(04)00014-5. doi:10.1016/S0896-6273(04)00014-5 [DOI] [PubMed] [Google Scholar]
- Skuse D.H. X-linked genes and mental functioning. Hum. Mol. Genet. 2005;14(Spec No. 1):R27–R32. doi: 10.1093/hmg/ddi112. doi:10.1093/hmg/ddi112 [DOI] [PubMed] [Google Scholar]
- Skuse D.H, Morris J.S, Dolan R.J. Functional dissociation of amygdala-modulated arousal and cognitive appraisal, in Turner syndrome. Brain. 2005;128:2084–2096. doi: 10.1093/brain/awh562. doi:10.1093/brain/awh562 [DOI] [PubMed] [Google Scholar]
- Slaughter V, McConnell D. Emergence of joint attention: relationships between gaze following, social referencing, imitation, and naming in infancy. J. Genet. Psychol. 2003;164:54–71. doi: 10.1080/00221320309597503. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral D.G. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J. Comp. Neurol. 2002;451:301–323. doi: 10.1002/cne.10339. doi:10.1002/cne.10339 [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Ql H.X, Kaas J.H. Projections of the superior colliculus to subdivisions of the inferior pulvinar in New World and Old World monkeys. Vis. Neurosci. 2000;17:529–549. doi: 10.1017/s0952523800174048. doi:10.1017/S0952523800174048 [DOI] [PubMed] [Google Scholar]
- Strange B.A, Henson R.N, Friston K.J, Dolan R.J. Brain mechanisms for detecting perceptual, semantic, and emotional deviance. Neuroimage. 2000;12:425–433. doi: 10.1006/nimg.2000.0637. doi:10.1006/nimg.2000.0637 [DOI] [PubMed] [Google Scholar]
- Surguladze S.A, Brammer M.J, Young A.W, Andrew C, Travis M.J, Williams S.C, Phillips M.L. A preferential increase in the extrastriate response to signals of danger. Neuroimage. 2003;19:1317–1328. doi: 10.1016/s1053-8119(03)00085-5. doi:10.1016/S1053-8119(03)00085-5 [DOI] [PubMed] [Google Scholar]
- Thomas K.M, Drevets W.C, Whalen P.J, Eccard C.H, Dahl R.E, Ryan N.D, Casey B.J. Amygdala response to facial expressions in children and adults. Biol. Psychol. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. doi:10.1016/S0006-3223(00)01066-0 [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Schwartz S. Emotional facial expressions capture attention. Neurology. 2001;56:153–158. doi: 10.1212/wnl.56.2.153. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony J.L, Driver J, Dolan R.J. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. doi:10.1016/S0896-6273(01)00328-2 [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony J.L, Clarke K, Husain M, Driver J, Dolan R.J. Neural response to emotional faces with and without awareness: event-related fMRI in a parietal patient with visual extinction and spatial neglect. Neuropsychologia. 2002;40:2156–2166. doi: 10.1016/s0028-3932(02)00045-3. doi:10.1016/S0028-3932(02)00045-3 [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony J.L, Driver J, Dolan R.J. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat. Neurosci. 2003;6:624–631. doi: 10.1038/nn1057. doi:10.1038/nn1057 [DOI] [PubMed] [Google Scholar]
- Wade A.M, Lawrence K, Mandy W, Skuse D. Charting the development of emotion recognition from 6 years of age. J. Appl. Stat. 2005;33:297–315. doi:10.1080/02664760500445756 [Google Scholar]
- Wager T.D, Phan K.L, Liberzon I, Taylor S.F. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. doi:10.1016/S1053-8119(03)00078-8 [DOI] [PubMed] [Google Scholar]
- Whalen P.J, Rauch S.L, Etcoff N.L, McInerney S.C, Lee M.B, Jenike M.A. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J. Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen P.J, et al. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306:2061. doi: 10.1126/science.1103617. doi:10.1126/science.1103617 [DOI] [PubMed] [Google Scholar]
- Williams L.M, et al. Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance recording. Neuroimage. 2001;14:1070–1079. doi: 10.1006/nimg.2001.0904. doi:10.1006/nimg.2001.0904 [DOI] [PubMed] [Google Scholar]
- Williams L.M, et al. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am. J. Psychiatry. 2004a;161:480–489. doi: 10.1176/appi.ajp.161.3.480. doi:10.1176/appi.ajp.161.3.480 [DOI] [PubMed] [Google Scholar]
- Williams L.M, Liddell B.J, Rathjen J, Brown K.J, Gray J, Phillips M, Young A, Gordon E. Mapping the time course of nonconscious and conscious perception of fear: an integration of central and peripheral measures. Hum. Brain Mapp. 2004b;21:64–74. doi: 10.1002/hbm.10154. doi:10.1002/hbm.10154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.A, Morris A.P, McGlone F, Abbott D.F, Mattingley J.B. Amygdala responses to fearful and happy facial expressions under conditions of binocular suppression. J. Neurosci. 2004c;24:2898–2904. doi: 10.1523/JNEUROSCI.4977-03.2004. doi:10.1523/JNEUROSCI.4977-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.M, Das P, Liddell B, Olivieri G, Peduto A, Brammer M.J, Gordon E. BOLD, sweat and fears: fMRI and skin conductance distinguish facial fear signals. Neuroreport. 2005a;16:49–52. doi: 10.1097/00001756-200501190-00012. doi:10.1097/00001756-200501190-00012 [DOI] [PubMed] [Google Scholar]
- Williams M.A, McGlone F, Abbott D.F, Mattingley J.B. Differential amygdala responses to happy and fearful facial expressions depend on selective attention. Neuroimage. 2005b;24:417–425. doi: 10.1016/j.neuroimage.2004.08.017. doi:10.1016/j.neuroimage.2004.08.017 [DOI] [PubMed] [Google Scholar]
- Wright C.I, Fischer H, Whalen P.J, McInerney S.C, Shin L.M, Rauch S.L. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12:379–383. doi: 10.1097/00001756-200102120-00039. doi:10.1097/00001756-200102120-00039 [DOI] [PubMed] [Google Scholar]