Abstract
Whether the motor cortex regulates voluntary movements by generating the motor pattern directly or by acting through subcortical central pattern generators (CPGs) remains a central question in motor control. Using the rat whisker system, an important model system of mammalian motor control, we develop an anesthetized preparation to investigate the interaction between the motor cortex and a whisking CPG. Using this model we investigate the involvement of a serotonergic component of the whisking CPG in determining whisking kinematics and the mechanisms through which drive from the CPG is converted into movements by vibrissa motor units. Consistent with an action of the vibrissa motor cortex (vMCx) on a subcortical CPG, the frequency of whisking evoked by intracortical microstimulation (ICMS) of vMCx differed significantly from the stimulation frequency, whereas whisking onset latencies correlated negatively with stimulation intensity. Further, ICMS-evoked whisking was suppressed by a serotonin receptor antagonist, supporting previous findings that the whisking CPG contains a significant serotonergic component. The amplitude of ICMS-evoked whisking was correlated with the number of active motor units—isolated from vibrissal EMGs or recorded directly from vibrissa motoneurons—and their activity level. In addition, whisking frequency was correlated with the firing rate of these motoneurons. These findings support the hypothesis that vMCx regulates whisking through its actions on a subcortical CPG.
INTRODUCTION
The mechanisms by which the motor cortex regulates voluntary movements remain uncertain, with some arguing that it exercises direct control over motoneurons and others maintaining that it acts by subcortical, premotoneuron cell assemblies (Iwaniuk and Whishaw 2000; Lemon and Griffiths 2005). In many motor pathways these subcortical targets function as central pattern generators (CPGs), capable of generating a rhythmic output in the absence of rhythmic inputs or sensory feedback (Arshavsky et al. 1997; Stein et al. 1997).
Palpatory vibrissa movements in rodents (Brecht et al. 1997; Vincent 1912) have emerged as an attractive model for motor control. Here, too, a controversy exists regarding the role of the motor cortex. There is evidence suggesting that direct inputs from the vibrissae representation in the motor cortex (vMCx; Grinevich et al. 2005) enable this structure to produce the patterned drive for individual whisks (Ahrens and Kleinfeld 2004; Berg and Kleinfeld 2003b). Other evidence suggests that vMCx does not directly drive vibrissa motoneurons (Carvell et al. 1996), but rather that vMCx acts through its interactions with a subcortical whisking CPG (Friedman et al. 2006; Hattox et al. 2003).
In many models of motor control, understanding of the role of the motor cortex has been advanced by the ability to generate naturalistic movements through stimulating the cortex of anesthetized animals, an approach that permits stable recordings from subcortical motor components (e.g., grasping: Graziano et al. 2002; suckling, Iriki et al. 1988; chewing: Katakura and Chandler 1990). However, similar progress in the vibrissal system has been hampered by the fact that cortical stimulation in anesthetized rats was reported to produce only small vibrissae movements, and often only retractions (Berg and Kleinfeld 2003b; Neafsey 1990; Weiss and Keller 1994). Motivated by the recent findings of Haiss and Schwarz (2005) in awake rats, we developed a preparation in which intracortical microstimulation (ICMS) of vMCx evokes whisking in anesthetized rats. This allowed us to test the relationships between vMCx outputs and outputs from vibrissae motor units and motoneurons. Our results are consistent with the hypothesis that vMCx regulates whisking through its actions on a serotonergic CPG.
METHODS
Surgical procedures
We used 20 female Sprague–Dawley rats (225–270 g) in this study. All procedures strictly adhered to institutional and federal guidelines. Under halothane (2.5%) anesthesia, a small incision was made in the face and a pair of bipolar EMG electrodes (76-μm Teflon-coated stainless steel wire) was tunneled subcutaneously into the deep intrinsic muscles. After infusion of local anesthetics to surgical sites, we performed craniotomies over the vibrissa motor cortex (vMCx) and, in experiments targeting vibrissa motoneurons (vMNs), the lateral facial nucleus (LVII). Saline baths were constructed over the craniotomies to prevent drying. In most experiments we monitored electrocorticograms (ECoGs) to ensure that the animal was in no pain or distress. During recordings, rats were maintained at anesthetic level III-2, characterized by an ECoG frequency between 5 and 7 Hz, a respiratory rate of about 100 breaths/min, and an absence of a withdrawal reflex to noxious stimuli (Friedberg et al. 1999). Because the threshold for evoking whisking depended on the depth of anesthesia, care was taken to maintain a consistent and stable level of anesthesia. Body temperature was maintained at 37°C with a servo-controlled heating blanket.
Stimulation
Custom-made platinum-in-quartz electrodes (tip ≈ 10 μm) were lowered to a depth of 1.5 mm, corresponding to layer V of vMCx. The rhythmic subregion (RS) of vMCx was identified by applying low-intensity current pulses (50 monophasic pulses at 50 Hz, 200-μs pulse width, 25 to 250 μA) to the area of vMCx identified by Haiss and Schwarz (2005). Stimulation in this region evoked rhythmic vibrissae movements, whereas stimulation outside of this region either generated small vibrissal retractions or failed to produce movements. RS was reliably located at anterioposterior 1.3 mm and mediolateral ± 1.3 mm, as previously documented (Haiss and Schwarz 2005).
Drug application
We applied the 5HT receptor antagonist, metergoline (100 μM to 1 mM, Sigma-Aldrich, St. Louis, MO), to vMNs in LVII using either microdialysis probes (n = 7, CMA Microdialysis, North Chelmsford, MA) or injection pipettes (n = 2). Injection pipettes were constructed from pulled borosilicate glass pipettes (Sutter Instruments, Novato, CA) with tip diameters of about 100 μm. Drug and vehicle delivery for the injection pipettes was controlled by a manually operated Picospritzer. Delivery through the microdialysis probes was controlled manually with a 1-ml syringe. Because both methods of drug application produced identical results, we combined data from all experiments. After recording a stable baseline of ICMS-evoked whisking, the antagonist was applied while continuing periodic ICMS of vMCx. In five of the microdialysis experiments, the antagonist was subsequently washed out by passing buffered saline through the probe.
Recording and analyses
To monitor vibrissae movements, we modified the optoelectronic method of Zeigler and collaborators (Bermejo et al. 1998). We trimmed all but a single vibrissa and used the analog output from a high-resolution (7 μm) charge-coupled detector (CCD) device (MetraLight, Sunnyvale, CA) to monitor its position as a function of time. The sensitivity of the CCD allowed us to reliably monitor these movements without attaching markers to the vibrissa. The vibrissae crossed the detectors about 15 mm from the face. Thus a linear deflection of 1 mm corresponds to movement through an arc of 3.8°. However, because the linear deflection measured in millimeters contains less uncertainty, these values were used in all analyses and figures. Analog signals were digitized at 40 kHz with an A/D board (ITC-18, Instrutech, Port Washington, NY) and stored on a computer running software written in Igor (Wavemetrics, Portland, OR).
Using the same hardware and software, EMG recordings were sampled at 40 kHz and filtered (1 Hz to 5 kHz). For motor unit isolation, data were high-pass filtered (corner frequency of 700 Hz) and decomposed using software written in Igor. The software operated as a time–amplitude window discriminator, with a single amplitude threshold and a two-point time window. In addition, all isolated waveforms were examined manually. All isolated waveforms representing the same unit were averaged and then subtracted from the EMG. This process was continued until all motor units were reliably isolated. We generated power spectral densities from the rectified, filtered EMG data, subsampled to 500 Hz. EMG envelopes were generated by smoothing the subsampled data using 14 repetitions of a sliding box algorithm with a width of 301 points. For data sampled at 40 kHz, the smoothing algorithm had a corner frequency of 59 Hz. Recordings of vibrissa position, monitored by the CCD device, were similarly smoothed; however, smoothing had no effect on the shape of the movement signal. Whisking onset was defined as the first increase in activity (measured from EMGs or the CCD) that significantly (≥99.7% confidence interval) exceeded the amplitude of baseline activity.
We obtained extracellular recordings from vibrissa motoneurons using platinum-in-quartz electrodes (2–5 MΩ) stereotaxically guided to LVII (anteroposterior 11.2 mm, lateromedial 2.4 mm, relative to bregma). After completion of the experiment, we marked the recording sites with an electrolytic lesion (5 μA for 10 s). The animals were then deeply anesthetized with sodium pentobarbital (60 mg/kg) and perfused transcardially with buffered saline followed by 4% paraformaldehyde. Lesion sites were verified in Nissl-stained coronal sections. We defined all cells recorded from LVII as vibrissa motoneurons (Hattox et al. 2002; Klein and Rhoades 1985); subsequent analysis (see RESULTS) confirmed that these neurons fired in phase with the ICMS-evoked EMG. Where present, stimulation artifacts were digitally removed from the electrophysiological recordings.
RESULTS
Kinematics of ICMS-evoked whisking
The vibrissa representation of the motor cortex (vMCx) has been divided into two functional subregions based on movements evoked by intracortical microstimulation (ICMS; Haiss and Schwarz 2005; Sanderson et al. 1984). In awake rats, stimulation within the retraction-face (RF) subregion evokes vibrissal retractions and concurrent activation of other facial muscles. Stimulation within the rhythmic-whisking (RW) subregion evokes rhythmic protractions of the vibrissae that mimic natural whisking (Haiss and Schwarz 2005). To investigate the mechanisms of cortical regulation of the whisking CPG in a mechanically stable platform, we developed an anesthetized preparation in which ICMS in the RW subregion evokes rhythmic whisking. A representative example of an ICMS-evoked whisking epoch in an anesthetized rat is shown in Fig. 1A. The bottom trace shows the EMG recorded from the intrinsic muscle of a single vibrissa, with the power spectral density (PSD) of the whisking epoch shown above. As we frequently observed during ICMS-evoked whisking, the rhythmic EMG activity outlasted the stimulation (see also Figs. 3, 4, 6, and 7). The spectral features of the EMG (5–15 Hz) mimic those recorded from behaving animals (Berg and Kleinfeld 2003b; Hattox et al. 2003), indicating that ICMS in the anesthetized rat evokes rhythmic vibrissae movements that closely resemble exploratory whisking.
FIG. 1.
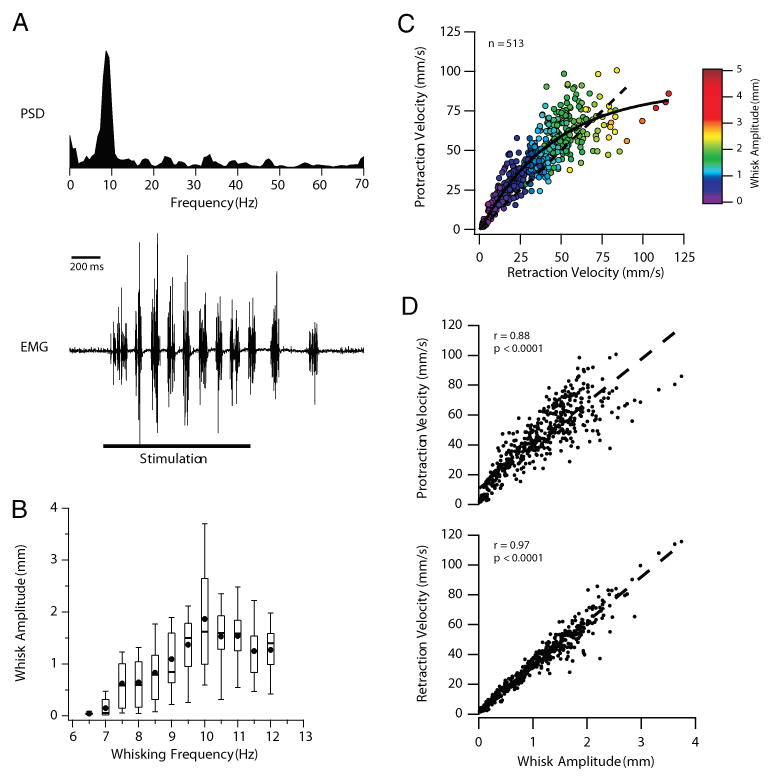
Intracortical microstimulation (ICMS)–evoked whisking in the anesthetized rat. A: power spectral density (PSD, top) of the intrinsic EMG (bottom)—evoked by ICMS of vibrissa motor cortex (vMCx)—shows a peak at 9 Hz. ICMS parameters: 200-μs pulses at 50 Hz, 125 μA for 1 s. Note the absence of a peak at the stimulation frequency of 50 Hz, indicating that the stimulation is not driving whisking on a cycle-by-cycle basis. Note also that rhythmic whisking outlasts the duration of the stimulation (indicated by the horizontal line). B: larger whisks were generated during higher-frequency whisking epochs. Amplitudes of 513 whisks were binned according to the frequency of their whisking epoch (bin size = 0.5 Hz). Box plots define the 25th and 75th percentiles, whereas the whiskers define the 10th and 90th percentiles. Medians are represented by a line through each box. Solid circles represent the means. C: relationship between retraction velocities and the velocities of the immediately preceding protractions (n = 513). Solid line is the exponential fit of the data. Colors indicate the amplitude of the corresponding whisk. At low whisk amplitudes, most retractions were slower than the preceding protractions (see dashed unity line). As amplitudes increased, retraction velocities exceeded protraction velocities. D: whisk amplitudes are highly correlated with the velocities of both whisk protractions (top) and retractions (bottom), but the correlation with retraction velocities is higher. Dashed lines are the linear regression for each data set.
FIG. 3.

Whisking kinematics are accurately predicted by the intrinsic electromyogram (EMG). A, top traces: spectrograms of ICMS-evoked whisking in response to 3 stimulation intensities. Below the spectrograms are recordings of vibrissa position as a function of time, measured by the charge-coupled device (CCD), and the corresponding vibrissa EMG. EMG envelope, produced by rectifying and smoothing the raw EMG, accurately predicts whisking kinematics. Small phase difference between the CCD and EMG reflects the brief delay between the depolarization of the muscle fibers (recorded by the EMG electrodes) and the movement of the vibrissa (recorded by the CCD). In each trace, positive deflections indicate a protraction, whereas negative deflections indicate a retraction. B: comparison of whisking kinematics measured from vibrissa movements (CCD) and those predicted from the EMG envelope. Values for normalized movement amplitude, velocity, and acceleration peaks are well correlated. R-values represent Pearson’s product moment correlation coefficient. Also shown are the regression lines for protraction (•) and retraction (▴) data.
FIG. 4.
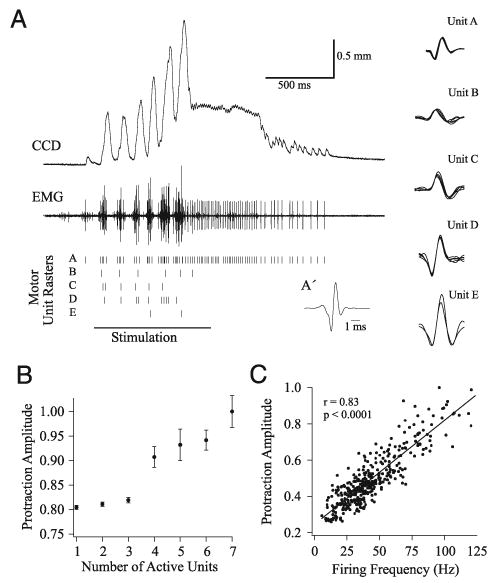
Decomposition of EMGs into constituent motor units reveals motoneuron activity during ICMS-evoked rhythmic whisking. A: representative example of EMG decomposition, where 5 motor units were isolated from the EMG. Rasters for each motor unit are shown below the EMG, and representative motor unit waveforms are to the right. Spike-triggered average (triggered by unit A) of the sustained protraction of the EMG is shown in A′. Motor units showed 2 general firing patterns: firing in a 1:1 manner with vibrissa deflections (e.g., unit B), or short trains of action potentials per whisk (e.g., unit A). B: group data (70 deflections over 3 stimulus epochs) reveal that the number of active motor units is correlated with the amplitude of vibrissa protraction. Amplitude is normalized to the largest evoked protraction. C: firing rate of a motor unit (371 whisks) is positively and significantly correlated with protraction amplitude (normalized to the largest protraction).
FIG. 6.

Activity of vibrissa motoneurons (vMNs) during ICMS-evoked whisking. A: intrinsic EMG (top) and extracellular recordings (bottom) from isolated vMNs show similar firing patterns as observed in motor unit recordings: firing single action potentials (unit A) or trains of action potentials (unit B) with each whisk. B: intervals between successive whisks are significantly and positively correlated with the firing intervals (interspike or interburst interval) of vMNs. C: vMN burst frequencies within a whisk are correlated with whisk amplitudes.
FIG. 7.

Serotonin receptor antagonist, metergoline, suppresses motor unit firing rates and whisking. A: ICMS-evoked vibrissal EMG recorded before (#1) and after (#2–#5) application of metergoline to vMNs. ICMS epochs (1-s duration) indicated by horizontal lines below each trace. Trace numbers correspond to the time points in the spectrogram below. B: spectrogram of ICMS-evoked whisking epochs showing the progressive reduction in frequency, and subsequent recovery, after metergoline application (at time indicated by arrowhead). Abscissa depicted in C. C: mean firing rates (calculated over the 1-s ICMS epoch) of motor units isolated from the EMG. Firing rates decrease reversibly after drug application. MUAP waveforms for each motor unit are shown in the insets. Note that the interstimulus periods between ICMS epochs (3–5 min) have been removed for clarity.
To quantify ICMS-evoked whisking amplitudes and velocities we used a CCD device to track the movements of individual vibrissae (see METHODS). In 513 whisks monitored by the CCD device whisk amplitudes ranged from 0.01 to 3.7 mm (approximately 0.04–14°). Larger whisks were generated during higher-frequency whisking epochs (Fig. 1B).
The relationship between protraction and retraction velocities is shown in Fig. 1C, where protraction velocities are plotted as a function of the subsequent retraction velocity. The color of each data point indicates the amplitude of the corresponding whisk. At low whisk amplitudes, most retractions were slower than the preceding protractions (see unity line Fig. 1C). As whisk amplitudes increased, retraction velocities exceeded protraction velocities, mimicking observations from voluntarily whisking rats (Bermejo et al. 1998; Carvell and Simons 1990). Whisk amplitudes and velocities were highly correlated (P < 0.0001), but these correlations were higher for retractions (r = 0.97) compared with protractions (r = 0.88; Fig. 1D).
Protraction set points often changed during ICMS-evoked whisking epochs, such that rhythmic whisking occurred on top of a sustained protraction (see Figs. 3 and 4). We never observed a net retraction of the vibrissa past the initial set point.
Stimulation of vibrissa motor cortex activates the whisking CPG
Epochs of ICMS-evoked whisking were consistently preceded by relatively long onset latencies. Further, whisking occurred at frequencies (5–15 Hz) that were significantly different from the stimulation frequencies (50–90 Hz). Both of these characteristics varied with the stimulation intensity. Onset latency decreased exponentially with increasing stimulation intensity (Fig. 2A); on average, the shortest latency (evoked by the highest stimulation intensity) was 200 ± 100 ms. As a result, the duration of whisking was proportional to the ICMS intensity.
FIG. 2.

ICMS parameters affect whisking kinematics. A: whisking onset latency decreases with stimulation intensity. Results for a single stimulation series (4 repetitions at each intensity) are shown. Inset: group data for 124 trials, normalized to the shortest latency (200 ± 100 ms,; range 30 to 300 ms) and threshold for evoking whisking. B: frequency of ICMS-evoked whisking increases with stimulation intensity, but is relatively unaffected by changes in ICMS frequency. Values in A and B are presented as means ± SE.
Evoked whisking frequency increased with stimulation intensity (Fig. 2B). In contrast to the effects of stimulation intensity, stimulation frequency had little effect on the evoked whisking frequency. This is evidenced by the large degree of overlap in the three traces in Fig. 2B, representing trials with stimulation at 50, 70, and 90 Hz.
Together, the relatively long onset latencies, the lack of a correlation between stimulation and whisking frequencies, and the ability of whisking to outlast the stimulation suggest that ICMS of vMCx is activating a CPG to evoke rhythmic whisking. These findings also suggest that vMCx activity regulates the output from the whisking CPG.
Activity of vibrissae motor units
Whisking, whether voluntary or evoked, is ultimately produced by the activity of vibrissae motor units, which consist of a motoneuron and the muscle fibers it innervates. Motor unit action potentials (MUAPs) can be extracted from the EMG by decomposition to reveal the activity of motoneurons contributing to a particular movement (Merletti and Parker 2004). Because motoneuron behavior reflects the drive they receive, analysis of their activity provides insights into the composition of the whisking CPG. If the EMG is also correlated with whisker movements, as suggested by Carvell et al. (1991), then the EMG can be used as a single metric to investigate both the drive from the whisking CPG and the conversion of this drive into movements by vibrissa motor units.
To ascertain that the EMG accurately reflects whisking kinematics we monitored ICMS-evoked movements of a single vibrissa with a CCD device, simultaneously with the corresponding vibrissal EMG from the intrinsic muscles (see METHODS). An example of such simultaneous recordings is shown in Fig. 3A, where we plot both the output from the CCD and the EMG records. Note that the EMG envelope—computed by rectifying and smoothing the raw EMG signal—closely resembles the output from the CCD. Rectifying the EMG is justified because ICMS did not produce retractions beyond the set point of each whisk (see above).
To quantify the relationship between vibrissa position and EMG recordings, we plotted the peak vibrissa protraction and retraction amplitudes measured by the CCD device against those measured from the corresponding EMG envelope. Both protraction and retraction peak amplitudes, determined from the two normalized signals, were significantly correlated (Fig. 3B). Similarly, peak vibrissa velocities and accelerations calculated from the two signals were also significantly correlated (Fig. 3B). These results indicate that the vibrissal EMG provides an accurate representation of whisking kinematics and that the activity of motor units extracted from the EMG can be reliably correlated with whisking kinematics.
To investigate the correlation between the activity of vibrissa motor units and whisking kinematics, we decomposed vibrissal EMGs into their constituent motor units (see METHODS). A representative example of EMG decomposition analysis is shown in Fig. 4A, where the MUAP waveforms for five motor units isolated from the EMG are shown to the right. Below the EMG are the MUAP rasters where each tick represents a discharge by that particular motor unit within the EMG. In this example, the epoch of rhythmic whisking was followed by a sustained protraction, and the latter was followed by several rhythmic protractions. During these events, a single motor unit was active in the EMG (motor unit A), suggesting that a single motor unit firing a single MUAP can produce measurable vibrissa movements. This conclusion is also supported by examining the spike-triggered average—triggered by unit A— of the sustained protraction phase of the EMG (Fig. 4A′).
Movements during the period of rhythmic whisking showed a gradual increase in amplitude and, during this period, motor unit activity changed in two mutually reinforcing ways: some motor units increased the number of MUAPs fired per whisk (e.g., motor unit A), whereas additional, previously silent motor units were recruited. We quantified this behavior by plotting the mean (n = 70 whisks) peak protraction amplitudes against the number of active motor units (Fig. 4B), revealing a direct correlation between these variables. To explore the relationship between firing rate and whisk amplitude we plot data from an experiment (371 whisks) in which EMG activity was dominated by a single motor unit (Fig. 4C); there was a significant, positive correlation between these variables. We observed similar relationships between whisking amplitudes and both the number of active units and their firing rates in all whisking epochs examined (n = 94).
Motor units also showed a stereotypical recruitment order such that, within a whisk, units with low-amplitude MUAPs tended to be recruited earlier than units with larger MUAPs. A representative example of this recruitment order is shown in Fig. 5A for five motor units from nine consecutive ICMS-evoked whisking epochs. Motor units are ranked according to the size of their MUAP with motor unit “A” having the smallest peak-to-peak amplitude and motor unit “E” having the largest. Figure 5A (left panel, “Order”) indicates which motor units were active during a whisk along with the order of their appearance. The diamonds above this panel represent the relative size of each motor unit. With only a single exception (whisk 6), motor units A, C, D, and E were recruited in order according to the size of their respective MUAPs. Motor unit B, however, was an exception to this recruitment order and was often recruited after larger motor units. Figure 5B summarizes the motor unit recruitment order for 73 whisks in which two to five motor units were active. Recruitment order obeyed the size principle in 73% of the whisks. This figure was highest (94%) when only two motor units participated. In the majority of the remaining trials (involving three to five motor units) no more than one of the units was recruited out of order (Fig. 5B).
FIG. 5.
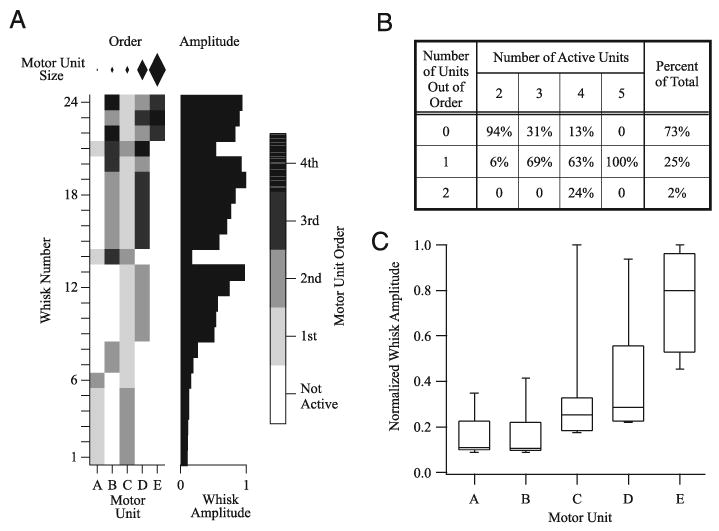
Recruitment order vibrissa motor units. A: behavior of 5 motor units (abscissa) isolated from EMG recorded during 24 whisks (ordinate). Diamonds at the top represent the relative peak-to-peak amplitudes of the motor units. Left panel (“Order”) depicts, in gray scale, the recruitment order of the motor units. With the exception of unit B, motor units were recruited according to their motor unit action potential (MUAP) amplitudes in 23 of 24 whisks. Right panel (“Amplitude”) plots the normalized amplitude of each whisk: larger whisks are associated with recruitment of additional and larger units. B: summary of motor unit recruitment order for 73 whisks. Motor units were recruited according to the size principle in the majority of whisks (73%). Only 25% of whisks had a single motor unit recruited out of order and only 2% had 2 units out of order. C: relationship between the size of a motor unit and the corresponding whisk amplitude for 73 whisks. Larger motor units were associated with larger-amplitude whisks. Median whisk amplitude is indicated by a line within each box. Box lower and upper limits represent the 25th and 75th percentiles of whisk amplitudes, whereas the whiskers encompass the 10th and 90th percentiles.
Motor units with larger-amplitude MUAPs were associated with larger-amplitude whisks. This relationship is seen in Fig. 5A (right panel, “Amplitude”) as an increase in whisk amplitude as the larger motor units (D and E) were recruited. Group data for 73 whisks (Fig. 5C) show the same relationship: as larger motor units were recruited, whisk amplitude increased. These results indicate that whisking amplitude is regulated through both the recruitment of motor units and the modulation of the motor unit firing frequencies within each whisk.
Activity of vibrissa motoneurons
We observed similar firing patterns in extracellular recordings from vibrissa motoneurons (vMNs; n = 7) during ICMS-evoked whisking. To our knowledge, these data constitute the first recordings obtained from vMNs in vivo. Representative examples of vMN activity during ICMS-evoked whisking are shown in Fig. 6A. As we observed in motor units isolated from the EMG (Fig. 4A), during each whisk vMNs fired either a single action potential or bursts of action potentials (Fig. 6A). The interspike or interburst intervals recorded from vMNs correlated significantly with interwhisk intervals (Fig. 6B), confirming that the firing rates of vMNs determine the whisking frequency. We found similar, high correlations (r > 0.99) in all seven vMNs. The firing rate of vMNs within each whisk was significantly correlated with protraction amplitudes in six of seven vMNs (Fig. 6C; r = 0.36–0.45, P < 0.05). This finding is consistent with the conclusion (see above) that whisking amplitude is modulated by the firing frequency of motor units.
Frequency of ICMS-evoked whisking depends on serotonin
Previous work in our laboratory demonstrated that serotonin (5HT), acting through 5HT2 or 5HT3 receptors, is necessary and sufficient for rhythmic whisking and that, in vitro, 5HT evokes rhythmic firing in vMNs at whisking frequencies (Hattox et al. 2003). These findings suggest that serotonergic drive from a component of the whisking CPG could establish the whisking frequency through its actions on vMNs. To test whether ICMS-evoked whisking operates through a similar mechanism, we infused the 5HT receptor antagonist metergoline onto vMNs in the lateral facial nucleus (LVII) during repeated epochs of ICMS-evoked whisking.
In eight of nine trials from five rats, metergoline (100 μM to 1 mM) caused a reversible suppression or a complete inhibition of ICMS-evoked whisking (Fig. 7). The effects of metergoline were manifest as a sharp reduction in spectral power of whisking frequencies (Fig. 7A). At higher drug concentrations (500 μM or 1 mM) suppression averaged 99 ± 1% (n = 3, range 98–100%, P < 0.012). At lower concentrations (100–200 μM, n = 5) the suppression averaged 77 ± 7% (range 66–94%, P < 0.011).
Before complete suppression, metergoline occasionally caused a reduction in the whisking frequency (Fig. 7B, immediately after drug infusion). This trend was particularly evident at the lower antagonist concentrations. Similarly, recovery to control whisking frequencies was preceded by a gradual increase in evoked whisking frequencies (Fig. 7B).
To determine the effects of metergoline on vMN firing, we isolated motor units from the EMG, as described above. Analysis of the two motor units that could be reliably isolated from the EMG in Fig. 7A reveals that metergoline caused a gradual and reversible suppression of their firing rates (Fig. 7C). We observed similar effects in all eight trials in which metergoline had an effect on evoked whisking.
Control infusions of buffered saline (n = 4) or placement of the probe without injection (n = 1) had no effect on evoked whisking.
These results suggest that 5HT, acting on 5HT receptors expressed by vMNs (Larkman et al. 1989; VanderMaelen and Aghajanian 1980), affect the firing rate of these neurons, thereby regulating whisking frequency.
DISCUSSION
The use of anesthetized preparations in which naturalistic movements can be elicited by cortical stimulation has helped elucidate the function of the motor cortex (Graziano et al. 2002; Iriki et al. 1988; Katakura and Chandler 1990). Motivated by the recent findings of Haiss and Schwarz (2005) in awake rats, we developed an anesthetized rat preparation in which cortical regulation of a well-defined behavior—whisking—can be investigated. Our results support the hypothesis that voluntary whisking is produced by a cortically modulated central pattern generator (CPG), and suggest that drive from this CPG regulates vibrissa motoneuron recruitment and firing frequencies to modulate whisking kinematics.
Kinematics of ICMS-evoked whisking
Whisking epochs evoked by ICMS of vMCx in the anesthetized rat mimicked the spatial and spectral characteristics of voluntary whisking. Evoked whisking amplitudes approached the lower ranges of voluntary whisking (Carvell and Simons 1990) and ICMS-evoked whisking in the awake rat (Berg and Kleinfeld 2003b; Haiss and Schwarz 2005), particularly during higher-frequency evoked whisking, where amplitudes reached 14°. Evoked whisking frequencies ranged from 5 to 15 Hz, a range characteristic of voluntary exploratory whisking (Berg and Kleinfeld 2003b; Hattox et al. 2003). Notably, the whisking frequency differed significantly from the stimulation frequency (50–90 Hz), suggesting activation of a subcortical CPG rather than direct cortical control of each whisk cycle.
Whisking is produced by the periodic activation of the intrinsic muscles to generate rhythmic protractions of the vibrissae (Dorfl 1982). Retraction of the vibrissae can occur either through the passive relaxation of tissue surrounding the vibrissa or through activation of extrinsic muscles (Berg and Kleinfeld 2003a; Dorfl 1982). During ICMS-evoked whisking, whisk amplitudes were more strongly correlated with retraction velocities than with protraction velocities (Fig. 1D). The lower variability between whisk amplitudes and retraction velocities could reflect: 1) more reliable recruitment of extrinsic motor units that generate more reproducible movements than intrinsic motor units or 2) dependency of retractions on a single variable: the passive relaxation of the surrounding tissue. Although we cannot exclude the involvement of extrinsic motor units, the observation that vibrissae did not retract past their initial starting position suggests that retractions during ICMS-evoked whisking were generated passively.
During voluntary whisking, retraction velocities typically exceed protraction velocities (Bermejo et al. 1998; Carvell and Simons 1990). We observed a similar relationship during large-amplitude ICMS-evoked whisks, whereas during low-amplitude whisks retraction velocities were frequently smaller in magnitude than the corresponding protraction velocities. Smaller protractions produce relatively little contractile tension in the whisker pad and therefore a passive retraction will exhibit low velocity. In contrast, after a large protraction, the higher tension in the tissue might accelerate the retraction phase of the whisk. The exponential relationship between protraction and retraction velocities (see Fig. 1C) suggests that as whisking amplitudes increase, retraction velocities exceed protraction velocities, matching observations from voluntary whisking.
ICMS-evoked whisking in the awake rat is accompanied by shifts in the whisking set point toward either a retracted or a protracted position, depending on the location of the stimulating electrode within vMCx (Haiss and Schwarz 2005). We also observed a shift in the set point during ICMS-evoked whisking in the anesthetized rat; however, shifts toward a retracted position were not observed. Such shifts toward a retracted state could result from current spread, or activation of intracortical connections, from the electrode in the RW region into the adjacent retraction-face (RF) region. The failure to observe a retracted shift in the whisking set point in the anesthetized animal might reflect anesthesia-dependent reduction in effective current spread from RW into RF.
We focused on whisking evoked through ICMS of the RW subregion of vMCx. Stimulation within this cortical region generates rhythmic activation of the intrinsic muscles and, consequently, rhythmic protractions. Stimulation in the surrounding RF region evokes complex facial movements, including a sustained retraction of the vibrissal pad (Haiss and Schwarz 2005), presumably through activation of extrinsic muscles. These two cortical regions might serve different functions during whisking: Activity in the RW region might regulate the output from the whisking CPG to modulate whisking kinematics, whereas the RF region may affect the set point around which the rhythmic whisking occurs.
Motor unit activity and whisking
To our knowledge, our experiments report the first recordings from vibrissa motoneurons (vMNs) and motor units obtained during whisking. These data, along with analyses of motor units isolated from the EMGs, indicate that whisking kinematics are modulated by the number of active motor units and their firing rates. In investigating this relationship between motor unit activities and whisking, we focused primarily on whisking frequency and amplitude because these variables provide a complete description of this behavior.
We found that during each whisk, some motor units often fire a single motor unit action potential (MUAP) and vMNs fire individual spikes. We also found that, barring contributions from undetected motor units, a single MUAP is sufficient for producing a vibrissa protraction without the need for fusion of a MUAP train (Fig. 4A). We suggest that this fundamental firing frequency of motoneurons is sufficient to establish the whisking frequency. Indeed, the interspike interval (or interburst interval for bursting vMNs) is strongly correlated with the interval between whisks (Fig. 6B).
During larger-amplitude whisks, the firing rate of active motor units increases and additional motor units are recruited (Fig. 4). These relationships suggest that vibrissa motor units use a combination of rate coding and motor unit recruitment to modulate protraction amplitudes, in a manner similar to force modulation in skeletal motor systems (De Luca et al. 1982; Merletti and Parker 2004).
Motor units recruited during larger-amplitude protractions tended to have larger-amplitude MUAP waveforms than those of units recruited during smaller movements (Fig. 5). Because the size of MUAPs is correlated with the twitch force of the motor unit (Goldberg and Derfler 1977) and the size of the motor unit is correlated with the size of the motoneuron, this finding suggests that vibrissa motor units are recruited according to the size principle proposed by Henneman et al. (1965). Because the size and shape of MUAPs recorded at the EMG electrode depends on factors such as the relationship between the motor unit and the recording electrode (Merletti and Parker 2004) we interpret these findings cautiously. However, several observations suggest that these potential confounds did not have a significant impact on our recordings. First, the shape of the MUAP from each motor unit remained constant throughout the ICMS-evoked whisking epoch, suggesting that the electrode did not shift during recordings. Second, this apparent recruitment order was observed in the majority of ICMS-evoked EMGs, making it unlikely that this observation was a result of random factors.
Serotonin and whisking
Serotonin (5HT) is necessary for voluntary whisking (Hattox et al. 2003). In line with this observation, we find that 5HT is also necessary for ICMS-evoked whisking. Thus whisking evoked by ICMS of vMCx likely activates the same circuitry active during voluntary whisking. Furthermore, the finding that the 5HT receptor antagonist metergoline reduces ICMS-evoked whisking frequency suggests that 5HT is not simply an ON–OFF switch that permits whisking. Rather, it suggests that the concentration of 5HT affects the whisking frequency.
Vibrissa motor units and motoneurons are capable of firing bursts of action potential during each whisk. Whether this burst frequency is established by 5HT as well, possibly by regulating the membrane properties of vMNs, or is produced independently by a second set of nonserotonergic inputs remains to be determined. In vitro, 5HT evokes single action potentials in vMNs at whisking frequencies (Hattox et al. 2003), suggesting that a second, independent drive might be responsible for bursting; however, if present, this additional drive remains to be identified.
The role of vMCx in whisking
Although it is widely assumed that vMCx participates in voluntary whisking, the mechanisms by which this occurs remain to be established. One line of evidence suggests that whisking might be controlled by the vMCx on a cycle-by-cycle basis. Berg and Kleinfeld (2003b) reported that stimulation of the vMCx at whisking frequencies evokes vibrissae movements entrained to the stimulation frequency. This result coupled with the recent findings that vibrissa motoneurons receive direct, albeit sparse, projections from vMCx (Grinevich et al. 2005) suggest that vMCx can, in principle, control whisking on a cycle-by-cycle basis.
Other data suggest that rhythmic whisking is generated by a subcortical central pattern generator (CPG), under modulatory control of the vMCx. Whisking persists after sensory denervation (Welker 1964), decerebration (Lovick 1972), or cortical ablation (Gao et al. 2003; Semba and Komisaruk 1984), indicating that vMCx is not necessary for rhythmic whisking. Extracellular recordings from single vMCx units in awake behaving rats failed to identify covariations between task-related discharges and vibrissae movements (Carvell et al. 1996). Furthermore, we recently demonstrated that vMCx activity precedes the onset of voluntary whisking and that rhythmic whisking outlasts vMCx activity, consistent with activation of a whisking CPG by vMCx (Friedman et al. 2006). Our results, in conjunction with those of Haiss and Schwarz (2005), also suggest that vMCx does not drive whisking on a cycle-by-cycle basis. We find that ICMS of the rhythmic subregion of vMCx evokes whisking at frequencies distinct from the stimulation frequency. In addition, our findings show that the frequency of ICMS-evoked whisking varies with stimulation intensity, that movements are preceded by relatively long onset latency, and that movement can outlast the stimulus. Furthermore, as is observed in voluntary whisking (Hattox et al. 2003), ICMS-evoked whisking also requires 5HT. These observations are consistent with the hypothesis that vMCx controls whisking by regulating a subcortical whisking CPG with an essential 5HT component.
Obviously, the two control strategies are not mutually exclusive (see Brecht 2004). Indeed, Brecht et al. (2004) found that stimulation of layer V vMCx neurons evokes vibrissae movements entrained to the stimulation frequency, whereas stimulation of layer VI neurons produces bouts of whisking that are out of phase across trials. Thus vMCx might use different control strategies to produce or modulate rhythmic whisking.
Acknowledgments
We are grateful to Y. Li and Dr. Larisa Sellers for excellent technical assistance and J. B. Swartz for helpful comments.
Footnotes
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-35360 to A. Keller and NS-053306 to N. P. Cramer.
References
- Ahrens KF, Kleinfeld D. Current flow in vibrissa motor cortex can phase-lock with exploratory rhythmic whisking in rat. J Neurophysiol. 2004;92:1700–1707. doi: 10.1152/jn.00020.2004. [DOI] [PubMed] [Google Scholar]
- Arshavsky YI, Deliagina TG, Orlovsky GN. Pattern generation. Curr Opin Neurobiol. 1997;7:781–789. doi: 10.1016/s0959-4388(97)80136-5. [DOI] [PubMed] [Google Scholar]
- Berg RW, Kleinfeld D. Rhythmic whisking by rat: retraction as well as protraction of the vibrissae is under active muscular control. J Neurophysiol. 2003a;89:104–117. doi: 10.1152/jn.00600.2002. [DOI] [PubMed] [Google Scholar]
- Berg RW, Kleinfeld D. Vibrissa movement elicited by rhythmic electrical microstimulation to motor cortex in the aroused rat mimics exploratory whisking. J Neurophysiol. 2003b;90:2950–2963. doi: 10.1152/jn.00511.2003. [DOI] [PubMed] [Google Scholar]
- Bermejo R, Houben D, Zeigler HP. Optoelectronic monitoring of individual whisker movements in rats. J Neurosci Methods. 1998;83:89–96. doi: 10.1016/s0165-0270(98)00050-8. [DOI] [PubMed] [Google Scholar]
- Brecht M. What makes whiskers shake? Focus on “current flow in vibrissa motor cortex can phase-lock with exploratory rhythmic whisking in rat. J Neurophysiol. 2004;92:1265–1266. doi: 10.1152/jn.00404.2004. [DOI] [PubMed] [Google Scholar]
- Brecht M, Preilowski B, Merzenich MM. Functional architecture of the mystacial vibrissae. Behav Brain Res. 1997;84:81–97. doi: 10.1016/s0166-4328(97)83328-1. [DOI] [PubMed] [Google Scholar]
- Brecht M, Schneider M, Sakmann B, Margrie TW. Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature. 2004;427:704–710. doi: 10.1038/nature02266. [DOI] [PubMed] [Google Scholar]
- Carvell G, Simons DJ. Biometric analyses of vibrissal tactile discrimination in the rat. J Neurosci. 1990;10:2638–2648. doi: 10.1523/JNEUROSCI.10-08-02638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvell GE, Miller SA, Simons DJ. The relationship of vibrissal motor cortex unit activity to whisking in the awake rat. Somatosens Mot Res. 1996;13:115–127. doi: 10.3109/08990229609051399. [DOI] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ, Lichtenstein SH, Bryant P. Electromyographic activity of mystacial pad musculature during whisking behavior in the rat. Somatosens Mot Res. 1991;8:159–164. doi: 10.3109/08990229109144740. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Behaviour of human motor units in different muscles during linearly varying contractions. J Physiol. 1982;329:113–128. doi: 10.1113/jphysiol.1982.sp014293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfl J. The musculature of the mystacial vibrissae of the white rat. J Anat. 1982;135:147–154. [PMC free article] [PubMed] [Google Scholar]
- Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. J Neurophysiol. 1999;81:2243–2252. doi: 10.1152/jn.1999.81.5.2243. [DOI] [PubMed] [Google Scholar]
- Friedman WA, Jones LM, Cramer NP, Kwegyir-Afful EE, Zeigler HP, Keller A. Anticipatory activity of motor cortex in relation to rhythmic whisking. J Neurophysiol. 2006;95:1274–1277. doi: 10.1152/jn.00945.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Hattox AM, Jones LM, Keller A, Zeigler HP. Whisker motor cortex ablation and whisker movement patterns. Somatosens Mot Res. 2003;20:191–198. doi: 10.1080/08990220310001622924. [DOI] [PubMed] [Google Scholar]
- Goldberg LJ, Derfler B. Relationship among recruitment order, spike amplitude, and twitch tension of single motor units in human masseter muscle. J Neurophysiol. 1977;40:879–890. doi: 10.1152/jn.1977.40.4.879. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Taylor CSR, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- Grinevich V, Brecht M, Osten P. Monosynaptic pathway from rat vibrissa motor cortex to facial motor neurons revealed by lentivirus-based axonal tracing. J Neurosci. 2005;25:8250–8258. doi: 10.1523/JNEUROSCI.2235-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiss F, Schwarz C. Spatial segregation of different modes of movement control in the whisker representation of rat primary motor cortex. J Neurosci. 2005;25:1579–1587. doi: 10.1523/JNEUROSCI.3760-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattox AM, Li Y, Keller A. Serotonin regulates rhythmic whisking. Neuron. 2003;39:342–352. doi: 10.1016/s0896-6273(03)00391-x. [DOI] [PubMed] [Google Scholar]
- Hattox AM, Priest CA, Keller A. Functional circuitry involved in the regulation of whisker movements. J Comp Neurol. 2002;442:266–276. doi: 10.1002/cne.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Iriki A, Nozaki S, Nakamura Y. Feeding behavior in mammals: corticobulbar projection is reorganized during conversion from sucking to chewing. Dev Brain Res. 1988;44:189–196. doi: 10.1016/0165-3806(88)90217-9. [DOI] [PubMed] [Google Scholar]
- Iwaniuk AN, Whishaw IQ. On the origin of skilled forelimb movements. Trends Neurosci. 2000;23:372–376. doi: 10.1016/s0166-2236(00)01618-0. [DOI] [PubMed] [Google Scholar]
- Katakura N, Chandler SH. An iontophoretic analysis of the pharmacologic mechanisms responsible for trigeminal motoneuronal discharge during masticatory-like activity in the guinea pig. J Neurophysiol. 1990;63:356–369. doi: 10.1152/jn.1990.63.2.356. [DOI] [PubMed] [Google Scholar]
- Klein BG, Rhoades RW. Representation of whisker follicle intrinsic musculature in the facial motor nucleus of the rat. J Comp Neurol. 1985;232:55–69. doi: 10.1002/cne.902320106. [DOI] [PubMed] [Google Scholar]
- Larkman PM, Penington NJ, Kelly JS. Electrophysiology of adult rat facial motoneurones: the effects of serotonin (5-HT) in a novel in vitro brainstem slice. J Neurosci Methods. 1989;28:133–146. doi: 10.1016/0165-0270(89)90018-6. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve. 2005;32:261–279. doi: 10.1002/mus.20333. [DOI] [PubMed] [Google Scholar]
- Lovick TA. The behavioral repertoire of precollicular decerebrate rats. J Physiol. 1972;224:4–6. [PubMed] [Google Scholar]
- Merletti R, Parker P. Electromyography. Hoboken, NJ: Wiley; 2004. p. 520. [Google Scholar]
- Neafsey EJ. The complete ratunculus: output organization of layer V of the cerebral cortex. In: Kolb B, Tees RC, editors. The Cerebral Cortex. Cambridge, MA: MIT Press; 1990. pp. 197–212. [Google Scholar]
- Sanderson KJ, Welker W, Shambes GM. Reevaluation of motor cortex and of sensorimotor overlap in cerebral cortex of albino rats. Brain Res. 1984;292:251–260. doi: 10.1016/0006-8993(84)90761-3. [DOI] [PubMed] [Google Scholar]
- Semba K, Komisaruk BR. Neural substrates of two different rhythmical vibrissal movements in the rat. Neurosci. 1984;12:761–774. doi: 10.1016/0306-4522(84)90168-4. [DOI] [PubMed] [Google Scholar]
- Stein PSG, Grillner S, Selverston AL, Stuart DG. Neurons, Networks, and Motor Behavior. Cambridge, MA: The MIT Press; 1997. p. 305. [Google Scholar]
- VanderMaelen C, Aghajanian G. Intracellular studies showing modulation of facial motoneurone excitability by serotonin. Nature. 1980;287:346–347. doi: 10.1038/287346a0. [DOI] [PubMed] [Google Scholar]
- Vincent SB. The function of the vibrissae in the behavior of the white rat. Behav Monogr. 1912;1:7–86. [Google Scholar]
- Weiss DS, Keller A. Specific patterns of intrinsic connections between representation zones in the rat motor cortex. Cereb Cortex. 1994;4:205–214. doi: 10.1093/cercor/4.2.205. [DOI] [PubMed] [Google Scholar]
- Welker WI. Analysis of sniffing of the albino rat. Behavior. 1964;22:223–244. [Google Scholar]


