Abstract
Peptidic mimics of the gut hormone glucagon-like peptide (GLP) 1, exemplified by the recently approved drug exenatide, show promise as therapies for type 2 diabetes. Such “incretin mimetics” regulate glucose appearance in the plasma and can restore glucose-stimulated insulin secretion without excess risk of hypoglycemia. The need for injection, which may limit the use of peptidic GLP-1 receptor (GLP-1R) agonists, has driven largely unsuccessful efforts to find smaller molecules. The failure to identify orally effective agonists has instead promoted the indirect approach of inhibiting the GLP-1-degrading enzyme dipeptidyl peptidase IV. Here we report a nonpeptidic GLP-1R agonist with sufficient activity to evoke effects in whole animals, including antidiabetic efficacy in db/db mice. Two substituted cyclobutanes (S4P and Boc5) were developed after screening a compound library against a cell line stably cotransfected with GLP-1R and a cAMP-responsive reporter. Each bound to GLP-1R and increased intracellular cAMP. Agonist effects were blocked by the GLP-1R antagonist exendin(9–39). Boc5 amplified glucose-stimulated insulin secretion in isolated rat islets. Both i.p. and oral administration of Boc5 dose-dependently inhibited food intake in mice, an effect that could be blocked by pretreatment with exendin(9–39). Daily injections of Boc5 into db/db mice reduced HbA1c to nondiabetic values, an effect not observed in ad libitum-fed or pair-fed diabetic controls. Thus, Boc5 behaved as a full GLP-1 mimetic in vitro and in vivo. The chemical genus represented by Boc5 may prompt the exploration of orally available GLP-1R agonists with potential utility in diabetes and obesity.
Keywords: diabetes, metabolism, therapeutics
The advancing epidemic of type 2 diabetes mellitus (1) now claims 9.3% of the U.S. population (2). The several antidiabetic effects of peptidic glucagon-like peptide (GLP) 1 receptor (GLP-1R) agonists, including glucose-dependent stimulation of insulin secretion (3), have promoted their development as therapeutics (4). Although GLP-1 itself can acutely (5) and chronically (6) normalize glucose in diabetic patients, its half-life is short (7), resulting in its abandonment as a drug in favor of longer-acting peptides such as exenatide and liraglutide. The considerable promise of peptidic “incretin mimetics” such as exenatide (BYETTA) with weight loss and near-normalization of HbA1c (8) may be diminished by their need for injection.
An alternate means to the development of orally available medicines is the inhibition of the enzyme primarily responsible for the degradation of endogenous GLP-1, dipeptidyl peptidase IV (9), but the efficacy of this approach may be limited by endogenous peptide secretion. There were several efforts to find nonpeptidic GLP-1R agonists. Although some small-molecule leads seemed to have at least partially activated the GLP-1 signaling pathway in vitro (10), there has been no report of effects in vivo. We here report an example of a nonpeptidic, orally available GLP-1R agonist with potentially beneficial effects on food intake, body weight, glucose tolerance, and HbA1c in db/db mice, a useful model of type 2 diabetes mellitus (11). Effects appear to be receptor-mediated and are blocked with the selective GLP-1R antagonist exendin(9–39) (12).
Results
Screening.
In an attempt to discover nonpeptidic small-molecule GLP-1R agonists, we screened a diverse library of 48,160 synthetic and natural compounds against HEK293 cells stably transfected with a rat GLP-1R vector and an multiple response element/cAMP response element-driven luciferase reporter plasmid (HEK293-rGLP-1R cells). The assay exhibited high signal-to-background and signal-to-noise ratios (39.6 and 236.7, respectively) and was amenable to high-throughput screening of GLP-1R agonists. Of five initial hits, two synthetic compounds (SH14800 and SH17249) were confirmed to invoke luciferase reactions, to elevate cAMP, and to displace [125I]GLP-1(7–36) amide from receptors [supporting information (SI) Fig. 5].
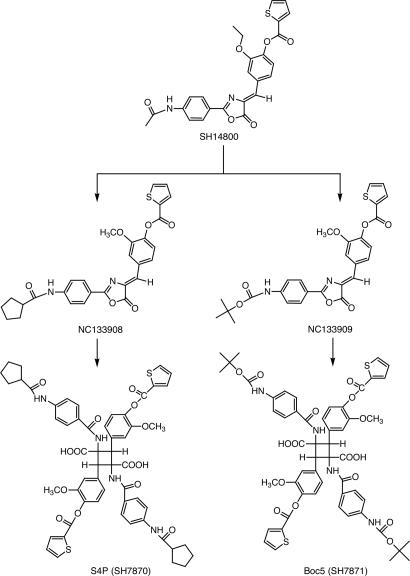
Minor structural modifications of SH14800 yielded compounds NC133908 and NC133909 (Fig. 1), with greater, but sporadic, biologic activity. It transpired that the bioactivity did not reside in these analogues themselves, but resulted from processes that occurred in DMSO solution and led to the formation of the substituted cyclobutane S4P (MW 1068) (Fig. 1), derived from NC133908. S4P, together with its analogue, Boc5 (MW 1076) (Fig. 1), derived from NC133909, were synthesized for further biological characterization.
Fig. 1.
Structures of SH14800, NC133908, NC133909, S4P, and Boc5.
In Vitro Characterization.
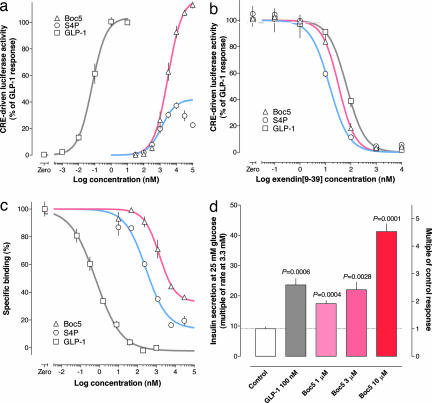
Both S4P and Boc5 dose-dependently elicited luciferase responses in HEK293-rGLP-1R cells with similar potencies. Whereas S4P at concentrations up to 10 μM evoked maximally only 37% of the GLP-1 response, Boc5 appeared to be a full agonist of GLP-1R (Fig. 2a). Neither compound nor GLP-1 affected vector control HEK293 cells expressing only an intact reporter system (which forskolin could directly activate) (13) (SI Fig. 6).
Fig. 2.
The effects of the substituted cyclobutanes are mediated through GLP-1R. (a) cAMP response element-driven luciferase activities were induced by different concentrations of GLP-1, S4P, and Boc5 in the HEK293-rGLP-1R cells. Estimated EC50 values were 68 pM, 1.08 μM, and 2.73 μM, respectively. (b) GLP-1R-mediated luciferase responses to Boc5 (10 μM), S4P (10 μM), and GLP-1 (0.05 nM) were each dose-dependently blocked by exendin(9–39). (c) Competitive inhibition of [125I]GLP-1(7–36) amide binding to GLP-1R by GLP-1, S4P, or Boc5. Estimated Ki values were 0.66 nM, 287 nM, and 1.47 μM, respectively. (d) The effect of Boc5 on the glucose-stimulated insulin secretion in rat isolated pancreatic islets. Symbols are means ± SEM in all panels. n = 4 measurements per symbol in a and d; n = 3 measurements per symbol in b and c.
The specificity of these ligands for GLP-1R was tested in HEK293 cells transiently transfected with human receptors for either of the related ligands, GLP-2 or glucagon. Like GLP-1, S4P and Boc5 did not activate these cells, whereas GLP-2 and glucagon each dose-dependently stimulated expression of their respective reporter genes. They were equally active at human and rat GLP-1Rs.
GLP-1R is a class B Gs-coupled membrane receptor, agonists of which activate adenylate cyclase and elevate intracellular cAMP content (14). GLP-1, S4P, and Boc5 each increased cAMP in HEK293-rGLP-1R cells, although the effects of two substituted cyclobutanes were less marked (SI Fig. 7). To further study whether cellular reactions were receptor-mediated, the GLP-1R antagonist exendin(9–39) was applied at different concentrations in the reporter assay before stimulation with GLP-1, S4P, or Boc5. Luciferase responses were completely blocked by exendin(9–39) at concentrations equal to or above 1 μM (Fig. 2b). Both S4P and Boc5 displaced [125I]GLP-1(7–36) amide from a GLP-1R-containing membrane preparation with similar affinities (Fig. 2c). Finally, we investigated the effects of Boc5 in a tissue preparation containing native GLP-1R, namely isolated rat pancreatic islets. Insulin secretion was increased 9.1-fold in control (vehicle-treated) islets when the superfusing glucose concentration was changed from 3.3 mM to 25 mM. This response was augmented by a factor of 2.6 after incubation with GLP-1 (100 nM). When incubated with Boc5 (up to 10 μM), glucose-stimulated insulin secretion was amplified by a factor of up to 4.5 (Fig. 2d).
Acute in Vivo Studies.
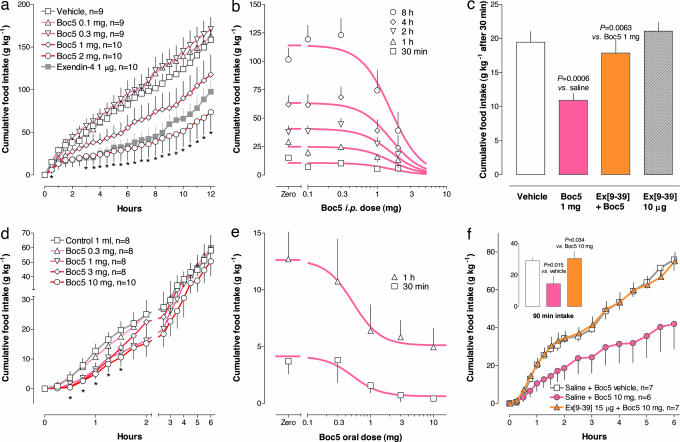
The reported effects of acutely injected GLP-1 mimetics include inhibition of food intake, slowing of gastric emptying, amplification of nutrient-stimulated insulin secretion, suppression of glucagon secretion, and restoration of impaired first-phase insulin secretion (15). To test for an acute anorectic effect Boc5 was injected i.p. into overnight-fasted female C57BL/6 (wild-type) mice. Boc5 dose-dependently inhibited food intake for at least 12 h (Fig. 3a). With the 2-mg dose, 12-h food intake was suppressed by 54 ± 13% (P = 0.0034). The estimated ED50 was 1.57 ± 0.09 mg (≈80 mg/kg) (Fig. 3b). When delivered orally in 1-ml gavage with 2% DMSO and 20% PEG400 in saline, Boc5 dose-dependently suppressed food intake for ≈2 h compared with vehicle controls, suggesting oral bioavailability (Fig. 3d). The ED50 for this anorectic effect was 0.53 ± 0.08 mg (Fig. 3e). The effects of i.p. and oral Boc5 were completely suppressed by pretreatment of mice with i.p. exendin(9–39) (Fig. 3 c and f).
Fig. 3.
The acute effect of Boc5 on food intake in female C57BL/6 mice. (a) Cumulative food intake up to 12 h after various i.p. doses of Boc5. Exenatide (1 μg) was used as control. (b) Dose–response characteristics. ED50 was estimated based on 9–10 measurements per group as a shared parameter for all time points. Hill slope was also shared. (c) Blockade of the 30-min anorectic effect of i.p. Boc5 at 1 mg by prior i.p. injection of exendin(9–39) at 10 μg. (d and e) Time course (d) and dose–response (e) of gavaged Boc5 on cumulative food intake. (f) Blockade of the 90-min anorectic effect of gavaged Boc5 (10 mg) by prior i.p. injection of exendin(9–39) at 15 μg. Symbols are means ± SEM, and asterisks indicate P < 0.05 in all panels. Ex(9–39), exendin(9–39).
Pharmacokinetic parameters were estimated after HPLC analysis of plasma sampled serially in three mice injected i.p. with 2 mg of Boc5. Terminal decay half-life was 7.5 ± 1.2 h (SI Fig. 8). Oral bioavailability was qualitatively affirmed after a 10-mg gavage by the appearance in plasma of Boc5 as a major peak.
Chronic in Vivo Studies.
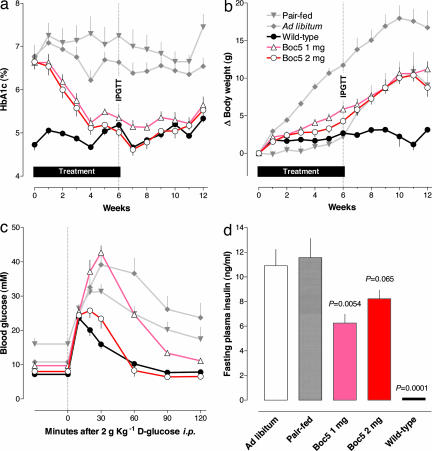
Chronic effects of peripherally administered GLP-1R agonists include weight loss in models of obesity, reduction in HbA1c and plasma lipids, restoration of β cell function, increases in insulin sensitivity in models of type 2 diabetes, and induction of β cell neogenesis and differentiation (15). Effects of chronically i.p. injected Boc5 and S4P were studied in C57BL/6J-m+/+ Leprdb (db/db) mice, a rodent model of type 2 diabetes. Based on the observation that HbA1c was robustly lowered with S4P administration (SI Fig. 9), it was used as the prime glycemic indicator for subsequent investigations with Boc5. By design, HbA1c was initially well matched in all db/db groups. In these groups it was significantly higher than in wild-type mice, in which it remained low throughout the experiment (Fig. 4a). HbA1c stayed high in db/db mice fed ad libitum. And even though their food intake was restricted by 24.9% (that is, to a level even less than that of wild-type mice) (SI Fig. 10), HbA1c continued to be high in pair-fed mice. In contrast, diabetic mice injected daily with either 1 mg or 2 mg of Boc5 i.p. exhibited a gradual reduction in HbA1c that became indistinguishable from that of wild-type mice after 4–6 weeks of dosing. HbA1c values after 6 weeks among wild-type and Boc5-treated (1 mg and 2 mg) groups were not different from each other but were significantly less than values in pair-fed or ad libitum-fed db/db mice (P < 0.0001 for all comparisons; Tukey–Kramer test). HbA1c remained normal for a further 6 weeks after cessation of Boc5 treatment in both dosage groups (Fig. 4a).
Fig. 4.
The effects of chronically administered Boc5 in diabetic db/db mice. (a) HbA1c measured weekly. (b) Body weight. (c) Excursion of plasma glucose after i.p. administration of 2 g of d-glucose after 6 weeks of treatment. (d) Fasting insulin concentration after 6 weeks of treatment. Symbols are means ± SEM in all panels. IPGTT, i.p. glucose tolerance testing.
Fasting blood glucose concentrations were expectedly more labile than HbA1c, but group responses were otherwise consistent with HbA1c results (SI Fig. 11). Glycemia, as reflected by either fasting glucose or HbA1c, was normalized with both 1-mg and 2-mg doses of Boc5.
Whereas body weight changed little in wild-type mice, it continually increased over the 6-week treatment period by ≈12 g in ad libitum-fed db/db mice, who consumed ≈23% more chow than wild-type mice (Fig. 4b). Weight changes in pair-fed, Boc5 (2 mg)-treated and wild-type mice were indistinguishable during the 6-week treatment period, and all showed less weight gain than did ad libitum-fed db/db mice (P < 0.0001 for all comparisons).
An i.p. glucose tolerance test was performed in half of the animals after 6 weeks of treatment and again 15 weeks after cessation of therapy. Glucose excursions after a 2-g glucose challenge were integrated as the area under the curve 0–120 min after glucose administration (Fig. 4c). After 6 weeks of therapy, the area under the curve was higher for ad libitum- and pair-fed db/db mice than for wild-type mice (P = 0.0075 and P = 0.0043, respectively). Mice treated with 2 mg of Boc5 demonstrated glucose excursions that were smaller than in ad libitum- and pair-fed controls (P = 0.0006 and P = 0.0006, respectively) and indistinguishable from those in wild-type mice (P = 0.64). Thus, glucose tolerance was normalized after chronic 2-mg Boc5 treatment. It was partly, but not fully, normalized with chronic administration of Boc5 at 1 mg per day. The effect to normalize glucose tolerance had reversed 15 weeks after cessation of Boc5 treatment. Glucose area under the curve was indistinguishable among all diabetic animals irrespective of prior treatment (P > 0.05 for all comparisons) but was different between wild-type mice and each diabetic group (ad libitum, P = 0.0026; pair-fed, P = 0.0153; 2 mg of Boc5, P = 0.0072; 1 mg of Boc5, P = 0.0052).
Fasting insulin concentrations in ad libitum-fed and pair-fed db/db mice were markedly higher than in wild-type mice (each P < 0.0001), indicating impaired insulin sensitivity. Fasting insulin in mice treated with 1 and 2 mg of Boc5 was lower, or trending lower, than in pair-fed controls (P = 0.0054 and P = 0.0653, respectively) (Fig. 4d).
Total cholesterol was reduced in terminal samples of the Boc5-treated cohort killed after 6 weeks (P = 0.0054 and P = 0.0002 for Boc5 at 2 mg vs. pair-fed and ad libitum-fed groups, respectively). This was principally driven by small decreases in low-density lipoprotein cholesterol (P = 0.045) and large reductions in high-density lipoprotein (P = 0.0005). The trend for reduction in triglycerides was not statistically significant.
No toxicities were detected with Boc5 in vitro [up to 300 μM in a HEK293 cell 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay] or in vivo (no elevation of the liver enzymes alanine aminotransferase and aspartate aminotransferase) in animals treated for 6 weeks.
Discussion
In Vitro Activity.
The molecule herein designated as Boc5 was derived from hits detected in a high-sensitivity screen for GLP-1R agonism. In the screening assay, which included artificially expressed GLP-1R in HEK293 cells, Boc5 behaved as a full agonist whereas the related molecule, S4P, behaved as a partial agonist. Agonism was inferred from an increase in reporter signal and also from an increase in cAMP. In contrast to the effects of forskolin, which acts directly on intracellular signaling (13), neither compound activated cells without GLP-1Rs. Likewise, neither compound activated cells expressing receptors for glucagon or GLP-2, which, like GLP-1, are also processed from pro-glucagon in a tissue-specific manner. As with GLP-1, cellular effects of S4P and Boc5 were blocked with exendin(9–39), a ligand reported to be a selective antagonist of GLP-1R (16). Finally, dose-dependent insulinotropic effects were observed in isolated rat pancreatic islets, a system in which endogenous GLP-1 sensitivity was manifest.
Although actions at yet-to-be-discovered receptors cannot be precluded, the most parsimonious explanation accounting for the concurrence of these findings is that S4P and Boc5 are agonists at GLP-1R. There have been several major pharmaceutical efforts to discover small-molecule GLP-1R agonists. Two patent disclosures report GLP-1 agonism in vitro (10, 17), but in neither of these was any in vivo activity reported. One publication describes a small-molecular-weight antagonist of GLP-1R.f The presence of in vivo activity was deemed important for these molecules to be considered as potential drug leads.
Acute in Vivo Activity.
A decrease in food intake in overnight-fasted mice may be used as a convenient measure of in vivo activity. However, similar effects could be obtained via receptors for a number of anorectic agonists, many of which are peptidic. Furthermore, reduction in food intake can be a manifestation of “sickness behavior” in response to a general toxic effect. However, agonism at alternate receptors, or anorexia due to a general toxicity, cannot be easily explained by abrogation of the response with the selective GLP-1R antagonist exendin(9–39). Thus, the anorectic effect of i.p. Boc5 is consistent with its behaving as a GLP-1R agonist in vivo. The effect of gavaged Boc5 to inhibit food intake could have been attributed to a behavioral response to the stress of handling. But the observation that it did not occur with vehicle treatment and was abrogated with prior treatment with exendin(9–39) suggests that it too is best explained as in vivo GLP-1R agonism. If this is indeed the case, then these experiments reveal a nonpeptidic GLP-1R agonist with acute in vivo activity.
Chronic in Vivo Activity.
As a further probe of in vivo activity, and of potential therapeutic utility, effects of chronic Boc5 administration were tested in db/db mice, a murine model of type 2 diabetes. These animals are characterized by hyperphagia due to defective leptin signaling (18), insulin resistance, and impaired β cell secretion and have been useful in characterizing potentially antidiabetic compounds (19).
Mice were used because of limited Boc5 supply, and i.p. injection was chosen as the route of administration because of uncertainty of oral bioavailability. Because red cell turnover is more rapid in rodents than in humans, HbA1c is sufficiently rapid to be a useful measure of glycemic changes (20) and was used here. The normalization of HbA1c with both 1-mg and 2-mg doses of Boc5 suggests that effects may have been near maximal and that efficacy may have been seen at lower doses. Differences in HbA1c comparable to those reported here were observed in db/db mice treated for 13 weeks with daily injections of exendin-4 (21).
The possibility that antidiabetic effects were related to reduced caloric intake (secondary, for example, to general malaise) was tested with the inclusion of a pair-fed control group. Despite being matched to the reduced food intake of the 2-mg Boc5-treated group, the pair-fed controls showed persistent diabetes. In another model of leptin signaling-deficient type 2 diabetes, the ZDF rat, pair-feeding similarly invoked only part of exendin-4's improvement in HbA1c and insulin sensitivity.g A comparable result was also seen in ZDF rats with the long-acting GLP-1 derivative NN2211 (liraglutide).h
Other aspects of metabolic improvement observed in the present study have been previously reported with peptidic GLP-1R agonists. Weight loss (20, 22, 23) is a feature of chronic GLP-1R agonist treatment, as are reductions in cholesterol,i triglycerides,j,k and other lipids (24). Cholesterol reduction was principally driven by small decreases in low-density lipoprotein cholesterol and large reductions in high-density lipoprotein, the latter being artifactually elevated in db/db mice and other models of disrupted leptin signaling (25).
Although insulin sensitivity was not measured directly in the present study, Boc5-mediated changes in fasting insulin concentration were consistent with an improvement in insulin sensitivity. Insulin sensitization is a feature of chronic (but not acute) exposure to GLP-1R agonism (20, 26–31).
Glucose tolerance after an i.p. glucose tolerance test was normalized in Boc5-treated animals and reverted upon withdrawal. This finding is consistent with a previous observation that chronic exenatide administration significantly improved glucose tolerance in db/db mice (32).
Durability of Antidiabetic Effect.
The antidiabetic effect of Boc5 extended well beyond the period of treatment in the present experiments. Although the mechanisms underlying this were not determined, potential explanations may include durable effects previously associated with chronic exenatide treatment. In those studies, in addition to an improvement in insulin sensitivity that extended beyond the period of treatment, there was an augmentation of the β cell mass appropriate for that degree of insulin sensitivity (33). Such an effect is consistent with reported effects of GLP-1R agonists to promote neogenesis and differentiation of β cellsl (34–36).
Summary.
The data presented here collectively support the substituted cyclobutane, Boc5, acting as a GLP-1R agonist. This interpretation is strengthened by blockade of both in vitro and in vivo effects with the specific GLP-1R antagonist exendin(9–39) and by the spectrum of biological actions observed in intact nondiabetic and diabetic animals. The actions reported here are consistent with this molecule being a nonpeptidic agent with sufficient activity at GLP1-R to evoke effects in whole animals. Although the observed effects point to a potential antidiabetic/antiobesity utility, an optimized drug will likely require greater potency. The findings reported here not only have the potential to spawn a new class of orally available GLP-1R agonists for treatment of metabolic diseases, but may also launch a wider general understanding of small-molecule agonism at other class B G protein-coupled receptors, therapeutic targets currently addressable only by injected macromolecules.
Materials and Methods
Construction of Plasmids, Cell Culture, and Stable Cell Lines.
The GLP-1R cDNA was amplified by RT-PCR from rat testis RNA and cloned into pCMV-Tag2B (Stratagene, La Jolla, CA). The sense and antisense primers used were 5′-GGGAATTCATGGCCGTCACCCCCAGCC-3′ and 5′-GGCTCGAGTCAGCTGCAGGA-ATTTTGGCAGGTGG-3′, respectively. The reporter gene was constructed by cloning three copies of multiple response element (5′-ATGCTAAAGGACGGTCACATTGCA-3′) and one copy of cAMP response element (5′-CGTCATACTGTGACGTC-3′) in front of the luciferase reporter plasmid, pGL3 vector (Promega, Madison, WI) (36). Three micrograms of pCMV-Tag2B-rGLP-1R and 1 μg of reporter gene plasmid were transfected into 1 × 105 HEK293 cells in a six-well plate. On the second day the cells were split into three 100-mm cell culture plates and 300 μg·ml−1 geneticin (Merck KGaA, Darmstadt, Germany) was added to the cell culture on the third day. The selection medium, i.e., DMEM containing 10% FBS in the presence of 400 μg·ml−1 geneticin, was changed every 3 days until colonies were formed. The single colony was picked up and expanded. The stable cell line expressing rGLP-1R was confirmed by a functional reporter assay in response to GLP-1 stimulation.
Reporter Gene Assay.
The HEK293-rGLP-1R cells were seeded onto 96-well cell culture plates with a density of 40,000 cells per well and incubated overnight. At the time of assaying, GLP-1(7–37) (Sigma, St. Louis, MO) or test compounds dissolved in DMSO were added (1–100 μl). In the blocking studies, cells were reacted with exendin(9–39) (Ana Spec, San Jose, CA) in DMSO (1 μl) for 10 min before the above procedure. After 6 h of incubation, they were lysed and quantified for luciferase activity by using the Steady-Glo luciferase assay system (Promega).
Receptor Binding Assay.
The membrane protein preparation was made from the HEK293-rGLP-1R cells as described previously (37). Seventeen micrograms of protein and 150 μg of FlashBlue beads (PerkinElmer, Boston, MA) were added to each well. After 5 h of incubation in the assay buffer at 4°C, 40 pM [125I]GLP-1(7–36) amide (Amersham, Piscataway, NJ), 2 μg·ml−1 aprotinin (Merck), 100 μM leupeptin (Merck), and different concentrations of GLP-1 or test compounds were added to give a final volume of 100 μl per well in a 96-well Isoplate (PerkinElmer). The plates were incubated at 4°C overnight and counted for radioactivity the following day.
Insulin Secretion from Isolated Pancreatic Islets.
The islets isolated from male Sprague–Dawley rats (38) were incubated first in 3.3 mM, then 25 mM glucose for 45 min in the presence or absence of 100 nM GLP-1 or different concentrations of Boc5. Insulin in the media was measured at the end of each incubation by using the mouse insulin ELISA kit (Linco Research, St. Charles, MO). The secretory response was calculated as the relative increase in 45-min insulin secretion under high- vs. low-glucose conditions. The insulinotropic effects of GLP-1 and Boc5 were expressed as the factor by which the secretory response was amplified.
Acute in Vivo Studies of Food Intake.
Overnight-fasted female C57BL/6 mice (6 weeks old, 18–20 g; Shanghai SLAC Laboratory Animals Co., Shanghai, China) were injected i.p. with vehicle or 0.1, 0.3, 1, or 2 mg of Boc5 dissolved in 1% DMSO and 20% PEG400 in saline. Individually caged mice were exposed to a preweighed food pellet, which was then reweighed every 15 or 30 min for 12 h to determine cumulative intake. A comparator group (n = 10) was injected i.p. with 1 μg of exendin-4 (Sigma) in saline. Vehicle alone (2% DMSO and 20% PEG400 in saline, 1 ml) or containing 0.3, 1, 3, or 10 mg of Boc5 was administered orally into mice prepared as above. Cumulative food intake was monitored for 6 h.
Chronic in Vivo Studies.
Eight-week-old C57BL/6J-m+/+ Leprdb (db/db) mice (Model Animal Research Center of Nanjing University, Nanjing, China) of both sexes were confirmed as diabetic and assigned into four treatment groups (n = 14–15 per group) with matched HbA1c (Glycosal HbA1c kit; Provalis Diagnostics, Deeside, U.K.; and DS1 Glycosal HbA1c Analyzer; Drew Scientific, Barrow in Furness, U.K.). One vehicle-treated control group was allowed to feed ad libitum. Two groups were injected i.p. once daily with 1 or 2 mg of Boc5 formulated as above for 42 days. To account for secondary effects that might be due to a drug-induced reduction in food intake, a pair-fed control group of db/db mice was presented 24 h later with the same quantity of food that the Boc5 2-mg-treated group had consumed. A further comparator group comprising the wild-type nondiabetic C57BL/6 littermates (n = 7) was used to index responses to normal values. HbA1c was measured weekly, and overnight fasting blood glucose was assayed twice per week by using a Freestyle Mini blood glucose monitoring system (TheraSense, Alameda, CA). Food intake and body weight were measured daily. After 42 days of treatment, half of the mice in each group (n = 7–8) were fasted overnight, measured for plasma insulin levels, and challenged i.p. with 2 g·kg−1 d-glucose (Sigma) followed by serial sampling of blood glucose. A terminal sample was taken for determination of total cholesterol, triglycerides, high-density lipoprotein, low-density lipoprotein, alanine aminotransferase, and aspartate aminotransferase. Drug treatment was stopped, and the remaining mice in each group were monitored for another 42 days. The pair-fed group was still constrained to a food intake as above.
Clinical Chemistry.
Plasma total cholesterol, triglycerides, high-density lipoprotein, low-density lipoprotein, alanine aminotransferase, and aspartate aminotransferase were analyzed by using a Hitachi (Tokyo, Japan) 7060 Automatic Analyzer with Roche (Indianapolis, IN) commercial kits.
Data Analysis.
Dose responses and concentration responses were analyzed by using Prism Version 4 (GraphPad Software, San Diego, CA) to fit four-parameter sigmoid functions. Pairwise comparisons were performed by using Tukey–Kramer tests for multiple comparisons and t tests for simple pairs by using InStat 3 (GraphPad). Data throughout are stated as means ± SEM. Two-tailed significance was tested at α = 0.05. Where possible, sample size was calculated from preliminary data by using power analysis, B = 0.8.
Supplementary Material
Acknowledgments
We thank Z. Zhang, H. Hu, W. L. Wang, H. Q. Zhang, X. Hui, J. Gao, L. Zhu, H. L. Lu, X. Y. Wu, and Q. R. Yang for technical assistance and D. E. Mais for critical review of the manuscript. This work was supported by grants from the Ministry of Science and Technology (2004CB518902), the Chinese Academy of Sciences (KSCX1-SW-11-2), the Natural Science Foundation of China (30540420135), the Shanghai Science and Technology Development Fund (03JC14022), and the K. C. Wong Education Foundation of Hong Kong.
Abbreviations
- GLP
glucagon-like peptide
- GLP-1R
GLP-1 receptor.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 689.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610173104/DC1.
Gronberg, A., Sol, E.-R., Danielsson, A., Colca, J. R., Bergsten, P. (2000) Diabetes 49:A251.
Jodka, C., Bhavsar, S., Gedulin, B., Krawiec, D., Tserlyuk, I. (1999) Diabetes 48(Suppl 1):A269.
Sturis, J., Jappe, M. B., Knudsen, L. B., Wilken, M., Gjedsted, A., Pimdahl, S., Gotfredsen, C. F. (2000) Diabetologia 43(Suppl 1):A145.
Gedulin, B., Smith, P., Young, A. (2001) Diabetologia 44(Suppl 1):A197.
Gotfredsen, C. F., Rolin, B., Sturis, J., Wilken, M., Nygaard, H., Carr, R. D., Knudsen, L. B. (2001) Diabetologia 44(Suppl 1):A196.
Kendall, D. M., Kim, D., Poon, T., Han, J., Schnabel, C., Fineman, M., Trautmann, M., Maggs, D. (2005) Diabetologia 48(Suppl 1):A389.
Bernard, C., Ilic, C., Guilbert, V., Ktorza, A. (1999) Diabetologia 42:A151.
References
- 1.Dove A. Nat Biotechnol. 2002;20:977–981. doi: 10.1038/nbt1002-977. [DOI] [PubMed] [Google Scholar]
- 2.Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, Saydah SH, Williams DE, Geiss LS, Gregg EW. Diabetes Care. 2006;29:1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 3.Mojsov S, Weir GC, Habener JF. J Clin Invest. 1987;79:616–619. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nauck MA, Meier JJ. Regul Pept. 2005;128:135–148. doi: 10.1016/j.regpep.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Diabetologia. 1993;36:741–744. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- 6.Rachman J, Barrow BA, Levy JC, Turner RC. Diabetologia. 1997;40:205–211. doi: 10.1007/s001250050664. [DOI] [PubMed] [Google Scholar]
- 7.Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Diabetes. 1995;44:1126–1131. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- 8.Ratner RE, Maggs D, Nielsen LL, Stonehouse AH, Poon T, Zhang B, Bicsak TA, Brodows RG, Kim DD. Diabetes Obes Metab. 2006;8:419–428. doi: 10.1111/j.1463-1326.2006.00589.x. [DOI] [PubMed] [Google Scholar]
- 9.Demuth HU, McIntosh CH, Pederson RA. Biochim Biophys Acta. 2005;1751:33–44. doi: 10.1016/j.bbapap.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Teng M, Truesdale LK, Bhumralkar D, Kiel D, Johnson MD, Thomas C, Jorgensen AS, Madsen P, Olesen PH, Knudsen LB, et al. 6,927,214. US Patent. 2005
- 11.Chang AY, Wyse BM, Gilchrist BJ, Peterson T, Diani AR. Diabetes. 1983;32:830–838. doi: 10.2337/diab.32.9.830. [DOI] [PubMed] [Google Scholar]
- 12.Göke R, Fehmann HC, Linn T, Schmidt H, Kraus M, Eng J, Göke B. J Biol Chem. 1993;268:19650–19655. [PubMed] [Google Scholar]
- 13.Kemp D, Habener J. Biochem Pharmacol. 2002;64:689–697. doi: 10.1016/s0006-2952(02)01212-1. [DOI] [PubMed] [Google Scholar]
- 14.Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Proc Natl Acad Sci USA. 1987;84:3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen LL, Young AA, Parkes DG. Regul Pept. 2004;117:77–88. doi: 10.1016/j.regpep.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 16.Schepp W, Schmidtler J, Riedel T, Dehne K, Schusdziarra V, Holst JJ, Eng J, Raufman JP, Classen M. Eur J Pharmacol. 1994;269:183–191. doi: 10.1016/0922-4106(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 17.Kopin AS, Beinborn M. WO2004103310. US Patent Appl. 2004
- 18.Lee G, Proenca R, Montez J, Carroll K, Darvishzadeh J, Lee J, Friedman J. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 19.Carroll MJ, Lister CA, Sennitt MV, Stewart-Long N, Cawthorne MA. Diabetes. 1985;34:1198–1204. doi: 10.2337/diab.34.11.1198. [DOI] [PubMed] [Google Scholar]
- 20.Young AA, Gedulin BR, Bhavsar S, Bodkin N, Jodka C, Hansen B, Denaro M. Diabetes. 1999;48:1026–1034. doi: 10.2337/diabetes.48.5.1026. [DOI] [PubMed] [Google Scholar]
- 21.Greig NH, Holloway HW, De Ore KA, Jani D, Wang Y, Zhou J, Garant MJ, Egan JM. Diabetologia. 1999;42:45–50. doi: 10.1007/s001250051111. [DOI] [PubMed] [Google Scholar]
- 22.Szayna M, Doyle ME, Betkey JA, Holloway HW, Spencer RG, Greig NH, Egan JM. Endocrinology. 2000;141:1936–1941. doi: 10.1210/endo.141.6.7490. [DOI] [PubMed] [Google Scholar]
- 23.Larsen PJ, Fledelius C, Knudsen LB, Tang-Christensen M. Diabetes. 2001;50:2530–2539. doi: 10.2337/diabetes.50.11.2530. [DOI] [PubMed] [Google Scholar]
- 24.Juntti-Berggren L, Pigon J, Karpe F, Hamsten A, Gutniak M, Vignati L, Efendic S. Diabetes Care. 1996;19:1200–1206. doi: 10.2337/diacare.19.11.1200. [DOI] [PubMed] [Google Scholar]
- 25.Silver DL, Jiang XC, Tall AR. J Biol Chem. 1999;274:4140–4146. doi: 10.1074/jbc.274.7.4140. [DOI] [PubMed] [Google Scholar]
- 26.Mizuno A, Kuwajima M, Ishida K, Noma Y, Murakami T, Tateishi K, Sato I, Shima K. Metab Clin Exp. 1997;46:745–749. doi: 10.1016/s0026-0495(97)90117-7. [DOI] [PubMed] [Google Scholar]
- 27.Sandhu H, Wiesenthal SR, Macdonald PE, Mccall RH, Tchipashvili V, Rashid S, Satkunarajah M, Irwin DM, Shi ZQ, Brubaker PL, et al. Diabetes. 1999;48:1045–1053. doi: 10.2337/diabetes.48.5.1045. [DOI] [PubMed] [Google Scholar]
- 28.Young AA. In: Insulin Resistance and Insulin Resistance Syndrome. Hansen B, Shafrir E, editors. London: Taylor and Francis; 2002. pp. 235–262. [Google Scholar]
- 29.Zander M, Madsbad S, Madsen JL, Holst JJ. Lancet. 2002;359:824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 30.Meneilly GS, Greig N, Tildesley H, Habener JF, Egan JM, Elahi D. Diabetes Care. 2003;26:2835–2841. doi: 10.2337/diacare.26.10.2835. [DOI] [PubMed] [Google Scholar]
- 31.Gedulin BR, Nikoulina SE, Smith PA, Gedulin G, Nielsen LL, Baron AD, Parkes DG, Young AA. Endocrinology. 2005;146:2069–2076. doi: 10.1210/en.2004-1349. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q, Brubaker PL. Diabetologia. 2002;45:1263–1273. doi: 10.1007/s00125-002-0828-3. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald LR, Mannan IJ, Dytko GM, Wu HL, Nambi P. Anal Biochem. 1999;275:54–61. doi: 10.1006/abio.1999.4295. [DOI] [PubMed] [Google Scholar]
- 34.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 35.Perfetti R, Zhou J, Doyle ME, Egan JM. Endocrinology. 2000;141:4600–4605. doi: 10.1210/endo.141.12.7806. [DOI] [PubMed] [Google Scholar]
- 36.Stoffers DA, Kieffer TJ, Hussain MA, Drucker DJ, Bonner-Weir S, Habener JF, Egan JM. Diabetes. 2000;49:741–748. doi: 10.2337/diabetes.49.5.741. [DOI] [PubMed] [Google Scholar]
- 37.Hui X, Gao J, Xie X, Suto N, Ogiku T, Wang MW. Acta Pharmacol Sin. 2005;26:1175–1180. doi: 10.1111/j.1745-7254.2005.00202.x. [DOI] [PubMed] [Google Scholar]
- 38.Lacy PE, Kostianovsky M. Diabetes. 1967;16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.