Abstract
In traditional screening with 96-well plates, microliters of substrates are consumed for each reaction. Further miniaturization is limited by the special equipment and techniques required to dispense nanoliter volumes of fluid. Plug-based microfluidics confines reagents in nanoliter plugs (droplets surrounded by fluorinated carrier fluid), and uses simple pumps to control the flow of plugs. By using cartridges pre-loaded with nanoliter plugs of reagents, only two pumps and a merging junction are needed to set up a screen. Screening with preloaded cartridges uses only nanoliters of substrate per reaction, and requires no microfabrication. The low cost and simplicity of this method has the potential of replacing 96-well and other multi-well plates, and has been applied to enzymatic assays, protein crystallization and optimization of organic reactions.
Introduction
This paper discusses recent advances in the miniaturization of chemical and biochemical screening techniques that rely on microfluidic cartridges preloaded with nanoliter plugs of reagents. 96-Well plates are widely used as reactors to screen a substrate against many reagents. The reagents and substrate are dispensed into the wells by manual pipetting or robotic liquid handler (Figure 1a). Screening in 96-well plates consumes microliters of solution per reaction, and further miniaturization is often needed to reduce the cost and to improve the productivity. The extent of miniaturization may be limited by the ability to handle small volumes of reagents, confining them within a reaction chamber without evaporation, and to analyze reactions with sufficient signal-to-noise. Analytical technology of amazing sensitivity has been developed and described in the literature, including methods based on fluorescence [1–4] and mass spectrometry [5,6]. These advances have been reviewed, and here we focus on limitations due to fluid handling and confining reagents.
Figure 1.
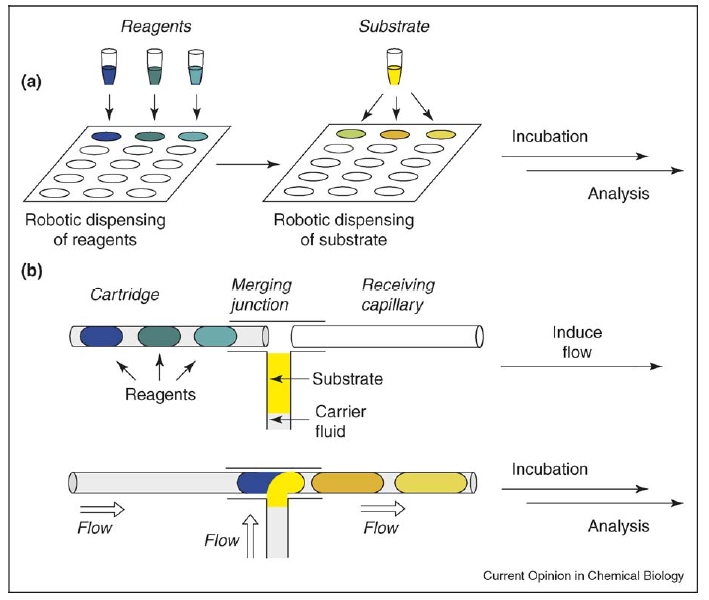
A comparison of screening in microtiter plates and screening with preloaded cartridges. (a) To set up the screen in microtiter plates, robotics is required to dispense nanoliters of both the reagents and the substrate. Solutions in the microwells may evaporate. (b) With preloaded cartridges, the reagents are confined in nanoliter plugs. When the flow is induced, defined nanoliter aliquots of the substrate solution are spontaneously dispensed into the plugs of reagents. Plugs of the reagents and the products are confined in capillaries and are surrounded by an impermeable fluorinated carrier fluid, preventing evaporation.
To miniaturize reactions in solution, microtiter plates of higher density and smaller well volume (384- and 1536-well plates) can be used to confine the reagents, and robotic systems may be used to dispense nanoliters of liquid [7,8] into the microwells. Such robotic systems may be too expensive to be used for screening by individual laboratories, and these approaches do not solve the problem of evaporation. Microfluidics has the potential to miniaturize reactions at low cost. Microfabricated valves may be used to control flow and to confine reagents for applications in protein crystallization [9], nucleic acid assays [10] and multistep reactions [11]. This approach is clearly attractive, but it may be too expensive and complicated to replace 96-well plates, because it still requires microfabrication of microvalves and the use of control equipment, and evaporation of fluids may still be a problem.
For applications that do not require sophisticated manipulations of fluids, a simpler strategy may be attractive: the use of nanoliter plugs in microchannels to confine reactions. This approach has been used in a variety of applications: enzyme kinetics [12], organic synthesis [13••], and protein crystallization [14]. Because generation and merging of plugs is spontaneous [15], using the plugs represents a unique opportunity to develop affordable and simple miniaturized screening technology.
Cartridges loaded with nanoliter plugs of reagents are promising tools for facile and low-cost screening in nanoliter volumes
The use of reagent-loaded cartridges simplifies screening experiments by separating them from the process of dispensing nanoliter volumes of reagents. This approach consists of three steps (Figure 1b). First, nanoliters of reagents can be dispensed as an array of plugs and stored in a capillary (reagent loaded cartridge) before the experiment. Second, to perform a screen, the plugs from the cartridge and a stream of the substrate solution are flowed into a merging junction. Every plug spontaneously combines with a constant small volume of the substrate solution. After merging, combined plugs containing both a reagent and the substrate proceed to enter the receiving capillary. Third, the receiving capillary is then detached from the merging junction, sealed and incubated. After incubation, the plugs are analyzed. These steps will be described in more detail below.
This cartridge-based screening format has three advantages. First, because the precious substrate is not involved until the last step of screening, the possibility of wasting the substrate is reduced. Second, the cost of screening may be reduced. If automated production of cartridges is centralized, only two pump-driven syringes and a merging junction are required to set up a screen. Third, reagents are stored in a capillary, so evaporation is largely reduced or eliminated.
The scale of reaction is determined by the volume of individual plugs, which is determined by the internal diameter of the capillary. For a capillary with an internal diameter of 200 μm, the volume of a plug is on the order of ~10 nanoliters. Cartridges can be fabricated by sequentially aspirating reagents into a capillary with a syringe. To keep the reagents separate from each other, gas segments can be used as spacers between reagents. Such liquid–gas two-phase cartridges have been used for immunoassay in capillaries, and the reagents were well separated by gas even after shipping [16••]. One potential problem of these liquid–gas two-phase cartridges is cross-contamination of reagents caused by a liquid film deposited by solutions of reagents on the wall of the capillary during flow. To solve this problem, a carrier fluid that preferentially wets the wall can be used. The plugs of reagents are completely surrounded by the carrier fluid [17] and cross-contamination is eliminated. Fluorinated oils are often used as the carrier fluid, because of their low solubility for most reagents [13••], and orthogonality to common organic and aqueous chemistry. Both Teflon and glass capillaries can be used. Teflon capillaries are excellent for fluid handling, because of the low interfacial tension between Teflon and fluorinated carrier fluid. Glass capillary can also be used, but surface fluorination is required to lower the interfacial tension with carrier fluid. Glass capillaries are especially attractive for eliminating evaporation of plugs. Plugs in glass capillaries have been stored for over six months without evaporation [18].
Merging of the plugs of reagents with the sample solution has been characterized in detail [19]. Previously, commercially available HPLC T-junctions [13••] or T-shaped microfluidic channels made of PDMS [20••] were used as the merging junctions, and were shown to combine the reagent and the sample in 1:1 volumetric ratio [20••]. Such merging junctions were convenient to use, but cross-contamination could happen due to reagents in one plug being deposited into the next plug during merging. Such cross-contamination can be significantly reduced by introducing plugs of buffer solution between the reagent plugs [13••,20••].
To interpret the results of screening, the identity of plugs needs to be tracked and the plugs need to be analyzed. The identity of a plug may be tracked by its order in the sequence, or by adding indexing plugs [21]. Analysis of plugs may be done in capillary (‘in situ’) or out of the capillary. For fluorescence assays, fluorescence can be measured in situ. In protein crystallization, plugs may be monitored optically for formation of crystals [14], and X-ray diffraction data could be obtained in situ to test the quality of crystals [18]. For organic reactions, MALDI-MS can be used to analyze products in the plugs [13••]. This method requires the plugs to be flowed out of the capillary and transferred onto a MALDI plate for analysis. Alternatively, online analysis technique such as capillary NMR [22] should also be promising for analysis of these plugs.
Fluorescence assay tested the activity of enzymes using 15 nl of each enzyme preloaded in a cartridge
A functional assay of enzymes was used to illustrate fluorescence assay with preloaded cartridges [20••] (Figure 2). Such assays may be used to identify proteins of desired activities after many proteins are produced in proteomic research. Fluorescein diphosphate (FDP) was used as the substrate to test the phosphatase activity of enzymes. Enzymes with phosphatase activity will cleave the substrate and release fluorescein, which is fluorescent. The cartridge was a glass capillary containing plugs of the enzymes in fluorinated carrier fluid. Every plug was ~15 nanoliters in volume. (Preliminary results showed that the error bar for the volume of plugs generated by splitting an array of plugs was less than 15%, and the error bar for the volumetric ratio between the plugs and the substrate stream during merging was ~3%.) As mentioned above, plugs of buffer solution were used between adjacent enzyme plugs to eliminate false positives caused by cross-contamination between plugs during merging. A gas bubble was also introduced between each plug. The gas bubbles prevent the plugs from coalescing, which may be caused by plugs of different viscosities moving at different velocities. After merging the plugs of enzymes with the FDP solution through a T-shaped PDMS channel, the results were checked by the fluorescence microscopy. Fluorescence was observed only in the plug of alkaline phosphotase, confirming that the buffer plugs were effective in reducing the contamination.
Figure 2.
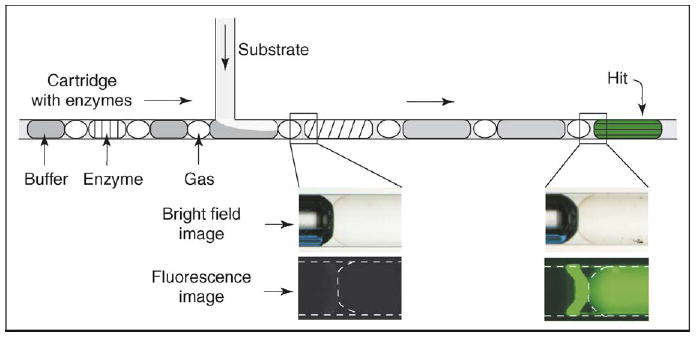
A schematic to illustrate a fluorescence assay using cartridges preloaded with plugs of enzymes [20••]. In the fluorescence images, dashed lines show the boundaries of the plugs and the capillary. Figure reprinted with minor modifications with permission from [20••]. Copyright 2005, Wiley-VCH.
Cartridges preloaded with nanoliter plugs of precipitants enable screening conditions for protein crystallization and in situ structure determination
Preloaded cartridges were also used to screen precipitants for protein crystallization, as reviewed previously [23]. Plugs of different precipitants were prepared in cartridges, merged with the protein solution, and the resulting plugs were transferred into a glass capillary, incubated and monitored for signs of crystallization. One recent advance in this approach is in situ structure determination [24]. After crystals were grown in X-ray transparent glass capillaries, X-ray diffraction data were collected at room temperature in situ. Because of radiation damage, diffraction data from multiple crystals were required to obtain a complete data set. The structure of a model protein was solved using this approach.
Reagent loaded cartridges with MALDI-MS detection optimize organic reaction on sub-microgram scale
High yields in organic reactions require optimization of the reaction conditions, including the choice of reagent, solvent, concentrations, temperature and reaction time. Such optimization necessitates performing many reactions, and reactions on the scale of microliters per reaction are no longer applicable to precious compounds such as advanced synthetic intermediates and organic molecules isolated from many natural sources.
Many reactions can be conducted in parallel, and the consumption of samples can be reduced to nanoliters per reaction by using cartridges preloaded with plugs of reagents (Figure 3). Multiple reactions can be set up by flowing plugs of reagents from the cartridge into a T-junction to combine with the substrate. Sub-microliter volume of substrate solution can be handled with no losses by filling the syringes and tubing with carrier fluid. After incubation, the resulting plugs can be analyzed by mass-spectrometry. MALDI-MS is a highly sensitive technique that is well suited for analyzing these nanoliter plugs. Information on the mass and relative concentrations of the products may be obtained with MALDI-MS. To resolve isomers, complementary techniques such as capillary NMR may potentially be used.
Figure 3.
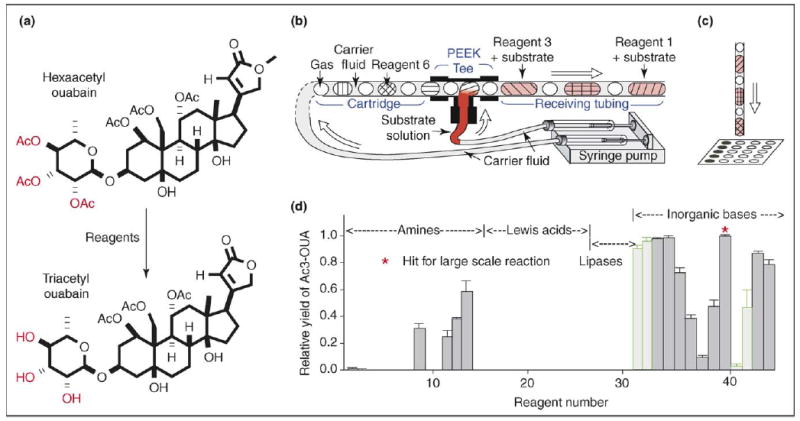
Optimizing organic reactions on a sub-microgram scale using preloaded cartridges of nanoliter plugs [13••]. (a) Deacetylation of hexaacetyl ouabain was optimized. (b) The experimental set up used for screening reagents. (c) After incubation, the plugs were deposited onto a MALDI plate for analysis. (d) The relative yield of triacetyl ouabain is shown against 44 deacetylation reagents. The green bars represent reactions in which most byproducts were due to excessive reaction, while the gray bars correspond to reactions in which most byproducts were due to inadequate reaction. The reaction time was 1 hour at 18 °C, and the 44 reactions χονσυμɛδ α τοταλ οφ ονλψ 20 μg of hexaacetyl ouabain. Figure reprinted with permission from [13••]. Copyright 2006, American Chemical Society.
This approach was illustrated [13••] in optimizing the conditions of a deacetylation reaction (Figure 3a). Using cartridges preloaded with plugs of hydrolytic reagents, different reagents were tested for selectively hydrolyzing hexaacetyl ouabain to triacetyl ouabain, and the results were analyzed by MALDI-MS. Only 20 μg of hexaacetyl ouabain were consumed to screen 44 deacetylation reactions, which corresponds to about 0.5 μg per reaction. Several reagents were found to give triacetyl ouabain with very high selectivity, and the validity of these results was confirmed by larger scale reactions [13••].
Conclusions: future improvements and applications
Four features make plug-based cartridges more attractive for high-throughput performance than 96-well or other multi-well formats. First, handling volumes in the picoliter to nanoliter range with very inexpensive equipment, and second, handling and incubating such volumes without evaporation. Control of evaporation enables pre-loading cartridges with plugs of reagents for long-term storage. Third, control of surface chemistry at the interface of the solution of reagent and the carrier fluid [25], and minimizing exposure of solutions to the atmosphere. Fourth, ability to utilize all of the volume of the reagent delivered for screening. Providers of chemicals typically do not distribute reagents to individual researchers in nanoliter volumes. Even when only a single nanoliter aliquot of a reagent is sufficient for an experiment, researchers purchase a 1000-times (μl) to 1,000,000-times (mL) larger volume of the reagent, and use only a small fraction of that volume for an experiment. An additional problem in miniaturization is loss of reagents in dead volumes during the introduction of the reagent solution into the miniaturized reactors, so more substrate than necessary is used to conduct the experiment. In plug-based microfluidics, this problem is solved by using carrier fluid to fill all the syringes and tubing, so no substrate is wasted.
There are two limitations of these systems. First, plug-based systems require non-zero surface tension between the carrier fluid and the solution of a reagent. This requirement may narrow the scope of solutions that can be used with this method (although even concentrated solutions of detergents used for crystallization of membrane proteins are compatible with this method). Second, because a plug is completely enclosed in the carrier fluid to eliminate evaporation, sampling an aliquot out of a plug may be more difficult than sampling an aliquot out of a multi-well plate. This limitation is not significant for assays with in situ analysis (optical or X-ray based). Methods exist for splitting off a small portion of a plug for analysis [15], but these methods may not be required for most applications.
Improvements to every step of the cartridge-based screening are desirable to fully utilize the potential of this approach. Methods to produce cartridges in large quantities need to be developed for their widespread use. With prefabricated cartridges, one single merging device can set up 86 400 reactions per day, assuming continuous operation with one reagent plug being merged with the substrate every second. Replacing gas bubbles used as spacers with an incompressible fluid will simplify handling and storage of cartridges. Merging of the droplets with substrate solution can be improved by designing merging devices that completely eliminate contamination from droplet to droplet. Indexing droplets with methods that simplify computer-based tracking of each droplet will be useful for screening even larger number of reagents. For miniaturizing organic reactions, analysis techniques that are complementary to mass spectrometry (capillary NMR [22] and capillary LC [26], for example) will further strengthen the capability of this method.
For industries heavily invested into robotic equipment for handling well plates, it is undesirable to switch away from the multiwell-plate format. This switch could occur more easily if cartridge-based techniques were integrated with this robotic equipment. Standardization and characterization of the performance of these cartridge-based systems would also be required for industry acceptance. On-site robotic systems for preparing large numbers of cartridges of proprietary reagents would be desirable as well. There is a growing consensus that the rate-limiting step in some screening applications is not in the numbers of experiments, but in running the right experiments, conclusive and well-controlled. Cartridge-based methods would become especially attractive if the control of evaporation, surface chemistry and control of atmospheric exposure were shown to result in more interpretable experiments.
Individual labs could benefit from cartridge-based methods more directly. Standardized, low-cost and simple to use kits of cartridges and merging junctions would accelerate acceptance of these systems by researchers, since simple pumps are already commercially available and are inexpensive. The biggest challenge is to develop an inexpensive source of cartridges containing libraries of reagents: ligands and catalysts for discovery of catalytic reactions; combinatorial libraries and reagents for building them; precipitants for protein crystallization; fluorescently labeled substrates; and the vast range of reagents used in molecular biology. In addition to commercial sources, it may be possible to develop a government-supported research resource that would produce cartridges and distribute them to the research community. This model is used successfully to maintain and distribute stocks of model organisms in biology, and could function well for distributing cartridges containing sets of common screening reagents.
As these improvements and developments become available, cartridges preloaded with nanoliter droplets of reagents should become a mainstream tool used in both industry and academia to perform screening experiments rapidly, inexpensively and conclusively.
Acknowledgments
The work described in this review has been supported by the National Institute for Biomedical Imaging and Bioengineering (R01 EB001903), by the National Institute of General Medical Sciences (R01 GM075827), by the Beckman Young Investigator Program and by the DuPont Young Professor Award. We thank our colleagues in the Ismagilov laboratory who have contributed to the work described here.
Footnotes
This review comes from a themed issue on Combinatorial chemistry and molecular diversity Edited by Scott K Silverman and Paul J Hergenrother
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Haustein E, Schwille P. Single-molecule spectroscopic methods. Curr Opin Struct Biol. 2004;14:531–540. doi: 10.1016/j.sbi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Johnson ME, Landers JP. Fundamentals and practice for ultrasensitive laser-induced fluorescence detection in microanalytical systems. Electrophoresis. 2004;25:3513–3527. doi: 10.1002/elps.200406086. [DOI] [PubMed] [Google Scholar]
- 3.Blom H, Kastrup L, Eggeling C. Fluorescence fluctuation spectroscopy in reduced detection volumes. Curr Pharm Biotechnol. 2006;7:51–66. doi: 10.2174/138920106775789629. [DOI] [PubMed] [Google Scholar]
- 4.Park HG, Song JY, Park KH, Kim MH. Fluorescence-based assay formats and signal amplification strategies for DNA microarray analysis. Chem Eng Sci. 2006;61:954–965. [Google Scholar]
- 5.Cooks RG, Ouyang Z, Takats Z, Wiseman JM. Ambient mass spectrometry. Science. 2006;311:1566–1570. doi: 10.1126/science.1119426. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin MA. Biological Mass Spectrometry. Methods Enzymol. Vol. 402. Elsevier Academic Press, Inc; 2005. Mass spectrometers for the analysis of biomolecules; pp. 3–48. [DOI] [PubMed] [Google Scholar]
- 7.Sauer S, Lange BMH, Gobom J, Nyarsik L, Seitz H, Lehrach H. Miniaturization in functional genomics and proteomics. Nat Rev Genet. 2005;6:465–476. doi: 10.1038/nrg1618. [DOI] [PubMed] [Google Scholar]
- 8.Ma H, Horiuchi KY, Wang Y, Kucharewicz SA, Diamond SL. Nanoliter homogenous ultra-high throughput screening microarray for lead discoveries and IC50 profiling. Assay Drug Dev Technol. 2005;3:177–187. doi: 10.1089/adt.2005.3.177. [DOI] [PubMed] [Google Scholar]
- 9.Hansen CL, Sommer MOA, Quake SR. Systematic investigation of protein phase behavior with a microfluidic formulator. Proc Natl Acad Sci USA. 2004;101:14431–14436. doi: 10.1073/pnas.0405847101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcus JS, Anderson WF, Quake SR. Parallel picoliter RT-PCR assays using microfluidics. Anal Chem. 2006;78:956–958. doi: 10.1021/ac0513865. [DOI] [PubMed] [Google Scholar]
- 11.Lee CC, Sui GD, Elizarov A, Shu CYJ, Shin YS, Dooley AN, Huang J, Daridon A, Wyatt P, Stout D, et al. Multistep synthesis of a radiolabeled imaging probe using integrated microfluidics. Science. 2005;310:1793–1796. doi: 10.1126/science.1118919. [DOI] [PubMed] [Google Scholar]
- 12.Song H, Ismagilov RF. Millisecond kinetics on a microfluidic chip using nanoliters of reagents. J Am Chem Soc. 2003;125:14613–14619. doi: 10.1021/ja0354566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.••.Hatakeyama T, Chen DLL, Ismagilov RF. Microgram-scale testing of reaction conditions in solution using nanoliter plugs in microfluidics with detection by MALDI-MS. J Am Chem Soc. 2006;128:2518–2519. doi: 10.1021/ja057720w. Using prefabricated cartridges to screen and optimize organic reaction conditions is described. Less than one microgram of substrate was used for each reaction, and the optimized conditions were confirmed by isolating the corresponding products in larger-scale reactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng B, Roach LS, Ismagilov RF. Screening of protein crystallization conditions on a microfluidic chip using nanoliter-size droplets. J Am Chem Soc. 2003;125:11170–11171. doi: 10.1021/ja037166v. [DOI] [PubMed] [Google Scholar]
- 15.Song H, Tice JD, Ismagilov RF. A microfluidic system for controlling reaction networks in time. Angew Chem Int Ed. 2003;42:768–772. doi: 10.1002/anie.200390203. [DOI] [PubMed] [Google Scholar]
- 16.••.Linder V, Sia SK, Whitesides GM. Reagent-loaded cartridges for valveless and automated fluid delivery in microfluidic devices. Anal Chem. 2005;77:64–71. doi: 10.1021/ac049071x. The authors fabricated cartridges consisted of reagents separated by air bubbles, and used the cartridges to perform immunoassays. [DOI] [PubMed] [Google Scholar]
- 17.Tice JD, Song H, Lyon AD, Ismagilov RF. Formation of droplets and mixing in multiphase microfluidics at low values of the Reynolds and the capillary numbers. Langmuir. 2003;19:9127–9133. [Google Scholar]
- 18.Zheng B, Tice JD, Roach LS, Ismagilov RF. A droplet-based, composite PDMS/glass capillary microfluidic system for evaluating protein crystallization conditions by microbatch and vapor-diffusion methods with on-chip X-ray diffraction. Angew Chem Int Ed. 2004;43:2508–2511. doi: 10.1002/anie.200453974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shestopalov I, Tice JD, Ismagilov RF. Multi-step synthesis of nanoparticles performed on millisecond time scale in a microfluidic droplet-based system. Lab Chip. 2004;4:316–321. doi: 10.1039/b403378g. [DOI] [PubMed] [Google Scholar]
- 20.••.Zheng B, Ismagilov RF. A microfluidic approach for screening submicroliter volumes against multiple reagents by using preformed arrays of nanoliter plugs in a three-phase liquid/liquid/gas flow. Angew Chem Int Ed. 2005;44:2520–2523. doi: 10.1002/anie.200462857. This paper demonstrated the use of preloaded cartridges to perform enzymatic assays and protein crystallization on nanoliter scale. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng B, Tice JD, Ismagilov RF. Formation of droplets of in microfluidic channels alternating composition and applications to indexing of concentrations in droplet-based assays. Anal Chem. 2004;76:4977–4982. doi: 10.1021/ac0495743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kautz RA, Goetzinger WK, Karger BL. High-throughput microcoil NMR of compound libraries using zero-dispersion segmented flow analysis. J Comb Chem. 2005;7:14–20. doi: 10.1021/cc0498940. [DOI] [PubMed] [Google Scholar]
- 23.Zheng B, Gerdts CJ, Ismagilov RF. Using nanoliter plugs in microfluidics to facilitate and understand protein crystallization. Curr Opin Struct Biol. 2005;15:548–555. doi: 10.1016/j.sbi.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yadav MK, Gerdts CJ, Sanishvili R, Smith WW, Roach LS, Ismagilov RF, Kuhn P, Stevens RC. In situ data collection and structure refinement from microcapillary protein crystallization. J Appl Cryst. 2005;38:900–905. doi: 10.1107/S002188980502649X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roach LS, Song H, Ismagilov RF. Controlling nonspecific protein adsorption in a plug-based microfluidic system by controlling interfacial chemistry using fluorous-phase surfactants. Anal Chem. 2005;77:785–796. doi: 10.1021/ac049061w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jemal M. High-throughput quantitative bioanalysis by LC/MS/MS. Biomed Chromatogr. 2000;14:422–429. doi: 10.1002/1099-0801(200010)14:6<422::AID-BMC25>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]


