Abstract
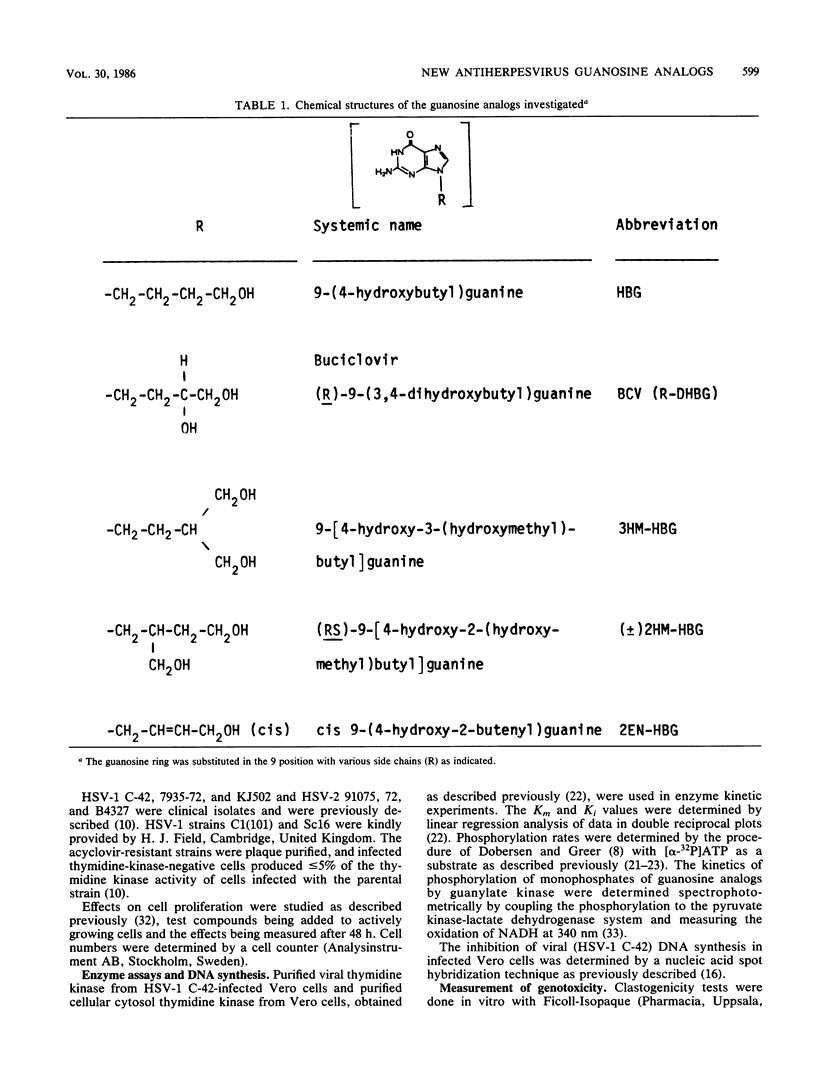
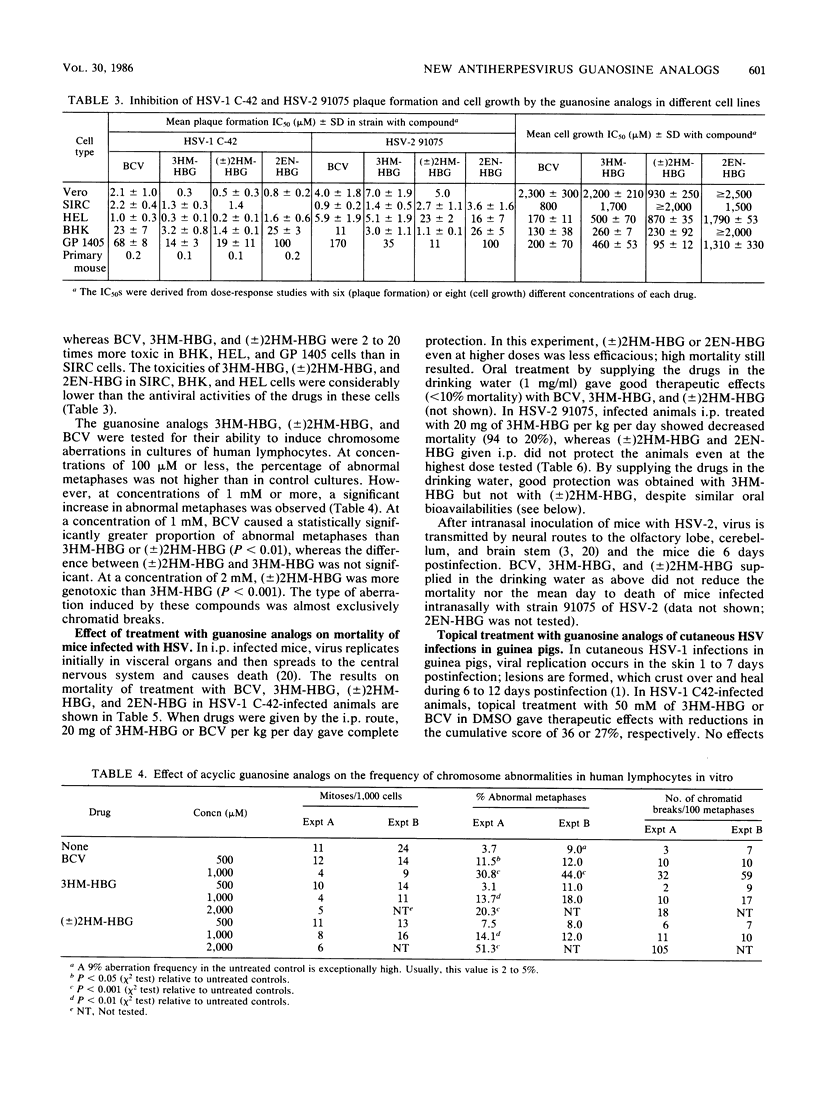
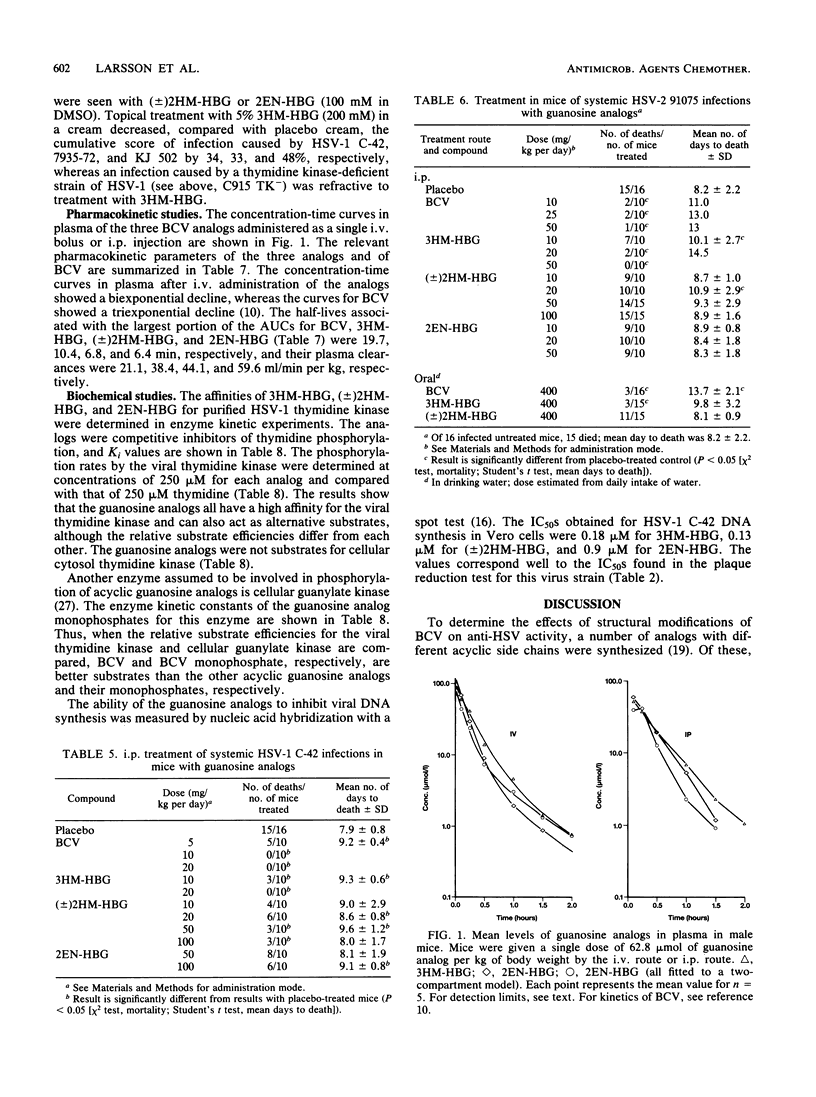
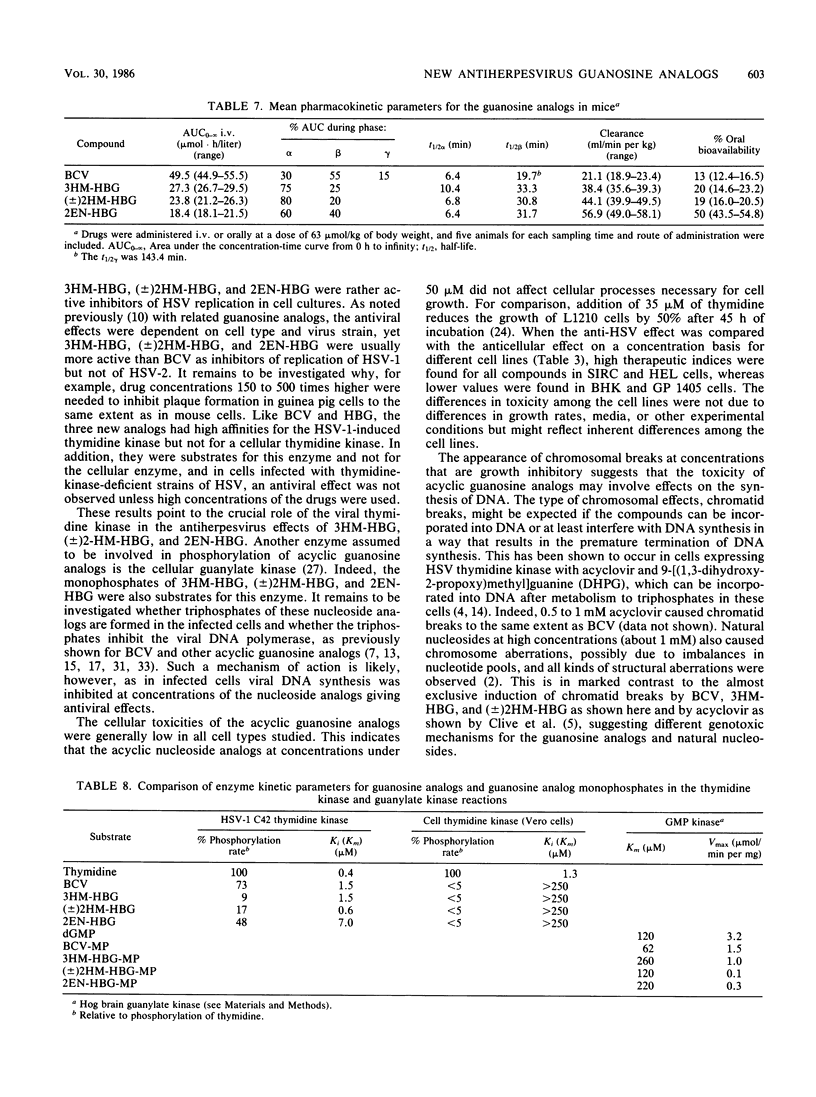
9-[4-Hydroxy-3-(hydroxymethyl)butyl]guanine (3HM-HBG), (RS)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine ([+/-]2HM-HBG), and cis-9-(4-hydroxy-2-butenyl)guanine (2EN-HBG), new acyclic guanosine analogs structurally related to buciclovir (BCV [(R)-9-(3,4-dihydroxybutyl)guanine]), were evaluated in parallel with buciclovir as anti-herpes simplex virus (HSV) agents. In cell cultures, replication of different strains of HSV type 1 (HSV-1) and HSV-2 was inhibited at nontoxic drug concentrations. The concentrations giving 50% inhibition of plaque formation were, however, dependent on virus strain and cell type. In most cell types, the order of activity against HSV-1 strains was 3HM-HBG greater than (+/-)2HM-HBG greater than BCV greater than 2EN-HBG, whereas the drugs showed an approximately equivalent activity against HSV-2 strains in different cells. The cytotoxic effects of the drugs were also cell type dependent, the order of activity being BCV greater than 3HM-HBG = (+/-)2HM-HBG greater than 2EN-HBG. At growth-inhibitory concentrations, the guanosine analogs BCV, 3HM-HBG, and (+/-)2HM-HBG showed clastogenic effects in human lymphocytes, mainly because of the induction of chromatid breaks. When evaluated for their anti-HSV effects in systemic HSV-1 infections in mice, the order of activity was BCV = 3HM-HBG greater than (+/-)2HM-HBG greater than 2EN-HBG, and in mice infected systemically with HSV-2, only BCV and 3HM-HBG showed efficacy. The differences between efficacy in vitro and in vivo could be explained in part by differences in kinetics of the drugs in mouse plasma, as the more efficacious drugs, BCV and 3HM-HBG, showed lower clearances and longer half-lives than the less efficacious ones, (+/-)2HM-HBG and 2EN-HBG. When used topically against a cutaneous HSV-1 infection in guinea pigs, 3HM-HBG showed an effect equivalent to that of BCV, whereas (+/-)2HM-HBG and 2EN-HBG were inactive. Mechanistically, the guanosine analogs were characterized by a high affinity for the viral thymidine kinase and a low affinity fo a cellular thymidine kinase and by their inhibition of viral DNA synthesis in infected cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alenius S., Oberg B. Comparison of the therapeutic effects of five antiviral agents on cutaneous herpesvirus infection in guinea pigs. Arch Virol. 1978;58(4):277–288. doi: 10.1007/BF01317820. [DOI] [PubMed] [Google Scholar]
- Anderson D., Richardson C. R., Davies P. J. The genotoxic potential of bases and nucleosides. Mutat Res. 1981 May;91(3):265–272. doi: 10.1016/0165-7992(81)90044-0. [DOI] [PubMed] [Google Scholar]
- Anderson J. R., Field H. J. The distribution of herpes simplex type 1 antigen in mouse central nervous system after different routes of inoculation. J Neurol Sci. 1983 Aug;60(2):181–195. doi: 10.1016/0022-510x(83)90061-8. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Grill S. P., Dutschman G. E., Nakayama K., Bastow K. F. Metabolism of 9-(1,3-dihydroxy-2-propoxymethyl)guanine, a new anti-herpes virus compound, in herpes simplex virus-infected cells. J Biol Chem. 1983 Oct 25;258(20):12460–12464. [PubMed] [Google Scholar]
- Clive D., Turner N. T., Hozier J., Batson A. G., Tucker W. E., Jr Preclinical toxicology studies with acyclovir: genetic toxicity tests. Fundam Appl Toxicol. 1983 Nov-Dec;3(6):587–602. doi: 10.1016/s0272-0590(83)80109-2. [DOI] [PubMed] [Google Scholar]
- Derse D., Cheng Y. C., Furman P. A., St Clair M. H., Elion G. B. Inhibition of purified human and herpes simplex virus-induced DNA polymerases by 9-(2-hydroxyethoxymethyl)guanine triphosphate. Effects on primer-template function. J Biol Chem. 1981 Nov 25;256(22):11447–11451. [PubMed] [Google Scholar]
- Dobersen M. J., Greer S. An assay for pyrimidine deoxyribonucleoside kinase using gamma-32P-labeled ATP. Anal Biochem. 1975 Aug;67(2):602–610. doi: 10.1016/0003-2697(75)90335-8. [DOI] [PubMed] [Google Scholar]
- Ericson A. C., Larsson A., Aoki F. Y., Yisak W. A., Johansson N. G., Oberg B., Datema R. Antiherpes effects and pharmacokinetic properties of 9-(4-hydroxybutyl) guanine and the (R) and (S) enantiomers of 9-(3,4-dihydroxybutyl)guanine. Antimicrob Agents Chemother. 1985 May;27(5):753–759. doi: 10.1128/aac.27.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. J., O'Riordan M. L. Human peripheral blood lymphocytes for the analysis of chromosome aberrations in mutagen tests. Mutat Res. 1975 Jun;31(3):135–148. doi: 10.1016/0165-1161(75)90082-5. [DOI] [PubMed] [Google Scholar]
- Field A. K., Davies M. E., DeWitt C., Perry H. C., Liou R., Germershausen J., Karkas J. D., Ashton W. T., Johnston D. B., Tolman R. L. 9-([2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine: a selective inhibitor of herpes group virus replication. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4139–4143. doi: 10.1073/pnas.80.13.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank K. B., Chiou J. F., Cheng Y. C. Interaction of herpes simplex virus-induced DNA polymerase with 9-(1,3-dihydroxy-2-propoxymethyl)guanine triphosphate. J Biol Chem. 1984 Feb 10;259(3):1566–1569. [PubMed] [Google Scholar]
- Furman P. A., McGuirt P. V., Keller P. M., Fyfe J. A., Elion G. B. Inhibition by acyclovir of cell growth and DNA synthesis of cells biochemically transformed with herpesvirus genetic information. Virology. 1980 Apr 30;102(2):420–430. doi: 10.1016/0042-6822(80)90109-9. [DOI] [PubMed] [Google Scholar]
- Furman P. A., St Clair M. H., Spector T. Acyclovir triphosphate is a suicide inactivator of the herpes simplex virus DNA polymerase. J Biol Chem. 1984 Aug 10;259(15):9575–9579. [PubMed] [Google Scholar]
- Gadler H., Larsson A., Sølver E. Nucleic acid hybridization, a method to determine effects of antiviral compounds on herpes simplex virus type 1 DNA synthesis. Antiviral Res. 1984 Apr;4(1-2):63–70. doi: 10.1016/0166-3542(84)90026-3. [DOI] [PubMed] [Google Scholar]
- Germershausen J., Bostedor R., Field A. K., Perry H., Liou R., Bull H., Tolman R. L., Karkas J. D. A comparison of the antiviral agents 2'-nor-2'-deoxyguanosine and acyclovir: uptake and phosphorylation in tissue culture and kinetics of in vitro inhibition of viral and cellular DNA polymerases by their respective triphosphates. Biochem Biophys Res Commun. 1983 Oct 31;116(2):360–367. doi: 10.1016/0006-291x(83)90530-2. [DOI] [PubMed] [Google Scholar]
- Kern E. R., Richards J. T., Overall J. C., Jr, Glasgow L. A. Alteration of mortality and pathogenesis of three experimental Herpesvirus hominis infections of mice with adenine arabinoside 5'-monophosphate, adenine arabinoside, and phosphonoacetic acid. Antimicrob Agents Chemother. 1978 Jan;13(1):53–60. doi: 10.1128/aac.13.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson A., Alenius S., Johansson N. G., Oberg B. Antiherpetic activity and mechanism of action of 9-(4-hydroxybutyl)guanine. Antiviral Res. 1983 Aug;3(2):77–86. doi: 10.1016/0166-3542(83)90028-1. [DOI] [PubMed] [Google Scholar]
- Larsson A., Sundqvist A., Parnerud A. M. Inhibition of herpes simplex virus-induced DNA polymerases and cellular DNA polymerase alpha by triphosphates of acyclic guanosine analogs. Mol Pharmacol. 1986 Jun;29(6):614–621. [PubMed] [Google Scholar]
- Larsson A., Tao P. Z. Phosphorylation of four acyclic guanosine analogs by herpes simplex virus type 2 thymidine kinase. Antimicrob Agents Chemother. 1984 Apr;25(4):524–526. doi: 10.1128/aac.25.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J. K., Grindey G. B. Inhibition of growth rate and deoxynucleoside triphosphate concentrations in cultured leukemia L1210 cells. Mol Pharmacol. 1976 Jan;12(1):177–184. [PubMed] [Google Scholar]
- Miller W. H., Miller R. L. Phosphorylation of acyclovir (acycloguanosine) monophosphate by GMP kinase. J Biol Chem. 1980 Aug 10;255(15):7204–7207. [PubMed] [Google Scholar]
- Oberg B., Johansson N. G. The relative merits and drawbacks of new nucleoside analogues with clinical potential. J Antimicrob Chemother. 1984 Aug;14 (Suppl A):5–26. doi: 10.1093/jac/14.suppl_a.5. [DOI] [PubMed] [Google Scholar]
- Schnürer J., Oberg B. Inhibitory effects of foscarnet on herpesvirus multiplication in cell culture. Arch Virol. 1981;68(3-4):203–209. doi: 10.1007/BF01314573. [DOI] [PubMed] [Google Scholar]
- Smee D. F., Boehme R., Chernow M., Binko B. P., Matthews T. R. Intracellular metabolism and enzymatic phosphorylation of 9-(1,3-dihydroxy-2-propoxymethyl)guanine and acyclovir in herpes simplex virus-infected and uninfected cells. Biochem Pharmacol. 1985 Apr 1;34(7):1049–1056. doi: 10.1016/0006-2952(85)90608-2. [DOI] [PubMed] [Google Scholar]
- St Clair M. H., Miller W. H., Miller R. L., Lambe C. U., Furman P. A. Inhibition of cellular alpha DNA polymerase and herpes simplex virus-induced DNA polymerases by the triphosphate of BW759U. Antimicrob Agents Chemother. 1984 Feb;25(2):191–194. doi: 10.1128/aac.25.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg K. Cellular toxicity of pyrophosphate analogues. Biochem Pharmacol. 1981 May 1;30(9):1005–1008. doi: 10.1016/0006-2952(81)90047-2. [DOI] [PubMed] [Google Scholar]
- Stenberg K., Larsson A., Datema R. Metabolism and mode of action of (R)-9-(3,4-dihydroxybutyl)guanine in herpes simplex virus-infected vero cells. J Biol Chem. 1986 Feb 15;261(5):2134–2139. [PubMed] [Google Scholar]


