Abstract
Background
Recent work indicates that mechanical force induces small-bowel growth, although methods reported do not have direct clinical application. We report a clinically feasible technique of enterogenesis and describe intestinal function in this model.
Methods
Using a pig model (n = 11), we stretched isolated small intestinal segments mechanically for 7 days in vivo with an intraluminal device. Control segments were not stretched. Morphology, histology, and epithelial proliferation were assessed. Absorption and epithelial barrier function were examined in an Ussing chamber.
Results
Stretch segments were significantly longer than Control segments and had nearly 2-fold greater surface area (P < .001). Mucosal thickness was much greater in Stretch than Control segments (772 ± 134 vs. 647 ± 75 μm, P = .02). Although villus height was reduced in Stretch and Control segments (353 ± 76 vs. 324 ± 76 μm, P = .6) versus native jejunum (522 ± 87, P < .0005), crypt depth was increased dramatically in Stretch (450 ± 95 μm) versus Control segments (341 ± 64, P = .005). This observation was accompanied by a 2-fold increase in cellular proliferation (26.3 ± 3.8 vs 12.1 ± 6.6 % bromodeoxyuridine+, P < .05). Barrier function was intact ([3H]-mannitol permeation, 0.16 ± 0.08%, vs native jejunum, 0.17 ± 0.08%, P = .81). Glucose-mediated sodium transport was similar in Stretch versus native jejunum segments (60.0 ± 23.5 vs 82.3 ± 47.3 μA/ cm2, P = .31), as was carbachol-induced chloride transport (82.4 ± 72.2 vs 57.2 ± 33.4 μA/cm2, P = .54) and alanine absorption (16.46 ± 12.94 vs 23.53 ± 21.31 μA/cm2, P = .53).
Conclusions
Mechanical stretching induces small intestinal growth, while maintaining function. Epithelial architecture does change, such that a decrease in villus height is offset by a marked increase in crypt depth and a 2-fold increase in epithelial proliferation. Epithelial barrier and absorptive functions remain intact. The device described may have direct clinical applicability.
SHORT BOWEL SYNDROME (SBS) is a complex and highly morbid condition in which small intestinal length is inadequate for proper nutrient absorption. Mortality from SBS may exceed 30%.1–5 Total or supplemental parenteral nutrition (PN) is required but carries long-term risks of sepsis, cholestatic liver disease, and bacterial overgrowth.5–7
Clinical studies have shown a strong correlation between the length of the remaining small bowel and patient outcomes.1 In a recent analysis of a large group of SBS patients, mortality was correlated closely with intestinal length less than 10% of the expected normal length.1 For this reason, therapy for SBS has been directed toward increasing bowel length.6–8 Several creative surgical procedures to elongate the intestine using the existing small bowel have been described. Although these surgical options succeed in some cases, they are limited to dilated (and potentially already dysfunctional) areas of bowel. In addition, risks include injury to the mesenteric vasculature and enteric leakage attributable to extensive anastomoses.7–9
In attempts to actually generate new intestinal tissue, other investigators have used growth hormones along with specific nutrients to stimulate bowel growth.10 To date, this approach has had limited effectiveness; and several obstacles including the long-term need for such factors and the potential risk of malignancy exist.11 Unfortunately, once these hormones are discontinued, the adaptive process reverses.12–14 As a result, many of these factors have not significantly improved somatic muscle mass or the ability to wean patients from PN.10,15
Recent reports have described successful elongation of the small intestine using mechanical force.16,17 These reports, however, were limited to rodents, and utilized an externally mounted device on the abdominal wall—an approach unlikely to be feasible in the clinical setting. However, the principle of stimulating tissue growth via mechanical force is consistent with other lines of research. Lengthening of skeletal bones in patients with limb length discrepancies is a well-known example,18 but the principle of distraction organogenesis is not limited to the skeletal system. Distracting forces have induced growth of cultured intestinal epithelial cells, and organ systems including bone, skin, nervous tissue, and muscle successfully.19–22 Biomechanical shear stress triggers expression of cell-surface integrins and activates cellular proliferation.22 Caco-2 cells, derived from colonic epithelium, demonstrate a similar responsiveness to mechanical shear stress.23 Taken together, these data strongly suggest that mechanical forces induce not only elongation but also actual growth of the small intestine. We therefore designed a device for bowel elongation in vivo, which could have direct clinical applicability, and tested intestinal function in segments of bowel undergoing longitudinal growth with this device.
METHODS
Animal models
All studies reported here conformed to the guidelines for the use and care of laboratory animals established by the University Committee on Use and Care of Animals of the University of Michigan, and protocols were approved by that committee (UCUCA #9026).
Study design
Female Yorkshire pigs (n = 11) weighing 15 to 25 kg were used. We created 2 isolated vascularized segments of jejunum in vivo, with the remaining bowel in continuity. A device was inserted intraluminally into the first isolated segment (Stretch segment), and the intestinal segment was closed surgically. One week postoperatively, the device was activated and began to elongate incrementally. An identical isolated segment of bowel was created adjacent to the Stretch segment, but the device inserted into this second bowel segment was not elongated (Control segment). After 7 days of Stretch segment elongation (0.5 mL per day), the pigs were euthanized, and tissue was harvested for analysis. As an additional control, at the time of the initial laparotomy, a segment of native jejunum in situ, adjacent to the isolated Stretch and Control segments, was marked with 2 seromuscular stitches placed on the antimesenteric border 12 cm apart. At the time of reexploration, these stitches were identified, and the distance between them was remeasured to assess the increase in length of native jejunum attributable to baseline growth rate.
Description of hydraulic-lengthening device
A hydraulic-driven dual concentric piston was designed (Fig 1). The device consisted of telescoping syringes (3 mL and 1 mL). The piston chambers of the syringes were connected internally. Fluid injected into the device produced incremental elongation; both ends of the device were coated with soft silicone bumpers to avoid bowel injury.
Fig 1.
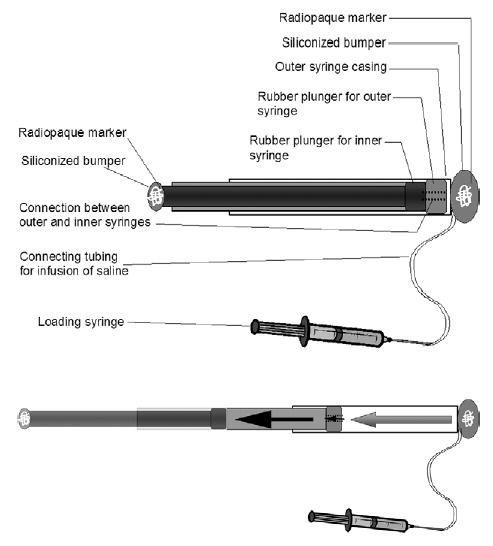
Schematic representation of the hydraulic lengthening device. Top, Device before extension. Note the radio-opaque markers within both ends embedded in silicon, allowing for visualization with abdominal radiographs. Bottom, Device in a nearly extended state. Note that the larger (3-mL) syringe is filled, and fluid has now entered through the channel in the 3-mL plunger, allowing the 1-mL syringe to start filling.
Anesthesia
Intramuscular injection of xylazine (2.2 mg/kg) and tiletamine/zolazepam (6–8 mg/ kg) was used for sedation. Glycopyrrolate (0.02 mg/kg) and cefazolin (50 mg/kg) were given before induction of anesthesia. Endotracheal intubation was performed, and a surgical plane of anesthesia was maintained with continuous inhaled isoflurane (2%–2.5%).
Surgical procedure
A midline abdominal incision was used (Fig 2). A section of mid-jejunum approximately 100 cm from the ligament of Treitz was taken out of luminal continuity while retaining its vascular supply. The remaining bowel was placed back into continuity with an end-to-end anastomosis. The isolated segment was divided in half, and a device was inserted intraluminally into each half. The ends of the segments were sutured shut. The fluid injection line for device lengthening exited the bowel segment through a purse-string suture; similarly, a drain catheter was placed to prevent accumulation of mucosal secretions. The device fluid line and the drain were tunneled subcutaneously and exited the skin between the scapulae. This also secured the isolated segment up against the parietal peritoneal surface to prevent volvulus. In this way, 2 identical intestinal segments were thus created so that one would serve as a Control (nonlengthened) and the other as a Stretch segment for elongation. In addition, a third portion of jejunum, not excised but simply left in situ, was marked with stitches 12 cm apart on the antimesenteric border. This third segment of bowel served as an additional control for the growth rate of the bowel remaining in enteric continuity. No device of any sort was applied to this nonoperated segment of native jejunum. The abdomen was then closed in standard fashion.
Fig 2.
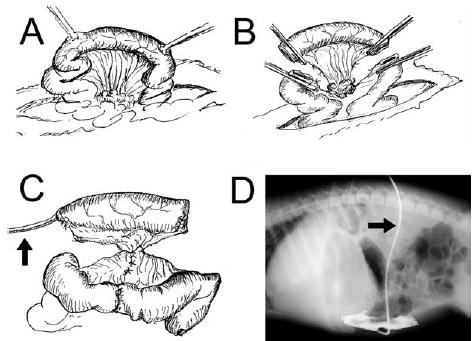
Surgical creation of isolated intestinal segments. A, Mid-jejunum was utilized. B, A segment of intestine was taken out of continuity, while preserving its mesenteric pedicle. C, The device for bowel elongation is inserted into the lumen with the tubing to activate the device exiting the bowel (arrow). Note: For simplicity, only 1 segment is shown. For experimental purposes, 2 such intestinal segments were created in each animal to provide an in situ Control segment as well as an elongated segment. D, The isolated segments of bowel also were drained via a soft catheter (arrow) to prevent accumulation of mucus. Radio-opaque contrast injection demonstrates normal diameter and morphology of the isolated segment in vivo.
Outcomes
Of 11 experiments, 8 were successful. There were 2 technical failures. One animal required euthanasia before completion of the experiment because of the development of a small-bowel obstruction before device elongation.
Sample collection
The pig was reanesthetized on postoperative day 14, and a repeat laparotomy was performed. The Stretch and Control segments, as well as the adjacent segment of normal jejunum, were excised while the animal was alive and were placed immediately into RPMI 1640 tissue culture medium (Gibco BRL, Gaithersburg, Md) with 10 mmol/L glutamine at 4°C. Studies were performed immediately after tissue harvest; samples were maintained in oxygenated 4°C tissue medium at all times. After sample collection, the animal was euthanized while still under general anesthesia by an intracardiac injection of KCl (2 mmol/kg) followed by cardiectomy to prevent inadvertent resuscitation.
Morphology of intestinal segments
Mesenteric fat was excised rapidly, and segments were opened longitudinally, with removal of the hydraulic device. Circumference of each segment, as well as mesenteric and antimesenteric length, was measured in the tension-free state. Samples were kept on ice, gently blotted, weighed, and returned immediately to glutamine-enriched tissue culture medium at 4°C for completion of electrophysiologic studies. A small portion was taken for histology, protein, and nucleic acid quantification.
Measurement of crypt-villus axis
Formalin-fixed specimens were stained with hematoxylineosin. Villus height and crypt depth were measured with an optical micrometer at ×100 magnification. Specimens were oriented meticulously at right angles to the long axis of the bowel, such that a true longitudinal view of crypts and villi was obtained. Only crypts and villi sectioned in such a way that the whole length of the villus/crypt could be seen were included in measurements.24 Multiple separate sections from each sample were evaluated in this way. Measurements were performed by an observer blinded to the identity of tissue specimens. A minimum of 10 crypts and villi were measured per slide.
Epithelial proliferation
Proliferative rates of epithelial cells were assessed with the use of in vivo bromodeoxyuridine (BrdU) labeling as described previously.25 Briefly, a single dose of 5-bromodeoxyuridine (50 mg/kg; Sigma Aldrich, St. Louis, Mo) was administered intravenously 4 hours before harvest of the intestinal segments. Histologic samples were fixed in 10% formalin immediately after surgical excision. Sections 5 μm thick were stained with the BrdU In-Situ Detection kit according to the manufacturer’s instructions (BD PharMingen, San Diego, Calif). The mean number of proliferating cells in 10 crypts per histologic section was calculated. Epithelial cell proliferation was expressed as the percentage of cells incorporating BrdU.
Protein and nucleic acid content
As an additional index of cellular activity, total tissue protein content was quantified by using a modified Lowry method. Protein was expressed as micrograms per milligram of wet tissue weight. Extraction of total cellular RNA and DNA has been described previously.26 Briefly, total RNA was quantified with the use of TRIzol reagent (Gibco BRL) according to the manufacturer’s instructions. Total DNA was purified with ethanol precipitation and ultracentrifugation at 12,000g. RNA and DNA content was expressed as micrograms per milligram of protein.
Baseline intestinal ion transport
Modified Ussing chambers (Physiologic Instruments, San Diego, Calif) were used to measure ion transport and epithelial barrier function as described previously.26,27 Mucosal and serosal compartments filled with 5 mL of Krebs buffer were maintained at 37°C at a pH of 7.4. Each chamber was oxygenated continuously with O2/CO2 (95%/5%). The serosal buffer included 10 mmol/L glucose as an energy source and was balanced osmotically with 10 mmol/L mannitol on the mucosal side. The spontaneous potential difference across the intestinal membrane was maintained at 5 mV by an automated voltage clamp, and the injected short circuit current (Isc) was monitored continuously as an indication of net active ion transport. Baseline Isc (an indication of the ion transport state of the tissue) was recorded after a 20-minute equilibration period.
Stimulated ion transport
Glucose uptake into enterocytes is linked to serosal sodium absorption, thus leading to changes in Isc with glucose absorption. To assess glucose-stimulated sodium absorption, 5 mL of 10 mmol/L D-glucose was added to the mucosal chamber. The change in Isc was measured by subtracting the basal current from the peak current after the addition of D-glucose. Second, alanine was used as an indicator of sodium-coupled amino acid absorption.27 Alanine increases Isc by stimulating mucosal-to-serosal sodium flux through a sodium-amino acid cotransporter. After equilibration, 5 mL of L-alanine (10 mmol/L) was added to the mucosal chamber, and 5 mL of fresh Krebs buffer was added to the serosal side. The change in Isc was measured as described above. Finally, carbachol (a secretory Cl− agonist) was used to measure chloride ion transport. Basal Isc was measured after equilibration, and 5 mL of 10-μmol/L carbachol was added to the serosal chamber. The change in Isc was measured by subtracting the basal current from the peak current after addition of carbachol.
Epithelial permeability
Mucosal permeability was assessed by 2 methods: transepithelial resistance (TER) and the transepithelial passage of 3H-mannitol. TER was determined by using Ohm’s law. After a 30-minute equilibration period, 3H-mannitol (3 μCi/mL, Sigma-Aldrich) was added to the mucosal compartment. One-milliliter samples were taken from the serosal compartment every 10 minutes for 90 minutes and were analyzed for 3H in a scintillation counter. Results are expressed as the percentage of transepithelial passage of [3H]-mannitol after 90 minutes.
Statistical analysis
Data are reported as mean ± SD. For comparison of multiple groups, analysis of variance with a post hoc Tukey test was used. A 2-tailed Student t test was used for comparison of paired groups. P less than .05 was considered significant.
RESULTS
Increased intestinal length and surface area
Stretch segments were significantly longer than Control segments (Table I). Measurements were taken in the tension-free state with intestinal segments split longitudinally. The Stretch group also had significantly greater surface area (nearly a 2-fold increase) as well as greater mass than Control segments (Table I). Intestinal tissue mass per square centimeter of surface area did not differ between Stretch and Control groups, demonstrating that the observed bowel lengthening was actual tissue growth—not merely a redistribution of tissue (Table I). In addition, while Stretch segments showed significant growth, Control segments actually atrophied (Table I). The fact that true growth of Stretch segments did occur was also shown by comparison of the Stretch segment to the native jejunal segment. This native jejunal segment (marked with stitches ~12 cm apart) demonstrated only a small increase in length over the course of the experiment, far less than the growth seen in the actively elongated Stretch segments (Table I).
Table I.
Intestinal length, tissue mass, and surface area increased with mechanical bowel lengthening*
| Stretch segment | Control segment | Jejunum (native bowel, not isolated)† | P value (Stretch vs Control) | P value (Stretch vs native jejunum) | |
|---|---|---|---|---|---|
| Initial length (cm) | |||||
| Mesenteric | 11.83 ± 1.05 | 12.33 ± 1.60 | N/A‡ | .53 | N/A |
| Antimesenteric | 12.13 ± 1.38 | 12.33 ± 1.60 | 11.50 ± 1.14 | .82 | .47 |
| Final length (cm) | |||||
| Mesenteric | 15.88 ± 2.38 | 9.24 ± 2.38 | N/A‡ | .0002 | N/A |
| Antimesenteric | 17.58 ± 2.42 | 10.41 ± 3.22 | 11.95 ± 0.42 | .0007 | .002 |
| Change in length (%; note direction of change) | |||||
| Mesenteric | + 35.0 ± 22.0 | − 24.1 ± 19.2 | N/A‡ | .0002 | N/A |
| Antimesenteric | + 45.3 ± 15.5 | − 14.9 ± 24.2 | + 4.69 ± 11.04 | .0002 | .002 |
| Rate of growth or atrophy (cm/d) | |||||
| Mesenteric | + 0.86 ± 0.51 | − 0.22 ± 0.21 | N/A‡ | .0002 | N/A |
| Antimesenteric | + 1.20 ± 0.46 | − 0.14 ± 0.25 | + 0.03 ± 0.08 | .00001 | .001 |
| Wet weight of segment (g) | 29.4 ± 2.3 | 17.4 ± 4.5 | N/A‡ | <.001 | N/A |
| Mucosal surface area (cm2) | 86.7 ± 19.8 | 46.1 ± 13.7 | N/A‡ | <.001 | N/A |
| Mass per unit area (g/cm2) | 0.33 ± 0.10 | 0.28 ± 0.03 | 0.23 ± 0.04 | .34 | .09 |
The increased length of stretched bowel was not merely a redistribution of tissue. There was actually an increase in surface area as well as an increase in mass. In addition, while Stretch segments showed significant growth, Control segments atrophied.
A segment of native jejunum, adjacent to the isolated Stretch and Control segments, was marked with 2 seromuscular stitches placed on the antimesenteric border approximately 12 cm apart. The distance between stitches was then measured carefully at the time of initial operation. On reexploration 2 weeks later, the stitches were identified again, and the distance between them was remeasured, to indicate the increase in length of native jejunum attributable to growth.
Only the antimesenteric side of the nonoperated jejunum in situ was marked thus with stitches.
Crypt-villus morphology
In addition to macroscopic growth, mucosal thickness also was significantly greater in Stretch than Control segments (Table II). However, villus height was reduced in both Stretch and Control segments, compared with the normal jejunum. In contrast, crypt depth was increased markedly in Stretch segments versus Control (P = .005) and was even somewhat greater than native jejunum (Table II). Thus, the total mucosal thickness in Stretch bowel was equal to native jejunum, because the decrease in villus height was offset by a significant increase in crypt depth in Stretch segments. In addition, both Stretch and Control segments demonstrated markedly increased thickness of the muscularis propria (Table II).
Table II.
Histologic changes with bowel segment isolation and elongation*
| A. Native jejunum | B. Stretch segment | C. Control segment | P value | |
|---|---|---|---|---|
| Villus height (μm) | 522 ± 87 | 353 ± 76† | 324 ± 76† | .0005 vs A† |
| Crypt depth (μm) | 365 ± 43 | 450 ± 95† | 341 ± 64 | .005 vs C†, .08 vs A† |
| Total mucosal thickness (μm) | 842 ± 75 | 772 ± 134† | 647 ± 75 | .02 vs C†, .39 vs A† |
| Villus width (μm) | 133 ± 15 | 143 ± 23 | 149 ± 24 | NS, all groups |
| Ratio of villus height to crypt depth | 1.47 ± 0.45† | 0.80 ± 0.21 | 0.98 ± 0.31 | .01 vs B and C† |
| Muscularis propria (μm) | 586 ± 118† | 1099 ± 172 | 1012 ± 229 | .05 vs B and C† |
Data are mean ± SD.
Comparisons made with analysis of variance and Tukey post hoc test.
Epithelial cell proliferation
The intestinal crypts are the primary sites of epithelial proliferation. Therefore, cellular proliferation in the crypts (BrdU nuclear staining) was assessed. Epithelial proliferation in Stretch segments was more than 2-fold greater than in Controls (26.3% ± 3.8% vs 12.1% ± 6.6%, P < .01) and also was greater than that of native jejunum (15.7% ± 7.2%, P = .05 versus Stretch) (Figs 3 and 4). Thus, a 2-fold increase in epithelial proliferation was consistent with the observed increase in crypt depth in Stretch segments.
Fig 3.
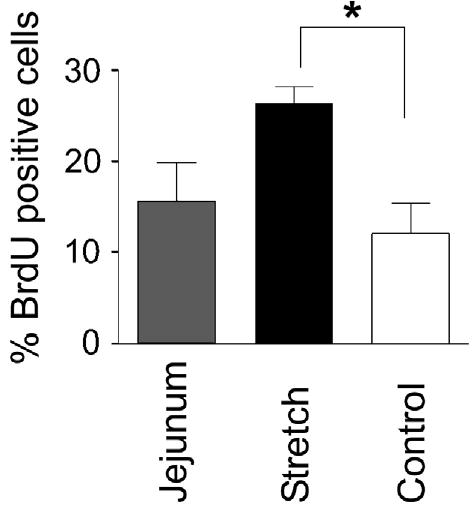
Epithelial cell proliferation was detected by measuring BrdU incorporation into actively dividing cells. Proliferation is expressed as the mean percent of BrdU-positive cells per crypt (±SD). Stretch segment proliferation was more than 2-fold greater than that of Control segment (*P < .01) and approached significance, compared with native jejunum (P = .05).
Fig 4.
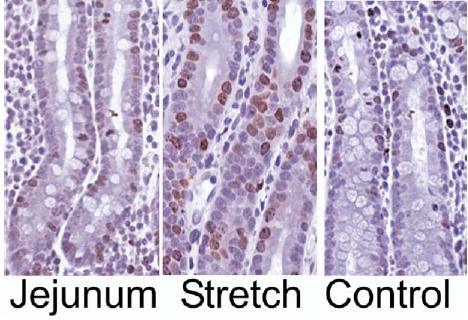
Representative histologic figures from nonoperated Jejunum, Stretch, and Control segments of pig intestine. All slides were stained for BrdU as a measure of proliferation. Note the marked increase in BrdU-positive crypt cells (brown) in the Stretch segment.
Protein and nucleic acid content
Protein, DNA, and RNA content were measured to control for a possible disparity in tissue cellularity. Protein, DNA, and RNA content did not differ between groups (Table III). Similar to the proliferation data, this finding supports that the lengthening process is due to progressive intestinal growth—not simply stretching of the bowel.
Table III.
Tissue content of DNA, RNA, and protein*
| Native jejunum | Stretch segment | Control segment | |
|---|---|---|---|
| Protein (μg per mg tissue) | 61.39 ± 10.98 | 56.73 ± 6.34 | 59.01 ± 11.15 |
| RNA (μg per mg protein) | 2.06 ± 0.95 | 1.33 ± 0.38 | 1.41 ± 0.65 |
| DNA (μg per mg protein) | 2.42 ± 0.95 | 1.95 ± 0.42 | 2.59 ± 0.82 |
No significant differences are found between any groups, demonstrating equivalent tissue cellularity.
Epithelial barrier function
Stretch segments demonstrated intact barrier function in comparison with native jejunum (cumulative % of transepithelial passage of [3H]-mannitol after 90 minutes, 0.16% ± 0.08%, vs normal jejunum, 0.17% ± 0.08%, P = .81) (Fig 5). Transepithelial resistance of the Stretch segments was virtually identical to the native bowel (Fig 6), providing another indication of normal epithelial barrier function in Stretch intestinal segments.
Fig 5.
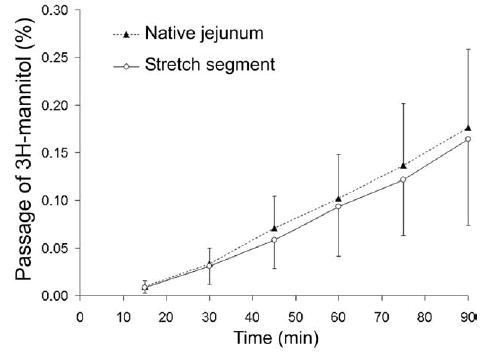
Cumulative transmucosal permeation of 3H-mannitol (mean ± SD%) during 90 minutes incubation in Ussing chambers. Time 0 represents the start of the incubation with 3H-mannitol after a 20-minute equilibration period. There was a steady linear permeation increment in transmucosal passage of 3H-mannitol over 90 minutes. Stretch segment of intestine did not differ from native jejunum, demonstrating intact mucosal barrier function.
Fig 6.
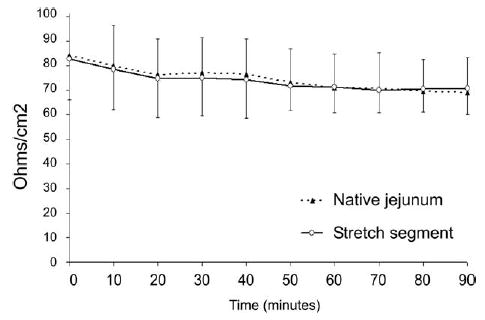
Epithelial barrier function as measured by trans-epithelial resistance (TER, ω/cm2) is shown. TER was virtually identical between Stretch segments of intestine, compared with native jejunum, indicating normal mucosal barrier function.
Epithelial ion transport
Baseline transepithelial current (Isc) was less in the Stretch intestine than in native jejunum (Fig 7), although the absolute difference was small. This finding may indicate a downregulation of baseline ion transport in the lengthened bowel. However, the Stretch bowel did not differ from native jejunum in terms of responsiveness to stimulated ion transport. Glucose-mediated sodium transport was similar in the Stretch group, compared with normal jejunum (60.0 ± 23.5 vs 82.3 ± 47.3 μA/cm2, P = .31), showing a normal response to glucose stimulation. Importantly, this result is calculated per square centimeter of mucosal surface area. Thus, the significant increase in mucosal surface area in Stretch segments (see above) resulted in an actual increase in the actual net amount of stimulated ion transport in Stretch segments. Similarly, carbachol-induced chloride transport was actually slightly greater in the Stretch group, compared with the normal jejunum per square centimeter (82.4 ± 72.2 vs 57.2 ± 33.4 μA/cm2, P = .54), and, again, although the difference per square centimeter was not significant, this finding would indicate a net increase in stimulated chloride transport. Finally, the same result was found for sodium-amino acid cotransport. Alanine absorption per square centimeter of mucosal surface area was equivalent between Stretch segments and native jejunum (16.46 ± 12.94 vs 23.53 ± 21.31 μA/cm2, P = .53). Thus, Stretch epithelium demonstrated overall stimulated ion transport equivalent to that of native jejunum per square centimeter, indicating that total stimulated ion transport increased in proportion to the increase in surface area.
Fig 7.
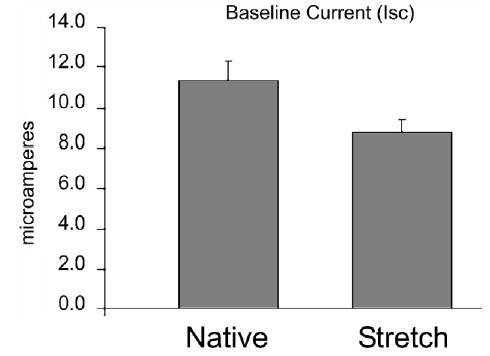
Averaged baseline short circuit current (Isc, μA/ cm2) during the first 20 minutes after mounting intestinal samples in the Ussing chamber. Baseline Isc was significantly lower in Stretch intestinal segments than in native jejunum (P < .0001). However, stimulated ion transport in response to various agonists did not differ between Stretch and native jejunum (see text).
DISCUSSION
Short-bowel syndrome remains a debilitating condition in which the small intestinal length is far less than that required for proper nutrient absorption.2–5 Despite advancements in nutrition support and critical care, mortality rates as high as 30% persist.1–4 Clearly, there is a great need for an approach that can treat short-bowel syndrome successfully.
Distracting forces have been applied successfully to promote growth in cultured cells as well as several organ systems, including bone, skin, nervous tissue, and muscle.18–21 Therefore, we hypothesized that application of distracting forces to the intestine would induce growth. This approach, in fact, has been studied since the mid-1990s with good but limited results. An obstructive model in pig small intestine resulted in mucosal proliferation.28 However, this model also caused marked intestinal dilation, leading to bacterial overgrowth and bowel injury. Application of an externally mounted device in rabbits produced bowel lengthening and normal histology but a decline in absorptive enzymes.29 Unfortunately, it is unclear whether this study achieved true bowel lengthening versus a mere stretching of the intestine.29 In 2 very recent reports using a rat model, forces were applied with the use of an external screw device mounted onto the abdominal wall and advanced by a few millimeters each week into an isolated limb of small bowel. This approach resulted in a 149%17 to 210%16 increase in small-bowel length, with either an increase or preservation of intestinal function. However, the applicability of these devices to the clinical setting is questionable. Nevertheless, these reports validated the idea of intestinal growth in response to mechanical stress. The current study was performed to demonstrate both the practicality of an implantable device in a large animal model, as well as intestinal function from successful enterogenesis.
This current study demonstrated that the intestine responds to distractive forces in a much more rapid fashion than appreciated previously. Over an 8-day period, the isolated intestinal segment increased in length by almost 2-fold. This increase in length occurred at the same time that the control segment atrophied and lost length (after removal of Control devices, the Control segment tended to recoil to a shorter length than its original length). This finding strongly suggests that absence of mechanical forces, when a segment of bowel is taken out of continuity, may play a major role in the development of intestinal atrophy. Loss of luminal nutrition in the isolated segment also may trigger atrophy. Furthermore, the increase in length of the Stretch segment was far greater than the length increase attributable to natural bowel growth. The native segment of jejunum (marked with stitches) did increase in length slowly over 2 weeks, but its growth was 10-fold less than that seen in the actively elongated segment of bowel.
In addition to an increase in length, both the wet weight and surface area of the intestine increased by a similar degree in Stretch segments. Intestinal mass per square centimeter did not differ between control and lengthened segments. This finding demonstrates that bowel lengthening was not merely the result of stretching or thinning out of the tissues. To further confirm this, we examined the histology of the elongated segments of intestine. Both mucosal thickness and crypt depth increased in the lengthened group, compared with the control group. Although villus height was reduced in both study groups, this finding was not surprising given that these segments had no intraluminal nutrition. Epithelial cell proliferation studies further confirmed that a true enterogenesis occurred in the lengthened group. Therefore, the increase in crypt depth in Stretch segments was consistent with an increase in cellular proliferation.
Physiologic measurement of intestinal function showed a preservation of intestinal barrier function and retention of transporter function in the Stretch segments. It was interesting to note that baseline ionic current was decreased in the lengthened intestinal segment. Whether this difference may be attributable to the change in crypt-villus architecture (deeper crypts and lower villi) or perhaps to downregulation of ion transporters is not clear. In addition, the duration of our study was limited: It is possible that mucosal function of the lengthened segment may approximate native jejunum more closely given more time. Future studies will need to be performed after the lengthened segment is replaced back into continuity with the remaining bowel. However, the fact that sodium-coupled alanine transport, sodium-glucose cotransport, and stimulated chloride transport in Stretch segments were equivalent to native jejunum per square centimeter of gross mucosal surface area is significant. The villi comprise the major absorptive surface of the intestine. Thus, the decreased villus height in Stretch segments might have been expected to result in a decreased absorptive capacity per unit area, but this did not actually occur. Instead, stimulated ionic transport was maintained and—in some cases—was greater than native jejunum. The fact that the actual gross surface area of the Stretch segment nearly doubled would indicate that the overall net absorptive capacity of the Stretch segment also doubled.
We are proposing our model as a potential future approach for clinical distraction enterogenesis. The pigs in our study tolerated the implanted device very well. They had no difficulty feeding or ambulating. No restraints were necessary. The device did not cause pain. The isolated case of small-bowel obstruction was due to intraperitoneal adhesions—a known risk of any intraperitoneal surgery—and was not due directly to the device per se. There are no foreign bodies directly in contact with the peritoneal cavity in this model, because the segment of bowel containing the device is brought into contact with the anterior abdominal wall. The control line and the drain to the device exit the isolated bowel segment directly into the abdominal wall. We believe that this model could indeed be performed successfully in patients. The question that must be answered first is whether the amount of bowel that can be generated in this fashion justifies the risks of the surgical procedure involved.
As both the frequency of the distracting force and the strength of the stimulus can affect the degree of cellular proliferation,30 future work will be needed to better understand how to maximize the extent of enterogenesis. One potential pathway that may guide enterogenesis is the activation of surface integrins. 31 These integrins subsequently activate phosphoinositol 3-kinase and mediate cellular proliferation,31 a process that may be mediated through epidermal growth factor, a key modulator of mucosal adaptation in short-bowel syndrome.32,33
CONCLUSION
The use of mechanical distracting forces can lead to significant small intestinal growth over a short period of time. Mucosal barrier function and stimulated ion transport were well preserved, while epithelial cell proliferation increased in concert with mucosal architectural rearrangements. Bowel lengthening with mechanical distraction may eliminate many of the obstacles associated with current modalities to treat SBS. This approach may offer a safer and more effective therapy for SBS than what is available currently.
The authors would like to thank Gail Rising, LVT, LATG, for technical assistance.
Footnotes
Presented at the 67th Annual Meeting of the Society of University Surgeons, First Annual Academic Surgical Congress, February 7–11, 2006, San Diego, California.
Supported in part by grant support of the Research Advisory Committee, Department of Surgery, University of Michigan.
References
- 1.Spencer AU, Neaga A, West B, et al. Pediatric short bowel syndrome: redefining predictors of success. Ann Surg. 2005;242:403–12. doi: 10.1097/01.sla.0000179647.24046.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warner BW, Ziegler MM. Management of the short bowel syndrome in the pediatric population. Pediatr Clin North Am. 1993;40(6):1335–50. doi: 10.1016/s0031-3955(16)38664-3. [DOI] [PubMed] [Google Scholar]
- 3.Barksdale EM, Stanford A. The surgical management of short bowel syndrome. Curr Gastroenterol Rep. 2002;4(3):229–37. doi: 10.1007/s11894-002-0068-1. [DOI] [PubMed] [Google Scholar]
- 4.Vanderhoof JA. Short bowel syndrome in children. Curr Opin Pediatr. 1995;7(5):560–8. doi: 10.1097/00008480-199510000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Messing B, Crenn P, Beau P, et al. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology. 1999;117(5):1043–50. doi: 10.1016/s0016-5085(99)70388-4. [DOI] [PubMed] [Google Scholar]
- 6.Kim HB, Fauza D, Garza J, et al. Serial transverse enteroplasty (STEP): a novel bowel lengthening procedure. J Pediatr Surg. 2003;38(3):425–9. doi: 10.1053/jpsu.2003.50073. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi A. Longitudinal intestinal lengthening and tailoring: results in 20 children. J Royal Soc Med. 1997;90(8):429–32. doi: 10.1177/014107689709000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianchi A. Experience with longitudinal intestinal lengthening and tailoring. Eur J Pediatr Surg. 1999;9(4):256–9. doi: 10.1055/s-2008-1072258. [DOI] [PubMed] [Google Scholar]
- 9.Bueno J, Guiterrez J, Mazariegos GV, et al. Analysis of patients with longitudinal intestinal lengthening procedure referred for intestinal transplantation. J Pediatr Surg. 2001;36(1):178–83. doi: 10.1053/jpsu.2001.20047. [DOI] [PubMed] [Google Scholar]
- 10.Scolapio JS. Effect of growth hormone and glutamine on the short bowel: five years later. [comment] Gut. 2000;47(2):164. doi: 10.1136/gut.47.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrne TA, Wilmore DW, Iyer K, et al. Growth hormone, glutamine, and an optimal diet reduces parenteral nutrition in patients with short bowel syndrome: a prospective, randomized, placebo-controlled, double-blind clinical trial. Ann Surg. 2005;242:655–61. doi: 10.1097/01.sla.0000186479.53295.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tavakkolizadeh A, Shen R, Jasleen J, et al. Effect of growth hormone on intestinal Na+/glucose cotransporter activity. JPEN: J Parent Enteral Nutr. 2001;25(1):18–22. doi: 10.1177/014860710102500118. [DOI] [PubMed] [Google Scholar]
- 13.Brubaker P, Crivici A, Izzo A, et al. Circulating and tissue forms of the intestinal growth factor, glucagon-like peptide-2. Endocrinology. 1997;138(11):4837–43. doi: 10.1210/endo.138.11.5482. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Wildhaber BE, Teitelbaum DH. Keratinocyte growth factor improves epithelial function after massive small-bowel resection. JPEN J Parent Enteral Nutr. 2003;27(3):198–206. doi: 10.1177/0148607103027003198. discussion 206–7. [DOI] [PubMed] [Google Scholar]
- 15.Jeppesen PB, Hartmann B, Thulesen J, et al. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology. 2001;120(4):806–15. doi: 10.1053/gast.2001.22555. [DOI] [PubMed] [Google Scholar]
- 16.Park J, Puapong D, Wu BM, et al. Enterogenesis by mechanical lengthening: morphology and function of the lengthened small intestine. J Pediat Surg. 2004;39(12):1823–7. doi: 10.1016/j.jpedsurg.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Safford S, Freemerman A, Safford K, et al. Longitudinal mechanical tension induces growth in the small-bowel of juvenile rats. Gut. 2005;54:1085–90. doi: 10.1136/gut.2004.061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilizarov G. Clinical application of the tension-stress effect for limb lengthening. Clin Orthop. 1990;250:8–26. [PubMed] [Google Scholar]
- 19.Chen C, Tan JT, Tien J. Mechanotransduction at cell-matrix and cell-cell contacts. Annu Rev Biomed Eng. 2004;6:275–302. doi: 10.1146/annurev.bioeng.6.040803.140040. [DOI] [PubMed] [Google Scholar]
- 20.Tong L, Buchman S, Ignelzi MJ, et al. Focal adhesion kinase expression during mandibular distraction osteogenesis: evidence for mechanotransduction. Plast Reconstr Surg. 2003;111(1):211–24. doi: 10.1097/01.PRS.0000033180.01581.9A. [DOI] [PubMed] [Google Scholar]
- 21.Neumann C. The expansion of an area of skin by progressive distention of a subcutaneous ballon: use of the method for securing skin for subtotal reconstruction of the ear. Plast Reconstr Surg. 1957;19:124–30. doi: 10.1097/00006534-195702000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Nelson C, Jean R, Tan J, et al. Emergent patterns of growth controlled by multicellular form and mechanics. Proc Natl Acad Sci USA. 2005;102:11594–9. doi: 10.1073/pnas.0502575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han O, Li G, Sumpio B, Basson M. Strain induces Caco-2 intestinal epithelial proliferation and differentiation via PKC and tyrosine kinase signals. Am J Physiol. 1998;275:G534–41. doi: 10.1152/ajpgi.1998.275.3.G534. [DOI] [PubMed] [Google Scholar]
- 24.Helmrath MA, VanderKolk WE, Can G, et al. Intestinal adaptation following massive small-bowel resection in the mouse. J Am Coll Surg. 1996;183:441–9. [PubMed] [Google Scholar]
- 25.Yang H, Antony PA, Wildhaber BE, Teitelbaum DH. Intestinal intraepithelial lymphocyte gammadelta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol. 2004;172(7):4151–8. doi: 10.4049/jimmunol.172.7.4151. [DOI] [PubMed] [Google Scholar]
- 26.Yang H, Finaly R, Teitelbaum DH. Alteration in epithelial permeability and ion transport in a mouse model of total parenteral nutrition. Crit Care Med. 2003;31(4):1118–25. doi: 10.1097/01.CCM.0000053523.73064.8A. [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Kiristioglu I, Fan Y, et al. Interferon-gamma expression by intraepithelial lymphocytes results in a loss of epithelial barrier function in a mouse model of total parenteral nutrition. Ann Surg. 2002;236(2):226–34. doi: 10.1097/00000658-200208000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins J, 3rd, Vicente Y, Georgeson K, Kelly D. Partial intestinal obstruction induces substantial mucosal proliferation in the pig. J Pediatr Surg. 1996;31(3):415–9. doi: 10.1016/s0022-3468(96)90750-2. [DOI] [PubMed] [Google Scholar]
- 29.Printz H, Schlenzka R, Requadt P, et al. Small-bowel lengthening by mechanical distraction. Digestion. 1997;58(3):240–8. doi: 10.1159/000201450. [DOI] [PubMed] [Google Scholar]
- 30.Iqbal J, Saidi M. Molecular regulation of mechanotransduction. Biochem Biophys Res Commun. 2005;328:751–5. doi: 10.1016/j.bbrc.2004.12.087. [DOI] [PubMed] [Google Scholar]
- 31.Morla A, Mogford J. Control of smooth muscle cell proliferation and phenotype by integrin signaling through focal adhesion kinase. Biochem Biophys Res Commun. 2000;272:298–302. doi: 10.1006/bbrc.2000.2769. [DOI] [PubMed] [Google Scholar]
- 32.Helmrath MA, Shin CE, Erwin CR, Warner BW. The EGF/ EGF-receptor axis modulates enterocyte apoptosis during intestinal adaptation. J Surg Res. 1998;77(1):17–22. doi: 10.1006/jsre.1998.5362. [DOI] [PubMed] [Google Scholar]
- 33.Kuwada SK, Li X. Integrin alpha5/beta1 mediates fibronectin-dependent epithelial cell proliferation through epidermal growth factor receptor activation. Mol Biol Cell. 2000;11(7):2485–96. doi: 10.1091/mbc.11.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]


