Abstract
The addition of the strongly π-bonding ligands CO or tert-butyl isocyanide to the low-spin five-coordinate iron(II) nitrite species, [Fe(TpivPP)(NO2)]−, (TpivPP = picket fence porphyrin) gives two new six-coordinate species [Fe(TpivPP)(NO2)(CO)]− and [Fe(TpivPP)-(NO2)(t-BuNC)]−. These species have been characterized by single-crystal structure determinations and by UV-vis, IR and Mössbauer spectroscopies. All evidence shows that in the mixed-ligand iron(II) porphyrin species, [Fe(TpivPP)(NO2)(CO)]−, the two trans, π-accepting ligands CO and nitrite compete for π density. The CO ligand however dominates the bonding. The Fe–N(NO2) bond lengths for the two independent anions in the unit cell at 2.006(4) and 2.009(4) Å are lengthened compared to other nitrite species with either no trans ligands or non-π-accepting trans ligands to nitrite. The Fe–C(CO) bond lengths are 1.782(4) Å and 1.789(5) Å for the two anions. The two Fe–C–O angles at 175.5(4)° and 177.5(4)° are essentially linear in both anions. The quadrupole splitting for [Fe(TpivPP)(NO2)(CO)]− was determined to be 0.32 mm/s and the isomer shift was 0.18 mm/s at room temperature in zero applied field. Both of the Mössbauer parameters are much smaller than those found for six-coordinate low-spin iron(II) porphyrinates with neutral nitrogen-donating ligands as well as iron(II) nitro complexes. However, the Mössbauer parameters are typical of other six-coordinate CO porphyrinates signifying that CO is the more dominant ligand. The CO stretching frequency of 1974 cm−1 is shifted only slightly to higher energy compared to six-coordinate CO complexes with neutral nitrogen-donor ligands trans to CO. Crystal data for [K(222)][Fe(TpivPP)(NO2)− (CO)]·1/2C6H5Cl: monoclinic, space group P21/c, Z = 8, a = 33.548(6) Å, b = 18.8172(15) Å, c = 27.187(2) Å, β = 95.240(7)°, V = 17091(4) Å3.
Introduction
The binding of the diatomic molecules O2, CO, and NO to heme proteins is extremely important to mammalian physiology, while the binding of nitric oxide (NO), nitrite (NO2−) and nitrate (NO3−) to hemes is involved in important denitrification processes. Hence, the study of the bonding interaction between these biologically significant small molecules and others to hemes has been and continues to be an active area of research.1–3 However, despite great advances in understanding the many biological functions of these molecules, there remains considerable controversy about the mechanisms and identities of possible intermediates involved in their numerous reactions. For instance, the discrimination in binding of O2 over CO to hemes in respiration is not completely understood.4, 5 In addition, it is proposed that many of the intermediates in the inorganic nitrogen cycle involve the interaction of hemes with small nitrogen containing molecules; the exact identities of several are only speculative. In a recent mechanistic study6 of the reduction of nitrite to ammonia by cytochrome c nitrite reductase (ccNiR) it was proposed that the binding of nitrite to an iron(II) heme which starts the reaction cycle depends on the strong pi bond between nitrite and iron(II). The π bond leads to a strong Fe–N bond and a weakened N–O bond which can be cleaved heterolytically. Hence, the nature of the bond between nitrite and iron is critically important to the activation of this enzyme. Clearly, gaining insight into the bonding interaction between these small molecules and hemes through structural and physical studies is of interest.
Experimental Section
General Information
All manipulations for the preparation of the iron(II) six-coordinate porphyrin derivatives (see below), were carried out under argon using a double-manifold vacuum line, Schlenkware and cannula techniques. Chlorobenzene was purified by washing with concentrated sulfuric acid, then with water until the aqueous layer was neutral, then dried with MgSO4, and then distilled twice over P2O5.29 Hexanes were distilled from sodium/benzophenone. KNO2 was recrystallized twice from hot distilled water, dried overnight at about 75°C and stored under argon. Kryptofix-222 (Aldrich) was recrystallized from benzene (distilled from sodium/benzo-phenone) and stored under argon in the dark. The free base, (H2TpivPP), and the corresponding iron(III) chloro and triflato derivatives were synthesized by literature methods.30,31 UV-vis spectra were recorded on a Perkin-Elmer Lambda 19 UV/vis/near-IR spectrometer and IR spectra were recorded on a Perkin-Elmer Paragon 10000 FT-IR spectrometer as KBr pellets. Mössbauer measurements were performed on a constant acceleration spectrometer from 4.2 K to 300 K with optional small field and in a 9 T superconducting magnet system (Knox College). A sample of [K(222)][Fe(TpivPP)(NO2)(CO)]·1/2C6H5Cl for Mössbauer spectroscopy was prepared by immobilization of the crystalline material (crystals not ground but washed with water under argon) in Apiezon M grease.
Synthesis of [K(222)][Fe(TpivPP)(NO2)(CO)]·1/2C6H5Cl
[Fe(TpivPP)(SO3CF3)-(H2O)] (20 mg, 0.016 mmol) and ~1 mL of zinc amalgam in 8 mL of chlorobenzene were stirred for about 1 hour under argon. The deep red solution of [Fe(TpivPP)] was filtered into a second solution prepared by stirring (overnight) 80 mg of Kryptofix-222 (0.2 mmol) and 185 mg of KNO2 (2.2 mmol) in 7 mL of chlorobenzene. A stream of CO gas was passed (for about 15 min) through the dark red-yellow solution of the five-coordinate (nitro)iron(II) species, [Fe(TpivPP)(NO2)]−.20 The color changes slightly to light red as a result of forming the six-coordinate product [Fe(TpivPP)(NO2)(CO)]−. Single crystals of this complex were prepared by slow diffusion of hexanes into the chlorobenzene solution. UV-vis in C6H5Cl: λmax, nm (log ε); 414 (sh) (4.67); 434 (5.30); 546 (3.98). IR (KBr): ν(CO) 1974 (m) cm−1; ν(NO2−) 1383
Results
Reaction of the five-coordinate iron(II) species, [Fe(TpivPP)(NO2)]−, with CO or tert-butyl isocyanide gives the new anionic, six-coordinate iron(II) complexes [Fe(TpivPP)(NO2)(CO)]− and [Fe(TpivPP)(NO2)(t-BuNC)]−. Care must be taken to eliminate halide impurities that are known to coordinate strongly to iron(II) species. Hence, chlorobenzene was washed with concentrated sulfuric acid and Kryptofix-222 and KNO2 were recrystallized. The two six-coordinate species were characterized in solution with UV-visible spectroscopy using a specialized inert-atmosphere cell with 1 and 10-mm path lengths. Crystalline anionic iron porphyrinates were obtained as potassium-(Kryptofix-222) salts. Both species were characterized by infrared spectroscopy. The carbonyl stretching frequency for [Fe(TpivPP)(NO2)(CO)]− appears at 1974 cm−1. The C–N stretch is at 2101 cm−1 for [Fe(TpivPP)(NO2)(t-BuNC)]−. The solid-state Mössbauer quadrupole splitting, ΔEQ, for [K(222)][Fe(TpivPP)(NO2)(CO)]·1/2C6H5Cl was found to be 0.32 mm/s and the isomer shift, δ, was 0.18 mm/s at room temperature in zero applied field.
Discussion
Over the past several years, we have synthesized a number of (nitro)iron(II) porphyrin species. The successful syntheses of these complexes results from the strategic use of picket fence porphyrin and cryptate (or macrocyclic crown ether) cations. The five-coordinate anionic iron(II) nitrite species, [Fe(TpivPP)(NO2)]−, is prepared by addition of cryptand-solubilized KNO2 to the highly air-sensitive four-coordinate species [Fe(TpivPP)]. All of the characterized six-coordinate (nitro)iron(II) species result from the anaerobic addition of gaseous NO or CO or by addition of excess neutral ligand such as pyridine, pentamethylene sulfide, or t-butyl iso-cyanide to [Fe(TpivPP)(NO2)]− in solution. As shown by electronic spectroscopy, all of these six-coordinate species persist in solution under anaerobic conditions. In fact, the spectroscopic characterization of [Fe(TpivPP)(NO2)]− and the resulting six-coordinate species [Fe(TpivPP)-(NO2)(L)]− was our first indication that nitrite is a very unusual ligand. We begin our discussion with the effects of the ligands NO2− and CO on the electronic spectrum.
The electronic spectrum of [Fe(TpivPP)(NO2)]− has a Soret band at 444 nm. As can be seen from the electronic spectral data summarized in Table 2, this 444 nm Soret band for low-spin five-coordinate [Fe(TpivPP)(NO2)]− is strongly red-shifted compared to all of the other five- or six-coordinate iron(nitro) species thus far characterized. It is also strongly red-shifted compared to the spectrum of five-coordinate [Fe(TPP)(CO)]. The addition of a neutral ligand such as PMS or pyridine to [Fe(TpivPP)(NO2)]− causes the Soret band to blue shift by more than 10 nm. A similar blue shift in the Soret occurs upon addition of NO to the five-coordinate nitrite species. The Soret band at 407 nm for the low-spin, five-coordinate species [Fe(TpivPP)(NO)] is very blue shifted compared to [Fe(TpivPP)(NO2)]−. So what shift in the position of the Soret band should one expect when CO is added trans to nitrite? Probably the most well-known porphyrin CO-related spectroscopic change occurs when CO is added to five-coordinate high-spin thiolate hemes. These are the P450-type porphyrin derivatives, so-called because of the intense red-shifted Soret band at ~450 nm. A small red shift is also seen when a CO ligand is added to the five-coordinate [Fe(TPP)(CO)] species to give [Fe(TPP)(CO)2].
Table 2.
Electronic Spectral Data for Selected Iron(II) and Iron(III) Tetraarylporphyrins a
| λmax (nm) | |||||||
|---|---|---|---|---|---|---|---|
| Complex | Soret region (log ε) | α, β region (log ε) | ref. | ||||
| Five-Coordinate Iron(II) | |||||||
| [Fe(TPP)(NO)]b | 406 (4.97) | 475(sh) | 538 (4.00) | 604 (3.45) | 36 | ||
| [Fe(TpivPP)(NO)]c | 407 (5.15) | 477(sh) (4.35) | 539 (4.12) | 607 (3.62) | 25 | ||
| [Fe(TMP)(NO)]d | 408 (4.96) | 477(sh) | 539 (3.04) | 611 (3.84) | 37 | ||
| [Fe(TPP)(CO)]e | 419 | 537 | 570 | ~610 | 38 | ||
| [Fe(TpivPP)(NO2)]− c | 433(sh) (4.94) | 444 (5.12) | 543(sh) (3.89) | 567 (4.02) | 608 (3.59) | 20,22 | |
| Six-Coordinate Iron(II) Nitro | |||||||
| [Fe(TpivPP)(NO2)(CO)]−c | 414 (sh) (4.67) | 434 (5.30) | 546 (3.98) | tw | |||
| [Fe(TpivPP)(NO2)(t-BuNC)]−c | 441 (5.52) | 541 (4.20) | 572 (3.45) | tw | |||
| [Fe(TpivPP)(NO2)(NO)]− c | 433 (5.17) | 543 (4.14) | 21 | ||||
| [Fe(TpivPP)(NO2)(Py)]− c | 413(sh) (4.83) | 430 (5.23) | 533 (4.21) | 560(sh) (3.66) | 653 (2.83) | 22 | |
| [Fe(TpivPP)(NO2)(PMS)]− c | 418(sh) (4.88) | 432 (5.07) | 537 (4.18) | 558(sh) (3.81) | 653 (3.12) | 22 | |
| Six-Coordinate Iron(II) Other | |||||||
| [Fe(TPP)(1-MeIm)(NO)]b | 415 (5.26) | 460 (4.26) | 545 (3.99) | 580(sh) (3.78) | 643 (3.05) | 39 | |
| [Fe(TPP)(4-NMe2Py)(NO)]d | 416 | 554 | 586 | 40 | |||
| [Fe(TPP)(1-MeIm)(NO)]d | 416 | 543 | 583 | 641 | 40 | ||
| [Fe(TPP)(4-MePip)(NO)]d | 417 | 463 | 554 | 591 | 40 | ||
| [Fe(TPP)(1-VinIm)2]d | 424 (5.22) | 533 (4.24) | 564 (3.67) | 41 | |||
| [Fe(TPP)(1-BzlIm)2]d | 426 (5.23) | 533 (4.24) | 565 (3.63) | 41 | |||
| [Fe(TPP)(1-AcIm)2]d | 422 (5.14) | 533 (3.99) | 563 (3.62) | 41 | |||
| [Fe(TPP)(1-MeIm)2]d | 425 (5.09) | 533 (4.26) | 564 (3.75) | 41 | |||
| [Fe(TpivPP)(1-MeIm)2]c | 430 | 536 | 42 | ||||
| [Fe(TpivPP)(1-MeIm)2]e | 432 (5.36) | 537 (4.32) | 562(sh) | 43 | |||
| [Fe(TpivPP)(Im−)(HIm)]−c | 442 (4.00) | 544 (3.47) | 44 | ||||
| [Fe(TPP)(1-MeIm)(CO)]e | 427 (5.50) | 542 (3.95) | 43 | ||||
| [Fe(TPP)(1-MeIm)(CO)]d | 422 | 544 | 45 | ||||
| [Fe(TpivPP)(1-MeIm)(CO)]c | 425 | 542 | 42 | ||||
| [Fe(TPP)(CO)2]e | 426 | 551 | 578 | 38 | |||
| Iron(II) Thiolates | |||||||
| [Fe(TpivPP)(SEt)]−c | 421 | 533 | 575 | 625 | 46 | ||
| [Fe(TpivPP)(SEt)(CO)]−c | 392,467 | 565 | 612 | 46 | |||
| [Fe(TpivPP)(SPh)]−c | 421 | 536 | 575 | 625 | 46 | ||
| [Fe(TpivPP)(SPh)(CO)]−c | 403,464 | 555 | 605 | 46 | |||
| Six-Coordinate Iron(III) Nitro | |||||||
| [Fe(TpivPP)(NO2)2]− c | 364(sh) | 426 (5.11) | 464(sh) | 553 (4.15) | 23 | ||
| [Fe(TpivPP)(NO2)(Py)]c | 422 (5.26) | 459 (sh) | 550 (4.11) | 24 | |||
| [Fe(TPP)(NO2)(NO)]b | 433 (5.34) | 510(sh) (3.70) | 545 (4.20) | 577(sh) (3.70) | 47 | ||
| [Fe(Tp-OCH3PP)(NO2)(NO)]c | 437 | 549 | 586 | 26 | |||
| [Fe(TpivPP)(NO2)(NO)]e | 433 | 543 | 581 | 26 | |||
All spectra were taken at 25°C.
Chloroform solution.
Chlorobenzene solution.
Dichloromethane solution.
Toluene solution.
The average equatorial Fe–Np bond length for [Fe(TpivPP)(NO2)(CO)]−(1) is 1.992(7) Å and for [Fe(TpivPP)(NO2)(CO)]−(2) is 1.997(6) Å. These average bond lengths are the same as those found for the six-coordinate nitro species [Fe(TpivPP)(NO2)(PMS)]−,22 [Fe(TpivPP)-(NO2)(Py)]−,22 and [Fe(TpivPP)(NO2)(NO)]−.21 The average distance for these six-coordinate species is 0.02 Å longer than in the five-coordinate nitro species, [Fe(TpivPP)(NO2)]−. It is also slightly shorter (by 0.01 Å) than the Fe–Np bond length average of other CO complexes (confer Table 3).
Table 3.
Bond Distances (Å) for (Nitro)iron Porphyrin Derivatives
| Iron(II) Complex | Fe–Np | Fe–N NO2 | Fe–L | ref. |
|---|---|---|---|---|
| [Fe(TpivPP)(NO2)(CO)]−(1) | 1.992 (7) | 2.006 (4) | 1.782 (4) | twa |
| [Fe(TpivPP)(NO2)(CO)] − (2) | 1.997 (6) | 2.009 (4) | 1.789 (5) | tw |
| [Fe(TpivPP)(NO2)] − | 1.970 (4) | 1.849 (6) | 20,22 | |
| [Fe(TpivPP)(NO2)(PMS)] − | 1.990 (6) | 1.937 (3) | 2.380 (2) | 22 |
| [Fe(TpivPP)(NO2)(Py)] − | 1.990 (15) | 1.951 (5) | 2.032 (5) | 22 |
| [Fe(TpivPP)(NO2)(NO)] −b | 1.988 (6) | 2.086 (8) | 1.792 (8) | 21 |
| [Fe(TpivPP)(NO2)(NO)] −c | 1.986 (6) | 2.060 (7) | 1.840 (6) | 21 |
| Iron(III) Complex | ||||
| [Fe(TpivPP)(NO2)(HIm)] | 1.974 (2) | 1.949 (10) | 2.037 (10) | 24 |
| [Fe(TpivPP)(NO2)(Py)] | 1.985 (3) | 1.960 (5) | 2.093 (5) | 24 |
| [Fe(TpivPP)(NO2)2] − | 1.992 (1) | 1.970 (5) | 2.001 (6) | 23 |
| [Fe(TpivPP)(NO2)(SC6HF4)] − | 1.980 (7) | 1.990 (7) | 2.277 (2) | 25 |
| [Fe(TpivPP)(NO2)(NO)]d | 2.000 (5) | 2.002 (2) | 1.668 (2) | 26 |
| [Fe(TpivPP)(NO2)(NO)]e | 1.996 (4) | 1.998 (2) | 1.671 (2) | 26 |
This work.
⊥ form.
|| form.
C2/c form.
P21/n form.
The iron–axial ligand bond lengths are sensitive to bonding changes and the largest structural changes occur upon coordination of a sixth ligand. The Fe–N(NO2) bond lengths for [Fe(TpivPP)(NO2)(CO)]−(1) and [Fe(TpivPP)(NO2)(CO)]−(2) are 2.006(4) Å and 2.009(4) Å, respectively. These are 0.16 Å longer than the Fe–N(NO2) bond length
Supplementary Material
Figure 1.
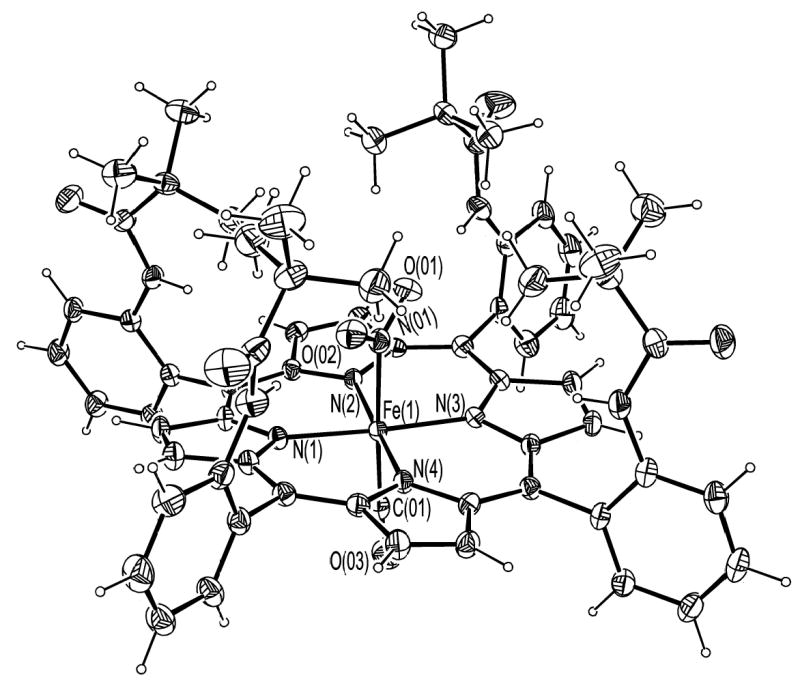
ORTEP diagram of [Fe(TpivPP)(NO2)(CO)]−(1) showing the coordination at iron. 50% probability ellipsoids are depicted.
Figure 2.
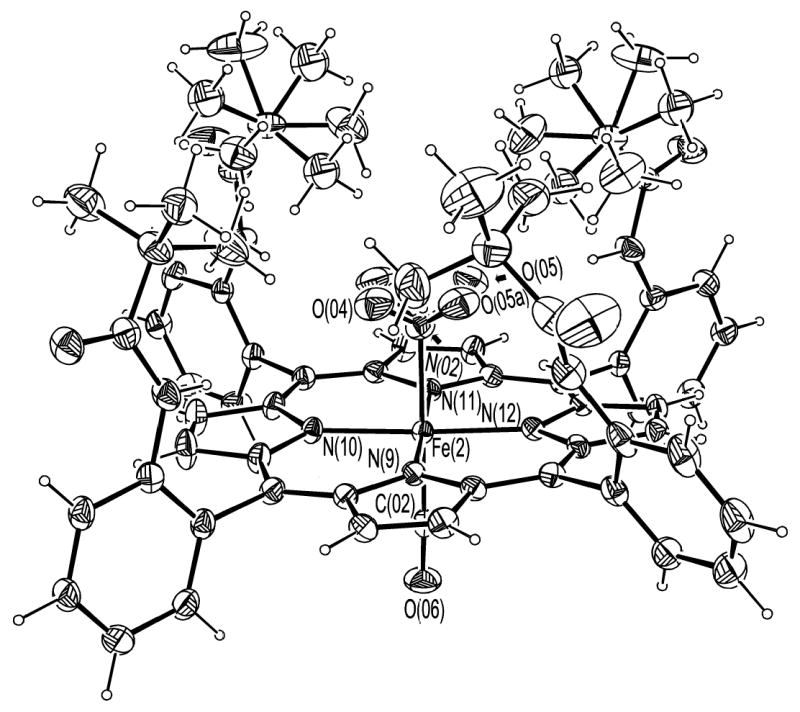
ORTEP diagram of [Fe(TpivPP)(NO2)(CO)]−(2). 50% probability ellipsoids are depicted. The rotational disorder in the nitro group and the pivalamide groups are shown.
Figure 3.
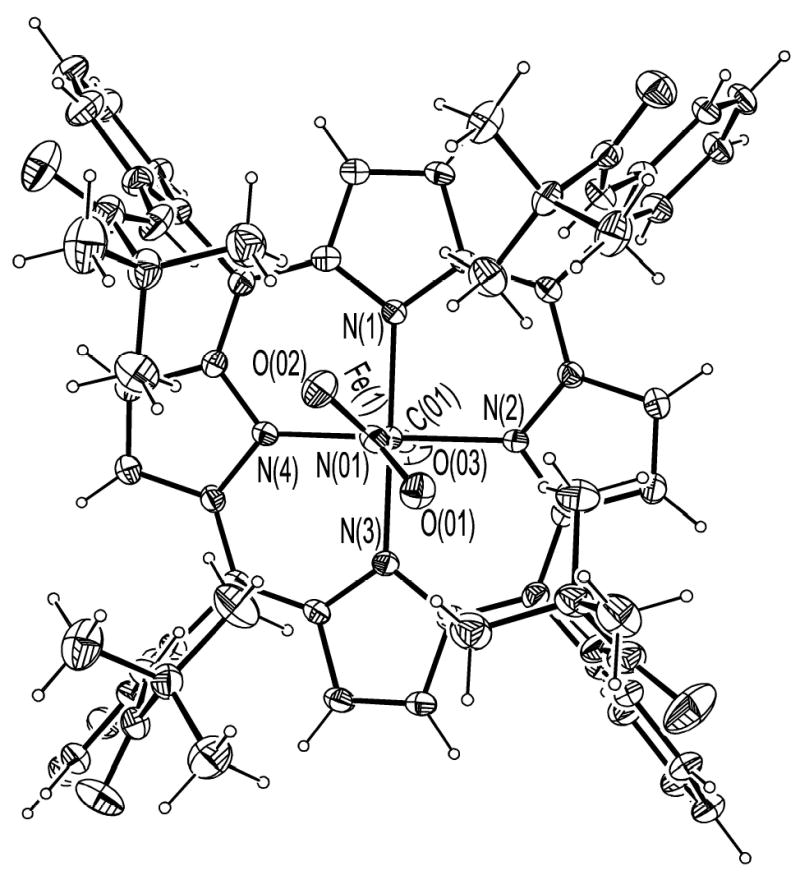
ORTEP diagram of [Fe(TpivPP)(NO2)(CO)]−(1) looking down on the porphyrin plane.
Figure 4.
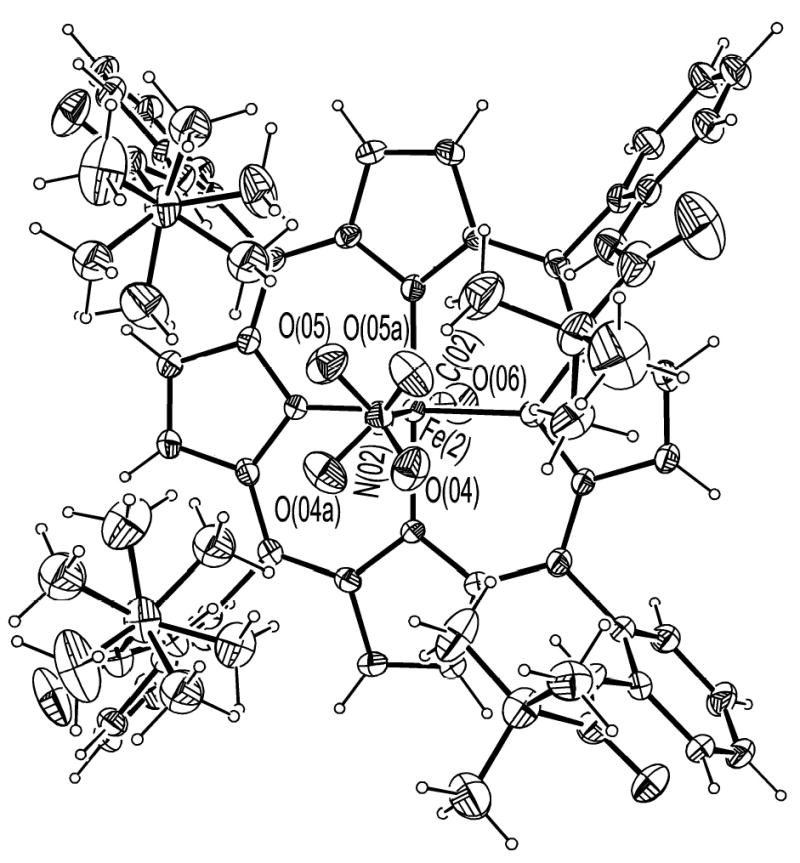
ORTEP diagram of [Fe(TpivPP)(NO2)(CO)]−(2) looking down on the porphyrin plane.
Figure 5.
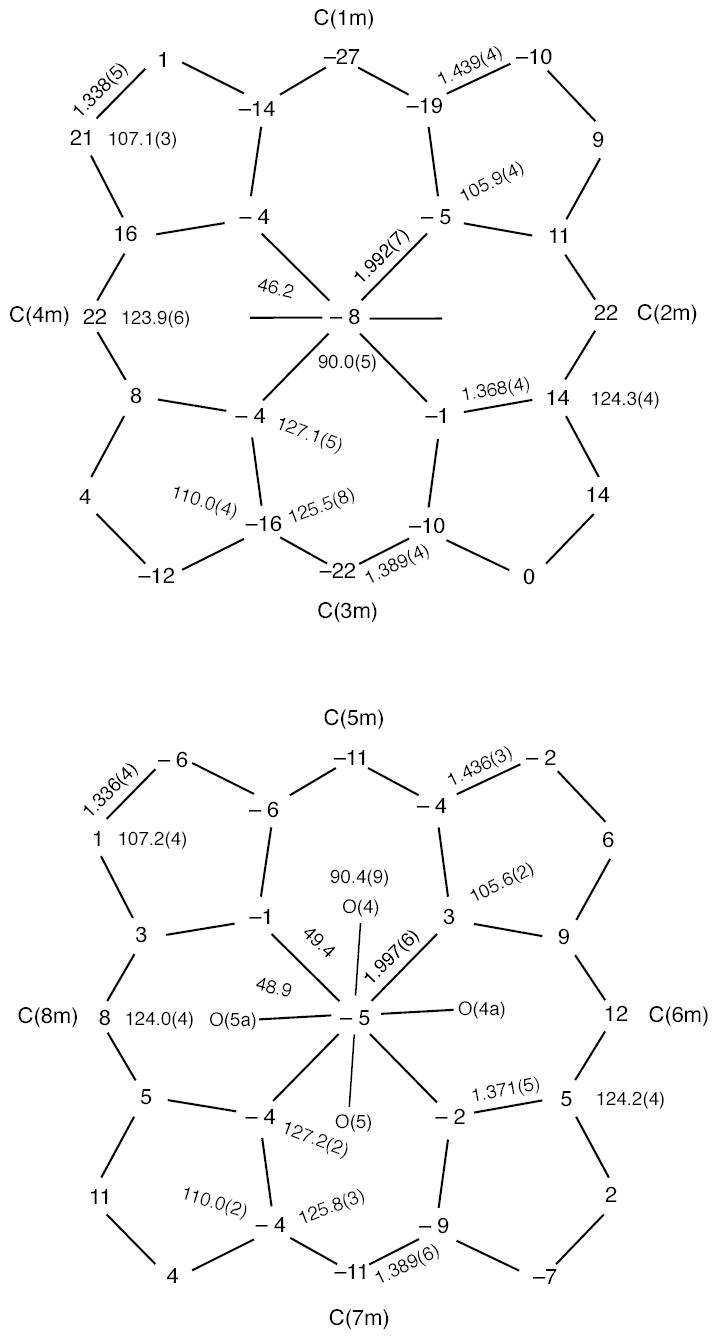
Formal diagrams of the porphyrinato cores of [Fe(TpivPP)(NO2)(CO)]−(1) (top) and [Fe(TpivPP)(NO2)(CO)]−(2) (bottom). Illustrated are the displacements of each atom from the mean plane of the 24-atom porphyrin cores in units of 0.01 Å. Positive values of displacement are toward the NO2 ligand in each anion. The major orientation (54%) of the nitrite in anion 2 is the horizontal orientation. The diagram also gives the averaged values of each distinct bond distance and angle in the porphyrinato cores.
Figure 6.
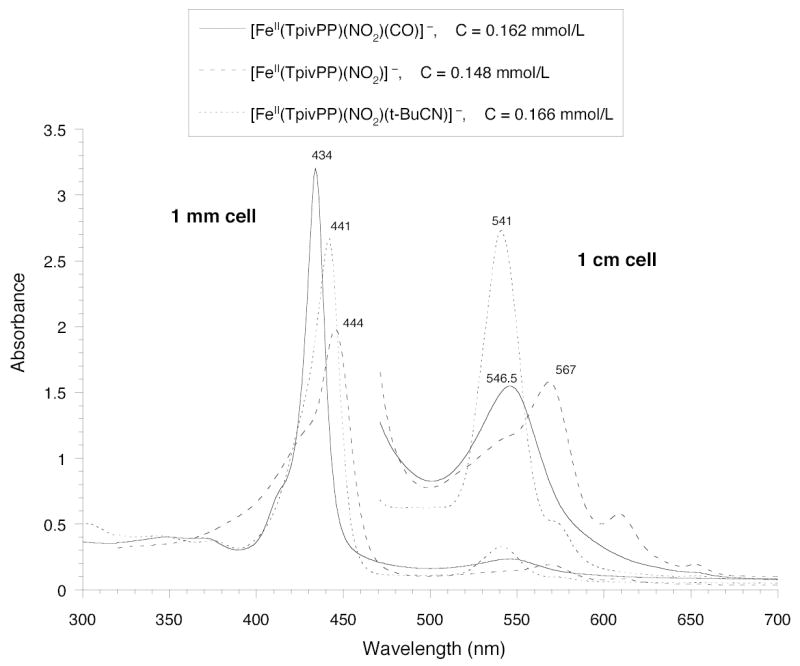
UV-vis spectra of [Fe(TpivPP)(NO2)]−, [Fe(TpivPP)(NO2)(CO)]−, and [Fe-(TpivPP)(NO2)(t-BuNC)]− taken under argon in chlorobenzene.
Figure S1.
ORTEP diagram of the K(222) cryptand, cation 1.
Figure S2.
ORTEP diagram of the K(222) cryptand, cation 2.
Figure S3.
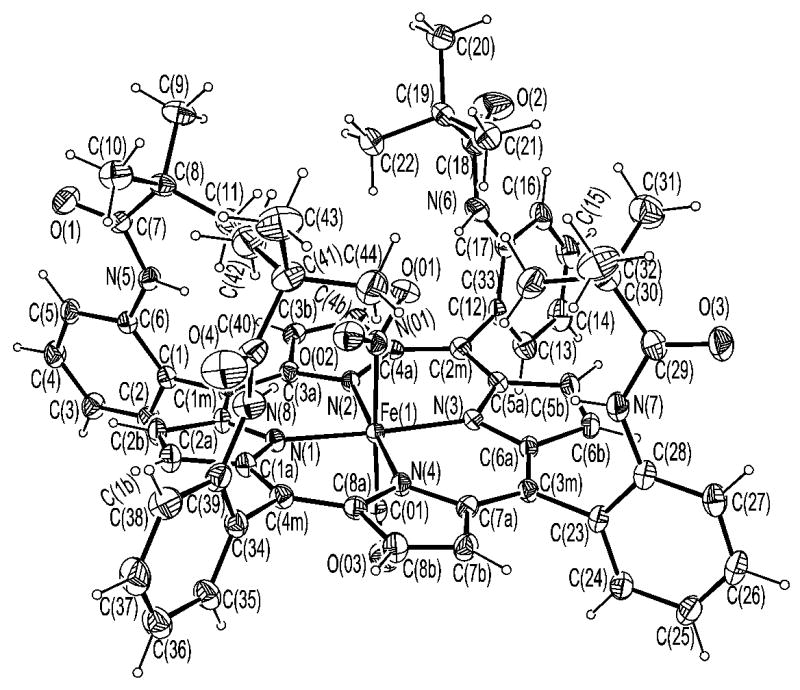
ORTEP diagram of [Fe(TpivPP)(NO2)(CO)]−(1) showing the coordination at iron. 50% probability ellipsoids are depicted and all atomic label are given.
Figure S4.
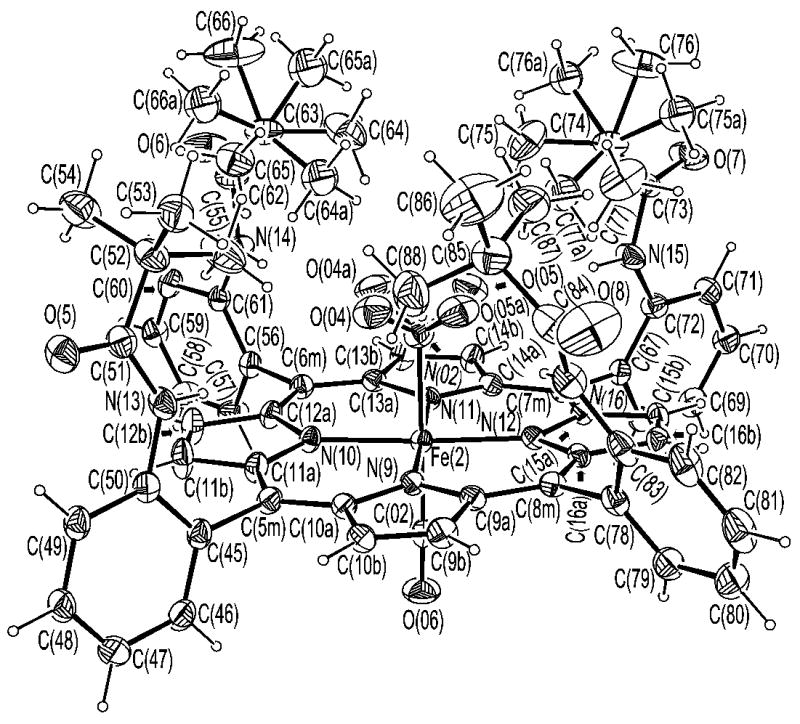
ORTEP diagram of [Fe(TpivPP)(NO2)(CO)]−(2). 50% probability ellipsoids are depicted. The rotational disorder in the nitro group and the pivalamide groups are shown.
Figure S5.
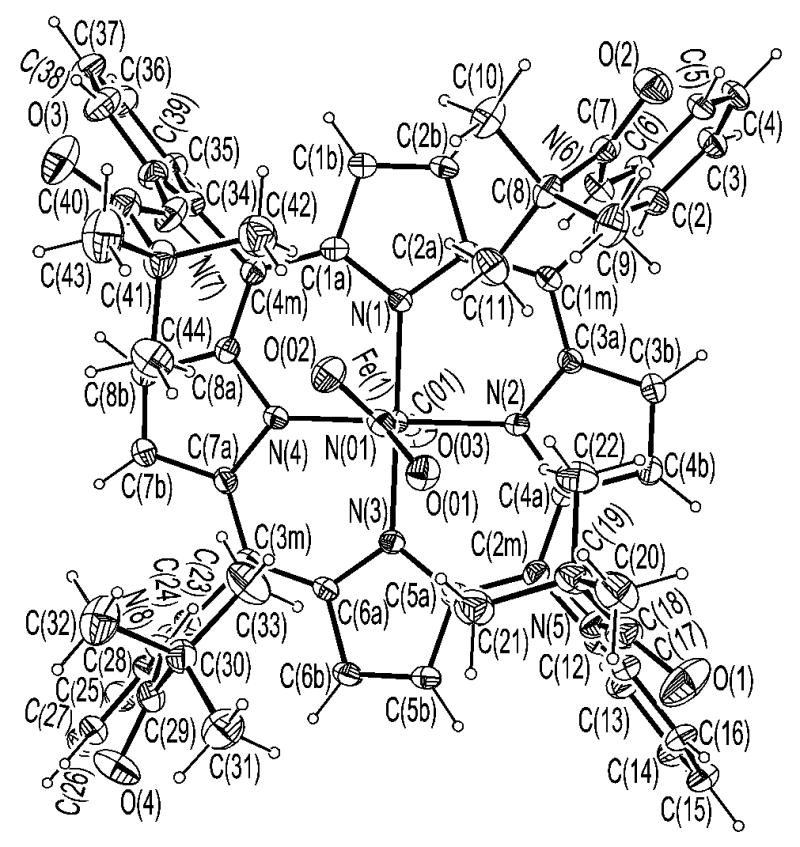
ORTEP of [Fe(TpivPP)(NO2)(CO)]−(1) looking down on the porphyrin plane.
Figure S6.
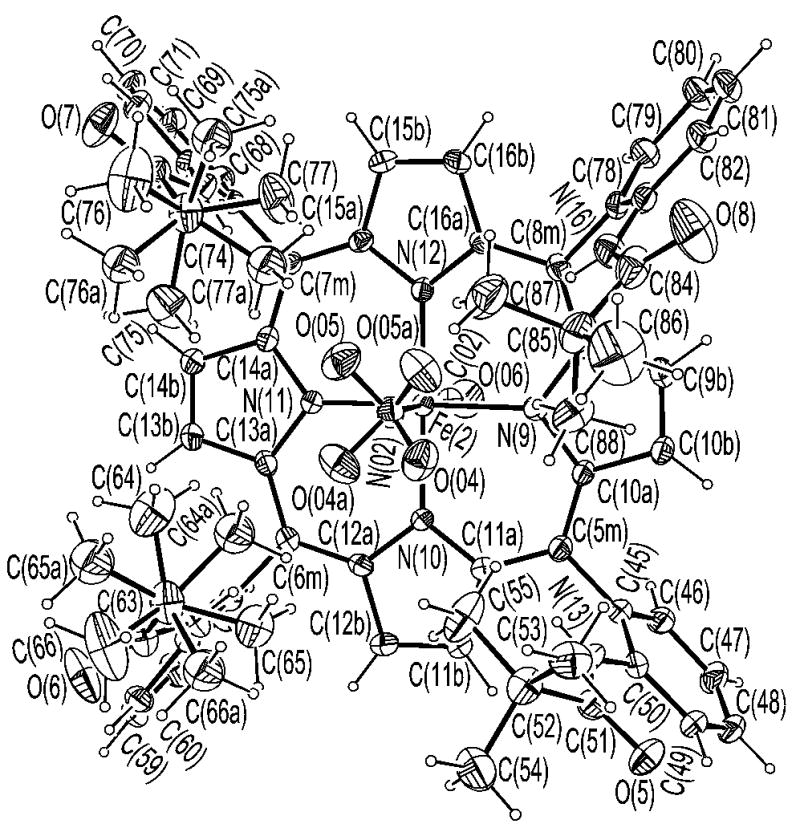
ORTEP of [Fe(TpivPP)(NO2)(CO)]−(2) looking down on the porphyrin plane.
Table 1.
Crystallographic details for [K(222)][Fe(TpivPP)(NO2)(CO)]·1/2C6H5Cl
| formula | C89H105ClFeKN11O13 |
| FW | 1667.24 |
| a, Å | 33.548(6) |
| b, Å | 18.8172(15) |
| c, Å | 27.187(2) |
| β, deg | 95.240(7) |
| V , Å3 | 17091(4) |
| Z | 8 |
| space group | P21/c |
| Dc, g/cm3 | 1.296 |
| F(000) | 7056 |
| μ, mm−1 | 0.325 |
| crystal dimens, mm | 0.24 × 0.12 × 0.08 |
| absorption correction | DIFABS |
| λ, Å | 0.71073 |
| T, K | 130(2) |
| total data colld | 52417 |
| unique data | 32440 (Rint = 0.0759) |
| unique obsd data [I > 2 σ(I)] | 21632 |
| refinement method | on F2 (SHELXL) |
| final R indices [I > 2 σ(I)] | R1 = 0.0775, wR2 = 0.1850 |
| final R indices [for all data] | R1 = 0.1224, wR2 = 0.2200 |
Table 4.
Bond Parameters for (Carbonmonoxy)iron Porphyrin Derivatives
| Complex | Fe–Npa | Fe–C(CO)a | Fe–La | Fe–C–Ob | C–Oa | ref. |
|---|---|---|---|---|---|---|
| [Fe(TpivPP)(NO2)(CO)]− (1) | 1.992 (7) | 1.782 (4) | 2.006 (4) | 175.5 (4) | 1.150 (5) | twc |
| [Fe(TpivPP)(NO2)(CO)] − (2) | 1.997 (6) | 1.789 (5) | 2.009 (4) | 177.5 (4) | 1.140 (5) | tw |
| [Fe(TPP)(Py)(CO)] | 2.02 (3) | 1.77 (2) | 2.10 (1) | 179 (2) | 1.12 (2) | 10 |
| [Fe(Deut)(THF)(CO)] | 1.98 (3) | 1.706 (5) | 2.127 (4) | 178.3 (14) | 1.144 (5) | 13 |
| [Fe(TPP)(SEt)(CO)] | 1.993 (4) | 1.78 (1) | 2.352 (2) | NR | 1.17 (1) | 14 |
| [Fe(TPP)(1-MeIm)(CO)] | 1.999 (7) | 1.756 (2) | 2.059 (1) | 178.91 (17) | 1.147 (2) | d |
| 2.003 (5) | 1.793 (3) | 2.071 (2) | 179.3 (3) | 1.061 (3) | 11 | |
| [Fe(OEP)(1-MeIm)(CO)] | 2.000 (3) | 1.744 (5) | 2.077 (3) | 175.1 (4) | 1.158 (5) | 12 |
| [Fe(TPpiv2C12P)(1-MeIm)(CO)] | 1.999 (3) | 1.728 (6) | 2.062 (5) | ~180 | 1.149 (6) | 15 |
| [Fe(β-PocpivP)(1,2-Me2Im)(CO)] | 1.973 (8) | 1.768 (7) | 2.079 (5) | 172.5 (6) | 1.148 (7) | 16 |
| [Fe(C2-Cap)(1-MeIm)(CO)] | 1.990 (7) | 1.742 (7) | 2.043 (6) | 172.9 (6) | 1.161 (8) | 17 |
| 1.988 (13) | 1.748 (7) | 2.041 (5) | 175.9 (6) | 1.158 (8) | 17 | |
| [Fe(OC3OPor)(1-MeIm)(CO)] | 1.996 (12) | 1.748 (7) | 2.027 (5) | 173.9 (9) | 1.171 (8) | 18 |
| [Fe(OC3OPor)(1,2-Me2Im)(CO)] | 2.00 (2) | 1.713 (8) | 2.102 (6) | 180e | 1.161 (10) | 18 |
| [Fe(C3-Cap)(1-MeIm)(CO)] | 1.99 (2) | 1.800 (13) | 2.046 (10) | 178.0 (13) | 1.107 (13) | 19 |
Value in Å
Value in degrees.
This work.
An, J.; Beatty, A.; Scheidt, W. R., unpublished result.
Required by symmetry.
Table 5.
Solid-State Mössbauer Parameters for [Fe(TpivPP)(NO2)(CO)]− and Related Complexes
| ΔEQ, mm/s | δFe, mm/s | T, K | ref | |
|---|---|---|---|---|
| [Fe(TpivPP)(NO2)(CO)]− | 0.32 | 0.18 | 293 | twa |
| 0.28 | 0.28 | 15 | tw | |
| Iron(II) Complexes | ||||
| [Fe(TMP)(Py)2] | 1.24 | 0.45 | 4.2 | 58 |
| [Fe(TPP)(Py)2] | 1.15 | 0.40 | 77 | 59 |
| [Fe(OEP)(Py)2] | 1.13 | 0.46 | 4.2 | 60 |
| [Fe(TPP)(1-VinIm)2] | 1.00 | 0.43 | 4.2 | 41 |
| [Fe(TPP)(Pip)2] | 1.44 | 0.51 | 4.2 | 61 |
| [Fe(TpivPP)(1-MeIm)2] | 1.02 | 0.46 | 4.2 | 61 |
| [Fe(TMP)(1-MeIm)2]b | 1.11 | 0.45 | 77 | 62 |
| [Fe(OEP)(1-MeIm)2]b | 0.96 | 0.46 | 77 | 62 |
| [Fe(TMP)(PMe3)(1-MeIm)]b | 0.75 | 0.38 | 77 | 62 |
| [Fe(TMP)(PMe3)2]b | 0.47 | 0.36 | 77 | 62 |
| [Fe(OEP)(PMe3)2]b | 0.35 | 0.36 | 77 | 62 |
| Iron(II) CO and CS Complexes | ||||
| [Fe(TPP)(1-MeIm)(CO)] | 0.35 | 0.20 | 293 | 63 |
| [Fe(TPP)(Py)(CO)] | 0.57 | 0.28 | 293 | 63 |
| [Fe(Tp-OCH3PP)(Py)(CO)] | 0.49 | 0.19 | 298 | 64 |
| [Fe(Tp-OCH3PP)(HIm)(CO)] | 0.36 | 0.18 | 298 | 64 |
| [Fe(TpivPP)(1-MeIm)(CO)] | 0.27 | 0.27 | 4.2 | 30 |
| MbCO | 0.35 | 0.27 | 4.2 | 65 |
| [Fe(OEP)(4-CNPy)(CS)] | 0.80 | 0.19 | 4.2 | 66 |
| [Fe(OEP)(Py)(CS)] | 0.57 | 0.15 | 4.2 | 66 |
| [Fe(OEP)(Pip)(CS)] | 0.65 | 0.19 | 4.2 | 66 |
| [Fe(OEP)(4-NMe2Py)(CS)] | 0.44 | 0.19 | 4.2 | 66 |
| [Fe(OEP)(1-MeIm)(CS)] | 0.42 | 0.14 | 4.2 | 66 |
| Iron(II) Nitro Complexes | ||||
| [Fe(TpivPP)(NO2)]− | 2.28 | 0.41 | 4.2 | 22 |
| [Fe(TpivPP)(NO2)(PMS)]− | 1.18 | 0.42 | 4.2 | 22 |
| [Fe(TpivPP)(NO2)(Py)]− | 0.93 | 0.41 | 4.2 | 22 |
| {FeNO}7 Complexes | ||||
| [Fe(TpivPP)(NO2)(NO)]−c | 1.78 | 0.22 | 200 | 21 |
| [Fe(TpivPP)(NO2)(NO)]−d | 1.20 | 0.35 | 4.2 | 21 |
| [Fe(TPP)(NO)] | 1.24 | 0.35 | 4.2 | 21 |
| [Fe(OEP)(NO)] | 1.26 | 0.35 | 100 | 67 |
| [Fe(Deut)(NO)] | 1.47 | 0.39 | 4.2 | 68 |
| [Fe(TPP)(1-MeIm)(NO)] | 0.73 | 0.35 | 4.2 | 40 |
| [Fe(TPP)(4-MePip)(NO)] | 0.91 | 0.37 | 4.2 | 40 |
| Iron(III) Nitro Complexes | ||||
| [Fe(TpivPP)(NO2)2]− | 2.1 | 0.25 | 4.2 | 24 |
| [Fe(TpivPP)(NO2)(Py)] | 2.2 | 0.26 | 4.2 | 24 |
| [Fe(TpivPP)(NO2)(SC6HF4)]− | 2.12 | 0.22 | 293 | 25 |
| 2.30 | 0.28 | 4.2 | 25 | |
| {FeNO}6 Complexes | ||||
| [Fe(OEP)(Iz)(NO)]+ | 1.99 | −0.07 | 293 | 69 |
| 1.92 | 0.02 | 4.2 | 69 | |
| [Fe(OEP)(2-MeHIm)(NO)]+ | 1.88 | 0.05 | 4.2 | 70 |
| [Fe(OEP)(1-MeIm)(NO)]+ b | 1.64 | 0.02 | 4.2 | 71 |
| [Fe(OEP)(NO)]ClO4 | 1.55 | 0.13 | 293 | 69 |
| 1.64 | 0.20 | 4.2 | 69 | |
| [Fe(Tp-OCH3PP)(NO2)(NO)] | 1.43 | 0.04 | 293 | 26 |
| [Fe(TpivPP)(NO2)(NO)] | 1.48 | 0.01 | 293 | 26 |
| 1.43 | 0.09 | 4.2 | 26 | |
| [Fe(TPP)(NO2)(NO)] | 1.37 | 0.02 | 293 | 26 |
| 1.36 | 0.13 | 4.2 | 26 | |
| 1.36 | 0.13 | 77 | 72 | |
| [Fe(OEP)(C6H4-p-F)(NO)] | 0.56 | 0.05 | 293 | 73 |
| 0.57 | 0.14 | 4.2 | 73 | |
This work.
In dimethylacetamide solution.
form.
|| form.
Acknowledgments
We thank the National Institutes of Health for support of this research under Grant GM-38401. Funds for the purchase of the FAST area detector diffractometer were provided through NIH Grant RR-06709 to the University of Notre Dame.
Footnotes
Contribution from The Department of Chemistry and Biochemistry, University of Notre Dame, Notre Dame, Indiana 46556, Faculté des Sciences de Monastir, Avenue de l’environnement, 5019 Monastir, Tunisia and Department of Physics, Knox College, Galesburg, Illinois 61401
Supporting Information Available: Tables S1–S6, giving complete crystallographic details, atomic coordinates, bond distances and angles, anisotropic temperature factors, and fixed hydrogen atom positions for [K(222)][Fe(TpivPP)(NO2)(CO)]·1/2C6H5Cl and Figures of K(222) cations and complete labelled diagrams of the [Fe(TpivPP)(NO2)(CO)]− anions. (PDF). An X-ray crystallographic file, in CIF format, is available. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Wyllie GRA, Scheidt WR. Chem Rev. 2002;102:1067. doi: 10.1021/cr000080p. [DOI] [PubMed] [Google Scholar]
- 2.Averill BA. Chem Rev. 1996;96:2951. doi: 10.1021/cr950056p. [DOI] [PubMed] [Google Scholar]
- 3.Scheidt WR, Ellison MK. Acc Chem Res. 1999;32:350. [Google Scholar]
- 4.Spiro TG, Kozlowski PM. J Biolog Inorg Chem JBIC. 1997;2:516. A JBIC commentary has appeared. J. Biolog. Inorg. Chem. JBIC, 19972, 515–552. The parts are: (a) [Google Scholar]; Slebodnick C, Ibers JA. J Biolog Inorg Chem JBIC. 1997;2:521. (b) [Google Scholar]; Vangberg T, Bocian DF, Ghosh A. J Biolog Inorg Chem JBIC. 1997;2:526. (c) [Google Scholar]; Lim M, Jackson TA, Anfinrud PA. J Biolog Inorg Chem JBIC. 1997;2:531. (d) [Google Scholar]; Sage JT. J Biolog Inorg Chem JBIC. 1997;2:537. (e) [Google Scholar]; Olson JS, Phillips GN., Jr J Biolog Inorg Chem JBIC. 1997;2:544. (f) [Google Scholar]
- 5.Kachalova GS, Popov AN, Bartunik HD. Science. Washington, D.C; 1999. pp. 284–473. [DOI] [PubMed] [Google Scholar]
- 6.Einsle O, Messerschmidt A, Huber R, Kroneck PMH, Neese F. J Am Chem Soc. 2002;124:11737. doi: 10.1021/ja0206487. [DOI] [PubMed] [Google Scholar]
- 7.Quillin ML, Arduini RM, Olson JS, Phillips GN., Jr J Mol Biol. 1993;234:140. doi: 10.1006/jmbi.1993.1569. [DOI] [PubMed] [Google Scholar]
- 8.Kuriyan J, Wilz S, Karplus M, Petsko GA. J Mol Biol. 1986;192:133. doi: 10.1016/0022-2836(86)90470-5. [DOI] [PubMed] [Google Scholar]
- 9.Vojtechivský J, Chu K, Berendzen J, Sweet RM, Schlicting I. Biophys J. 1999:2153. doi: 10.1016/S0006-3495(99)77056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng SM, Ibers JA. J Am Chem Soc. 1976;98:8032. doi: 10.1021/ja00441a025. [DOI] [PubMed] [Google Scholar]
- 11.Salzmann R, Ziegler CJ, Godbout N, McMahon MT, Suslick KS, Oldfield E. J Am Chem Soc. 1998;120:11323. [Google Scholar]
- 12.Salzmann R, McMahon MT, Godbout N, Sanders LK, Wojdelski M, Oldfield E. J Am Chem Soc. 1999;121:3818. [Google Scholar]
- 13.Scheidt WR, Haller KJ, Fons M, Mashiko T, Reed CA. Biochemistry. 1981;20:3653. doi: 10.1021/bi00515a054. [DOI] [PubMed] [Google Scholar]
- 14.Caron C, Mitschler A, Rivère G, Ricard L, Schappacher M, Weiss R. J Am Chem Soc. 1979;101:7401. [Google Scholar]
- 15.Ricard L, Weiss R, Momenteau M. J Chem Soc, Chem Commun. 1986:818. [Google Scholar]
- 16.Kim K, Fettinger J, Sessler JL, Cyr M, Hugdahl J, Collman JP, Ibers JA. J Am Chem Soc. 1989;111:403. [Google Scholar]
- 17.Kim K, Ibers JA. J Am Chem Soc. 1991;113:6077. [Google Scholar]
- 18.Slebodnick C, Duval ML, Ibers JA. Inorg Chem. 1996;35:3607. [Google Scholar]
- 19.Slebodnick C, Fettinger JC, Peterson HB, Ibers JA. J Am Chem Soc. 1996;118:3216. [Google Scholar]
- 20.Nasri H, Wang Y, Huynh BH, Scheidt WR. J Am Chem Soc. 1991;113:717. [Google Scholar]
- 21.Nasri H, Ellison MK, Chen S, Huynh BH, Scheidt WR. J Am Chem Soc. 1997;119:6274. [Google Scholar]
- 22.Nasri H, Ellison MK, Krebs C, Huynh BH, Scheidt WR. J Am Chem Soc. 2000;122:10795. [Google Scholar]
- 23.Nasri H, Goodwin JA, Scheidt WR. Inorg Chem. 1990;29:185. [Google Scholar]
- 24.Nasri H, Wang Y, Huynh BH, Walker FA, Scheidt WR. Inorg Chem. 1991;30:1483. [Google Scholar]
- 25.Nasri H, Haller KJ, Wang Y, Huynh BH, Scheidt WR. Inorg Chem. 1992;31:3459. [Google Scholar]
- 26.Ellison MK, Schulz CE, Scheidt WR. Inorg Chem. 1999;38:100. doi: 10.1021/ic000789e. [DOI] [PubMed] [Google Scholar]
- 27.Abbreviations: Porph, a generalized porphyrin dianion; Tp-OCH3PP, dianion of meso-tetra-p-methoxyphenylporphyrin; TPP, dianion of meso-tetraphenylporphyrin; TMP, meso-tetramesitylporphyrin; OEP, dianion of 2,3,7,8,12,13,17,18-octaethylporphyrin; Deut, deuteroporphyrin IX dimethyl ester;TpivPP, dianion of (α,α,α,α-tetrakis(o-pival-amidophenyl)porphyrin; TPpiv2C12P, dianion of 5,15-[2,2′-(dodecanediamido)diphenyl]-α,α-10,20-bis(o-pivaloylaminophenylporphyrin; β-PocpivP, dianion of “pocket” porphyrin; C2-Cap and C3-Cap, dianion of “capped” porphyrins; OC3OPor, dianion of “benzene capped” porphyrin; PMS, pentamethylene sulfide; Py, pyridine; THT, tetrahydrothio-phene; THF, tetrahydrofuran; HIm, imidazole; 1-MeIm, 1-methylimidazole; 2-MeHIm, 2-methylimidazole; 1,2-Me2Im, 1,2-dimethylimidazole; 4-NMe2Py, 4-N-dimethylamino-pyridine; Pip, piperidine; 4-MePip, 4-methylpiperidine; Pz, pyrazole; 1-VinIm, 1-vinyl-imidazole; 1-BzlIm, 1-benzylimidazole; 1-AcIm, 1-acetylimidazole; Iz, indazole (benzo-pyrazole); SC6HF4, 2,3,5,6-tetrafluorophenyl thiolate; SPh, phenyl thiolate; PMe3,trimethyl phosphine; Kryptofix-222 or 222, 4,7,13,16,21,24-hexaoxo-1,10-diazabicyclo-[8.8.8]hexacosane; Np, porphyrinato nitrogen.
- 28.Finnegan MG, Lappin AG, Scheidt WR. Inorg Chem. 1990;29:181. [Google Scholar]
- 29.Armagero WLF, Perrin DD. Purification of Laboratory Chemicals. 4th ed. Woburn, MA: Butterworth-Heinemann; 1997. p. 140. [Google Scholar]
- 30.Collman JP, Gagne RR, Halbert TR, Lang G, Robinson WT. J Am Chem Soc. 1975;97:1427. doi: 10.1021/ja00839a026. [DOI] [PubMed] [Google Scholar]
- 31.Gismelseed A, Bominaar EL, Bill E, Trautwein AX, Winkler H, Nasri H, Doppelt P, Mandon D, Fischer J, Weiss R. Inorg Chem. 1990;29:2741. [Google Scholar]
- 32.Scheidt WR, Turowska-Tyrk I. Inorg Chem. 1994;33:1314. [Google Scholar]
- 33.Sheldrick GM. Acta Crystallogr. 1990;A46:467. [Google Scholar]
- 34.Sheldrick GM. J Appl Cryst. in preparation. [Google Scholar]
- 35.R1 = ∑||Fo| − |Fc||/∑|Fo| and wR2 = {∑ [w (Fo2 − Fc2)2]/∑[wFo4]}1/2 The conventional R-factors R1 are based on F, with F set to zero for negative F2 The criterion of F2 > 2σ (F2) was used only for calculating R1 R-factors based on F2 (wR2) are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger.
- 36.Scheidt WR, Frisse ME. J Am Chem Soc. 1975;97:17. doi: 10.1021/ja00834a005. [DOI] [PubMed] [Google Scholar]
- 37.Mu XH, Kadish KM. Inorg Chem. 1988;27:4720. [Google Scholar]
- 38.Wayland BB, Mehne LF, Swartz J. J Am Chem Soc. 1978;100:2379. [Google Scholar]
- 39.Scheidt WR, Piciulo PL. J Am Chem Soc. 1976;98:1913. doi: 10.1021/ja00423a044. [DOI] [PubMed] [Google Scholar]
- 40.Wyllie GRA, Schulz CE, Scheidt WR. Inorg Chem. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Safo MK, Scheidt WR, Gupta GP. Inorg Chem. 1990;29:626. [Google Scholar]
- 42.Ellison, M. K.; Scheidt, W. R., unpublished results.
- 43.Collman JP, Brauman JI, Doxsee KM, Halbert TR, Bunnenberg E, Linder RE, LaMar GN, Del Guadio J, Lang G, Spartalian K. J Am Chem Soc. 1980;102:4182. [Google Scholar]
- 44.Mandon D, Ott-Woelfel F, Fisher J, Weiss R, Bill E, Trautwein AX. Inorg Chem. 1990;29:2442. [Google Scholar]
- 45.Roth, A.; Scheidt, W. R., unpublished results.
- 46.Schappacher M. Thesis. Université de Strasbourg; Strasbourg, France: 1982. Ph. D. [Google Scholar]
- 47.Yoshimura T. Inorg Chim Acta. 1984;83:17. [Google Scholar]
- 48.Stolzenberg AM, Strauss SH, Holm RH. J Am Chem Soc. 1981;103:4763. [Google Scholar]
- 49.Rougee M, Brault D. Biochemistry. 1975;14:4100. [Google Scholar]
- 50.Wang CM, Brinigar WS. Biochemistry. 1979;18:4960. doi: 10.1021/bi00589a026. [DOI] [PubMed] [Google Scholar]
- 51.All other five-coordinate iron(II) porphyrinates with a single anionic axial ligand are high spin. Some examples include the anions ethanethiolate,14,52 chloride,53 tetrafluorophenyl-thiolate,53 phenoxide,54 2-methylimidazolate,44 and acetate.55
- 52.Schappacher M, Ricard L, Fischer J, Weiss R, Montiel-Montoya R, Bill E, Trautwein AX. Inorg Chem. 1989;28:4639. doi: 10.1111/j.1432-1033.1987.tb13436.x. [DOI] [PubMed] [Google Scholar]
- 53.Schappacher M, Ricard L, Weiss R, Montiel-Montoya R, Gonser U, Bill E, Trautwein AX. Inorg Chim Acta. 1983;78:L9. [Google Scholar]
- 54.Nasri H, Fischer J, Weiss R, Bill E, Trautwein AX. J Am Chem Soc. 1987;109:2549. [Google Scholar]
- 55.Bominaar EL, Ding X, Gismelseed A, Bill E, Winkler H, Trautwein AX, Nasri H, Fischer J, Weiss R. Inorg Chem. 1992;31:1845. [Google Scholar]
- 56.The large change in Fe–N(NO)2 bond length is not merely a result of change in coordination number. See reference 22 for a detailed discussion.
- 57.The only exceptions are two isomorphous forms of [Fe(TpivPP)(NO2)(NO)] for which the synthesis was designed to place the anionic ligand on the open face. The actual result was disorder of the axial ligands with a 57% occupancy of a nitro group on the open face of the picket fence porphyrin and a linear nitrosyl group inside the pocket. In the second case there was a 26% occupancy of the nitro out of the pocket.26
- 58.Safo MK, Nesset MJM, Walker FA, Debrunner PG, Scheidt WR. J Am Chem Soc. 1997;119:9438. [Google Scholar]
- 59.Kobayashi H, Maeda Y, Yanagawa Y. Bull Chem Soc Jpn. 1970;43:2342. [Google Scholar]
- 60.Dolphin D, Sams JR, Tsin TB, Wong KL. J Am Chem Soc. 1976;98:6970. doi: 10.1021/ja00438a037. [DOI] [PubMed] [Google Scholar]
- 61.Collman JP, Hoard JL, Kim N, Lang G, Reed CA. J Am Chem Soc. 1975;97:2676. doi: 10.1021/ja00843a015. [DOI] [PubMed] [Google Scholar]
- 62.Polam JR, Wright JL, Christensen KA, Walker FA, Flint H, Winkler H, Grodzicki M, Trautwein AX. J Am Chem Soc. 1996;118:5272. [Google Scholar]
- 63.Havlin RH, Godbout N, Salzmann R, Wojdelski M, Arnold W, Schulz CE, Oldfield E. J Am Chem Soc. 1998;120:3144. [Google Scholar]
- 64.Conner WM, Straub DK. Inorg Chem. 1976;15:2289. [Google Scholar]
- 65.Maeda Y, Harami T, Morita Y, Trautwein AX, Gonser U. J Chem Phys. 1981;75:36. [Google Scholar]
- 66.Cao C, Dahal S, Shang M, Beatty AM, Hibbs W, Schulz CE, Scheidt WR. Inorg Chem. 2003;42:5202. doi: 10.1021/ic030043r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bohle DS, Debrunner P, Fitzgerald J, Hansert B, Hung CH, Thompson AJ. J Chem Soc, Chem Commun. 1997:91. [Google Scholar]
- 68.Wyllie GRA, Scheidt WR. Inorg Chem. 2003;42:4259. doi: 10.1021/ic034364e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ellison MK, Schulz CE, Scheidt WR. Inorg Chem. 2000;39:5102. doi: 10.1021/ic000789e. [DOI] [PubMed] [Google Scholar]
- 70.Ellison MK, Schulz CE, Scheidt WR. J Am Chem Soc. 2002;124:13833. doi: 10.1021/ja0207145. [DOI] [PubMed] [Google Scholar]
- 71.Schünemann V, Benda R, Trautwein AX, Walker FA. Isr J Chem. 2000;40:9. [Google Scholar]
- 72.Settin MF, Fanning JC. Inorg Chem. 1988;27:1431. [Google Scholar]
- 73.Richter-Addo GB, Wheeler RA, Hixson CA, Chen L, Khan MA, Ellison MK, Schulz CE, Scheidt WR. J Am Chem Soc. 2001;123:6314. doi: 10.1021/ja010276m. [DOI] [PubMed] [Google Scholar]
- 74.Paulsen H, Krockel M, Grodzicki M, Bill E, Trautwein AX, Leigh GJ, Silver J. Inorg Chem. 1995;34:6244. [Google Scholar]
- 75.The quadrupole splittings (ΔEQ) for the two geometric forms of [Fe(TpivPP)(NO2)(NO)]− are significantly different. Although the Mössbauer spectra for the two forms were taken at two different temperatures, any temperature-dependent effect on ΔEQ is unlikely to make the two values more equivalent.
- 76.Li M, Bonnet D, Bill E, Neese F, Weyhermüller T, Blum N, Sellman D, Wieghardt K. Inorg Chem. 2002;41:3444. doi: 10.1021/ic011243a. [DOI] [PubMed] [Google Scholar]
- 77.Neese F. Inorg Chim Acta. 2002;337:181. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


