Abstract
Advanced heart failure may be refractory despite aggressive support with inotropic agents and intra-aortic balloon pumping. Implantable left ventricular assist devices are increasingly being used as bridges to cardiac transplantation or as destination therapy because of the limited availability of donor organs. We report the 1st use of the TandemHeart® percutaneous ventricular assist device as a short-term bridge to cardiac transplantation.
Key words: Cardiomyopathy; circulatory assist devices; circulatory assistance, temporary; shock, cardiogenic/therapy; heart catheterization; heart failure; transplantation, heart
Over the last decade, mechanical devices have been used extensively to support end-stage heart failure patients awaiting cardiac transplantation. Inotropic support and intra-aortic balloon pump (IABP) insertion are the mainstays of aggressive treatment for the failing heart; however, acute advanced heart failure may be refractory to such therapy. We report the 1st use of the TandemHeart® percutaneous ventricular assist device (pVAD) (CardiacAssist, Inc.; Pittsburgh, Pa) (Fig. 1) as a short-term bridge to cardiac transplantation.
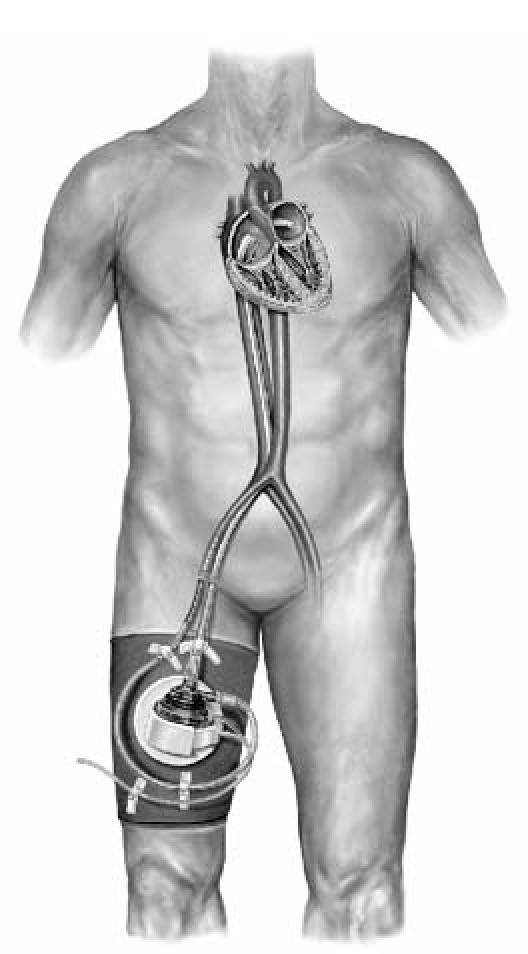
Fig. 1 The TandemHeart percutaneous ventricular assist device.
Case Report
In June 2005, a 60-year-old man in cardiogenic shock and respiratory failure was transferred to our institution from another hospital. He required prompt intubation and mechanical ventilation. He had a history of cardiomyopathy secondary to sarcoidosis. An echocardiogram showed severe mitral regurgitation and an ejection fraction of 0.10. The patient also had acute deterioration of his chronic renal failure (baseline creatine level, 2 mg/dL). On transfer to our institution, he had a low-grade fever, was receiving multiple inotropic agents, and was on IABP support (1:1 ratio).
A fiberoptic Swan-Ganz catheter was inserted for continuous monitoring. Despite 48 hours of inotropic support (dopamine, 10 μg/kg per min and intravenous milrinone, 0.5 μg/kg per min), the patient's hemodynamic status continued to deteriorate, with a capillary wedge pressure that increased to 22 mmHg and pulmonary edema that required 100% fraction of inspired oxygen (FIO2) ventilation. The patient was approved for cardiac transplantation and was classified as United Network for Organ Sharing (UNOS) status 1-A (the highest priority status).
A TandemHeart pVAD was placed by means of standard transseptal catheterization. The procedure was well tolerated, and intravenous heparin treatment was initiated to keep the activated clotting time above 300 seconds. The patient's hemodynamic status and organ function stabilized over the next 6 days of pVAD support. He was to have received an implantable left ventricular assist device as a bridge to transplantation. However, a suitable donor heart that could not be used for other status 1-A recipients became available, and he underwent successful cardiac transplantation. At that time, the TandemHeart and the IABP were removed.
The postoperative course was complicated by bleeding into the right tracheobronchial tree and by atelectasis of the right lower lobe. We performed aggressive clot removal with repeated bronchoscopy and local flushing. A tracheostomy and prolonged mechanical ventilatory support (15 days) were required. Pathologic examination of the explanted heart confirmed the diagnosis of sarcoidosis. The subsequent clinical course was uneventful, and the patient was transferred to a rehabilitation facility 34 days after undergoing transplantation.
Comment
We used the percutaneously inserted TandemHeart device to improve the hemodynamic status of a patient awaiting cardiac transplantation. The device was able to effectively unload the left ventricle. The left atrial wedge pressure decreased, and a chest roentgenogram showed improvement. The device provided up to 4 L/min of flow, thus ameliorating end-organ function.
The TandemHeart pVAD is a continuous-flow centrifugal assist device placed outside the body. Cannulas are inserted percutaneously through the femoral vein and are advanced across the interatrial septum into the left atrium. The pump, which weighs 8 oz, withdraws oxygenated blood from the left atrium, propels it by means of a magnetically driven 6-bladed impeller through the outflow port, and returns it to one or both femoral arteries via arterial cannulas.
The TandemHeart has been used mainly during high-risk percutaneous coronary interventions.1,2 It provides complete circulatory support, irrespective of residual cardiac function. This device has also been used for patients in postcardiotomy cardiogenic shock3 and has proved to be more effective than the IABP in reversing hemodynamic and metabolic abnormalities.4 The pVAD provides short-term support, lasting from a few hours up to 14 days, which gives the heart time to potentially recover its native function.
Because the TandemHeart is percutaneous, it can be used in even the most ill patients. According to our protocol, a long-term device would normally be used as a bridge to transplantation in order to provide end-organ recovery and improve native heart function. In the patient described here, however, the TandemHeart allowed quick restoration of hemodynamic stability. When a suitable donor heart became available, transplantation was performed.
In the rapidly advancing field of device technology, pVADs may soon become an excellent alternative for hemodynamically unstable patients who are high-risk surgical candidates. Currently, 17 centers in the United States, including the Texas Heart Institute, use the TandemHeart clinically as a bridge to high-risk coronary intervention, to high-risk coronary bypass surgery, and to long-term support with a left ventricular assist device. Further research is needed to identify the role of pVADs as bridges to cardiac transplantation.
Footnotes
Address for reprints: Igor D. Gregoric, MD, Texas Heart Institute, MC 3-147, P.O. Box 20345, Houston, TX 77225-0345. E-mail: kstephan@heart.thi.tmc.edu
References
- 1.Aragon J, Lee MS, Kar S, Makkar RR. Percutaneous left ventricular assist device: “TandemHeart” for high-risk coronary intervention. Catheter Cardiovasc Interv 2005;65:346–52. [DOI] [PubMed]
- 2.Kar B, Butkevich A, Civitello AB, Nawar MA, Walton B, Messner GN, et al. Hemodynamic support with a percutaneous left ventricular assist device during stenting of an unprotected left main coronary artery. Tex Heart Inst J 2004;31: 84–6. [PMC free article] [PubMed]
- 3.Pitsis AA, Dardas P, Mezilis N, Nikoloudakis N, Filippatos G, Burkhoff D. Temporary assist device for postcardiotomy cardiac failure. Ann Thorac Surg 2004;77:1431–3. [DOI] [PubMed]
- 4.Thiele H, Sick P, Boudriot E, Diederich KW, Hambrecht R, Niebauer J, Schuler G. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial in-farction complicated by cardiogenic shock. Eur Heart J 2005; 26:1276–83. [DOI] [PubMed]


