Abstract
A 69-year-old white woman presented at our emergency room with right-side pleuritic chest pain, fever, and tachycardia. Results of the physical examination, routine laboratory tests, and chest radiography were unexceptional. An electrocardiogram showed ST elevation in leads V1 through V3 with T-wave inversion. Because of the chest pain and the ST elevation, the patient underwent emergency cardiac catheterization, which showed no coronary artery stenosis. A computed tomographic scan of the chest showed pulmonary infiltration in the right middle lobe and the lingula of the left upper lobe; pneumonia was diagnosed, and appropriate antibiotic therapy was started.
The electrocardiographic changes met the criteria for type-1 Brugada pattern. Brugada syndrome is an arrhythmogenic disease caused in part by mutations in the cardiac sodium channel gene SCN5A. When the sodium current is disrupted, the outward transient current at the end of phase 1 of the action potential becomes unopposed. This creates a voltage gradient between the epicardium and endocardium, especially in the right ventricular wall, which leads to J-point elevation in leads V1 through V3. Fever exaggerates this defect in sodium channels. In our patient, the pleuritic chest pain was caused by the pneumonia, and the ST elevation was probably related to Brugada syndrome, unmasked by the febrile episode. Brugada syndrome can be associated with ventricular tachycardia or fibrillation; the only treatment proven to prevent sudden death is placement of an implantable cardioverter defibrillator, which is recommended in symptomatic patients or in those with ventricular tachycardia induced during electrophysiologic studies.
Key words: Action potentials; arrhythmia/physiopathology; Brugada syndrome; death, sudden cardiac/etiology; defibrillators, implantable; electrocardiography; ST elevation; sodium channels; tachycardia, ventricular; ventricular fibrillation
Brugada syndrome (BrS) is associated with life-threatening ventricular arrhythmias and is responsible for 20% of sudden cardiac deaths (SCDs) in patients with a structurally normal heart. The pathogenesis of the disease is a sodium channelopathy in the right ventricular epicardium that presents with a speciic abnormal electrocardiographic (ECG) pattern with ST elevation in leads V1 through V3. The Brugada pattern is a dynamic ECG finding that can be uncovered by fever and certain drugs.
Case Report
A 69-year-old white woman presented at our emergency department, experiencing chest pain. Localized to the right side of the chest, the pain had begun a few hours earlier and became worse upon deep inspiration. The pain did not radiate to the arms, jaw, or neck, nor did it correlate with exertion or position. A review of the patient's systems revealed no other pertinent indings. Her relevant medical history included hyperlipidemia and osteoporosis. She was taking risedronate, multivitamins, calcium supplements, and atorvastatin calcium. She had a temperature of 100.3 °F; blood pressure, 159/71 mmHg; pulse rate, 120 beats/min; respirations, 20/min; and oxygen saturation, 99% on room air. Upon pulmonary auscultation, she had no rales, and upon cardiac auscultation, no heart murmur or irregular rhythm. There was no swelling of her legs. The chest pain was not reproducible on palpation.
Routine laboratory tests, including a complete blood count, comprehensive metabolic panel, and coagulation studies, produced results within normal limits. Chest radiography revealed no abnormality. Electrocardiography (Fig. 1) showed significant ST elevation in leads V1 through V3, with T-wave inversion and no reciprocal ST depression.
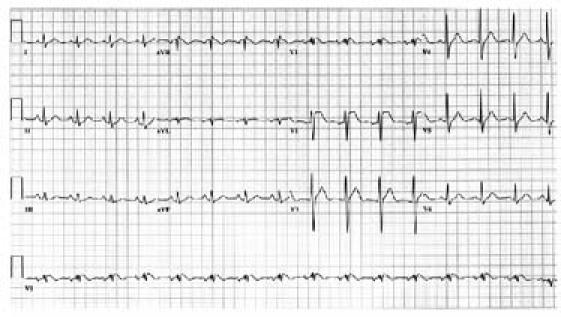
Fig. 1 The patient's electrocardiogram shows type-1 Brugada pattern, evidenced by down-sloping ST elevation from leads V1 through V3 with T-wave inversion and no reciprocal ST depression.
Emergency cardiac catheterization for possible myocardial infarction showed patent coronary arteries. Cardiac biomarkers, including the troponin level and the MB isoenzyme of creatine kinase, were normal. A computed tomographic scan of the chest showed no evidence of pulmonary embolism, but there was pulmonary iniltration in the right middle lobe and the lingula of the left upper lobe.
Because of the fever, the pleuritic chest pain, and the infiltrate on computed tomography, pneumonia was diagnosed. Repeat ECG confirmed the ST elevation in leads V1 through V3 with the same pattern; this met the ECG criteria for diagnosis of type-1 Brugada pattern.
In the absence of relevant symptoms and ventricular tachycardia (VT) or a family history of either BrS or SCD, the patient was discharged from the hospital and given antibiotic therapy for pneumonia.
Discussion
Brugada syndrome is associated with life-threatening ventricular arrhythmias and SCD. This syndrome was first described by Brugada and Brugada in 1992,1 after which it attracted great interest, as evidenced by the number of cases reported subsequently. The disease is characterized by a specific abnormal ECG pattern with ST elevation in leads V1 through V3.
In approximately one fourth of Brugada patients, a mutation has been reported in the cardiac sodium-channel gene (SCN5A) on chromosome 3, which encodes for the a-subunit of the cardiac sodium channel.2 The mode of inheritance is autosomal dominant, and the mutated gene is seen more often in familial than in sporadic cases. More than 60 different mutations of this gene have been reported to produce BrS, and some may cause overlapping conditions, since this is the same gene in which different mutations can lead to a congenital form of long QT syndrome and Lenegre's disease.3 The relationship between specific mutations and the risk of arrhythmic events is under investigation, but genetic testing can provide support to confirm the diagnosis and to detect other family members at risk.
Because of sodium-channel malfunction, the transient outward current at the end of phase 1 of the action potential will be unopposed. More exaggerated in the right ventricular wall, this altered balance results in increased dispersion in the action potential between the involved epicardium and the endocardium and causes J-point elevation in leads V1 through V3. Losing the action potential dome in some but not all parts of the epicardium leads to local re-excitation via phase 2 re-entry. This re-excitation may result in the development of closely coupled extrasystoles and in the triggering of VT or ventricular ibrillation.3
The transient outward current at the end of phase 1 is more prominent in men than in women. This explains why, despite an autosomal transmission, the symptomatic phenotype is about 10 times more common in men.3
Any condition or medication that increases outward currents or decreases inward currents may precipitate or unmask BrS.4 Therefore, sodium-channel blockers such as procainamide or flecainide can unmask the syndrome by decreasing the inward current, as can calcium-channel blockers, β-blockers, cocaine, and antidepressants. Vagotonic agents and hypokalemia have the same effect, but they work by enhancing the outward current. A normal sodium channel may be inactivated prematurely by above-normal body temperatures, and it has been observed that this inactivation is exaggerated in BrS. This may explain the frequency with which BrS and polymorphic VT have been unmasked in febrile patients.3
Because ECG changes in BrS are dynamic and transient, it is dificult to know the syndrome's true prevalence and incidence, but it is common in South Asia and Japan. It most often initially presents in the 4th decade of life, although it has been reported in children as young as 2 days and in adults as old as 84 years. Brugada syndrome is the most common cause of SCD in patients with normal heart structure and is responsible for an estimated 4% to 12% of all sudden deaths.3
The most common clinical presentation of BrS is syncope.3,5 Three types of changes may appear on 12-lead ECG. In type-1 Brugada pattern, ECG shows J-point elevation ≥2 mm with a down-sloping coved ST segment and usually a negative T wave. Types 2 and 3 show the same J-point elevation, but the positive T wave gives a saddleback appearance to the ST-T segment. In type-2 BrS, the terminal portion of the ST segment is elevated 1 mm or more and the T wave may be positive or biphasic, whereas the terminal ST elevation in type 3 is less than 1 mm and the T wave is not biphasic, but only positive.3,6 Brugada and Brugada1 initially described a right bundle branch block (RBBB) pattern among their 8 cases of BrS. Thereafter, it was shown that, whereas the presence of the RBBB pattern is not necessary in reaching a diagnosis of BrS, it is supportive.6
Because the Brugada ECG pattern is dynamic, all of the above changes may be seen in the same patient at different times. Placing the right precordial ECG leads at an upper position, such as the 2nd intercostal space, increases the sensitivity of ECG to detect all types of the syndrome; however, it decreases speciicity.3
The diagnosis of BrS is considered deinite when 1 of the ECG criteria and 1 of the clinical criteria are present. The ECG criteria are type-1 ECG changes or the conversion of type 2 or 3 to type 1 after administration of a sodium-channel blocker. Type-2 and -3 changes are considered nonspecific and nondiagnostic for BrS3,7 unless they convert to a type-1 pattern after the sodium-blocker challenge test. Even after conversion to type 1 upon challenge, types 2 and 3 remain less specific for diagnostic purposes than a type-1 ECG pattern.
One of the following clinical criteria must be present to confirm the diagnosis: syncope, history of VT or ventricular fibrillation, family history of SCD or type-1 ECG changes, or inducibility of VT via electrophysiologic study (EPS).
Because ST elevation can accompany many underlying conditions, it is good practice to exclude other causes of ST elevation by considering clinical and laboratory information. The most common and important causes of ST elevation on ECG in the differential diagnosis of BrS are acute myocardial infarction, atypical RBBB, Prinzmetal's angina, hyperkalemia, early repolarization, and acute pericarditis.3,7 Some rarer conditions have also been reported to mimic the Brugada ECG changes, such as hypothermia8 and a mediastinal tumor with mechanical compression on the right ventricle.9
To date, the only treatment proven effective for BrS is placement of an implantable cardioverter defibrillator (ICD).3,7,10 A large multicenter trial10 showed that ICD placement was 100% effective in preventing SCD after 5 years of follow-up of 690 Brugada patients. In that study, appropriate shocks were delivered in 51% of the patients who initially presented with syncope and in 37% of the patients who were initially asymptomatic. In a recent clinical trial, it was shown that ICD therapy provided full protection from SCD in Asian male patients who had survived an episode of Sudden Unexplained Death Syndrome.11
The ICD is recommended for 2 groups of Brugada patients: symptomatic patients with type-1 ECG changes (with or without sodium-channel-blocker challenge), and asymptomatic patients who have inducible ventricular arrhythmias after undergoing EPS. Regarding the 1st group, it is important to rule out other causes of syncope or seizure before attributing the symptoms to BrS. Asymptomatic patients with type-1 ECG changes should undergo EPS if there is a family history of SCD; ICD placement is recommended if ventricular arrhythmias are reproducible during EPS. There is controversy about whether to perform EPS in asymptomatic patients who show type-1 changes spontaneously but have no family history of SCD3,4; in this situation, we suggest considering other conditions, such as the patient's age and comorbidities. Those asymptomatic patients who show type-1 ECG changes only after sodium-channel-blocker challenge may not need EPS, but they should be monitored because of the spontaneous fluctuations between diagnostic and nondiagnostic ECGs in BrS.12
Alternative treatments are pacemaker placement, radiofrequency ablation, and some antiarrhythmic medications. Pharmacologic therapies seek to rebalance the current at the end of phase 1 of the action potential and may have a role when ICD is not available, or they can supplement ICD therapy when ICD discharges are frequent and inconvenient. Experimental models have shown that quinidine decreases ST elevation and prevents phase 2 re-entry and associated VT.13,14 Isoproterenol, which is a β-agonist, and cilostazol,15 a phosphodiesterase inhibitor, also may be helpful. Ablation of the premature ventricular beats that trigger VT and ventricular fibrillation may be a treatment option as well.16 Because SCDs that occur during sleep or rest are usually associated with a slow heart rate, a pacemaker is a possible treatment.3 None of the above-mentioned treatment options are as effective as ICD therapy to prevent SCD.
Although our patient's chest pain and ST elevation prompted us to send her to the catheterization laboratory for investigation of a possible ST-elevation myocardial infarction, her chest pain was pleuritic. Unmasked by the fever associated with pneumonia, the ST elevation met the criteria for type-1 Brugada pattern. However, our patient had no family history of BrS, VT, or SCD. Although EPS is recommended in type-1 ECG patients,4 the value of EPS in induced cases is still unknown. Our patient had experienced no cardiac event in her 69 years; therefore, because of the normalization apparent on the electrocardiogram, she was discharged from the hospital on antibiotic therapy for pneumonia.
Footnotes
Address for reprints: Ali A. Sovari, MD, Department of Internal Medicine, University of Illinois, COM-UC, 611 West Park St., Forum Bldg., Urbana, IL 61801. E-mail: alizadeh@uiuc.edu
References
- 1.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographical syndrome. A multicenter report. J Am Coll Cardiol 1992;20:1391–6. [DOI] [PubMed]
- 2.Keller DI, Rougier JS, Kucera JP, Benammar N, Fressart V, Guicheney P, et al. Brugada syndrome and fever: genetic and molecular characterization of patients carrying SCN5A mutations. Cardiovasc Res 2005;67:510–9. [DOI] [PubMed]
- 3.Antzelevitch C, Brugada P. The Brugada syndrome: from bench to bedside. Malden (MA): Blackwell Publishers; 2005. p. 1–22.
- 4.Grant AO. Electrophysiological basis and genetics of Brugada syndrome. J Cardiovasc Electrophysiol 2005;16 Suppl 1:S3–7. [DOI] [PubMed]
- 5.Brugada J, Brugada R, Antzelevitch C, Towbin J, Nademanee K, Brugada P. Long-term follow-up of individuals with the electrocardiographic pattern of right bundle-branch block and ST-segment elevation in precordial leads V1 to V3. Circulation 2002;105:73–8. [DOI] [PubMed]
- 6.Wilde AA, Antzelevitch C, Borggrefe M, Brugada J, Brugada R, Brugada P, et al. Proposed diagnostic criteria for the Brugada syndrome: consensus report. Circulation 2002;106:2514–9. [DOI] [PubMed]
- 7.Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, et al. Brugada syndrome: report of the second consensus conference [published erratum appears in Heart Rhythm 2005;2:905]. Heart Rhythm 2005;2:429–40. [DOI] [PubMed]
- 8.Fish JM, Antzelevitch C. Link between hypothermia and the Brugada syndrome. J Cardiovasc Electrophysiol 2004; 15:942–4. [DOI] [PMC free article] [PubMed]
- 9.Tarin N, Farre J, Rubio JM, Tunon J, Castro-Dorticos J. Brugada-like electrocardiographic pattern in a patient with a mediastinal tumor. Pacing Clin Electrophysiol 1999;22: 1264–6. [DOI] [PubMed]
- 10.Brugada J, Brugada R, Brugada P. Right bundle-branch block and ST-segment elevation in leads V1 through V3: a marker for sudden death in patients without demonstrable structural heart disease. Circulation 1998;97:457–60. [DOI] [PubMed]
- 11.Nademanee K, Veerakul G, Mower M, Likittanasombat K, Krittayapong R, Bhuripanyo K, et al. Defibrillator versus beta-blockers for unexplained death in Thailand (DEBUT): a randomized clinical trial. Circulation 2003;107:2221–6. [DOI] [PubMed]
- 12.Veltmann C, Schimpf R, Echternach C, Eckardt L, Kuschyk J, Streitner F, et al. A prospective study on spontaneous fluctuations between diagnostic and non-diagnostic ECGs in Brugada syndrome: implications for correct phenotyping and risk stratification. Eur Heart J 2006;27:2544–52. [DOI] [PubMed]
- 13.Belhassen B, Glick A, Viskin S. Efficacy of quinidine in high-risk patients with Brugada syndrome. Circulation 2004; 110:1731–7. [DOI] [PubMed]
- 14.Belhassen B, Viskin S, Fish R, Glick A, Setbon I, Eldar M. Effects of electrophysiologic-guided therapy with Class IA antiarrhythmic drugs on the long-term outcome of patients with idiopathic ventricular fibrillation with or without the Brugada syndrome. J Cardiovasc Electrophysiol 1999;10: 1301–12. [DOI] [PubMed]
- 15.Tsuchiya T, Ashikaga K, Honda T, Arita M. Prevention of ventricular fibrillation by cilostazol, an oral phosphodiesterase inhibitor, in a patient with Brugada syndrome. J Cardiovasc Electrophysiol 2002;13:698–701. [DOI] [PubMed]
- 16.Haissaguerre M, Extramiana F, Hocini M, Cauchemez B, Jais P, Cabrera JA, et al. Mapping and ablation of ventricular fibrillation associated with long-QT and Brugada syndromes [published erratum appears in Circulation 2005;111:378]. Circulation 2003;108:925–8. [DOI] [PubMed]


