Abstract
The complication rates and late clinical follow-up of patients who experience stent hyperexpansion have not been well studied. We designed this prospective study to evaluate the influence of stent hyperexpansion on clinical outcomes in patients with coronary artery disease.
Patients who underwent coronary stenting were divided into 2 groups according to whether or not their stents hyperexpanded (defined as stent/artery luminal diameter ratio of ≥1.1/1.0 with no residual stenosis) during implantation. Clinical, angiographic, and procedural characteristics were evaluated at baseline, and clinical outcomes were analyzed in-hospital and at 1 year. The primary endpoint comprised 1-year major adverse cardiovascular events (MACE): death, myocardial infarction, and target-vessel revascularization.
Clinical characteristics were not statistically different between patients with hyperexpansion (n=94) and those without (n=542; controls). The hyperexpansion group had significantly smaller mean target-vessel diameters and less severe stenoses; their stents were implanted at higher pressures (13.51 ± 12.93; P=0.01); and they had higher balloon/artery ratios (1.07 U vs 0.99 U; P <0.0001) and higher aggressiveness scores (14.5 U vs 12.79 U; P <0.0001). Rates of angiographic success and in-hospital MACE were similar, although the hyperexpansion group more often had occlusions of large side branches (5.3% vs 1.5%; P=0.03). At 1 year, the groups had similar rates of MACE (10.8% vs 10.7%), including target-vessel revascularization (8.2% vs 6.5%). Multivariate analysis revealed associations between stent hyperexpansion and higher aggressiveness scores, higher balloon/artery ratios, and narrower target vessels; the hyperexpansion group also had more target-vessel large-side-branch occlusions. Hyperexpansion was not associated with lower rates of MACE.
Key words: Angioplasty, transluminal, percutaneous coronary/adverse effects;, blood vessel prosthesis/implantation, coronary angiography, coronary artery occlusion/therapy, hemodynamics, percutaneous coronary intervention, prospective studies, stents/hyperexpansion, treatment outcome
Optimal implantation of a coronary stent, defined as implantation that results in clinically effective expansion of the stent and residual stenosis ≤10%, is associated with lower rates of angiographic restenosis and subacute thrombosis than non-optimal procedures.1–5 To achieve these results, it is often necessary to use aggressive approaches, such as high implantation pressures, oversized balloons, or both.6–12 These methods may, however, cause a stent to extend beyond the reference diameter of the target vessel, or “hyperexpand,” resulting in negative residual stenosis and the angiographic phenomenon known as the “step-up, step-down” effect. Several clinical studies have compared the clinical efficacy of high versus low implantation pressure,7–12 but none have studied the complication rates and late clinical follow-up of patients in whom stent hyperexpansion has occurred. In this prospective study, we compared clinical outcomes after 1 year in patients who experienced stent hyperexpansion, as opposed to optimal stent implantation with no hyperexpansion.
Patients and Methods
Patients
Eligible for inclusion in this study were all patients undergoing percutaneous coronary intervention (PCI) with coronary stents as treatment for symptomatic coronary artery disease at the Instituto de Cardiologia do Rio Grande do Sul (Porto Alegre, Brazil) from April 1996 through December 2000. Excluded from the study were patients who received 1st-generation stents (coil or tubular), underwent angiographically unsuccessful procedures, or had residual stenosis >10% after stent implantation.
Clinical Presentation
Stable angina was defined as angina that had remained stable during the last 2 months before stent implantation. Unstable angina was defined as angina that had worsened or intensified, with or without chest pain at rest, during the last 2 months before stent implantation. Acute myocardial infarction (AMI) was defined as ongoing chest pain and ST-segment elevation that prompted referral for percutaneous revascularization of an infarct-related artery.
Indications for Stenting
Indications for stenting included elective procedures, provisional stenting procedures, and salvage procedures. Elective procedures were those that were planned. Provisional stenting procedures were those necessitated by suboptimal results of a coronary angioplasty procedure (large nonocclusive dissections, residual lesions greater than 50%, or elastic recoil). Salvage procedures were those performed during an episode of acute occlusion.
Implantation Procedure
All patients were receiving oral platelet inhibitors—aspirin (100 mg daily) and ticlopidine (250 mg twice daily)—at the time of PCI. In urgent cases, these agents were administered during or soon after stenting. Intravenous boluses of heparin were administered during the implantation procedure, and intracoronary nitroglycerin was routinely administered before angiography in all patients. Standard PCI techniques were used13,14 to place the following stents: 313 Multilink Duet or Tristar (Guidant/Advanced Cardiovascular Systems; Santa Clara, Calif), 260 Tenax (Biotronik; Berlin, Germany), and 103 BX Velocity (Cordis, Johnson & Johnson Interventional; Miami Lakes, Fla). In most patients, treatment involved balloon dilation followed by stent placement. In each instance, the treating physician decided on the type and number of stents to use, and whether to use high pressure. A stenting procedure was considered successful if it resulted in no major adverse cardiovascular events (MACE) during the patient's hospital stay.
Angiographic Analysis
All angiographic analyses were performed by experienced operators, with use of manual pachymeters. Target-vessel diameter was defined as the mean diameter of the luminal segments proximal and distal to the lesion. The severity of stenosis was measured in 2 orthogonal views. Lesion length was measured “shoulder to shoulder.” Longer, interrupted lesions were considered to be a single lesion only when the normal segment that lay between them was <10 mm long. Stenoses were classified according to criteria established by the American College of Cardiology.15 Balloon/artery ratio was defined as the ratio between the nominal diameter of the balloon used to implant the stent, according to the data supplied by the balloon's manufacturer, and the target-vessel diameter. Aggressiveness score was defined as the product of the balloon/artery ratio multiplied by the maximum pressure (atm) used to implant the stent, as proposed by Hoffmann and coworkers.7 High-pressure stent implantation was defined as the use of pressures ≥14 atm.
Study subjects were classified into 2 groups on the basis of stent hyperexpansion. The occurrence of hyperexpansion was determined by measuring mid-stent luminal diameter immediately after stent deployment and comparing it with the target-vessel diameter. Hyperexpansion was defined as a postimplantation stent/artery luminal diameter ratio of ≥1.1/1.0 and residual stenosis of at least −10%. Optimal implantation with no hyperexpansion was defined as a postimplantation stent/artery luminal diameter ratio of <1.1/1.0 and residual stenosis ranging from −10% to 10%.
Follow-Up and Study Endpoints
Patients were followed up for 1 year by clinical evaluation in an outpatient clinic, by contact with the attending physician, or by telephone contact. Follow-up angiography was performed only when symptoms or signs of recurrent myocardial ischemia were present.
The primary endpoint of the study was a composite of MACE, which comprised cardiac-related death, Q-wave or non-Q-wave myocardial infarction, and target-vessel revascularization (including coronary artery bypass grafting and PCI) 1 year after the index stenting procedure. Clinical, procedural, and angiographic characteristics and in-hospital follow-up data regarding the study population were recorded and prospectively entered into a dedicated database. All MACE that occurred during the in-hospital period and within the 1st year after stenting were recorded in the database. Other adverse events recorded were subacute thrombosis (defined as in-stent occlusion occurring >24 hours but ≤30 days after stent implantation) and occlusion of large side branches (≥2.0 mm).
Statistical Analysis
Categorical variables were expressed as percentiles; continuous variables were expressed as the mean ± standard deviation (SD). Differences between the 2 study groups were evaluated by the χ2 test (categorical variables) and by Student's t-test (continuous variables). The rate of MACE-free survival during the 1-year follow-up period was analyzed by the life-table method, and the difference between survival curves was calculated by the log-rank method. Multivariate analysis was used to correct for differences between the groups that could have influenced 1-year MACE rates. Multivariate analysis was also used to identify predictors of hyperexpansion, and the Hosmer-Lemeshow goodness-of-fit test was used to determine the model that most appropriately fit the data.16 For all other tests, a P value ≤0.05 was considered to be statistically significant.
Results
Patients' Characteristics
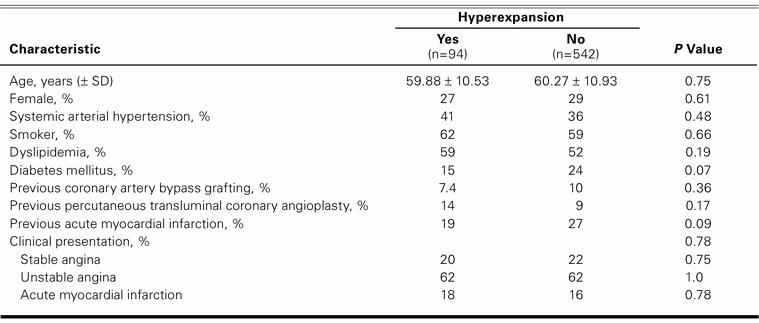
There were no statistically significant differences between the hyperexpansion group (n=94) and the control group (n=542) in terms of age, sex, or frequency of cardiac risk factors (Table I). The clinical presentation, previous AMI, previous myocardial revascularization, and mean left ventricular ejection fraction were also similar between groups.
TABLE I. Clinical Characteristics of Patient Population (n=636) According to Stent Hyperexpansion after Implantation
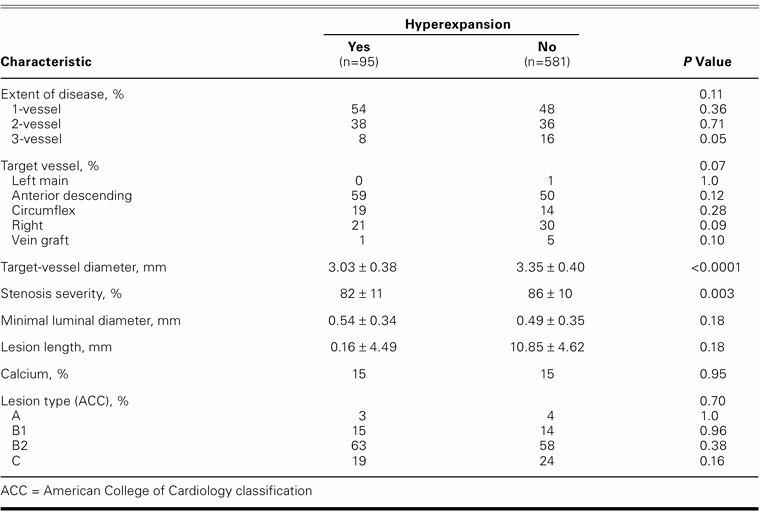
Patients with hyperexpansion less frequently had 3-vessel disease (8% vs 16%; P=0.05) (Table II). The mean target-vessel diameter was significantly lower in the hyperexpansion group than in the control group (3.03 ± 0.38 mm vs 3.35 ± 0.40 mm; P <0.0001), as was the mean severity of stenosis before stent implantation (82% ± 11% vs 86% ± 10%; P=0.003). There were no other statistically significant differences regarding the target vessels. Lesion length, calcium seen on angiography, and American College of Cardiology lesion type were similar in both groups.
TABLE II. Angiographic Characteristics According to Stent Hyperexpansion after Implantation (n=676)
Procedural Characteristics
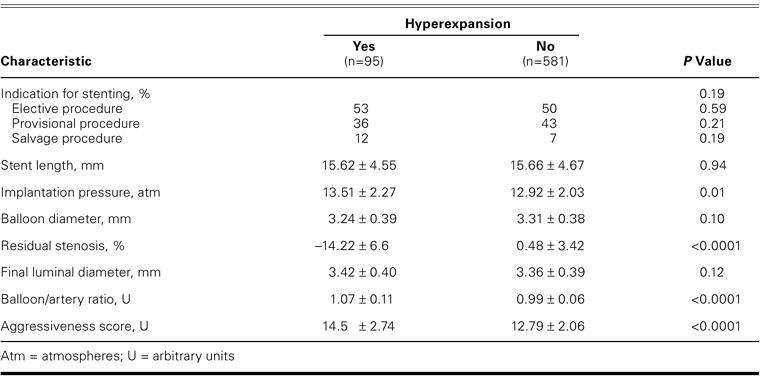
The indications for stenting were similar in both groups (Table III). Patients treated with Multilink stents had a greater proportion of hyperexpanded stents (17.7%) than did those patients treated with Tenax stents (10.9%) or BX Velocity stents (11.1%) (P=0.046). However, when the influence of the stent type on the probability of hyperexpansion was adjusted for the other important covariates, it no longer remained associated with hyperexpansion. There were no statistically significant differences between the 2 groups in terms of mean stent length, mean nominal balloon diameter, or final luminal diameter. The mean residual stenosis was significantly less in the hyperexpansion group (−14.22% ± 6.6% vs 0.48% ± 3.42%; P <0.0001), as expected on the basis of the definitions that we used. The mean implantation pressure was significantly higher in the hyperexpansion group (13.51 ± 2.27 atm vs 12.92 ± 2.03 atm; P=0.01), as were the mean balloon/artery ratio (1.07 ± 0.11 vs 0.99 ± 0.06; P <0.0001) and the aggressiveness score (14.5 ± 2.74 U vs 12.79 ± 2.06 U; P <0.0001). The independent predictors of hyperexpansion were aggressiveness score, balloon/artery ratio, and target-vessel diameter. When separate models were compared by means of the Hosmer-Lemeshow goodness-of-fit test, none reached statistical significance. The aggressiveness score remained as the strongest predictor of hyperexpansion (aggressiveness score: χ2=9.2832, P=0.319; balloon/artery ratio: χ2=14.3192, P=0.0738; and target-vessel diameter: χ2=10.9032, P=0.2072).
TABLE III. Procedural Factors According to Stent Hyperexpansion after Implantation (n=676)
Study Outcomes
One-year clinical follow-up data were obtained in 97% (617/636) of our study population. In 33% (210/636), angiography was performed at follow-up because of recurrent symptoms or myocardial ischemia. The hyperexpansion and the control groups had similar clinical success rates (98.9% vs 98.3%; P=NS). One in-hospital death (1.1%) occurred in the hyperexpansion group, and 6 (1.1%) occurred in the control group. No in-hospital AMIs occurred in the hyperexpansion group; 3 (0.6%) occurred in the control group. Occlusion of large side branches occurred more often in the hyperexpansion group (5.3% vs 1.5%; P=0.02) (Fig. 1). Subacute thrombosis occurred with similar frequency in both groups (none in the hyperexpansion group vs 0.7% in the control group; P=NS).
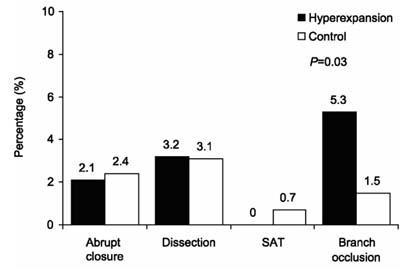
Fig. 1 Periprocedural complications of stent implantation.
SAT = subacute thrombosis
At 1-year follow-up, the hyperexpansion and control groups had similar rates of MACE (10.8% vs 10.7%), target-vessel revascularization (8.2% vs 6.5%), coronary angioplasty (4.2% vs 4.3%), coronary artery bypass grafting (4.2% vs 2.4%), AMI (2.1% vs 1.2%), and cardiac mortality rate (3.2% vs 1.5%) (Figs. 2 and 3). In those patients who underwent angiography on follow-up, angiographic restenosis occurred with similar frequency in both the hyperexpansion group and the control group (12.0% vs 12.8%). The differences were not statistically significant for any of these variables.
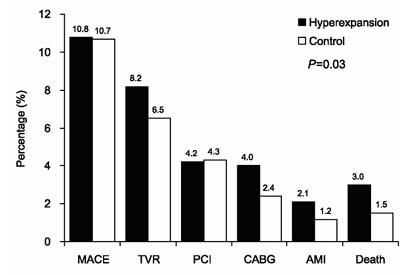
Fig. 2 Clinical events at 1-year follow-up (n=617).
AMI = acute myocardial infarction; CABG = coronary artery bypass grafting; MACE = major adverse cardiovascular events; PCI = percutaneous coronary intervention; TVR = target-vessel revascularization
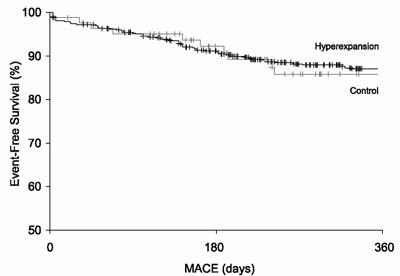
Fig. 3 Survival free of major adverse cardiovascular events (MACE) at 1 year.
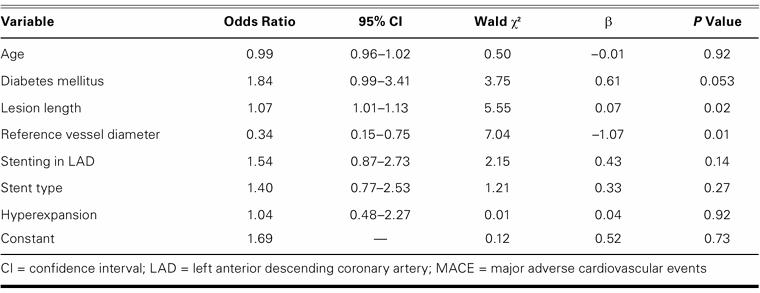
By multivariate analysis, the presence of hyperexpansion was not associated with 1-year MACE. Independent predictors of 1-year MACE were target-vessel diameter, lesion length, and diabetes mellitus, as shown in Table IV.
TABLE IV. Multivariate Analysis of Candidate Variables Associated with the Occurrence of 1-Year MACE
Discussion
In this study, the clinical outcomes after stent hyperexpansion were generally similar to those after optimal stent implantation without hyperexpansion. Patients in the hyperexpansion group experienced MACE and target-vessel revascularization just as frequently as did patients in the control group. However, patients in the hyperexpansion group experienced side-branch occlusions more frequently. The aggressiveness score was the variable most strongly associated with hyperexpansion.
Clinical Outcomes
During the last decade, the “bigger-is-better” approach has been the hallmark of guided intravascular stent implantation. This approach has gained additional acceptance as the association between optimal stent expansion and lower rates of subacute thrombosis and restenosis has become clear. However, the bigger-is-better approach may now have to be revised in light of recent experimental and clinical findings. Studies in animals and human beings have shown that intimal hyperplasia increases when stent struts are more deeply embedded in the arterial wall during implantation.17,18 The results of several clinical studies have suggested that excessive trauma to the vessel wall should be avoided. In one of those studies, Hoffmann and coworkers7 reported an association between aggressiveness score and increased neointimal volume in 102 patients evaluated by intracoronary ultrasonography. In another, Uretsky and colleagues11 showed that the systematic use of very high pressures (20 atm) at implantation increases procedural complications and does not lower the rate of target-vessel revascularization. Randomized clinical trials9,10 comparing the effects of high- versus low-pressure strategies on target-vessel revascularization rates have shown no clinical benefits of routinely using higher pressures, even when those higher pressures result in less residual stenosis and larger cross-sectional areas. The idea that excessive vascular trauma occurs when higher pressures are used may also explain why, in 1 randomized clinical trial, routine ultrasonographic guidance did not lead to better clinical outcomes despite better angiographic results.19
Although the effects of implantation pressures and oversized balloons on clinical outcomes have been studied extensively, their direct effects on the arterial wall (that is, the step-up, step-down effect of hyperexpansion) have not. The present findings should help fill this gap in knowledge: in our study, hyperexpansion neither worsened nor improved clinical outcomes. These results suggest that aggressive implantation strategies should not be used simply to achieve an angiographic step-up, step-down effect.
Occlusion of Side Branches
Side-branch occlusion occurs after stent implantation in approximately 10% of cases when the stent struts cover the ostium of a branch vessel. The mechanisms responsible for this adverse effect include the redistribution and displacement of atherosclerotic plaque toward the branch ostium (the so-called “snow-plow effect”), embolization of atherosclerotic débris, thrombosis, vessel dissection, spasm, and blockage of the ostium of the branch by the stent struts.20–22 The outcome of side-branch occlusion has traditionally been considered benign,21–23 and the regression of branch stenoses or occlusions has been shown in some late follow-up angiographic studies.20,22,24 Other studies have shown higher rates of non-Q-wave myocardial infarction and a slightly increased release of creatine kinase and its MB isoenzyme (CK-MB) after side-branch occlusions, although the clinical import of the increased CK-MB release is as yet unknown.25
In the present study, we found that large side-branch occlusions occurred more often in the group with stent hyperexpansion than in the control group (5.3% vs 1.5%; P=0.03). In previous studies, the use of higher pressures and larger balloons was also associated with the type of stent used, side-branch occlusion, side-branch ostial lesions, and dissection toward the plane of the branch ostium.20,21
Mechanisms of Hyperexpansion
The expansion of intravascular stents into the artery wall is the result of complex interactions between balloon diameter, material, and implantation pressure; stent type; stent material; and vascular compliance.26–28 Vascular compliance is determined by the characteristics of the arterial wall and plaque, such as calcium content, eccentricity, and presence of positive or negative vascular remodeling.29,30 The final stent diameter is related linearly to the balloon/artery ratio and implantation pressure; this diameter is largest when the maximum compliance of the vessel is achieved.2,6,9 A stent's configuration and material also influence its expansion and stability (absence of retraction) once deployed.28 The differences between stent models appear to be more important among 1st-generation stents. Nevertheless, recent studies have shown that even the latest-generation stents expand in vivo to only 60% to 70% of the diameter expected by their manufacturers.31,32 The plaque burden, as evaluated by intracoronary ultrasonography, appears to be related to incomplete stent expansion, even in the case of the latest-generation prostheses.32
We found that aggressiveness score, balloon/artery ratio, and target-vessel diameter were all associated with stent hyperexpansion. However, as shown by the Hosmer-Lemeshow goodness-of-fit tests, the aggressiveness score most appropriately fit our data. This not only emphasizes the role of aggressive implantation strategies in the genesis of stent hyperexpansion, but also suggests that stent hyperexpansion is more closely related to implantation strategy than it is to either the intrinsic characteristics of the target lesion or the compliance of the target vessel. Still, it is important to note that target vessels were, on average, narrower in the hyperexpansion group, which suggests that patients with smaller vessels are more frequently treated with oversized balloons. Other studies have noted this, as well.8,33
Study Limitations
One limitation of our study was that some of the differences between the 2 study groups may have influenced outcome independently of hyperexpansion. For example, patients in the hyperexpansion group were more likely to have narrower vessels, and were less likely to have 3-vessel disease and lesions that were more severe. A 2nd limitation was that our angiographic analyses did not include quantitative online angiography. However, this limitation was offset in part by having experienced operators review all angiographic data. Intravascular ultrasonography, which might have provided useful insights in stent hyperexpansion, was not included in the study protocol. A 3rd limitation was the unavailability of specific information regarding the pharmacological therapy given to each patient, although all patients who undergo PCI in our laboratory receive standard-of-care therapy.
Conclusion
This study shows that stent hyperexpansion is associated with more aggressive implantation strategies and narrower target vessels. When compared with optimal stent implantation, stent hyperexpansion does not reduce the rate of target-vessel revascularization or MACE and is associated with more frequent periprocedural occlusion of large side branches of the target vessel. These findings suggest that stent hyperexpansion should not be pursued during the procedure, since clinical benefit was not shown in our study, nor has it been shown in any other study to date.
Footnotes
Address for reprints: Alexandre Quadros, MD, MSc, Unidade de Pesquisa do IC/FUC, Avenida Princesa Isabel, 370 Santana 90620-001, Porto Alegre–RS, Brazil. E-mail: alesq@terra.com.br
References
- 1.Kuntz RE, Gibson CM, Nobuyoshi M, Baim DS. Generalized model of restenosis after conventional balloon angioplasty, stenting and directional atherectomy. J Am Coll Cardiol 1993;21:15–25. [DOI] [PubMed]
- 2.Colombo A, Hall P, Nakamura S, Almagor Y, Maiello L, Martini G, et al. Intracoronary stenting without anticoagulation accomplished with intravascular ultrasound guidance. Circulation 1995;91:1676–88. [DOI] [PubMed]
- 3.Serruys PW, Kay IP, Disco C, Deshpande NV, de Feyter PJ. Periprocedural quantitative coronary angiography after Palmaz-Schatz stent implantation predicts the restenosis rate at six months: results of a meta-analysis of the BElgian NEtherlands Stent study (BENESTENT) I, BENESTENT II Pilot, BENESTENT II and MUSIC trials. Multicenter Ultrasound Stent In Coronaries. J Am Coll Cardiol 1999;34: 1067–74. [DOI] [PubMed]
- 4.Gottschall CA, Miler VV, Yordi LM, Cardoso CR, Rodrigues LC. Detection of restenosis after percutaneous transluminal coronary angioplasty by an angiographic score. J Invasive Cardiol 1998;10:1–11. [PubMed]
- 5.Cutlip DE, Baim DS, Ho KK, Popma JJ, Lansky AJ, Cohen DJ, et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation 2001;103:1967–71. [DOI] [PubMed]
- 6.Stone GW, St Goar FG, Hodgson JM, Fitzgerald PJ, Alderman EL, Yock PG, et al. Analysis of the relation between stent implantation pressure and expansion. Optimal Stent Implantation (OSTI) Investigators. Am J Cardiol 1999;83:1397–400, A8. [DOI] [PubMed]
- 7.Hoffmann R, Mintz GS, Mehran R, Kent KM, Pichard AD, Satler LF, Leon MB. Tissue proliferation within and surrounding Palmaz-Schatz stents is dependent on the aggressiveness of stent implantation technique. Am J Cardiol 1999; 83:1170–4. [DOI] [PubMed]
- 8.Goldberg SL, Di Mario C, Hall P, Colombo A. Comparison of aggressive versus nonaggressive balloon dilatation for stent deployment on late loss and restenosis in native coronary arteries. Am J Cardiol 1998;81:708–12. [DOI] [PubMed]
- 9.Hoffmann R, Haager P, Mintz GS, Kerckhoff G, Schwarz R, Franke A, et al. The impact of high pressure versus low pressure stent implantation on intimal hyperplasia and follow-up lumen dimensions; results of a randomized trial. Eur Heart J 2001;22:2015–24. [DOI] [PubMed]
- 10.Dirschinger J, Kastrati A, Neumann FJ, Boekstegers P, Elezi S, Mehilli J, et al. Influence of balloon pressure during stent placement in native coronary arteries on early and late angiographic and clinical outcome: A randomized evaluation of high-pressure inflation. Circulation 1999;100:918–23. [DOI] [PubMed]
- 11.Uretsky BF, Rosanio S, Lerakis S, Wang FW, Smiley M, Stouffer GA, et al. A prospective evaluation of angiography-guided coronary stent implantation with high versus very high balloon inflation pressure. Am Heart J 2000;140:804–12. [DOI] [PubMed]
- 12.Johansson B, Allared M, Borgencrantz B, Brorson L, Geijer H, Kellerth T, et al. Standardized angiographically guided over-dilatation of stents using high pressure technique optimize results without increasing risks. J Invasive Cardiol 2002;14:221–6. [PubMed]
- 13.Holmes DR Jr, Hirshfeld J Jr, Faxon D, Vlietstra RE, Jacobs A, King SB 3rd. ACC Expert Consensus document on coronary artery stents. Document of the American College of Cardiology. J Am Coll Cardiol 1998;32:1471–82. [DOI] [PubMed]
- 14.Carrozza JP, Baim DS. Coronary stenting. In: Baim DS, Grossman W, editors. Grossman's cardiac catheterization, angiography, and intervention. 6th ed. Philadelphia: Lippincott, Williams & Wilkins; 2000. p. 637–66.
- 15.Ellis SG, Vandormael MG, Cowley MJ, DiSciascio G, Deligonul U, Topol EJ, Bulle TM. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel Angioplasty Prognosis Study Group. Circulation 1990;82:1193–202. [DOI] [PubMed]
- 16.Hosmer DW Jr, Lemeshow S. Applied logistic regression. New York: Wiley; 1989. p. 135–75.
- 17.Schwartz RS, Huber KC, Murphy JG, Edwards WD, Camrud AR, Vlietstra RE, Holmes DR. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol 1992;19:267–74. [DOI] [PubMed]
- 18.Farb A, Sangiorgi G, Carter AJ, Walley VM, Edwards WD, Schwartz RS, Virmani R. Pathology of acute and chronic coronary stenting in humans. Circulation 1999;99:44–52. [DOI] [PubMed]
- 19.Mudra H, di Mario C, de Jaegere P, Figulla HR, Macaya C, Zahn R, et al. Randomized comparison of coronary stent implantation under ultrasound or angiographic guidance to reduce stent restenosis (OPTICUS Study). Circulation 2001; 104:1343–9. [DOI] [PubMed]
- 20.Fischman DL, Savage MP, Leon MB, Schatz RA, Ellis S, Cleman MW, et al. Fate of lesion-related side branches after coronary artery stenting. J Am Coll Cardiol 1993;22:1641–6. [DOI] [PubMed]
- 21.Aliabadi D, Tilli FV, Bowers TR, Benzuly KH, Safian RD, Goldstein JA, et al. Incidence and angiographic predictors of side branch occlusion following high-pressure intracoronary stenting. Am J Cardiol 1997;80:994–7. [DOI] [PubMed]
- 22.Alfonso F, Hernandez C, Perez-Vizcayno MJ, Hernandez R, Fernandez-Ortiz A, Escaned J, et al. Fate of stent-related side branches after coronary intervention in patients with in-stent restenosis. J Am Coll Cardiol 2000;36:1549–56. [DOI] [PubMed]
- 23.Iniguez A, Macaya C, Alfonso F, Goicolea J, Hernandez R, Zarco P. Early angiographic changes of side branches arising from a Palmaz-Schatz stented coronary segment: results and clinical implications. J Am Coll Cardiol 1994;23:911–5. [DOI] [PubMed]
- 24.Pan M, Medina A, Suarez de Lezo J, Romero M, Melian F, Pavlovic D, et al. Follow-up patency of side branches covered by intracoronary Palmaz-Schatz stent. Am Heart J 1995;129: 436–40. [DOI] [PubMed]
- 25.Bhargava B, Waksman R, Lansky AJ, Kornowski R, Mehran R, Leon MB. Clinical outcomes of compromised side branch (stent jail) after coronary stenting with the NIR stent. Catheter Cardiovasc Interv 2001;54:295–300. [DOI] [PubMed]
- 26.Ahmed JM, Mintz GS, Weissman NJ, Lansky AJ, Pichard AD, Satler LF, Kent KM. Mechanism of lumen enlargement during intracoronary stent implantation: an intravascular ultrasound study. Circulation 2000;102:7–10. [DOI] [PubMed]
- 27.Hjemdahl-Monsen CE, Ambrose JA, Borrico S, Cohen M, Sherman W, Alexopoulos D, et al. Angiographic patterns of balloon inflation during percutaneous transluminal coronary angioplasty: role of pressure-diameter curves in studying distensibility and elasticity of the stenotic lesion and the mechanism of dilation. J Am Coll Cardiol 1990;16:569–75. [DOI] [PubMed]
- 28.Bermejo J, Botas J, Garcia E, Elizaga J, Osende J, Soriano J, et al. Mechanisms of residual lumen stenosis after high-pressure stent implantation: a quantitative coronary angiography and intravascular ultrasound study. Circulation 1998;98:112–8. [DOI] [PubMed]
- 29.Grewe PH, Thomas D, Machraoui A, Barmeyer J, Muller KM. Coronary morphologic findings after stent implantation. Am J Cardiol 2000;85:554–8. [DOI] [PubMed]
- 30.Finet G, Weissman NJ, Castagna MT, Kent KM, Satler LF, Laird JR, Pichard AD. Influence of coronary atherosclerotic remodeling and plaque characteristics on mechanisms of lumen enlargement during stent implantation. J Am Coll Cardiol 2001;37(Suppl A):79A.
- 31.Takano Y, Yeatman LA, Higgins JR, Currier JW, Ascencio E, Kopelson KA, Tobis JM. Optimizing stent expansion with new stent delivery systems. J Am Coll Cardiol 2001;38:1622–7. [DOI] [PubMed]
- 32.Yoon SC, Laskey WK, Assadourian A, Kelly D, Gellman J, Herzog W, Stafford JL. Assessment of contemporary stent deployment using intravascular ultrasound. Catheter Cardiovasc Interv 2002;57:150–4. [DOI] [PubMed]
- 33.Hsieh IC, Chien CC, Chang HJ, Chern MS, Hung KC, Lin FC, Wu D. Acute and long-term outcomes of stenting in coronary vessel > 3.0 mm, 3.0–2.5 mm, and < 2.5 mm. Catheter Cardiovasc Interv 2001;53:314–22. [DOI] [PubMed]


