Abstract
Acute massive pulmonary embolism after cardiac surgery is very rare. Although accurate diagnosis and rapid treatment are crucial to a successful outcome, there is no standard treatment option. Thrombolytic therapy and catheter embolectomy are the usual treatment options, but they are associated with risks, especially in patients who experience massive pulmonary embolism after coronary artery bypass surgery. Open pulmonary embolectomy may be the best choice for treating these patients. This report describes our use of emergency pulmonary embolectomy along with cardiopulmonary bypass as an effective therapeutic approach in 2 cases of massive pulmonary embolism that occurred after on-pump coronary artery bypass grafting.
Key words: Coronary artery bypass, echocardiography, embolectomy/methods, pulmonary embolism/diagnosis/mortality/surgery/therapy, risk factors, treatment outcome
Selecting an optimal treatment for acute massive pulmonary embolism (PE) is challenging. Thrombolytic therapy and catheter embolectomy are widely used therapeutic interventions. Although thrombolysis is effective, it can cause bleeding, especially after surgery. Percutaneous intervention also carries risks, such as fragmentation of a large clot.
Open pulmonary embolectomy, in our judgment, is the safest and most effective treatment. Herein, we report the successful surgical management of massive PEs in 2 patients who had undergone coronary artery bypass grafting (CABG).
Case Reports
Two men (ages, 66 and 76 years) underwent CABG in our clinic. Their postoperative periods were uneventful; each was discharged on the 7th postoperative day with instructions to take aspirin. On their 12th and 24th postoperative days, respectively, they were admitted to our emergency unit with sudden-onset respiratory distress and chest pain. Their vital signs were borderline, but the hematologic values (all biochemical markers except for blood-gas samples) were optimal in both patients.
Diagnoses of PE were made on the basis of the patients' arterial blood gas levels and were confirmed by transthoracic echocardiography (TTE), which revealed massive pulmonary emboli associated with moderate tricuspid regurgitation. Oxygen therapy and continuous infusions of heparin were initiated at 1,000 U/hr.
Patient 1 (the 66-year-old) had a pulmonary arterial pressure of 65 mmHg, and computed tomography (CT) revealed a large saddle embolus at the bifurcation of the main pulmonary artery. The embolus protruded into the left and right pulmonary arteries, totally occluding the left branch and almost totally occluding the right (Fig. 1). In Patient 2 (the 76-year-old), TTE revealed massive thrombi in the right atrium and in the proximal segment of the main pulmonary artery.
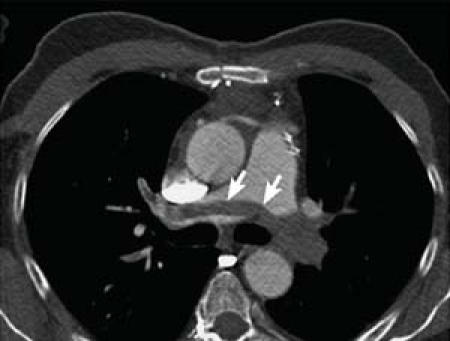
Fig. 1 Patient 1. Computed tomographic scan of the massive pulmonary embolism (arrows).
The patients were taken to the operating room in their 5th and 7th hours, respectively, after admission. After the administration of general anesthesia, the diagnoses of PE were confirmed by transesophageal echocardiography (TEE). Cardiopulmonary bypass was established in both patients by use of femoral artery, femoral vein, and selective superior vena cava cannulation. The aortas were not cross-clamped. Transverse arteriotomies were made in each patient's main pulmonary artery, and, with simple forceps, massive saddle emboli were extracted en bloc, under direct view, from the main pulmonary arteries and from the right and left pulmonary branches (Fig. 2). A 5F Fogarty catheter was used to check the distal arterial branches for residual emboli. An incision was made in the right atrium of Patient 2, and a thrombus adhering to the eustachian valve was resected. By palpation, we determined that the bypass grafts were patent in both patients. Both operations were completed without complications.
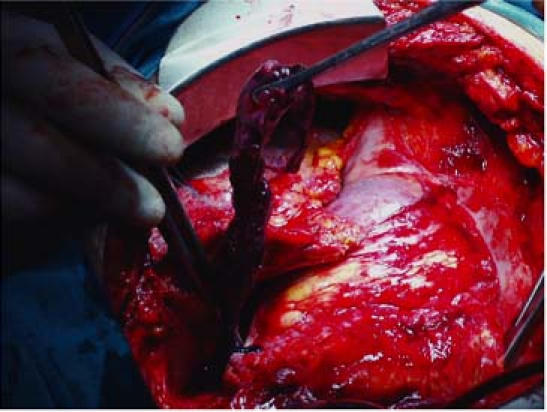
Fig. 2 Patient 2. Intraoperative view of the extraction of the large saddle embolus from the main pulmonary artery.
Postoperative TEE revealed no residual thrombi. The patients were extubated in their 10th and 8th postoperative hours, and warfarin treatment was begun on the 1st postoperative day. During the patients' hospitalization, venous Doppler ultrasonography revealed no source of thrombi in the lower extremities. The subsequent recovery periods were uneventful, and the patients were discharged on their 8th and 9th postoperative days, respectively.
The patients' follow-up periods lasted for 12 and 14 months without adverse event. At last follow-up, both patients were free from dyspnea and met the criteria of New York Heart Association functional class I. Thoracic CT scans revealed no residual thrombi.
Discussion
Massive PE is a serious condition that requires prompt diagnosis and intervention. Treatment options are not standardized. In patients who have recently undergone major surgery, such as coronary bypass, thrombolytic therapy can produce negative outcomes; the data suggest that medically treated patients have a higher death rate, an increased risk of major hemorrhage, and an increased recurrence of PE.1–3 Another treatment, percutaneous intervention, is also associated with some risks.4 The time needed to prepare for percutaneous intervention is not always tolerated, especially by hemodynamically unstable patients; in addition, fragmentation of a large thrombus in the main pulmonary artery may result in showering of the embolytic fragments distally into smaller arteries. The embolectomy may then be incomplete, leading to pulmonary hypertension, which decreases quality of life and overall survival rates. These considerations led us to choose open pulmonary embolectomy as an alternative for our 2 patients.
Although there are reports of successful pulmonary embolectomies in various situations,2,3 surgical management of massive PE after CABG is rare.5 We consider emergency open pulmonary embolectomy more advantageous than medical therapy in these cases. Because massive PE occurring after CABG can cause patients' clinical status to deteriorate at any time, surgical intervention should occur as soon as possible.
We confirmed our patients' diagnoses of PE by use of CT, TTE, and TEE. Although pulmonary angiography is considered the gold standard for the evaluation of pulmonary embolism, it is associated with some sequelae.6 Computed tomography and TEE are reliable methods for diagnosing PE. With 90% to 95% sensitivity and 100% specificity in diagnosis,7 TEE can also be used in the operating room after surgery to check for residual emboli.
Patients may benefit from cardiopulmonary bypass itself, which helps to correct acidemia and provides oxygenation to improve the postoperative stability of the heart and perfusion of the vital organs. It has been reported that open embolectomy with cross-clamping of the aorta and application of cardioplegia will help avert ischemic injury to a stunned right ventricle.2 We decided to perform open embolectomies without aortic cross-clamping and without cardioplegia, because we detected no right ventricular failure in either of our patients. Femoral artery, femoral vein, and selective superior vena cava cannulation enabled us to establish cardiopulmonary bypass safely without manipulating the aorta and right atrium.
The combination of rapid diagnosis by CT, TTE, and TEE with the aggressive surgical approach of open pulmonary embolectomy offers great advantages over thrombolytic therapy and percutaneous intervention. We consider open embolectomy the best option for treating acute massive PE, particularly in patients who have recently undergone CABG.
Footnotes
Address for reprints: Tankut Hakki Akay, MD, PK 56, 06552 Cankaya, Ankara, Turkey. E-mail: tankutakay@gmail.com
References
- 1.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999; 353:1386–9. [DOI] [PubMed]
- 2.Georghiou GP, Brauner R, Berman M, Stamler A, Glanz L, Vidne BA, Erez E. Successful resuscitation of a patient with acute massive pulmonary embolism using emergent embolectomy. Ann Thorac Surg 2004;77:697–9. [DOI] [PubMed]
- 3.Yalamanchili K, Fleisher AG, Lehrman SG, Axelrod HI, Lafaro RJ, Sarabu MR, et al. Open pulmonary embolectomy for treatment of major pulmonary embolism. Ann Thorac Surg 2004;77:819–23. [DOI] [PubMed]
- 4.Brady AJ, Crake T, Oakley CM. Percutaneous catheter fragmentation and distal dispersion of proximal pulmonary embolus. Lancet 1991;338:1186–9. [DOI] [PubMed]
- 5.Ralph-Edwards AC, Feindel CM, Glynn MF. Successful treatment of massive pulmonary embolism after coronary artery bypass grafting due to heparin-induced thrombocytopenia. Ann Thorac Surg 1994;57:1326–8. [DOI] [PubMed]
- 6.Dalen JE, Brooks HL, Johnson LW, Meister SG, Szucs MM Jr, Dexter L. Pulmonary angiography in acute pulmonaryembolism: indications, techniques, and results in 367 patients. Am Heart J 1971;81:175–85. [DOI] [PubMed]
- 7.Douketis JD, Kearon C, Bates S, Duku EK, Ginsberg JS. Risk of fatal pulmonary embolism in patients with treated venous thromboembolism. JAMA 1998;279:458–62. [DOI] [PubMed]


