Abstract
Increasing evidence points to a role for circulating CD34-positive (CD34+) cells in vascular maintenance and neovascularization. Although there are established methods for evaluating absolute numbers of CD34+ cells in bone marrow or mobilized peripheral blood, there is no convenient and highly reproducible method for quantifying low numbers of CD34+ cells in blood samples, such as those from the peripheral blood of patients with cardiovascular disease. With current commonly used methods, the mean percentage of CD34+ cells in leukocyte fractions from such patients was only 0.02%, and the cumulative intra-assay coefficient of variation was ∼30%. With use of the protocol described herein, actual counts of CD34+ cells increased ∼5-fold and cumulative intra-assay coefficients of variation were reduced to ∼7%. The new method is useful to precisely measure low numbers of CD34+ cells in samples, and it has potential as a screening tool to evaluate cardiovascular risk in large patient populations.
Key words: Antigens, CD34/analysis; blood cell count; cardiovascular diseases; cell sorter, fluorescence-activated; CD34-positive cells; coefficients of variation; endothelium, vascular/cytology; stem cells
In vasculature maintenance and neovascularization, there is increasing evidence of a role for circulating endothelial progenitor cells (EPCs)—including the populations of CD34-positive (CD34+) cells that are present in peripheral blood.1 As a source of numerous growth and angiogenesis factors at ischemic loci, CD34+ cells also contribute to vascular homeostasis.2 Furthermore, initial clinical trials of cell transplantation in treating ischemia of the hind limb3 and myocardium4 have shown promising results. On the basis of these observations, circulating EPCs5 and CD34+ cells6 have been evaluated in patients with cardiovascular disease, and strong correlations of their levels with vascular function have been reported. However, procedures to evaluate EPCs and CD34+ cells are not simple5; because of low numbers of circulating CD34+ cells, routine FACS (luorescence-activated cell sorter) analysis7 of CD34+ cell counts in patients with cardiovascular disease is not feasible. In this report, we demonstrate a new method that facilitates determination of the absolute number of circulating CD34+ cells in patients with low levels of CD34+ cells.
Patients and Methods
This study was approved by the Human Assurance Committee of the National Cardiovascular Center and Osaka Minami Medical Center, and all subjects provided written informed consent. Results of experiments are reported as mean ± standard error.
Analysis of Peripheral Blood
Three milliliters of heparinized peripheral blood were obtained from 20 patients who had histories of cardiovascular disease: 14 had sustained myocardial infarction, and 9 had sustained cerebral infarction (3 had histories of both). Patients who had experienced vascular events within 30 days of measurement were excluded. The study group included 12 men and 8 women, with a mean age of 74 ± 1.7 years (range, 59–87 yr). Medicines taken by study subjects included anticoagulants (aspirin, 17); anti-hypertensive agents, including calcium-channel antagonists, angiotensin-converting enzyme (ACE) inhibitors, or both (14); and sulfonylureas for glycemic control (5). Patients who were taking HMG-CoA reductase inhibitors (statins) were excluded from the study.
First, we counted circulating CD34+ cells with ProCount™ (BD Bioscience; San Jose, Calif) and Stem-Kit™ (Beckman Coulter; Marseilles, France), according to the manufacturers' protocols. (These protocols are based on International Society of Hematotherapy and Graft Engineering (ISHAGE) Guidelines7 and are frequently used for quantiication of CD34+ cells that have mobilized into peripheral blood.) Next, to increase the reproducibility of CD34+ cell counts, the Stem-Kit protocol was modified as follows: the blood sample volume, antibodies, and lysing solution were doubled. After adding 30 μL of internal control particles (stem count: Beckman Coulter), samples were centrifuged for 5 min at 450 G, and 3,860 μL of supernatant was removed carefully with a pipette. Samples were analyzed by Coulter CYTOMICS™ FC500 & XL-system II software (Beckman Coulter) for 6 min each (Fig. 1).
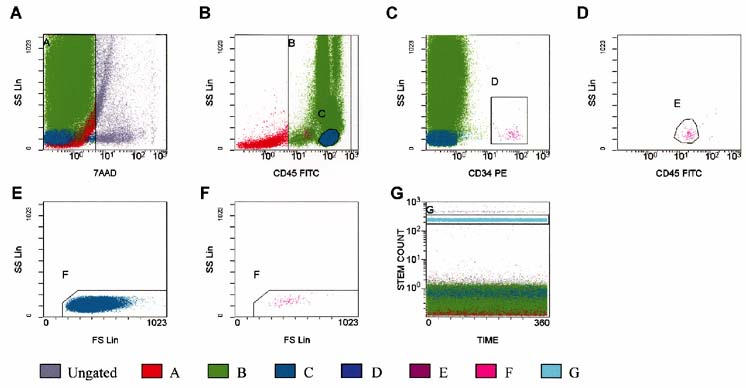
Fig. 1 Quantification of circulating CD34+ cells by fluorescence-activated cell sorter analysis using our modified, improved protocol. A) All events: 7-aminoactinomycin-D viability dye-positive cells (dead cells) were excluded from region A. B) Events from region A: All CD45+ cells (leukocytes) were included in region B. Region C was adjusted to include only lymphocytes (bright CD45, low side-scatter). C) Events from regions A and B: Region D was adjusted to include CD34+ hematopoietic progenitor cells (HPC). D Events from regions A, B, and D: Region E was adjusted to include cells forming a cluster with characteristic CD34+ HPC (low side-scatter and low-tointermediate CD45 staining). Brightly stained events were excluded from region E. E) Events from regions A and C: Region F was adjusted to include lymph/blast cells, excluding platelet aggregates if present. F) Events from A, B, D, and E: Lymph/blast region F identified a cluster of events that met all the fluorescence and light-scattering criteria of ISHAGE Guidelines for CD34+ HPC. G) All events: Region G was adjusted to enclose the internal control.
7AAD = 7-aminoactinomycin-D; CD34 PE = cluster of differentiation 34 phycoerythrin; CD45 FITC = cluster of differentiation 45 fluorescein isothiocyanate; FS Lin = forward-scatter linear scale; ISHAGE = International Society of Hematotherapy and Graft Engineering; SS Lin = side-scatter linear scale
Results
Increase of CD34+ Cell Counts
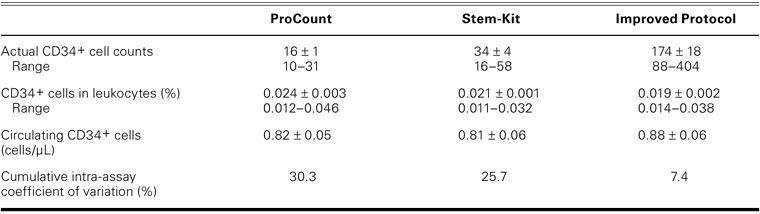
The mean percentage of CD34+ cells in the leukocyte fraction obtained from mobilized peripheral blood has been reported to be about 0.2% to 0.5%.7 First, we used the ProCount and Stem-Kit protocols to count circulating CD34+ cells that had been obtained from patients with cardiovascular disease (Table I). The mean percentages of CD34+ cells in the leukocyte fraction were 0.024% ± 0.003% (range, 0.012%–0.046%) for ProCount and 0.021% ± 0.001% (range, 0.011%–0.032%) for Stem-Kit. The actual CD34+ cell counts per analysis were only 16 ± 1 (range, 10–31) for ProCount and 34 ± 4 (range, 16–58) for Stem-Kit. Because the absolute cell count is a major factor in the reproducibility of the measurement,8 our approach was to modify the original protocol in order to obtain higher numbers of actual CD34+ cells per count. However, simply increasing sample volume or measurement time does not improve reproducibility, because of these factors: adhesion of internal control particles to cells in the patient sample, and precipitation and aggregation of cells in the sample. Our approach, as outlined under Methods, was to seek a method that produces higher cell counts while maintaining short measurement times. Through the use of our method, the mean CD34+ cell count increased to a level of 174 ± 18 (range, 88–404) per analysis, and the mean percentage of CD34+ cells in the leukocyte fraction was 0.019 ± 0.002 (range, 0.014%–0.038%), which was consistent with measurements using the standard method (Table I). The supernatant that was removed during the procedure was also analyzed and was found not to contain either cells or internal control particles.
TABLE I. Reduction in the Coefficient of Variation Using Our Modified, Improved Protocol for Determining CD34+ Cell Levels in Peripheral Blood
Improvement in the Cumulative Intra-Assay Coefficient of Variation
In mobilized peripheral blood, the coeficients of variation have been reported to be about 8% and 4% using ProCount and Stem-Kit, respectively, on the basis of the manufacturers' published information. However, in non-mobilized peripheral blood of patients with cardiovascular disease, the mean percentage of CD34+ cells in the leukocyte fraction was less than 10%, compared with mobilized blood, and the calculated cumulative intra-assay coeficients of variation were 30.3% and 25.7%, as evaluated by ProCount and Stem-Kit, respectively. Our method increased the absolute number of CD34+ cells by about 5-fold during the same measurement period, and it resulted in a reduced (7.4%) cumulative intra-assay coefficient of variation (Table I).
Discussion
Although our modified method for quantifying CD34+ cells in blood was similar to established methods for the calculation of mean circulating CD34+ cell counts, our method substantially improved reproducibility of the measurement.
The coeficient of variation of CD34+ cell counts is inversely proportional to the square root of the number of CD34+ cells detected in the sample. A minimum of 100 CD34+ cells is required to ensure a coeficient of variation in the range of 10%.8 Our modiied protocol yielded more than 100 CD34+ cells in each count (conirmed by duplicate counting), and the coeficient of variation was reduced to 7%. Simply increasing the sample volume or lengthening the time for measurement of cell numbers does not necessarily improve reproducibility of the counts.8 Our results indicate that absolute numbers of circulating CD34+ cells in peripheral blood of patients who have low levels of such cells can now be quantiied precisely using a modiication of the ISHAGE protocol. This easy method enables precise measurement of the CD34+ cell population of stem cells in peripheral blood and can be broadly applied to screening patients for cardiovascular risk.
Footnotes
Address for reprints: Akihiko Taguchi, MD, Department of Cerebrovascular Disease, National Cardiovascular Center, 5-7-1 Fujishiro-dai, Suita, Osaka 565-8565, Japan. E-mail: ataguchi@res.ncvc.go.jp
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Health, Labor, and Welfare, Japan.
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–7. [DOI] [PubMed]
- 2.Majka M, Janowska-Wieczorek A, Ratajczak J, Ehrenman K, Pietrzkowski Z, Kowalska MA, et al. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood 2001;97:3075–85. [DOI] [PubMed]
- 3.Taguchi A, Ohtani M, Soma T, Watanabe M, Kinosita N. Therapeutic angiogenesis by autologous bone-marrow transplantation in a general hospital setting. Eur J Vasc Endovasc Surg 2003;25:276–8. [DOI] [PubMed]
- 4.Hamano K, Nishida M, Hirata K, Mikamo A, Li TS, Harada M, et al. Local implantation of autologous bone marrow cells for therapeutic angiogenesis in patients with ischemic heart disease: clinical trial and preliminary results. Jpn Circ J 2001;65:845–7. [DOI] [PubMed]
- 5.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003;348:593–600. [DOI] [PubMed]
- 6.Taguchi A, Matsuyama T, Moriwaki H, Hayashi T, Hayashida K, Nagatsuka K, et al. Circulating CD34-positive cells provide an index of cerebrovascular function. Circulation 2004;109:2972–5. [DOI] [PubMed]
- 7.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by low cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother 1996;5:213–26. [DOI] [PubMed]
- 8.Sutherland DR, Keeney M, Gratama JW. Enumeration of CD34+ hematopoietic stem and progenitor cells. In: Robinson JP, Darzynkiewicz Z, Dobrucki J, Hyun WC, Nolan JP, Orfao A, Rabinovitch PS, editors. Current protocols in cytometry. New York: John Wiley and Sons, Inc.; 2003. Unit 6.4, p. 1–23. [DOI] [PubMed]


