Abstract
Prostatitis is a common medical diagnosis. The etiology of this symptomatic syndrome can be an acute or chronic bacterial infection, a noninfectious initiator (the most common cause), or iatrogenic heat or radiation; the syndrome may coexist with benign prostatic hyperplasia. Alpha-blockers have a role in the treatment of the prostatitis syndromes. In Category I, acute bacterial prostatitis, α-blockers have been shown to possibly ameliorate obstructive and irritative voiding symptoms. In Category II, chronic bacterial prostatitis, α-blockers seem to reduce the risk of clinical and bacteriological recurrence. In Category III, chronic pelvic pain syndrome, α-blockers improve symptoms and quality of life. Alpha-blockers also seem to ameliorate the symptoms and reduce the risk of acute urinary retention in patients who suffer from either heat- or radiation-induced prostatic inflammation. Alpha-blockers improve lower urinary tract symptoms, including pain, in patients who are diagnosed with both prostatitis and benign prostatic hyperplasia. Evidence has proven there is definitely a role for α-blockers in the management of the prostatitis syndromes.
Key words: Alpha-blockers, Prostatitis syndromes, Lower urinary tract symptoms, Benign prostatic hyperplasia, CP/CPPS
The efficacy of α-blocker therapy for the treatment of lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH) is accepted by the medical community, and this remains the most common medical therapy for BPH. Alpha-blockers have also been used for urologic conditions other than LUTS associated with BPH. These include bladder neck obstruction, neurogenic bladders, interstitial cystitis, female LUTS, and LUTS in men too young to have developed BPH. However, almost no data exist to substantiate the claim of efficacy for the use of α-blockers in these conditions and, for the most part, the evidence cited is anecdotal. The next most common use of α- blockers in urologic conditions other than BPH is for the prostatitis syndromes. All of the clinically recognized prostatitis syndromes are associated with pain (genitourinary pain can be considered a type of LUTS) in the suprapubic area, perineum, pelvis, penis, and testes. In addition, all of the clinically symptomatic prostatitis syndromes are to some extent associated with storage (irritative) symptoms, such as increased frequency, urgency, and nocturia, and voiding (obstructive) symptoms, such as hesitancy, slow stream, intermittency, and terminal dribbling.
Alpha-blocker therapy has been advocated, with various levels of evidence, as a treatment modality for all categories of the prostatitis syndromes. It has been suggested (ie, anecdotal evidence) that α-blockers ameliorate the severe obstructive voiding symptoms associated with Category I acute bacterial prostatitis. Weak-to-moderate evidence has shown that combining α-blocker therapy with antibiotics in Category II chronic bacterial prostatitis reduces the risk of recurrence when compared with antibiotics alone. Moderate evidence exists from uncontrolled series and older randomized, placebo-controlled trials without validated outcome measures that suggest α- blockers would be helpful in Category III chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS). However, recently published randomized, placebo-controlled trials have confirmed that α-blockade results in clinically significant amelioration of LUTS and pain/discomfort, as well as improvement in quality of life of patients suffering from Category III CP/CPPS.
Prostatic inflammation and its associated clinical scenario of irritative and obstructive voiding symptoms, dysuria, and genitourinary discomfort can be induced by heat therapy for BPH and radiotherapy for prostate cancer. Neoadjuvant and adjuvant α-blocker therapies seem to provide benefits in terms of amelioration of iatrogenic prostato-urethritis-related LUTS in men who have undergone transurethral microwave thermal therapy (TUMT) or radiotherapy.
This review will evaluate available data that exist in the literature regarding the role of α-blockers in the treatment of the various prostatitis syndromes.
What Are the Prostatitis Syndromes?
The generally accepted classification of the prostatitis syndromes has become the system advocated by the National Institutes of Health (NIH).1 Category I (acute bacterial prostatitis) is associated with acute bacterial infection of the prostate gland; Category II (chronic bacterial prostatitis) is associated with chronic infection of the prostate gland, characterized by recurrent lower urinary tract infections; Category III (CP/CPPS) is characterized by chronic pain/discomfort of more than 3 months’ duration in the pelvic/perineal area, with negative bacterial cultures according to standard microbiological techniques; and Category IV (asymptomatic inflammatory prostatitis) is diagnosed in patients with prostatic inflammation without symptoms. Other prostatitis-like syndromes exist that are difficult to classify under the NIH classification system. These include iatrogenic prostatic inflammation resulting from heat treatment (eg, TUMT) for BPH and radiation for prostate cancer (external beam radiotherapy and brachytherapy). These treatment-induced syndromes are characterized by obstructive and irritative voiding symptoms and significant risk of acute urinary retention. These conditions are sometimes included under Category III CP/CPPS; however, in this review we will evaluate the evidence for this group separately because TUMT and radiation therapy were not eligible for the clinical trials evaluating α-blockers in CP/CPPS.
How Common Is Prostatitis?
The prevalence of men experiencing prostatitis-like symptoms can range up to 6% to 9%, depending on the definition used in the specific survey.2–4 The incidence of men with a concurrent or previous diagnosis of prostatitis ranges from 11% to 14%.5–7 Prostatitis constitutes 3% to 8% of male outpatient visits to urologists in North America8,9 and up to 12% in Europe.10 The quality of life of a patient diagnosed with a chronic prostatitis syndrome is dismal, significantly worse than that of patients suffering from BPH, as well as that of most patients with prostate cancer.11
How Are the Prostatitis Syndromes Diagnosed?
Acute bacterial prostatitis is diagnosed clinically and confirmed by a positive urine culture. Chronic bacterial prostatitis is suspected in men with recurrent lower urinary tract infection and confirmed culture of uropathogenic bacteria in prostate-specific specimens (expressed prostatic secretion or post-prostatic massage urine). Category III CP/CPPS is a diagnosis of exclusion (no demonstrable infection) in men presenting with chronic pelvic/perineal pain and variable voiding symptoms. North American and international consensus groups have established criteria for the diagnosis and evaluation of men presenting with suspected CP/CPPS.12 Medical history, physical examination, and urinalysis/urine culture are considered mandatory for the evaluation of all patients with CP/CPPS. Recommended evaluations include a lower urinary tract localization test, symptom inventory or index (NIH-Chronic Prostatitis Symptom Index [NIH-CPSI]),13 flow rate, residual urine determination, and urine cytology. Optional evaluations include semen analysis and cultures, urethral swab for culture, pressure flow studies, video urodynamics (including flow electromyography), cystoscopy, transrectal ultrasound, pelvic imaging (pelvic ultrasound), computed tomography scan, magnetic resonance imaging, and prostatic-specific antigen in selected patients.
Category IV (asymptomatic inflammatory prostatitis) is diagnosed by the observation of excessive leukocytosis in specimens of expressed prostatic secretions, post-prostatic massage urine sediment, or semen. Histologic evidence of prostatic inflammation in patients who do not suffer prostatitis-like symptoms is also considered to indicate Category IV prostatitis.
Iatrogenic prostatic inflammation is diagnosed in patients presenting with acute exacerbation of irritative and obstructive voiding symptoms (even acute urinary retention) after treatment of BPH or prostate cancer with thermotherapy or radiation. Urine culture should be sterile, and if examined (which is not mandatory) the prostate may feel swollen and tender. Generally, leukocytosis in the prostate-specific specimens is not determined.
Why Should Alpha-Blocker Therapy Work in the Prostatitis Syndromes?
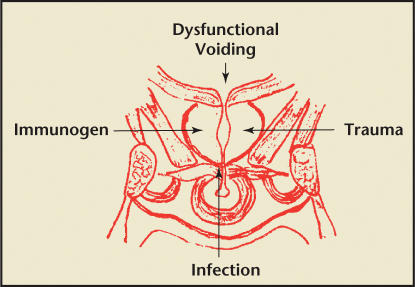
Initiators of the prostatitis syndromes include infection (culturable organisms in Categories I and II and possibly nonculturable or posteradication in Category III) and, in the case of Category III CP/CPPS nonspecific inflammation, a nondefined allergen or immunogen or trauma may be implicated. In Categories I, II, and III prostatitis, dysfunctional high-pressure voiding and possibly related intraprostatic ductal reflux in an anatomically susceptible man (secondary to functional or anatomical obstruction) is believed to be implicated (Figure 1).14
Figure 1.
Initiators of the prostatitis syndrome.
Alpha-receptors are present in the prostate, bladder neck, and central nervous system. Alpha-blockers have been demonstrated to improve bladder outlet obstruction and subsequent LUTS in older men with BPH. Men with all of the symptomatic prostatitis syndromes usually present not only with pain but also with irritative and obstructive voiding symptoms, with significant overlap with BPH-associated LUTS. It is hypothesized that α-blocker therapy may improve the dysfunctional voiding implicated in the pathogenesis of CP/CPPS. Over time, improvement in voiding function and LUTS may lead to less inflammation and/or pressure, eventually resulting in less pain.15 If the initiator is not treated early in the course of the presentation, the condition becomes chronic and then peripheral nervous system sensitization and, subsequently, central nervous system sensitization evolve. Alphareceptors in the central nervous system may be implicated in long-term pain syndromes, and the delayed pain amelioration seen in some patients taking α-blockers may be secondary to central α-blockade mechanisms.14
Are Alpha-Blockers Effective in the Prostatitis Syndromes?
Category I: Acute Bacterial Prostatitis
Treatment of acute bacterial prostatitis is the use of immediate wide-spectrum antibiotics, usually starting with parenteral antibiotics, supportive therapy, and if the patient presents with urinary retention, bladder drainage (either urethral or suprapubic catheter). Alpha-blocker therapy has been suggested for patients who are not in retention but suffer from associated obstructive voiding symptoms.16 There are no data, only unsubstantiated anecdotal experience, to support the use of α-blockers in Category I prostatitis.
Category II: Chronic Bacterial Prostatitis
Treatment of Category II prostatitis is the use of long-term antibiotics (fluo-roquinolones seem to be the most effective class of antimicrobial). It has been suggested that combining α- blockers with antibiotics to ameliorate voiding parameters may not only improve therapy but also reduce the risk of recurrence when compared with antibiotics alone. However, the only data to substantiate this particular claim are from a single, retrospective, uncontrolled study of α-blockers and antibiotics in patients with prostatitis.17 In this study, 64 patients with Category II chronic bacterial prostatitis were treated with antibiotics; 32 of these patients were also treated with α-blockers. The rate of both clinical and bacteriologic recurrence was significantly reduced by combination therapy of antibiotics and α- blockers. There are no phase III clinical studies available to corroborate this observation.
Category III: CP/CPPS
Comprehensive reviews of traditional treatments available for CP/CPPS paint a dismal picture.18–20 Except for the case of α-blockers, none of these traditional therapies have proven to be superior to placebo in large, multicenter, randomized studies. With accumulating data from randomized, placebo-controlled studies, it is apparent that α-blockers have become an important therapeutic tool in the physicians’ armamentarium for the treatment of CP/CPPS.
The use of α-blockers in CP/CPPS had been originally justified by a number of uncontrolled trials that evaluated alfuzosin17 and terazosin,21 and a few small, poorly controlled, randomized, placebo-controlled trials that evaluated phenoxybenzamine,22,23 alfuzosin,24 terazosin,25,26 and tamsulosin.25 All of these studies strongly suggested that α-blockers were effective in improving the symptoms associated with CP/CPPS. Unfortunately, the researchers used different definitions of the prostatitis symptoms, used variable inclusion/exclusion criteria, and did not have an available validated outcome parameter, such as a symptom index. Therefore, these trials are confusing and cannot be compared with one another.
The NIH-CPSI13 was developed and subsequently validated to assess symptoms and responses in patients diagnosed with CP/CPPS. The NIHCPSI can be scored for a total score of 0 to 43, or the 3 major domains of the prostatitis experience can be scored separately. The pain domain (0–21) measures the location, frequency, and severity of pain/discomfort; the voiding domain (0–10) measures obstructive and irritative voiding symptoms; and the impact/quality-of-life domain (0–12) measures the specific impact of prostatitis on the patient’s quality of life. Four contemporary randomized, placebo-controlled trials that used the NIH-CPSI to evaluate α-blockers in Category III CP/CPPS have been published in the peer-reviewed literature. We can now compare the change in the various treatment and placebo groups and, even more importantly, the difference in the change between the treatment and placebo groups in each study (ie, the treatment effect).27 Each trial also calculated a responder analysis; however, because each study defined a responder in a slightly different way, the comparison is more difficult.
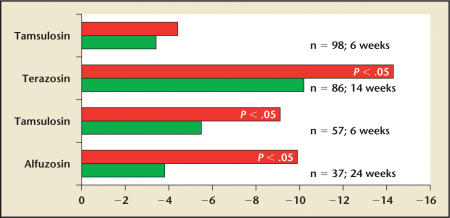
Cheah and colleagues28 randomized 86 patients with chronic prostatitis to either terazosin or placebo for 14 weeks. Compared with baseline, a significant improvement was noted for both the treatment and placebo groups, with a calculated treatment effect of −4.1. Patients taking terazosin had a 50% reduction in the mean symptom score, compared with a 37% reduction in the placebotreated group (P = .001).
Mehik and colleagues29 randomized 40 CP/CPPS patients to 6 months of alfuzosin or placebo treatment. Patients in the alfuzosin group had significant amelioration of symptoms compared with both the placebo and the standard therapy group; this improvement was evident at 4 months and became even more clinically significant by 6 months. At 6 months, the treatment effect was −6.1, with an alfuzosin response rate of 65% compared with a placebo response rate of 42%. At the end of the 6-month active-treatment phase, the improvement in both groups deteriorated; however, deterioration occurred more quickly in placebo patients.
Nickel and colleagues30 randomized 58 men with CP/CPPS to treatment with tamsulosin 0.4 mg or placebo for 6 weeks after a 2-week placebo run-in phase. Patients treated with tamsulosin had a statistically significant treatment effect (−3.6; P = .04), compared with placebo patients. Fifty-two percent of tamsulosin-treated patients were considered responders, compared with 33% of placebo-treated patients. An interesting observation seen in post hoc analyses was that the patients with moderate-to-severe CP/CPPS had a more pronounced treatment effect than patients who presented with mild symptoms.
The NIH-Chronic Prostatitis Collaborative Research Network (NIHCPCRN) conducted a randomized, placebo-controlled, phase III trial that compared placebo, ciprofloxacin, tamsulosin, and ciprofloxacin plus tamsulosin in 196 patients with CP/CPPS.31 In contrast to the other 3 positive α-blocker trials mentioned above, the results from this 6-week study failed to show any improvement in patients treated with tamsulosin (with or without ciprofloxacin) compared with patients treated with placebo. However, compared with the 3 positive α-blocker trials, patients enrolled in this NIH-CPCRN study had chronic, long-term symptoms and were heavily pretreated (including previous treatment with α-blockers).
Analyses of the pooled estimates from these 4 randomized, placebo-controlled trials of α-blockers in CP/CPPS noted evidence of moderate efficacy (pooled relative risk for improvement was 0.57 [95% CI, 0.24–0.91; Q = 10.70; P = .10; I2 = 43.9%]).20 The absolute risk difference was 0.18 (95% CI, 0.005–0.35), translating into a number needed to treat of 6 (95% CI, 2.8–200). However, we cannot really combine the results from these 4 studies. The recruited populations were different in terms of duration of condition and prevalence of pretreatment with α-blockers, and the studies were of various lengths. Figure 2 shows the different changes in NIHCPSI scores observed in each study.
Figure 2.
Results from the 4 randomized, placebo-controlled trials available in the literature comparing α-blocker therapy with placebo in chronic prostatitis/chronic pelvic pain syndrome. The bar graphs describe the delta between the end-of-treatment National Institutes of Health-Chronic Prostatitis Symptom Index score and the baseline score in each group. Data from Alexander RB et al,31 Cheah PY et al,28 Nickel JC et al,30 and Mehik A et al.29
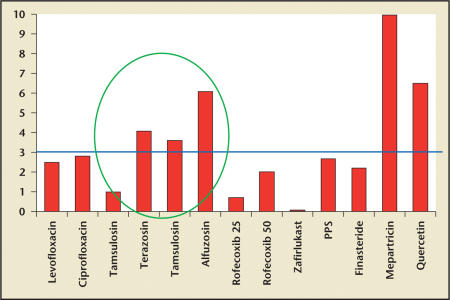
Figure 3 shows the comparative treatment effect of the α-blocker trials compared with other randomized, placebo-controlled trials in CP/CPPS available in the literature.
Figure 3.
The comparative treatment effect (delta between change in the National Institutes of Health-Chronic Prostatitis Symptom Index score in the treatment group compared with the placebo group) seen in the available randomized, placebo-controlled trials available in the literature. Except for the α-blocker studies and 2 small (fewer than 30 patients in the mepartricin and quercetin trials) single-center studies, none of the usual medications that are employed clinically for the prostatitis syndromes demonstrated a significant treatment effect. PPS, pentosan polysulfate. Data from Nickel JC,14 Nickel JC,19 and Dimitrakov JD et al.20
The important lesson learned from examining these 4 very well designed and well-implemented studies separately was that α-blockers provided significant symptom amelioration only after more than 6 weeks of therapy in less heavily treated patients with recent onset of moderate-to-severe symptoms. The NIH-CPCRN is presently conducting an important trial evaluating 12 weeks of alfuzosin in 280 α-blocker-naïve men with a recent diagnosis of CP/CPPS. This definitive study will show the real response in this population of men presenting with CP/CPPS.
Category IV: Asymptomatic Inflammatory Prostatitis
There is no indication at present for the use of α-blockers for Category IV asymptomatic inflammatory prostatitis. However, recent data implicating pathological prostatic inflammation in BPH as a very strong predictor of poor long-term clinical outcome, such as acute urinary retention,32 suggest another role, or mechanism, for 7ga- blockade in BPH.
Does Alpha-Blocker Therapy Benefit Men With Heat- and Radiation-Induced Prostatitis?
TUMT is associated with an increase of irritative and obstructive voiding symptoms and an appreciable risk of acute urinary retention after the procedure. It may take many weeks for symptoms to resolve. Djavan and colleagues33 randomized 81 patients to TUMT alone or TUMT plus tamsulosin (2 weeks before and 12 weeks after therapy). The TUMT group treated with α-blockade had improved velocity of symptom relief in the first 6 to 12 weeks after therapy. Patients treated with α-blocker therapy also experienced less acute urinary retention after the procedure (2%) compared with those treated with TUMT alone (12%).
Radiation for prostate cancer induces prostato-urethritis in many patients. Approximately 50% of patients treated with conformal external beam radiation therapy and 95% of patients treated with interstitial radiation therapy develop nocturia, frequency, hesitancy, and decreased flow-symptoms that impact quality of life. This is usually a self-limited process, and over time patients’ symptoms improve without therapy; however, for many patients these symptoms severely affect quality of life in the posttreatment period. Prosnitz and colleagues34 treated 26 patients with radiation-induced prostato-urethritis with tamsulosin. The investigators noted an improved symptomatic control of their symptoms with α-blockade.
Almost all patients develop storage/voiding symptoms after prostate brachytherapy for prostate cancer. Many patients develop acute urinary retention (an estimated 3%– 22% of patients35), and the risk of acute urinary retention correlates with the severity of the preimplant symptom score. Merrick and colleagues35 initiated α-blocker therapy in patients undergoing transperineal ultrasound guided prostate brachytherapy for prostate cancer. The investigators described a higher rate of successful same-day catheter removal and quicker return of preimplant level voiding symptoms than they had experienced in their anecdotal series before the use of α-blocker therapy. Most patients had the catheter successfully removed on the day of brachytherapy. The International Prostatitis Symptom Score returned to preimplant levels quicker than the investigators had expected from clinical experience. A subsequent prospective but uncontrolled study by the same investigators,36 of 130 patients undergoing brachytherapy, showed that patients receiving prophylactic α-blockers had significantly less urinary morbidity than those patients who either did not receive an α- blocker or received an α-blocker after implantation because of development of urinary symptoms. In a single-institution, double-blind, placebo-controlled trial of prophylactic α-blockade in 126 brachytherapy patients, Elshaikh and colleagues37 demonstrated that although α-blockers did not significantly affect urinary retention rates, they did have a positive effect on urinary morbidity for the first 5 weeks after implantation. These studies would suggest that neoadjuvant and adjuvant α-blockade may benefit patients undergoing brachytherapy, especially those suffering from moderate- to-severe BPH-like symptoms.
Do Alpha-Blockers Ameliorate LUTS and Pain in Men With Prostatitis Associated With BPH?
It is estimated that LUTS suggestive of BPH may affect 50% of men over the age of 50 years. Prostatitis is the third most common urologic diagnosis in men over 50 years of age.9 In older men, the overall prevalence of a prostatitis diagnosis is 11% to 16%.2,7 Prostatitis and BPH both present with LUTS, but prostatitis, particularly CP/CPPS, is differentiated from BPH by symptoms of pain/discomfort localized to the pelvis, perineum, and external genitalia, and/or associated with ejaculation.38 An increase in smooth muscle tone in the bladder neck and prostate, mediated by the sympathetic nervous system (in particular the α-adrenergic receptors), is implicated in the etiology of both BPH and CP/CPPS. Furthermore, recent analyses of the placebo group in the Medical Therapy of Prostatic Symptoms study indicated that patients identified with prostatitis inflammation in their prestudy prostate biopsy specimen had a significantly increased risk of BPH-related complications. In particular, the risk of developing acute urinary retention was significantly higher in the inflammation group compared with the group without inflammation.32 It is estimated that almost 1 in 5 patients with BPH may also suffer from prostatitis.39
Alpha-blockers have generally been accepted as the most effective first-line medical therapy for the treatment of LUTS associated with BPH. As discussed in the previous section, prospective, randomized, placebo-controlled trials have indicated that the α-blockers alfuzosin,29 terazosin, 28 and tamsulosin30 showed efficacy compared with placebo in treating symptoms associated with prostatitis. An analysis of a 6-month open-label study of 4857 men with BPH-related LUTS suggests that alfuzosin 10 mg once daily significantly improves LUTS, quality of life, and sexual function in the 20% of men identified with prostatitis-like symptoms. 40 The initiation of α-blocker therapy in men with both BPH and prostatitis-related LUTS is appropriate.
Summary: Does the Evidence Support a Role for Alpha- Blockers in the Management of the Prostatitis Syndromes?
In Category I, acute bacterial prostatitis, α-blockers possibly ameliorate obstructive and irritative voiding symptoms. In Category II, chronic bacterial prostatitis, α-blockers appear to reduce the risk of clinical and bacteriological recurrence. In Category III, CP/CPPS, α-blockers ameliorate symptoms and improve quality of life. This has now been demonstrated in at least 3 independent, randomized, placebo-controlled trials using similar inclusion/exclusion criteria and validated outcome parameters. Alphablockers also seem to ameliorate the symptoms and reduce the risk of acute urinary retention in patients who suffer from either heat- or radiation-induced prostatic inflammation. Alpha-blockers ameliorate LUTS, including pain, in patients who are diagnosed with both prostatitis and BPH. Table 1 reviews clinical evidence to support the use of α-blockers in these conditions. There is definitely a role for α-blockers in the management of the prostatitis syndromes.
Table 1.
Clinical Utility of Alpha-Blockers in the Various Prostatitis Syndromes
| Clinical Utility of | |||
|---|---|---|---|
| Category | Clinical Definition | Alpha-Blocker Therapy | Evidence |
| National Institutes of | |||
| Health prostatitis categories | |||
| Category I | Acute infection of the prostate | Adjuvant therapy (with antibiotics) | Weak |
| Acute bacterial prostatitis | to improve obstructive urinary symptoms | ||
| Category II | Chronic infection of the prostate | Adjuvant therapy (with antibiotics) to | Weak |
| Chronic bacterial prostatitis | characterized by recurrent lower | reduce recurrence rate | |
| urinary tract infections | |||
| Category III | Genitourinary pain associated | Long-term therapy for amelioration | Strong |
| Chronic prostatitis/chronic | with negative bacterial cultures | of symptoms | |
| pelvic pain syndrome | |||
| Category IV | Inflammation noted in prostate | No clinical utility; however, may | None |
| Asymptomatic inflammatory | specimens in men with no | be useful in the future in patients | |
| prostatatis | specific prostatitis symptoms | with co-occurring BPH | |
| Iatrogenic treatment-induced | |||
| prostatitis | |||
| TUMT induced | Prostatitis-urethritis occurring | Neoadjuvant and adjuvant therapy | Weak |
| after TUMT | to decrease post-treatment LUTS | ||
| and risk of acute urinary retention | |||
| Radiation therapy induced | Prostatitis-urethritis occurring | Neoadjuvant and adjuvant therapy | Weak |
| after external beam radiotherapy | to decrease risk of acute urinary | ||
| or brachytherapy radiation | retention in men with pretreatment LUTS | ||
| Can be used to treat radiation | |||
| cystoprostatourethritis | |||
| Co-occurring prostatitis | |||
| and BPH | |||
| Prostatitis and BPH | Prostatitis-like symptoms (eg, | Ameliorates LUTS associated with | Moderate |
| pain on ejaculation) in patient | BPH and prostatitis, including | ||
| with BPH-related LUTS | pain in some men |
BPH, benign prostatic hyperplasia; TUMT, transurethral microwave thermal therapy; LUTS, lower urinary tract symptoms.
Main Points.
Alpha-blocker therapy for the treatment of lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH) is the most common medical therapy for BPH. Other than for BPH, the most common use of α-blockers in urological conditions is for the treatment of the prostatitis syndromes.
Alpha-blocker therapy has been advocated-with various levels of evidence-as a treatment modality for the 4 categories of the prostatitis syndromes advocated by the National Institutes of Health (NIH): Category I (acute bacterial prostatitis), Category II (chronic bacterial prostatitis), Category III (chronic prostatitis/chronic pelvic pain syndrome [CP/CPPS]), and Category IV (asymptomatic inflammatory prostatitis). Other prostatitis-like syndromes also exist; however, they are difficult to categorize under the NIH classification system.
There is the possibility for α-blockers to ameliorate the prostatitis-like symptoms of Categories I and II, although data to corroborate this claim are either unsubstantiated or limited. However, with accumulating data from randomized, placebo-controlled studies of Category III, it is apparent that α-blockers have become an important therapeutic tool in the treatment of CP/CPPS.
Several studies have also shown that α-blocker therapy seems to improve the symptoms and reduce the risk of acute urinary retention in patients who suffer from either heat- or radiation-induced prostatic inflammation.
Men with all of the symptomatic prostatitis syndromes usually present not only with pain but also irritative and obstructive voiding symptoms, with significant overlap with BPH-related LUTS. An analysis of a 6-month, open-label study of 4857 men with BPH-associated LUTS suggests that 10 mg once daily of the α-blocker alfuzosin significantly improves LUTS, quality of life, and sexual function in the 20% of men identified with prostatitis-like symptoms.
Clinical evidence exists to support the role of α-blockers in the management of the prostatitis syndromes.
References
- 1.Krieger JN, Nyberg L, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282:236–237. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 2.Roberts RO, Jacobson DJ, Girman CJ, et al. Prevalence of prostatitis-like symptoms in a community based cohort of older men. J Urol. 2002;168:2467–2471. doi: 10.1016/S0022-5347(05)64170-5. [DOI] [PubMed] [Google Scholar]
- 3.Tan JK, Png DJ, Lieuw LC, et al. Prevalence of prostatitis-like symptoms in Singapore: a population based study. Sing Med J. 2002;43:189– 193. [PubMed] [Google Scholar]
- 4.Nickel JC, Downey J, Hunter D, Clark J. Prevalance of prostatitis-like symptoms in a population based study employing the NIH-chronic prostatitis symptom index (NIH-CPSI) J Urol. 2001;165:842–845. [PubMed] [Google Scholar]
- 5.Mehik A, Hellstrom P, Lukkarinen O, et al. Epidemiology of prostatitis in Finnish men: a population-based cross-sectional study. BJU Int. 2000;86:443–448. doi: 10.1046/j.1464-410x.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- 6.McNaughton-Collins M, Meigs JB, Barry MJ, et al. Prevalence and correlates of prostatitis in the Health Professionals Follow-up Study Cohort. J Urology. 2002;167:1363–1366. [PubMed] [Google Scholar]
- 7.Roberts RO, Lieber MM, Rhodes T, et al. Prevalence of a physician-assigned diagnosis of prostatitis: the Olmsted County Study of Urinary Symptoms and Health Status Among Men. Urology. 1998;51:578–584. doi: 10.1016/s0090-4295(98)00034-x. [DOI] [PubMed] [Google Scholar]
- 8.Nickel JC, Teichman JMH, Gregoire M, et al. Prevalence, diagnosis, characterization, and treatment of prostatitis, interstitial cystitis, and epidymitis in outpatient urological practice: The Canadian PIE study. Urology. 2005;66:935– 940. doi: 10.1016/j.urology.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 9.McNaughton-Collins M, Stafford RS, O’Leary MP, Barry MJ. How common is prostatitis? A national survey of physician visits. J Urol. 1998;159:1224– 1228. [PubMed] [Google Scholar]
- 10.Rizzo M, Marchetti F, Travaglini F, et al. Prevalence, diagnosis and treatment of prostatitis in Italy: a prospective urology outpatient practice study. BJU Int. 2004;23:61–66. doi: 10.1111/j.1464-410x.2003.04520.x. [DOI] [PubMed] [Google Scholar]
- 11.McNaughton-Collins M, Pontari MA, O’Leary MP, et al. Quality of life is impaired in men with chronic prostatitis: the chronic prostatitis collaborative research network. J Gen Intern Med. 2001;16:656–662. doi: 10.1111/j.1525-1497.2001.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nickel JC. Clinical evaluation of the man with chronic prostatitis/chronic pelvic pain syndrome. Urology. 2003;60(suppl 6A):20–23. doi: 10.1016/s0090-4295(02)02298-7. [DOI] [PubMed] [Google Scholar]
- 13.Litwin MS, McNaughton Collins M, Fowler FJ, et al. The National Institutes of Health Chronic Prostatitis Symptom Index: development and validation of a new outcome measure. J Urol. 1999;162:369–375. doi: 10.1016/s0022-5347(05)68562-x. [DOI] [PubMed] [Google Scholar]
- 14.Nickel JC. Urology Update Series. Baltimore, MD: AUA Press; Prostatitis: state of the art. In press. [Google Scholar]
- 15.Barbalias GA. Why alpha-blockers in prostatitis? Eur Urol Suppl. 2003;2:27–29. [Google Scholar]
- 16.Neal DE. Treatment of acute prostatitis. In: Nickel JC, editor. Prostatitis. Oxford: Isis Medical Media Ltd; 1999. pp. 279–284. [Google Scholar]
- 17.Barbalias GA, Nikiforidis G, Liatsikos EN. Alphablockers for the treatment of chronic prostatitis in combination with antibiotics. J Urol. 1998;159:883–887. [PubMed] [Google Scholar]
- 18.McNaughton Collins M, MacDonald R, Wilt TJ. Diagnosis and treatment of chronic abacterial prostatitis: a systematic review. Ann Intern Med. 2000;133:367–381. doi: 10.7326/0003-4819-133-5-200009050-00013. [DOI] [PubMed] [Google Scholar]
- 19.Nickel JC. The three A’s of chronic prostatitis therapy: antibiotics, alpha-blockers, and antiinflammatories. What is the evidence? BJU Int. 2004;94:1230–1233. doi: 10.1111/j.1464-410X.2004.05148.x. [DOI] [PubMed] [Google Scholar]
- 20.Dimitrakov JD, Kaplan SA, Kroenke K, et al. Management of chronic prostatitis/chronic pelvic pain syndrome: an evidence-based approach. Urology. 2006;67:881–888. doi: 10.1016/j.urology.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neal DE, Moon TD. Use of terazosin in prostatodynia and validation of a symptom score questionnaire. Urology. 1994;43:460–465. doi: 10.1016/0090-4295(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 22.Osborn DE, George NJ, Rao PN, et al. Prostatodynia- psychological characteristics and rational management with muscle relaxants. Br J Urol. 1981;53:621–623. doi: 10.1111/j.1464-410x.1981.tb03275.x. [DOI] [PubMed] [Google Scholar]
- 23.Dunzendorfer U, Kruschwitz K, Letzel H. Effects of phenoxybenzamine on clinical picture, laboratory test results and spermatogram in chronic abacterial prostatitis. Therapiewoche. 1983;33:4694–4705. [Google Scholar]
- 24.de la Rosette JJ, Karthaus HF, van Kerrebroeck PE, et al. Research in ‘prostatitis syndromes’: the use of alfuzosin (a new alpha-1 blocking agent) in patients presenting with micturition complaints of an irritative nature and confirmed urodynamic abnormalities. Eur Urol. 1992;22:222–227. doi: 10.1159/000474760. [DOI] [PubMed] [Google Scholar]
- 25.Lacquaniti S, Destito A, Servello C, et al. Terazosine and tamsulosin in non bacterial prostatitis: a randomized placebo-controlled study. Arch Ital Urol Androl. 1999;71:283–285. [PubMed] [Google Scholar]
- 26.Gül O, Eroğlu M, Özok U. Use of terazosine in patients with chronic pelvic pain syndrome and evaluation by prostatitis symptom score index. Int Urol Nephrol. 2001;32:433–436. doi: 10.1023/a:1017504830834. [DOI] [PubMed] [Google Scholar]
- 27.Propert KJ, Alexander RB, Nickel JC, et al. Design of a multicenter randomized clinical trial for chronic prostatitis/chronic pelvic pain syndrome. Urology. 2002;59:870–876. doi: 10.1016/s0090-4295(02)01601-1. [DOI] [PubMed] [Google Scholar]
- 28.Cheah PY, Liong ML, Yuen KH, et al. Terazosin therapy for chronic prostatitis/chronic pelvic pain syndrome: a randomized, placebo controlled trial. J Urol. 2003;169:592–596. doi: 10.1097/01.ju.0000042927.45683.6c. [DOI] [PubMed] [Google Scholar]
- 29.Mehik A, Alas P, Nickel JC, et al. Alfuzosin treatment for chronic prostatitis/chronic pelvic pain syndrome: a prospective, randomized, double-blind, placebo-controlled, pilot study. Urology. 2003;62:425–429. doi: 10.1016/s0090-4295(03)00466-7. [DOI] [PubMed] [Google Scholar]
- 30.Nickel JC, Narayan P, MacKay J, et al. Treatment of chronic prostatitis/chronic pelvic pain syndrome with tamsulosin: a randomized double blind trial. J Urol. 2004;171:1594–1597. doi: 10.1097/01.ju.0000117811.40279.19. [DOI] [PubMed] [Google Scholar]
- 31.Alexander RB, Propert KJ, Schaeffer AJ, et al. Chronic Prostatitis Collaborative Research Network. Ciprofloxacin or tamsulosin in men with chronic prostatitis/chronic pelvic pain syndrome: a randomized, double-blind trial. Ann Intern Med. 2004;141:581–589. doi: 10.7326/0003-4819-141-8-200410190-00005. [DOI] [PubMed] [Google Scholar]
- 32.Roehrborn CG, Kaplan SA, Noble WD, et al. The impact of acute or chronic inflammation in baseline biopsy on the risk of clinical progression of BPH: results from the MTOPS study [abstract 1277] J Urol. 2005;173 [Google Scholar]
- 33.Djavan B, Shariat S, Fakhari M, et al. Neoadjuvant and adjuvant alpha-blockade improves early results of high-energy transurethral microwave thermotherapy for lower urinary tract symptoms of benign prostatic hyperplasia: a randomized, prospective clinical trial. Urology. 1999;53:251–259. doi: 10.1016/s0090-4295(98)00538-x. [DOI] [PubMed] [Google Scholar]
- 34.Prosnitz RG, Schneider L, Monola J, et al. Tamsulosin palliates radiation-induced urethritis in patients with prostate cancer: results of a pilot study. Int J Radiat Oncol Biol Phys. 1999;45:563–566. doi: 10.1016/s0360-3016(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 35.Merrick GS, Butler WM, Lief JH, et al. Temporal resolution of urinary morbidity following prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2000;47:121–128. doi: 10.1016/s0360-3016(99)00525-8. [DOI] [PubMed] [Google Scholar]
- 36.Merrick GS, Butler WM, Wallner KE, et al. Brachytherapy-related dysuria. BJU Int. 2005;4:597–602. doi: 10.1111/j.1464-410X.2005.05346.x. [DOI] [PubMed] [Google Scholar]
- 37.Elshaikh MA, Ulchaker JC, Reddy CA, et al. Prophylactic tamsulosin (Flomax) in patients undergoing prostate I125 brachytherapy for prostate carcinoma: final report of a double-blind, placebo-controlled randomized study. Int J Radiat Oncol Biol Phys. 2005;62:164–169. doi: 10.1016/j.ijrobp.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 38.McNaughton-Collins M, Stafford RS, O’Leary MP, Barry MJ. Distinguishing chronic prostatitis and benign prostatic hyperplasia symptoms: the results of a national survey of physician visits. Urology. 1999;53:921–925. doi: 10.1016/s0090-4295(98)00636-0. [DOI] [PubMed] [Google Scholar]
- 39.Nickel JC, Elhilali M, Vallancien G. Benign prostatic hyperplasia (BPH) and prostatitis: prevalence of painful ejaculation in men with clinical BPH. BJU Int. 2005;95:571–574. doi: 10.1111/j.1464-410X.2005.05341.x. for the ALFONE Study Group. [DOI] [PubMed] [Google Scholar]
- 40.Nickel JC, Elhilali M, Emberton M, Vallancien G. Alfuzosin 10 mg OD shows a beneficial effect on LUTS, quality of life and sexual dysfunction in men with LUTS and painful ejaculation in real life practice. BJU Int. 2006;97:1242–1246. doi: 10.1111/j.1464-410X.2006.06171.x. for the ALF-ONE Study Group. [DOI] [PubMed] [Google Scholar]