Abstract
Although erectile dysfunction has recently become the most well-known aspect of male sexual dysfunction, the most prevalent male sexual disorders are ejaculatory dysfunctions. Ejaculatory disorders are divided into 4 categories: premature ejaculation (PE), delayed ejaculation, retrograde ejaculation, and anejaculation/anorgasmia. Pharmacologic treatment for certain ejaculatory disorders exists, for example the off-label use of selective serotonin reuptake inhibitors for PE. Unfortunately, the other ejaculatory disorders are less studied and not as well understood. This review revisits the physiology of the normal ejaculatory response, specifically explores the mechanisms of anejaculation, and presents emerging data. The neurophysiology of the ejaculatory reflex is complex, making classification of the role of individual neurotransmitters extremely difficult. However, recent research has elucidated more about the role of serotonin and dopamine at the central level in the physiology of both arousal and orgasm. Other recent studies that look at differing pharmacokinetic profiles and binding affinities of the α1-antagonists serve as an indication of the centrally mediated role of ejaculation and orgasm. As our understanding of the interaction between central and peripheral modulations and regulation of the process of ejaculation increases, the probability of developing centrally acting pharmaceutical agents for the treatment of sexual dysfunction approaches reality.
Key words: Retrograde ejaculation, Anejaculation, Ejaculatory disorders, Tamsulosin, Alfuzosin
For centuries, the field of male sexual dysfunction lacked evidence-based, scientific foundations, making both clinical diagnosis and management somewhat arbitrary. With the advent of effective pharmaceutical treatments (ie, phosphodiesterase type 5 [PDE-5] inhibitors) for the treatment of erectile dysfunction (ED), public awareness of the prevalence and therapies of sexual dysfunction has expanded. The notion that diminishing sexual function is exclusively a problem in the aging population is now being challenged. In a landmark 1999 study, Laumann and colleagues analyzed a cohort of young adult men aged 18 to 59 years and observed that 31% of this cohort suffered from some form of sexual dysfunction.1
Male sexual dysfunction can be divided into 3 main categories: hypogonadism, ED, and ejaculatory disorders. The latter can be further subdivided into premature ejaculation (PE), delayed ejaculation (DE), retrograde ejaculation (RE), and anejaculation (AE)/anorgasmia.
Male hypogonadism refers to a decrease in 1 of the 2 primary functions of the testes: production of sperm and testosterone. Hypogonadism can result from intrinsic disease of the testes (primary) or a dysfunction of the pituitary or hypothalamus (secondary). Testosterone deficiency can have multiple presentations depending on the age of onset.
ED is defined as the inability to attain or maintain an erection of sufficient rigidity for satisfactory sexual intercourse. With the success of the PDE-5 inhibitors (sildenafil, vardenafil, and tadalafil), ED has become the most publicly recognized male sexual dysfunction. Despite ED? notoriety, the most prevalent male sexual dysfunction is the ejaculatory dysfunction PE.2
The ejaculatory disorders fall into 1 of 4 major categories. Most experts agree that PE is defined as ejaculation occurring from a lack of ejaculatory control that interferes with sexual or emotional well-being in 1 or both partners. DE is characterized by a man’s inability to ejaculate in a reasonable period under normal sexual stimulation. RE occurs when the semen intended for propulsion out the urethral meatus is directed backwards into the urinary bladder. This is most commonly due to intrinsic problems with the internal sphincter of the bladder. AE and anorgasmia are often mistakenly used interchangeably. AE specifically refers to the lack of ejaculation that may or may not be coupled with an orgasm. Anorgasmia is simply the lack of orgasm that is not necessarily a coupled response with ejaculation. Orgasm can be perceived as physical (most commonly occurring at the time of ejaculation), psychological, or emotional, or a combination of these.
Pharmacologic treatments for PE exist, and off-label therapy with selective serotonin reuptake inhibitors (SSRIs) is currently the most favored clinical approach. SSRIs exhibit the well-established side effect of delaying ejaculation and, at higher doses, causing AE and anorgasmia. Further research on the development of US Food and Drug Administration-approved medications for the primary intent of controlling PE is ongoing. The other ejaculatory disorders—DE, RE, and AE/anorgasmia—are less studied and less well understood. The etiologies of these ejaculatory dysfunctions are numerous and multifactorial: psychogenic, congenital, anatomic, neurogenic, infectious, endocrinological, and iatrogenic factors secondary to medications may all play a role.
In a 2003 study, The Multinational Survey of the Aging Male (MSAM-7), Rosen and colleagues investigated the relationship between lower urinary tract symptoms (LUTS)/benign prostatic hyperplasia (BPH) and sexual problems in aging men (between the ages of 50 and 80 years).3 Specifically, 46% of the population able to achieve erections reported reduced ejaculation, and another 5% of these men noted AE.4,5 The prevalence of ejaculatory dysfunction showed a direct correlation to both increasing age and severity of BPH/LUTS. Interestingly, when asked, most of these men were equally bothered by their symptoms of ED and ejaculatory disorders, regardless of age.3
BPH/LUTS is a common condition in men aged 50 to 80 years and numerous reports have suggested a link between BPH/LUTS and sexual dysfunction. The recommended first-line therapy of BPH symptomatology is α1-blocker medications. The longstanding precept holds that α1-blockers exert their benefit by mediating smooth muscle relaxation in the prostate, urethra, and bladder neck.6 One agent in particular, tamsulosin, exhibits a much higher rate of RE and AE.7 The mechanism for this ejaculatory dysfunction has been proposed by many to be a selective α-receptor blockade and subsequent relaxation of the bladder neck region.
Although clinical advances have been made in the diagnosis and treatment of hypogonadism and ED, basic concepts on the physiology of ejaculation and treatment options for ejaculatory disorders have lagged. This article aims to review the physiology of the normal ejaculatory response; explore our current understanding of the mechanism of AE, especially in light of animal experimentation; and present emerging clinical trial data that bridge the gap in our knowledge about this important clinical subject.
Physiology of Ejaculation
The 2 major phases of normal ejaculation are emission and expulsion. These 2 processes are mediated by afferent, efferent, somatic, sympathetic, and parasympathetic fibers. Emission, the first phase of ejaculation, consists of a peristaltic contraction of the smooth muscles of the seminal tract until the ejaculate reaches the prostate, where it is deposited into the posterior urethra. Expulsion, the latter phase, occurs when the semen is rapidly and forcefully advanced forward through the urethra and out the penile meatus. Adequate propulsion of semen requires synchronized relaxation of the external urinary sphincter with concomitant bladder neck closure and rhythmic contractions of the striated muscles of the pelvic floor and the bulbospongiosus muscles.7 The process of ejaculation can be triggered in a number of ways, including tactile stimulation of the glans penis, as well as influences from various cortical stimuli (Figure 1).8
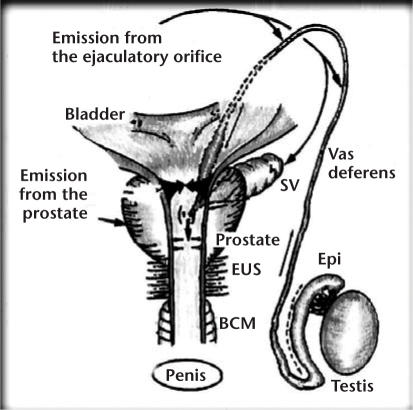
Figure 1.
The emission phase of ejaculation consists of the peristaltic contractions of the smooth muscles of the vas deferens from the base of the epididymis until its deposition into the posterior urethra through the ejaculatory ducts in the prostate. Once emission is complete, the ejaculate is ready for the forceful expulsion out the penile urethra. SV, seminal vesicle; EUS, external urethral sphincter; BCM, bulbocavernosus muscle. Reprinted with permission from Mann T and Lutwak-Mann C.32
Peripheral and central signals, as well as both sympathetic and parasympathetic signals, are integrated into the ejaculation center of the spinal cord through input from the thoracolumbar sympathetic, sacral parasympathetic, and somatic spinal pathways. This integration allows for a normal ejaculatory reflex, as coordinated signals are sequentially relayed to the muscles and structures of the pelvis and perineum and enable them to function in an orchestrated fashion (Figure 2).5
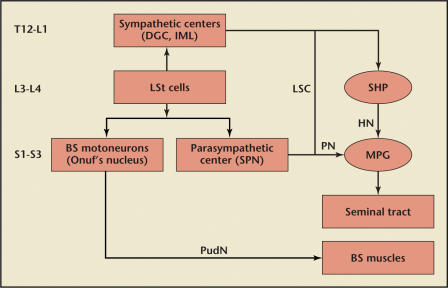
Figure 2.
Neural signal integration in the control of ejaculation in rats. Parasympathetic fibers project from the spinal ejaculation generator (LSt) to the sacral parasympathetic nucleus (SPN). Sympathetic fibers are projecting from the LSt to the dorsal gray commissure (DGC) and the intermediolateral cell column (IML). Parasympathetics are carried to the seminal tract by the pelvic nerve (PN) and the major pelvic ganglion (MPG). Sympathetics travel to the seminal tract by projections through the lumbar sympathetic chain (LSC) to the superior hypogastric plexus (SHP) and then on to MPG. The bulbospongiosus (BS) motor neurons responsible for expulsion will travel to the BS muscle through the motor branch of the pudendal nerve (PudN). HN, hypogastric nerve. Reproduced with permission from Giuliano F and Clement P.5
Control mechanisms that inhibit ejaculation originate at the supraspinal level in specialized brain structures known as the posteromedial bed nucleus of the stria terminalis, the posterodorsal medial amygdaloid nucleus, the posterodorsal preoptic nucleus, and the parvicellular part of the subparafascicular thalamus.9 Apparently, this cerebral network modulates and controls the final common output from all ejaculatory stimuli (Figure 3).
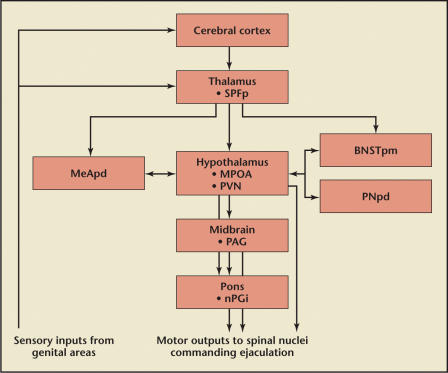
Figure 3.
Locations and pathways of the inhibitory central pathways of ejaculation. Gray structures are mediated by serotonin auto/heteroreceptors. SPFp, parvicellular part of the subparafascicular thalamus; MeAPD, posterodorsal medial amygdaloid nucleus; MPOA, medical preoptic area; PVN, paraventricular thalamic nucleus; BNSTpm, posteromedial bed nucleus of stria terminalis; PNpd, posterodorsal preoptic nucleus; PAG, periaqueductal grey; nPGi, paragigantocellular nucleus. Reproduced with permission from Giuliano F and Clement P.5
Multiple neurotransmitter systems at both the spinal and supraspinal regions have been implicated in regulation of the ejaculatory reflex. The most significant of these seem to be the central serotonergic and dopaminergic neurons.10 Acetylcholine, adrenaline, neuropeptides, oxytocin, γ-aminobutyric acid (GABA), and nitric oxide have all been shown to play a secondary role.11 The multifactorial and complex nature of the ejaculatory reflex, however, makes definition of the precise role of each individual neurotransmitter extremely difficult.
Dopamine levels in the medial preoptic area of the hypothalamus progressively increase during excitation and intercourse; thus, dopamine signaling has been implicated in the physiology of arousal and orgasm.10 GABA-receptor antagonists have demonstrated an inhibitory effect on sexual behavior in animal models, and muscular contractions during ejaculation seem to be mediated by oxytocin.11
The most studied neurotransmitter in the neurophysiology of ejaculation is 5-hydroxytryptamine (5-HT [serotonin]).11,12 To date, 14 different 5-HT receptor subtypes have been identified, each having a different neuroanatomical location and function.13 The 5-HT neurons comprise somatodendritic autoreceptors (5-HT1a), presynaptic autoreceptors (5-HT1b, 5-HT1d), signaling receptors (5-HT2c), and reuptake transporters. Each of these receptors mediates different aspects of cellular activation and signaling of the 5-HT system.14
The SSRIs inhibit the presynaptic reuptake of 5-HT in the central nervous system (CNS). What differentiates them from their predecessors, the tricyclic antidepressants, is that they have minimal or no effect on the reuptake of norepinephrine and dopamine. Further, the SSRIs do not significantly bind to α-adrenergic, histamine, or cholinergic receptors.
The 5-HT1a somatodendritic autoreceptors, which are present in the mesencephalic and medullary raphe nuclei, are responsible for decreasing 5-HT release into the synapse through a negative feedback mechanism and reducing ejaculatory latency.5,15 The 5-HT1b and 5-HT2c receptors, in contrast to the presynaptic 5-HT1a autoreceptors, are present in the postsynaptic membrane, and both have been shown to prolong ejaculatory latency. The relationship between CNS 5-HT1b receptors and their potential inhibitory effect on ejaculation has yet to be fully clarified. Given the relationship between the serotonergic receptors and their inhibitory and excitatory effects, it is likely that the pathophysiologic mechanism behind ejaculatory disorders is altered levels of 5-HT or altered 5-HT receptor sensitivity in the ejaculatory modulating centers of the CNS.15 Although this hypothesis requires further investigation, the imbalance of 5-HT synaptic concentration and varying receptor sensitivities may provide the pathophysiologic basis for many of the observed ejaculatory disorders (Figure 4).
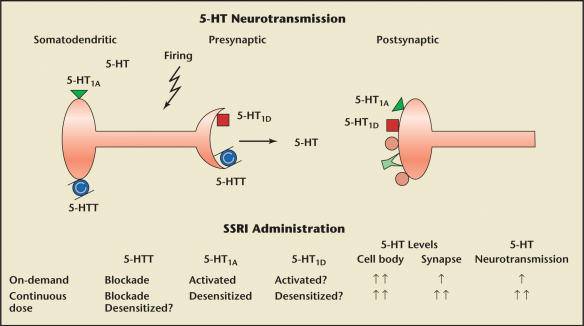
Figure 4.
Varying serotonin (5-hydroxytryptamine, 5-HT) receptor sensitivity and comparison of on-demand and continuously dosed selective serotonin reuptake inhibitors (SSRIs). Adapted with permission from Giuliano F and Clement P.5
Animal Models of Ejaculation
The most commonly studied animal model of ejaculation is the male rat. There have been many behavioral studies that have documented the effects of SSRIs on rat ejaculatory function. More specifically, recent studies have examined the differences between chronic and acute administration of these drugs.
The research consensus is that chronic SSRI usage inhibits copulatory behaviors without affecting sexual motivation. Specifically, ejaculatory latency and postejaculatory interval are both increased in animals receiving daily SSRI treatment.16 Despite relative consensus on chronic exposure models, acute treatment models show a number of conflicting results. Whereas the acute systemic delivery of medication (fluoxetine, paroxetine) has demonstrated increased ejaculatory latency in sexually experienced rats approaching sexual exhaustion, this was not observed in naïve rats or in sexually mature rats in their first ejaculatory series.17
The delay in ejaculatory function with chronic SSRI administration has also been well delineated in the rat.14,15,18 Recent clinical trials with on-demand dosing of a short-acting SSRI, dapoxetine, have shown tremendous promise.4,19 Giuliano and Clement recently set out to determine whether synaptic 5-HT levels were higher with acute versus chronic administration of an SSRI and, if so, whether this difference would result in a clinically relevant delay of ejaculation. These researchers’ rat experiments showed that inhibition of ejaculatory behavior by SSRIs was actually more pronounced when administered chronically versus acutely. They further believe that these results are likely secondary to long-term adaptive changes in neuromodulatory systems other than the serotonergic system.5
As previously discussed in the Physiology of Ejaculation section, activation of the presynaptic 5-HT1a receptors decreases the synaptic 5-HT concentration, which in turn decreases ejaculatory latency time. Therefore, antagonism and agonism of the 5-HT1a receptor can directly influence ejaculatory function. Multiple studies have documented a central mediation of 5-HT1a receptors by the agonist 8-hydroxy-2-di-npropylamino-tetralin (8-OH-DPAT). This prototypical 5-HT1a agonist induces rapid/PE in the rat model.20–22
Clement and colleagues, who created PE-like conditions in an animal model, have established that 8-OHDPAT has a dose-dependent, proejaculatory effect on the bulbospongiosus muscle, which increases contractility and expulsion of semen. In their experiments, the rat cerebral ventricles were cannulated stereotactically for central drug dosing. Administration of the 5-HT1a antagonist WAY100635 by this route failed to fully antagonize the action of 8-OH-DPAT. Of interest, intercerebral administration of the dopamine D2-receptor antagonists (raclopride and spiperone) significantly abolished contractions of the bulbospongiosus muscles and thereby prolonged ejaculatory latency. The D2-like receptor agonist quinelorane was then shown to induce rhythmic contractions of the bulbospongiosus muscles. These findings are a strong indication that the D2-like receptors in the CNS play an integral role in the ejaculatory response. The investigators concluded that the D2-like receptors are equally important to the 5-HT1a receptor in mediating ejaculation, if not more important.23 Hence, future pharmacologic manipulations of the ejaculatory reflex will need to focus more on the D2-like receptors.
In a 2003 study, Andersson and Wyllie examined the binding affinity of the α1-blockers for the 5-HT1a receptor. The investigators documented that whereas alfuzosin and doxazosin exhibit low binding affinity for the receptor, tamsulosin exhibits nearly equivalent binding affinity for the 5- HT1a receptor as for its intended target, the α1-receptor.24
These seminal animal experiments suggest that the ejaculatory reflex is mediated by central mechanisms, which allows us in turn to connect the CNS effects of the specific α1-blocker in question. Tamsulosin, for example, has nearly 10,000 times more binding affinity for the 5-HT1a and D2-like receptors than the other α1-blockers. Giuliano and colleagues used this fact to investigate the differences in bulbospongiosus muscle contractions after central administration of either tamsulosin or alfuzosin. Their research indicates that there is a significant decrease in the 8-OH-DPAT-induced contractions of the bulbospongiosus muscle with the α1-blocker tamsulosin.25
Additional experiments have revealed a differential blood-brain barrier passage between the different α1-blockers. Alfuzosin does not easily pass through the blood-brain barrier, thus accounting for less CNS exposure of this agent compared with tamsulosin.26
Human Pilot Studies
As previously noted, the MSAM-7 reflected a direct relationship between BPH/LUTS and ejaculatory disorders. A recent observational study by Nickel and colleagues examined a subset of the population with LUTS and painful ejaculation. This study revealed that 20% of men with LUTS complain of pain/discomfort with ejaculation. The analysis demonstrated that men who completed 6 months of therapy with alfuzosin had a significant decrease in painful ejaculation.27 Similarly, Van Moorselaar and colleagues examined the beneficial effects of long-term α1-blockers in men with BPH/LUTS. Specifically, their research showed that alfuzosin significantly improved the International Prostate Symptom Score and bother score from baseline. The perception of these improvements was increased in the men with more severe LUTS and more severe bother score at baseline.28 Furthermore, Van Moorselaar and colleagues evidence a positive correlation between IPSS and its appended bother score with the sexuality questions on the Danish Prostatic Symptom Score.28 These publications that focus on the benefits of α1-blockers in men with BPH/LUTS with regard to sexual and ejaculatory functions attest to the clinical importance and contemporary relevance of these agents.
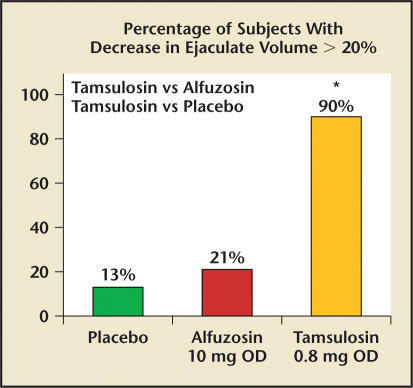
Patients who have undergone transurethral resection of the prostate (TURP) experience the well-documented side effect of RE secondary to decreased resistance of the prostatic urethra and bladder neck. Therefore, with relaxation of the bladder neck region by certain α1-blockers, it has been reasoned that RE is the mechanism responsible for reduced or absent external expulsion of semen. Recent studies with varying doses of tamsulosin have revealed that there is a significant dose-dependent decrease in ejaculate volume and diminished sensation of orgasm.29,30 Specifically, there was a 35% incidence of total AE with tamsulosin versus none with placebo or alfuzosin. Perhaps more impressive is the percentage of study subjects with a decrease in ejaculate volume of greater than 20%. Of the 48 men who completed the study by Hellstrom and Sikka,29 90% of the tamsulosin (0.8 mg) subjects had reduced ejaculate volume, compared with 21% taking alfuzosin (10 mg) and 13% taking placebo (Figure 5).29
Figure 5.
Graph showing decreased ejaculate volume in a head-to-head comparison of tamsulosin and alfuzosin. *Tamsulosin vs alfuzosin, P < .001; tamsulosin vs placebo, P < .001. OD, once daily. Data from Hellstrom WJ and Sikka SC.29
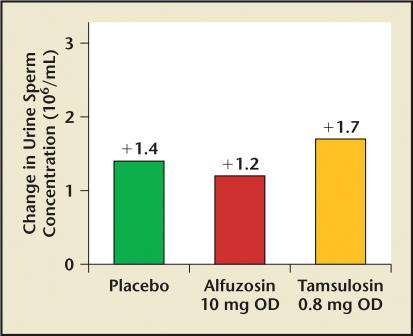
Examination of postejaculate urine serves as a reliable indicator of RE, as the semen pushed back into the bladder will be evident in the subsequent void. In the study by Hellstrom and Sikka29 and the study by Hisasue,30 sperm concentration in postejaculate urine showed no significant change (Figure 6).
Figure 6.
Graph showing postejaculation-postvoid sperm concentration from a head-to-head comparison of tamsulosin and alfuzosin. Samples were taken at first void after ejaculation. P = nonsignificant. OD, once daily. Data from Hellstrom and Sikka.29
These facts suggest that the mechanism of ejaculatory disorders observed with tamsulosin is more likely AE caused by a central etiology as opposed to a peripheral retrograde ejaculatory effect. Although central mediation involving the serotonergic and dopaminergic systems seems a likely possibility, other possibilities include differential effects of varied α1-blockers on the seminal vesicles and/or vas deferens, resulting in variable contractilities.29
Another finding of note in these pilot studies was related to the sense of diminished sexual pleasure experienced by men while taking tamsulosin. 29 Although men who have received TURP develop postoperative RE, the majority maintain orgasm and sense of pleasure. The implication again is that the control components of the sexual experience (orgasm, pleasure, and possibly arousal) may have central sources that allow for future potential pharmacologic interventions.
Conclusions
Male sexual dysfunction is a growing domain of medical science. Besides hypogonadism and ED, new inroads are being made in our understanding of ejaculatory disorders. Disorders of ejaculation, which encompass PE, DE, RE, and AE/anorgasmia, remain underdiagnosed and undertreated, largely because of a longstanding deficiency in our understanding and treatment.
Of the ejaculatory disorders, PE is the most prevalent and has been successfully treated off label with SSRI medications. Typification of the serotonergic role in the rat model has spawned recent human investigations yielding varied results on the efficacy of chronic/daily use of SSRIs and on-demand dosing of a newer, short-acting SSRI in the treatment of PE.
Current research suggests that AE/anorgasmia may some day be a pharmacologically controlled entity as well. Of note is the work of Clement and colleagues, which has provided evidence that both 5-HT1a and dopamine D2-like receptors play an integral role in the expulsion phase of ejaculation.23
BPH is a prevalent condition in aging men. Estimates indicate a nearly 50% prevalence in the age range of 51 to 60 years, increasing to 70% in the age range of 61 to 70 years.31 In recent years, therapy for BPH has shifted toward more long-term medical management. The mainstay of pharmacotherapy for BPH relies on the use of the α1-adrenergic blockers. RE has long been assumed to be a common side effect of specific α1-blocker therapy (ie, tamsulosin); however, recent investigations have shown that what was previously thought to be a peripheral effect causing RE is actually a centrally mediated process. This marks a paradigmatic shift in our understanding of the pathophysiology of the ejaculatory disorders.
Summary
Review of the data presented in this communication and from studies on animal models emphasizes the importance of central versus peripheral modulation and regulation of the process of ejaculation. Increased mechanistic understanding will, in turn, allow us to develop centrally acting pharmaceutical agents for the treatment of sexual dysfunction, including the ejaculatory dysfunctions PE, RE, and AE/anorgasmia.
Main Points.
Male sexual dysfunction can be divided into 3 main categories: hypogonadism, erectile dysfunction, and ejaculatory disorders. The latter can be further subdivided into premature ejaculation (PE), delayed ejaculation (DE), retrograde ejaculation (RE), and anejaculation (AE)/anorgasmia.
The most prevalent male sexual dysfunction is PE. Off-label therapy with selective serotonin reuptake inhibitors, which exhibit the well-established side effect of delaying ejaculation, is currently the most favored clinical treatment of this disorder. However, the other ejaculatory disorders, DE, RE, and AE/anorgasmia, are less studied and less well understood.
Given the relationship between the serotonergic receptors and their inhibitory and excitatory effects, it is likely that the pathophysiologic mechanism behind ejaculatory disorders is altered levels of serotonin (5-HT) or altered 5-HT receptor sensitivity in the ejaculatory modulating centers of the central nervous system (CNS).
Results of an animal study indicated that D2-like receptors in the CNS play an integral role in the ejaculatory response. The investigators concluded that D2-like receptors are equally important to the 5-HT1a receptor in modulating ejaculation, perhaps even more important.
Several seminal animal studies suggest that the ejaculatory reflex is mediated by central mechanisms, allowing investigators to connect the CNS effects of specific α1-blockers.
The mainstay of pharmacotherapy for benign prostatic hyperplasia has been the use of the α1-adrenergic blockers. Although RE has long been assumed to be a common side effect of specific α1-blocker therapy (ie, tamsulosin), recent investigations have shown that what was previously thought to be a peripheral effect causing RE is actually a centrally mediated process. This marks a paradigmatic shift in our understanding of the pathophysiology of the ejaculatory disorders.
References
- 1.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537–544. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 2.Rosen RC. Prevalence and risk factors of sexual dysfunction in men and women. Curr Psychiatry Rep. 2000;2:189–195. doi: 10.1007/s11920-996-0006-2. [DOI] [PubMed] [Google Scholar]
- 3.Rosen R, Altwein J, Boyle P, et al. Lower urinary tract symptoms and male sexual dysfunction: The Multinational Survey of the Aging Male (MSAM-7) Eur Urol. 2003;44:637–649. doi: 10.1016/j.eururo.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Shabsigh R, Broderick GA, Maja Miloslavsky M. Dapoxetine has long-term efficacy in the treatment of premature ejaculation [abstract 918]; American Urological Association 2006 Annual Meeting; May 20–25, 2006. [Google Scholar]
- 5.Giuliano F, Clement P. Serotonin and premature ejaculation: from physiology to patient management. Euro Urol. 2006;50:454–466. doi: 10.1016/j.eururo.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 6.Andersson KE. Mode of action of alpha 1 adrenoceptor antagonists in the treatment of lower urinary tract symptoms. BJU Int. 2000;85(suppl 2):12–18. doi: 10.1046/j.1464-410x.2000.00059.x. [DOI] [PubMed] [Google Scholar]
- 7.McMahon C, Abdo C, Incrocci M, et al. Disorders of orgasm and ejaculation in men. J Sex Med. 2004;1:58–65. doi: 10.1111/j.1743-6109.2004.10109.x. [DOI] [PubMed] [Google Scholar]
- 8.Hellstrom W. Current and future pharmacotherapies of premature ejaculation. J Sex Med. 2006;3(suppl 4):332–341. doi: 10.1111/j.1743-6109.2006.00309.x. [DOI] [PubMed] [Google Scholar]
- 9.Meisel RL, Sachs BD. The physiology of male sexual behavior. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 3rd ed. New York: Raven Press; 2005. pp. 3–105. [Google Scholar]
- 10.Hull EM, Du J, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J Neurosci. 1995;15:7465–7471. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bitran D, Hull EM. Pharmacological analysis of male rat sexual behavior. Neurosci Biobehav Rev. 1987;11:365–389. doi: 10.1016/s0149-7634(87)80008-8. [DOI] [PubMed] [Google Scholar]
- 12.Waldinger MD, Berendsen H, Blok BF, et al. Premature ejaculation and serotonergic antidepressantsinduced delayed ejaculation: the involvement of the serotonergic system. Behav Brain Res. 1995;92:111–118. doi: 10.1016/s0166-4328(97)00183-6. [DOI] [PubMed] [Google Scholar]
- 13.Pandey SC, Davis JM, Pandey GN. Phosphoinositide system-linked serotonin receptor subtypes and their pharmacologic properties and clinical correlates. J Psychiatry Neurosci. 1995;20:247–250. [PMC free article] [PubMed] [Google Scholar]
- 14.Frank JL, Hendricks SE, Olson GH. Multiple ejaculations and chronic fluoxetine: effects on male rat copulatory behavior. Pharmacol Biochem Behav. 2000;66:337–342. doi: 10.1016/s0091-3057(00)00191-x. [DOI] [PubMed] [Google Scholar]
- 15.Cantor JM, Binik YM, Pfaus JG. Chronic fluoxetine inhibits sexual behavior in the male rat: reversal with oxytocin. Psychopharmacology (Berl) 1999;144:355–362. doi: 10.1007/s002130051018. [DOI] [PubMed] [Google Scholar]
- 16.Olivier B, van Oorschot R, Waldinger MD. Serotonin, serotenergic receptors, selective serotonin reuptake inhibitors and sexual behaviour. Int Clin Psychopharmacol. 1998;13(suppl 6):S9–S14. doi: 10.1097/00004850-199807006-00003. [DOI] [PubMed] [Google Scholar]
- 17.Mos J, Mollet I, Tolbloom JT, et al. A comparison of the effects of different serotonin reuptake blockers on sexual behavior of the male rat. Eur Neuropsychopharmacol. 1999;9:123–135. doi: 10.1016/s0924-977x(98)00015-7. [DOI] [PubMed] [Google Scholar]
- 18.Waldinger MD, van De Plas A, Pattij T, et al. The selective serotonin re-uptake inhibitors fluvoxamine and paroxetine differ in sexual inhibitory effects after chronic treatment. Psychopharmacology (Berl) 2002;160:283–289. doi: 10.1007/s00213-001-0980-3. [DOI] [PubMed] [Google Scholar]
- 19.Smith W, Hellstrom WJ, Sikka SC. Effects of alpha blockers on effects of ejaculatory function in normal subjects [abstract] J Urol. 2005;173(suppl) [Google Scholar]
- 20.Haensel SM, Mos J, Olivier B, Slob AK. Sex behavior of male and female Wistar rats affected by the serotonin agonist 8-OH-DPAT. Pharmacol Biochem Behav. 1991;40:221–228. doi: 10.1016/0091-3057(91)90543-b. [DOI] [PubMed] [Google Scholar]
- 21.Schnur SL, Smith ER, Lee RL, et al. A component analysis of the effects of DPAT on male rat sexual behavior. Physiol Behav. 1989;45:897–901. doi: 10.1016/0031-9384(89)90212-6. [DOI] [PubMed] [Google Scholar]
- 22.Hillegaart V, Ahlenius S. Facilitation and inhibition of male rat ejaculatory behaviour by the respective 5-HT1A and 5-HT1B receptor agonists 8-OH-DPAT and anpirtoline, as evidenced by use of the corresponding new and selective receptor antagonists NAD-299 and NAS-181. Br J Pharmacol. 1998;125:1733–1743. doi: 10.1038/sj.bjp.0702239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clement P, Bernabe J, Kia HK. D2-like receptors mediate the expulsion phase of ejaculation elicited by 8-hydroxy-2-(di-N-propylamino) tetralin in rats. J Pharmacol Exp Ther. 2006;316:830–834. doi: 10.1124/jpet.105.092411. [DOI] [PubMed] [Google Scholar]
- 24.Andersson KE, Wyllie MG. Ejaculatory dysfunction: why all alpha-blockers are not equal. BJU Int. 2003;92:876–877. doi: 10.1111/j.1464-410x.2003.04590.x. [DOI] [PubMed] [Google Scholar]
- 25.Giuliano F, Bernabe J, Laurin M, et al. Tamsulosin impairs bulbospongiosus muscle contractions induced by central injection of 8-hydroxy-2-(di-n-propylamino) tetralin (8 OH-DPAT) in anesthetized rats while alfuzosin does not [abstract 1444]; 21st Congress of the European Association of Urology; April 5–8, 2006; Paris, France. [Google Scholar]
- 26.Rouquier L, Claustre Y, Benavides J. Alpha 1-adrenoceptor antagonists differentially control serotonin release in the hippocampus and striatum: a microdialysis study. Eur J Pharmacol. 1994;261:59–64. doi: 10.1016/0014-2999(94)90300-x. [DOI] [PubMed] [Google Scholar]
- 27.Nickel JC, Elhilalil M, Emberton M, Vallancien G. The beneficial effect of alfuzosin 10 mg once daily in ‘real-life’ practice on lower urinary tract symptoms (LUTS), quality of life and sexual dysfunction in men with LUTS and painful ejaculation. BJU Int. 2006;97:1242–1246. doi: 10.1111/j.1464-410X.2006.06171.x. [DOI] [PubMed] [Google Scholar]
- 28.Van Moorselaar RJA, Hartung R, Emberton M, et al. Alfuzosin 10 mg once daily improves sexual function in men with lower urinary tract symptoms and concomitant sexual dysfunction. BJU Int. 2005;95:603–608. doi: 10.1111/j.1464-410X.2005.05347.x. [DOI] [PubMed] [Google Scholar]
- 29.Hellstrom WJ, Sikka SC. Effects of acute treatment with tamsulosin versus alfuzosin on ejaculatory function in normal volunteers. J Urol. 2006;176(4 pt 1):1529–1533. doi: 10.1016/j.juro.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Hisasue S, Furuya R, Itoh N, et al. Ejaculatory disorder induced by alpha-adrenergic receptor blockade is not retrograde ejaculation [abstract] J Urol. 2005;173(suppl) [Google Scholar]
- 31.Walsh PC. Benign prostatic hyperplasia. In: Walsh PC, Retik AB, Stamey TA, Vaughan ED, editors. Campbell’s Urology. 6th ed. Philadelphia: WB Saunders Company; 1992. pp. 1007–1022. [Google Scholar]
- 32.Mann T, Lutwak-Mann C. Male Reproductive Function and Semen. Berlin: Springer-Verlag; 1981. Secretory function of the prostate, seminal vesicle, Cowper’s gland and other accessory organs of reproduction; pp. 171–193. [Google Scholar]