Abstract
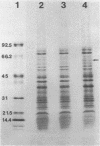
We describe an unusual type of erythromycin resistance (Emr) mediated by a plasmid designated pNE24 from Staphylococcus epidermidis. This 26.5-kilobase plasmid encodes resistance strictly to 14-membered macrolide antibiotics, erythromycin, and oleandomycin. Resistance to other macrolide-lincosamide-streptogramin B (MLS) antibiotics was not observed even after a prior induction stimulus with various MLS antibiotics. Plasmid pNE24 was found to express resistance constitutively and manifested a low to intermediate MIC (62.5 micrograms/ml) for erythromycin. The resistance gene, designated erpA, appears to mediate resistance by altering the permeability of the host cell for erythromycin, because the measured uptake of 14C-labeled erythromycin by strain 958-2 (containing pNE24) was lower than for the erythromycin-susceptible, isogenic strain 958-1. No inactivation of erythromycin in overnight broth culture supernatants could be detected. In addition, no significant loss in binding affinity between [14C]erythromycin and ribosome could be detected for ribosomes isolated from strain 958-2 relative to 958-1, indicating that pNE24 probably does not produce a modification of the bacterial ribosome. No other selectable marker was found associated with pNE24; however, a 60,000-dalton protein was present only in the membrane fractions of cells (958-2) containing pNE24 and may play a role in mediating resistance to erythromycin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad M. H., Rechenmacher A., Böck A. Interaction between aminoglycoside uptake and ribosomal resistance mutations. Antimicrob Agents Chemother. 1980 Nov;18(5):798–806. doi: 10.1128/aac.18.5.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthélémy P., Autissier D., Gerbaud G., Courvalin P. Enzymic hydrolysis of erythromycin by a strain of Escherichia coli. A new mechanism of resistance. J Antibiot (Tokyo) 1984 Dec;37(12):1692–1696. doi: 10.7164/antibiotics.37.1692. [DOI] [PubMed] [Google Scholar]
- Chopra I. Mechanisms of resistance to fusidic acid in Staphylococcus aureus. J Gen Microbiol. 1976 Oct;96(2):229–238. doi: 10.1099/00221287-96-2-229. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Plasmids, drug resistance, and gene transfer in the genus Streptococcus. Microbiol Rev. 1981 Sep;45(3):409–436. doi: 10.1128/mr.45.3.409-436.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. C., Chopra I., Shales S. W., Howe T. G., Foster T. J. Analysis of tetracycline resistance encoded by transposon Tn10: deletion mapping of tetracycline-sensitive point mutations and identification of two structural genes. J Bacteriol. 1983 Feb;153(2):921–929. doi: 10.1128/jb.153.2.921-929.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Ounissi H., Arthur M. Multiplicity of macrolide-lincosamide-streptogramin antibiotic resistance determinants. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):91–100. doi: 10.1093/jac/16.suppl_a.91. [DOI] [PubMed] [Google Scholar]
- Dorman C. J., Foster T. J. Posttranscriptional regulation of the inducible nonenzymatic chloramphenicol resistance determinant of IncP plasmid R26. J Bacteriol. 1985 Jan;161(1):147–152. doi: 10.1128/jb.161.1.147-152.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney D. F., Cundliffe E., Foster T. J. Chloramphenicol resistance that does not involve chloramphenicol acetyltransferase encoded by plasmids from gram-negative bacteria. J Gen Microbiol. 1981 Jul;125(1):113–121. doi: 10.1099/00221287-125-1-113. [DOI] [PubMed] [Google Scholar]
- Gots J. S. THE DETECTION OF PENICILLINASE-PRODUCING PROPERTIES OF MICROORGANISMS. Science. 1945 Sep 21;102(2647):309–309. doi: 10.1126/science.102.2647.309. [DOI] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder S. L., Streitfeld M. M. Inducible and constitutive resistance to macrolide antibiotics and lincomycin in clinically isolated strains of Streptococcus pyogenes. Antimicrob Agents Chemother. 1973 Sep;4(3):327–331. doi: 10.1128/aac.4.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Weisblum B. Altered methylation of ribosomal RNA in an erythromycin-resistant strain of Staphylococcus aureus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):856–860. doi: 10.1073/pnas.68.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson B. C., Parisi J. T. Naturally occurring Staphylococcus epidermidis plasmid expressing constitutive macrolide-lincosamide-streptogramin B resistance contains a deleted attenuator. J Bacteriol. 1986 May;166(2):479–483. doi: 10.1128/jb.166.2.479-483.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goffic F., Capmau M. L., Abbe J., Cerceau C., Dublanchet A., Duval J. Plasmid mediated pristinamycin resistance: PH 1A, a pristinamycin 1A hydrolase. Ann Microbiol (Paris) 1977 Nov-Dec;128B(4):471–474. [PubMed] [Google Scholar]
- Leclercq R., Carlier C., Duval J., Courvalin P. Plasmid-mediated resistance to lincomycin by inactivation in Staphylococcus haemolyticus. Antimicrob Agents Chemother. 1985 Sep;28(3):421–424. doi: 10.1128/aac.28.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J. C. Protein synthesis in a cell-free extract from Staphylococcus aureus. J Bacteriol. 1967 Jul;94(1):80–86. doi: 10.1128/jb.94.1.80-86.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J. C., Putterman M. Accumulation in gram-postive and gram-negative bacteria as a mechanism of resistance to erythromycin. J Bacteriol. 1968 Mar;95(3):1111–1117. doi: 10.1128/jb.95.3.1111-1117.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
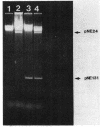
- Mao J. C., Putterman M. The intermolecular complex of erythromycin and ribosome. J Mol Biol. 1969 Sep 14;44(2):347–361. doi: 10.1016/0022-2836(69)90180-6. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Mitsuhashi S. New type of R factors incapable of inactivating chloramphenicol. J Bacteriol. 1972 Jan;109(1):1–7. doi: 10.1128/jb.109.1.1-7.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y., Abe H., Endou K., Matsuoka M. Resistance to macrolide antibiotics in Staphylococcus aureus susceptible to lincomycin and mikamycin B. J Antibiot (Tokyo) 1984 Jun;37(6):675–679. doi: 10.7164/antibiotics.37.675. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson W. C., Jr, Parisi J. T., Totten P. A., Baldwin J. N. Transduction of penicillinase production in Staphylococcus epidermidis and nature of the genetic determinant. Can J Microbiol. 1979 Apr;25(4):508–511. doi: 10.1139/m79-074. [DOI] [PubMed] [Google Scholar]
- Ounissi H., Courvalin P. Nucleotide sequence of the gene ereA encoding the erythromycin esterase in Escherichia coli. Gene. 1985;35(3):271–278. doi: 10.1016/0378-1119(85)90005-8. [DOI] [PubMed] [Google Scholar]
- Parisi J. T., Robbins J., Lampson B. C., Hecht D. W. Characterization of a macrolide, lincosamide, and streptogramin resistance plasmid in Staphylococcus epidermidis. J Bacteriol. 1981 Nov;148(2):559–564. doi: 10.1128/jb.148.2.559-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi J. T., Talbot H. W., Skahan J. M. Development of a phage typing set for Staphylococcus epidermidis in the United States. Zentralbl Bakteriol Orig A. 1978 Jul;241(1):60–67. [PubMed] [Google Scholar]
- Schneider D. R., Parker C. D. Effect of pyridines on phenotypic properties of Bordetella pertussis. Infect Immun. 1982 Nov;38(2):548–553. doi: 10.1128/iai.38.2.548-553.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumar A. G., Dubnau D. Characterization of a plasmid-specified ribosome methylase associated with macrolide resistance. Nucleic Acids Res. 1981 Jun 11;9(11):2549–2562. doi: 10.1093/nar/9.11.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmund C. D., Morgan E. A. Erythromycin resistance due to a mutation in a ribosomal RNA operon of Escherichia coli. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5602–5606. doi: 10.1073/pnas.79.18.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner R., Cundliffe E., Schmidt F. J. Site of action of a ribosomal RNA methylase responsible for resistance to erythromycin and other antibiotics. J Biol Chem. 1983 Oct 25;258(20):12702–12706. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stark M., Cundliffe E. On the biological role of ribosomal protein BM-L11 of Bacillus megaterium, homologous with Escherichia coli ribosomal protein L11. J Mol Biol. 1979 Nov 15;134(4):767–769. doi: 10.1016/0022-2836(79)90485-6. [DOI] [PubMed] [Google Scholar]
- TAUBMAN S. B., YOUNG F. E., CORCORAN J. W. ANTIBIOTIC GLYCOSIDES. IV. STUDIES ON THE MECHANISM OF ERYTHROMYCIN RESISTANCE IN BACILLUS SUBTILIS. Proc Natl Acad Sci U S A. 1963 Nov;50:955–962. doi: 10.1073/pnas.50.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Yamashita N., Konno M. Occurrence of a beta-lactam-inducible penicillin-binding protein in methicillin-resistant staphylococci. Antimicrob Agents Chemother. 1985 May;27(5):851–857. doi: 10.1128/aac.27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Graham M. Y., Gryczan T., Dubnau D. Plasmid copy number control: isolation and characterization of high-copy-number mutants of plasmid pE194. J Bacteriol. 1979 Jan;137(1):635–643. doi: 10.1128/jb.137.1.635-643.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Siddhikol C., Lai C. J., Demohn V. Erythromycin-inducible resistance in Staphylococcus aureus: requirements for induction. J Bacteriol. 1971 Jun;106(3):835–847. doi: 10.1128/jb.106.3.835-847.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi S., Nakajima Y., Inoue M., Oka Y. Decrease in accumulation of macrolide antibiotics as a mechanism of resistance in Staphylococcus aureus. Jpn J Microbiol. 1971 Jan;15(1):39–52. doi: 10.1111/j.1348-0421.1971.tb00549.x. [DOI] [PubMed] [Google Scholar]